Abstract
A decrease in intracellular pH (pHi) inhibits whole-cell Kir4.1 currents. To understand channel biophysical properties underlying this inhibition, single channel Kir4.1 currents were studied in inside-out patches using symmetric concentrations of K+ applied to each side of the plasma membrane. Under such conditions, inward rectifying currents were observed in about 2 of 3 patches. At pH 7.4, these currents showed a single channel conductance of 22 pS with a channel open-state probability (Popen) of ≈0.9.
The effects of intracellular protons on macroscopic Kir4.1 currents were examined in giant inside-out patches at various pH levels of internal solutions. Current amplitude increased with a modest acidification (pH 7.0 and 6.6), and decreased with further reductions in pHi. The Kir4.1 currents were completely suppressed at pH 5.4. These effects were fast and reversible.
Low pHi inhibited Popen and enhanced single channel conductance in a concentration-dependent manner with pK (midpoint pH value for channel inhibition) of 6.0 and 6.8, respectively. At pH 5.8, Popen was inhibited by 70 % and single channel conductance increased by 35 %. Washout brought both Popen and single channel conductance rapidly back to baseline levels.
Theoretical currents were calculated using percentage changes in Popen and single channel conductance at each pH level tested. The trajectory of these currents is very close to that of experimental currents recorded from giant patches. Thus, opposite effects of intracellular protons on Popen and single channel conductance are demonstrated, which are likely to result in changes of macroscopic Kir4.1 currents with low pH.
The inward rectifier K+ (Kir) channels are responsible for the maintenance of membrane potential and cellular excitability (Nichols & Lopatin, 1997). These channels are modulated by several membrane and cytosolic factors including G proteins, membrane potentials, second messengers and hydrogen ions (Coulter et al. 1995; Tsai et al. 1995; Cohen et al. 1996a, b; Fakler et al. 1996; Henry et al. 1996; Chuang et al. 1997; Huang et al. 1998; Zhu et al. 1999). The modulation of Kir channels by protons is significant, because changes in intra- and extracellular pH occur in a number of pathophysiological conditions during which a corresponding alteration in membrane excitability can be produced via these Kir channels. Indeed, there is experimental evidence indicating certain Kir channels are inhibited during hypercapnia and other types of acidosis (Wang et al. 1990; Ito et al. 1992; Schlatter et al. 1994; Zhou & Wingo 1994; Pineda & Aghajanian, 1997). Inhibition of cloned Kir currents by pH has recently been demonstrated in several members of Kir1, Kir2 and Kir4 families (Coulter et al. 1995; Tsai et al. 1995; Fakler et al. 1996; Shuck et al. 1997; Qu et al. 1999; Pearson et al. 1999). Kir1.1 (ROMK1) and Kir1.2 (ROMK2) channels are inhibited by a decrease in intracellular pH (Tsai et al. 1995; Fakler et al. 1996; Choe et al. 1997; McNicholas et al. 1998). This inhibition is mediated by a suppression of channel open-state probability (Popen) without affecting the single channel conductance (Choe et al. 1997; McNicholas et al. 1998). A member of the Kir2 subfamily, i.e. Kir2.3 or HIR, is pH sensitive. An inhibition of single channel conductance by extracellular pH and suppression of both Popen and conductance by intracellular pH account for the inhibition of whole-cell Kir2.3 currents (Coulter et al. 1995; Zhu et al. 1999). Low pH has also been shown to inhibit Kir4.1 channels, which have a high sequence similarity to ROMK channels including a lysine residue involved in proton sensing (Fakler et al. 1996; Shuck et al. 1997; Pearson et al. 1999). In contrast to ROMK and Kir2.3 channels, the biophysical mechanisms underlying Kir4.1 inhibition are not known (Shuck et al. 1997; Pearson et al. 1999). Moreover, there is a lack of information regarding whether the pH-dependent inhibition of Kir4.1 is an inherent property of the channel protein or a result of the involvement of other intermediate molecules. We therefore performed these experiments to study the effects of intracellular protons on single-channel Kir4.1 activity using cell-free excised patches.
METHODS
Oocytes from frogs (Xenopus laevis) were used in the present studies. All experimental procedures were subject to the Animal Welfare Assurance of Georgia State University (A97008). Frogs were anaesthetized by bathing in 0.3 % 3-aminobenzoic acid ethyl ester. A few lobes of ovaries were removed after a small abdominal incision (∼5 mm). The surgical incision was closed and the frogs were allowed to recover from the anaesthesia. Oocytes were removed not more than three times from any animal, with adequate time for healing between each procedure. Following the last collection the frogs were killed by overexposure to the anaesthetic. Xenopus oocytes were treated with 2 mg ml−1 of collagenase (Type I, Sigma Chemicals, St Louis, MO, USA) in OR2 solution containing (mM): NaCl, 82; KCl, 2; MgCl2, 1 and Hepes, 5; pH 7.4) for 90 min at room temperature. Then, cDNAs (25–50 ng in 50 nl double distilled water) were injected into the oocytes. The oocytes were then incubated at 18°C in ND-96 solution containing (mM): NaCl, 96; KCl, 2; MgCl2, 1; CaCl2, 1.8; Hepes, 5 and sodium pyruvate, 2.5 with geneticin added (100 mg l−1) (pH 7.4).
Brain Kir4.1 (BIRK10) cDNA was generously provided by J. Adelman (Vollum Institute, Portland, OR, USA; Bond et al. 1994). A vector for eukaryotic expression (pcDNA3.1, Invitrogen Inc., Carlsbad, CA, USA) was used to express Kir4.1 in the Xenopus oocytes. The Kir4.1 cDNA was removed from the pBF vector at EcoRI restriction sites, and subsequently subcloned into corresponding EcoRI site in the pCDNA3.1. The orientation and the flanking sequences were confirmed with DNA sequencing.
Patch clamp experiments were performed at room temperature (24–27°C) as described previously (Jiang et al. 1994; Zhu et al. 1999). In brief, fire-polished patch pipettes (2–4 MΩ) were made from 1.2 mm borosilicate capillary glass (Sutter P-97/PC puller). Single channel currents were recorded from conventional inside-out patches. Giant inside-out patches were also employed to study macroscopic currents in cell-free conditions using recording pipettes of 0.5–1.0 MΩ (Zhu et al. 1999). Current records were low-pass filtered (2 000 Hz, Bessel, 4-pole filter, −3 dB), digitized (10 kHz, 12-bit resolution), and stored on computer disk for later analysis (pCLAMP6, Axon Instruments). Junction potentials between bath and pipette solutions were appropriately nulled before seal formation.
Vitelline membranes of oocytes were mechanically removed after being exposed to hypertonic solution (400 mosmol l−1) for 5 min. Recordings were performed on the stripped oocytes using a bath solution containing (mM): KCl, 40; potassium gluconate, 75; potassium fluoride, 5; sodium vanadate, 0.1; potassium pyrophosphate, 10. EGTA, 1; adenosine diphosphate (ADP), 0.2; Pipes, 10; glucose, 10; and spermine, 0.1; (FVPP solution, pH 7.4). The pipette was filled with the same FVPP solution (pH 7.4). Data analyses were made using Clampfit and Fetchan and pStat software (Axon Instruments Inc.). Data were further filtered (0–1000 Hz) with a Gaussian filter. This filtering causes events shorter than 100 μs to be ignored. No correction was attempted for the missed events. Single channel conductance was measured as a slope conductance with at least two voltage points. Popen was calculated from 1 to 3 stretches of data with a total duration of 20–60 s as described previously (Jiang et al. 1994, Zhu et al. 1999). Mean open and closed times were measured from records with only one active channel.
A parallel perfusion system was used to administer agents to patches at a rate of ∼1 ml min−1 with no dead space (Zhu et al. 1999). Low pH was produced by exposing patches to the same internal FVPP solution that had been titrated to various pH levels using gluconic acid, N-methyl-D-glucamine or KOH. Osmotic pressure was adjusted to ∼300 mosmol l−1 after pH titration. No significant difference in single channel conductance was seen between N-methyl-D-glucamine and KOH titrations. Pipes buffer was used, because of its appropriate buffering range and membrane impermeability.
Data are presented as means ±s.e.m., (n being the number of patches). Differences in means were tested with Student's t test and were accepted as significant if P≤ 0.05.
RESULTS
Expressions of Kir currents were first identified in the two-electrode voltage-clamp mode in which inward rectifying currents as large as 20 μA were frequently seen. These currents were clearly different from oocyte endogenous currents that averaged 1.6 ± 8.2 % (mean ±s.e.m., n = 5) with an injection of the expression vector in 50 nl H2O. Inside-out patches were subsequently obtained from these oocytes. These patches were exposed to symmetric concentrations of K+ (150 mM) on each side of the plasma membranes. Command potentials from −140 mV to +100 mV were applied to these patches. When inward rectifying currents were seen, the slope conductance was measured (Fig. 1). These inward rectifying Kir4.1 currents had a single channel conductance of 21.5 ± 0.5 pS (n = 16) and were observed in most (∼2 of 3) patches. Other single channel properties were studied in patches with only one active channel. These channels showed a high baseline channel open-state probability (Popen 0.896 ± 0.005, n = 4; pH 7.4, membrane potential −120 mV) with long periods of opening and short periods of closures (Fig. 2). The mean open time of these Kir4.1 currents was 70.8 ± 1.5 ms (n = 4), and the mean closed time was 12.8 ± 1.6 ms (n = 4). Bursting activity was not observed.
Figure 1. Single channel conductance of Kir4.1 currents.
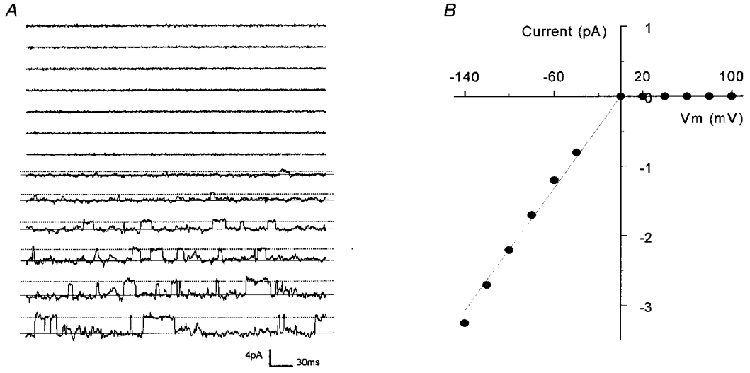
A, single channel current was recorded from an inside-out patch using symmetric concentrations of K+ (150 mM) on each side of the patch at various membrane potentials (Vm). While there is no channel activity at depolarizing potentials, an active channel is seen at hyperpolarizing Vm. Continuous line, open state; dotted line, closed state. B, single channel conductance calculated from the channel in A is linear at negative Vm. The straight line represents a slope conductance of 22 pS.
Figure 2. Single channel activity of Kir4.1 currents.
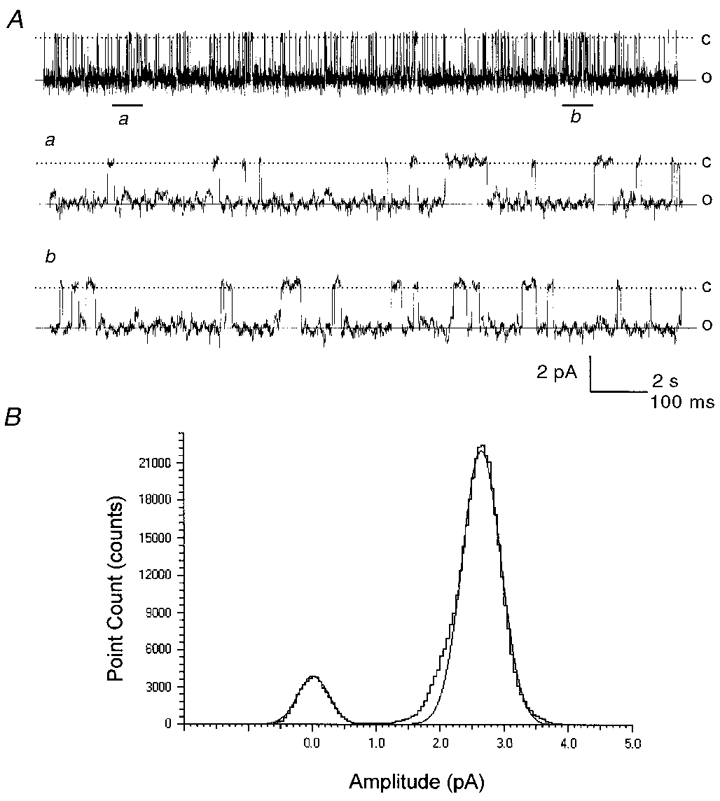
A, single channel activity was recorded from another inside-out patch under the same conditions as in Fig. 1. Although no channel opening was detected at a membrane potential (Vm) level of 100 mV (not shown), this channel showed a high channel activity at Vm of −120 mV with Popen at 0.887 (top). In long-lasting openings, brief closures can be seen which are better illustrated on extended time scales (a, b). Traces a and b are obtained from positions a and b indicated by lines below the top trace, respectively. Calibration: 2 s for the top trace and 100 ms for traces a and b; 2 pA for all. Labels on the right side: c, closed state; o, open state. B, all points histogram of Kir4.1 currents shows channel openings at 2.5 pA at a membrane potential of −120 mV. Data are obtained from A as a stretch of recording of 20 s which was fitted by Gaussian distributions with peaks at 0.00 and 2.49 pA.
The effect of intracellular pH (pHi) on macroscopic Kir currents was studied using giant patches. At baseline (pH 7.4), inward rectifying currents as large as 200 pA were seen in these patches at a membrane potential of −100 mV. Exposures of the internal surface of the patch membranes to solutions with various pH levels produced a biphasic response in these currents (Fig. 3A). The amplitude of Kir4.1 currents increased with modest drops in pHi. Kir4.1 currents increased by 5 ± 6 % (n = 6) at pH 7.0, and 15 ± 5 % (n = 6) at pH 6.6. Further acidification to pH 6.2 or 5.8 led to a rapid reduction in these Kir4.1 currents. At pH 5.4, the inward rectifying Kir4.1 currents were completely suppressed. The maximal effect of the inhibition was reached within 30 s. A complete recovery of the Kir currents was seen if the exposure was not longer than 2 min, while long-lasting exposure led to only a partial recovery. The relationship of current amplitude to various pHi levels can be described using the Hill equation with pK at 5.95 and a Hill coefficient (nH) of 2.7 (Fig. 3B). The effect of protons on Kir4.1 currents appeared to be voltage independent, because the slope of affected currents (obtained by subtraction of the remaining currents at pH 5.8 from those at pH 7.4 and scaled to the same amplitude of the baseline currents) was almost identical to that of baseline currents (not shown).
Figure 3. Effects of pHi on macroscopic Kir4.1 currents.
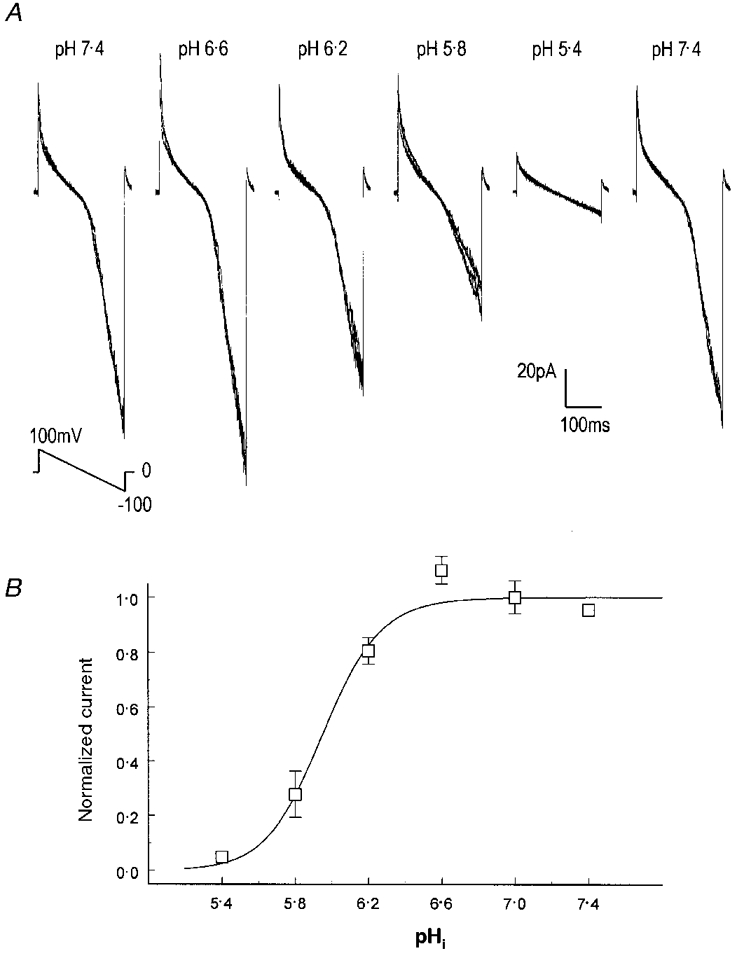
A, Kir4.1 currents were recorded from a giant patch with equal concentrations of K+ (150 mM) on each side of the membrane. Inward rectifying currents were seen at pH 7.4 using a slope command potential from +100 mV to −100 mV. When the pH in the internal solution was reduced to 6.6, the amplitude of these currents increased by 16 %. Further decreases in pHi caused strong inhibitions of these currents. Inward rectifying currents were totally suppressed at pH 5.4. Washout led to a recovery of these currents to the baseline level. Note that 4 superimposed traces are shown in each panel. B, the relationship of Kir4.1 currents with pHi. Kir4.1 currents increase with a modest intracellular acidification (pH 6.6, 7.0), and are strongly inhibited by further acidification. At pH 5.4–6.6, the relationship of Kir4.1 currents (I) to pHi can be expressed with the Hill equation (continuous line): I = 1/{1 + (pK/pHi)nH}, where pK is the midpoint pH value for channel inhibition, and nH the Hill coefficient. The pK and nH here are pH 5.95 and 2.7, respectively.
In conventional inside-out patches, Popen decreased with pHi levels in a concentration-dependent manner (Fig. 4). This inhibition was very different from that for the macroscopic currents observed in giant patches. There was no evident increase in Popen at pH 7.0 and 6.6. The relationship of Popen to pHi can be described with the same Hill equation as for the macroscopic currents (pK 5.95, nH 2.7) (Fig. 5). The inhibition of Popen was due to a decrease in channel mean open time and an increase in mean closed time, (26.1 ± 15.1 ms, n = 4; 34.4 ± 21.9 ms, n = 4, respectively, pHi 5.8).
Figure 4. Effects of pH on single channel activity.
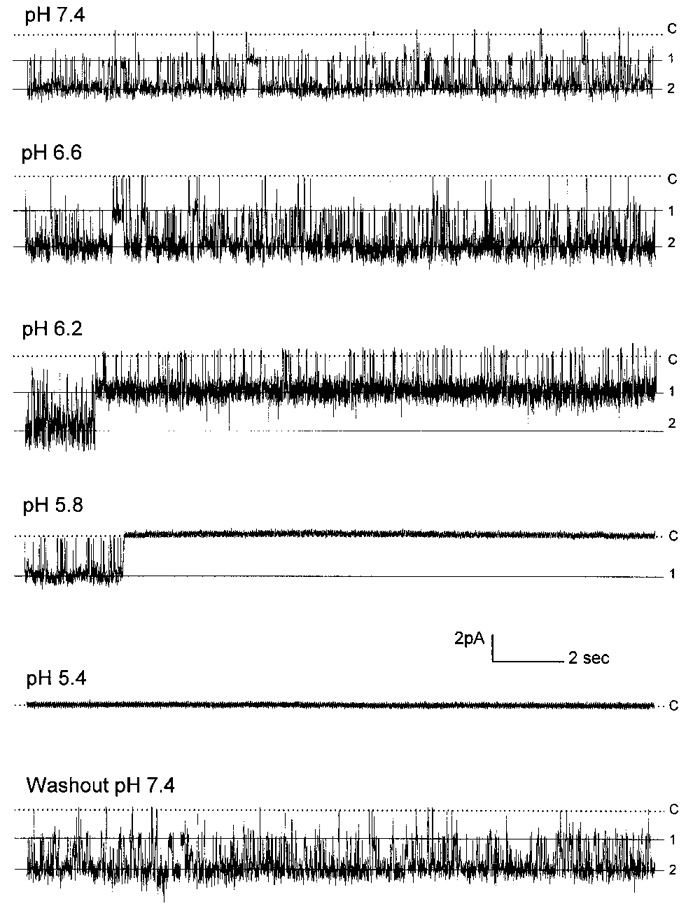
Concentration-dependent inhibition of single channel activity by acidic pH. Single channel currents were recorded from an inside-out patch under the same conditions as Fig. 1. At a Vm of −120 mV, two active channels were seen at pHi 7.4, both of which had a slope conductance of 22 pS (top, Popen = 0.873). These currents were completely inhibited at pHi 5.4 (middle, Popen = 0.000). Channel activity resumed after washout (bottom, Popen = 0.863). Labels on the right: c, closure; 1, the first opening; 2, the second opening.
Figure 5. pH-dependent changes in macroscopic currents, Popen and single channel conductance.
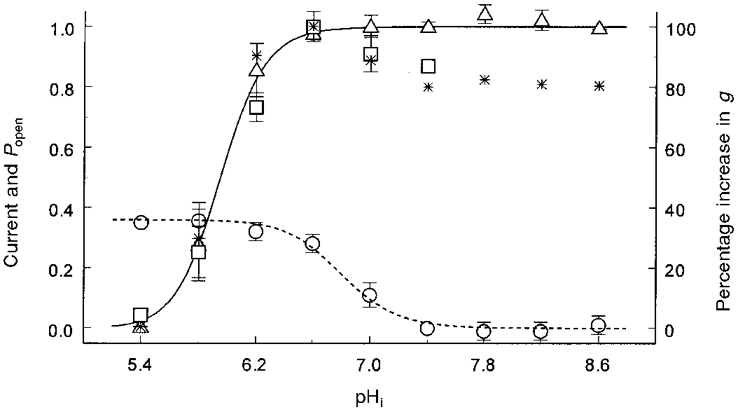
Whereas Popen (▵, n = 4) decreases with low pHi, the Kir4.1 currents (□, n = 5) increase with a modest intracellular acidification (pH 6.6, 7.0), and are strongly inhibited by further acidification. At pH 5.4–6.6, current response to low pH is almost the same as the Popen, both of which can be expressed with the same Hill equation (continuous line): Popen = 1/{1 + (pK/pHi)nH}, where pK is the midpoint pH value for channel inhibition, and nH the Hill coefficient. The pK and nH here are pH 5.95 and 2.7, respectively. In contrast to Popen, single channel conductance (g,^, n = 5) increases with low pHi. Its change can be also expressed using a Hill equation (dashed line) with pK 6.8 and nH 2.3. Based on the percentage changes in conductance and Popen, theoretical currents (⋆) are calculated at each pH point using Ohm's law and an equation: I = i PopenN, where I is the macroscopic current, i single channel currents, and N the number of channels. Data are presented as means ±s.e.m.
Single channel conductance was also studied during low pHi. Surprisingly, we found that a decrease in pHi augmented single channel conductance instead of suppressing it (Fig. 6). At the maximum effect of pHi, Kir4.1 conductance was enhanced by 35 % at pH 5.8 in comparison with its baseline value at pH 7.4 (Figs 5 and 6). The relationship of conductance to pH can also be expressed with the Hill equation with pK of 6.8 and nH of 2.3 (Fig. 5).
Figure 6. Augmentation of single channel conductance with intracellular acidification.
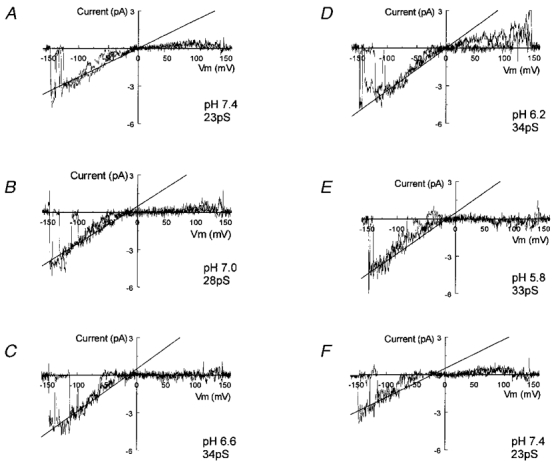
Single channel current was recorded from an inside-out patch with symmetric K+ concentrations (150 mM) applied to each side of the patch membrane. Ramp command potentials from +150 mV to −150 mV were given to the patch every 2 s. The internal membrane was exposed to solutions at various pH levels. A, under such conditions, a single active channel was isolated at pH 7.4. This channel had a slope conductance of 23 pS as indicated by the straight line. B, at pH 7.0, the conductance of this channel increased to 28 pS. C, the conductance became 34 pS at pH 6.6. D and E, the unitary conductance was stabilized at 33–34 pS when the internal solutions were acidified to pH 6.2 and 5.8. F, washout (pH 7.4) led to a complete recovery of the single channel conductance. Note that two superimposed traces are shown in each panel.
Using the equation I = iPopenN (where I is macroscopic current, i single channel current, and N is the number of channels) and Ohm's law, theoretical I was calculated at each pH point according to percentage changes in Popen and single channel conductance. The calculated I is fairly consistent to the experimental I recorded from oocytes (Fig. 5), suggesting that the biphasic changes in the macroscopic Kir4.1 currents are mediated by the pH effects on Popen and single channel conductance.
DISCUSSION
We have shown in these studies that brain Kir4.1 is inhibited by strong acidification and enhanced by moderate acidification. The inhibition is mediated by a suppression of Popen, whereas the enhancement is due to an augmentation of single channel conductance.
In cell-attached patches with 110 mM K+ in the external solutions, Kir4.1 has a single channel conductance of 12 pS (Pessia et al. 1996). This level is much lower than we have observed (22 pS) in inside-out patches with 150 mM K+ on each side of the patch membrane. It is likely that the different K+ concentrations used in these two separate studies caused this effect. In addition to conductance, we have examined several other single-channel properties of Kir4.1 such as voltage dependence, Popen, substate conductance and bursting activity. Kir4.1 has a high baseline activity with a long mean open time and a short mean closed time.
Several members of the Kir4 family have been demonstrated to be pH sensitive. Shuck et al. (1997) have shown that intracellular acidification inhibits a Kir channel cloned from the kidney (named Kir1.2) with a 97 % identity to the brain Kir4.1. The pK value for this Kir channel is pH 6.2 measured with extracellular acetate buffer (Shuck et al. 1997), which is higher than that we have seen in brain Kir4.1 (pK 5.95). What causes this difference is not clear. It may be a result of different Kir4 species or the potential controlling error of the pHi using extracellular acetic acid. Kir4.2 cloned from liver is also pH sensitive (Pearson et al. 1999). It has a 64 % homology in its amino acid sequence to brain Kir4.1, and is inhibited by 50 mM extracellular bicarbonate, although its pK value is unknown (Pearson et al. 1999). In the present studies, we have employed excised patches to have an easier access to the cytosolic side of membranes and a better control of intracellular pH than whole-cell recordings. We have found that intracellular acidification produces a biphasic response in Kir4.1 currents.
In cell-free excised patches when cytosolic soluble factors are generally washed out, we have seen the pH sensitivity of Kir4.1. This observation suggests that the inhibition of the Kir channels by low pHi is unlikely to be caused by changes in concentrations of cytosolic soluble factors such as second messengers and kinases. Channel phosphorylation does not seem to be a reason for inhibition of Kir4.1 currents by intracellular protons. Several blockers of phosphatase and phosphodiesterase such as vanadate, fluoride and pyrophosphate were used in the intracellular solution. Also, there was no Mg2+ or ATP in this intracellular solution. Under such conditions, the turnover of protein phosphorylation and dephosphorylation should not occur during our experimental period with low pH. Therefore, we believe that amino acid sequences and their tertiary structures in Kir4.1 channel proteins are the molecular basis for the Kir4.1 inhibition during intracellular acidification, similar to the effect of protons on Kir1.1, Kir1.2 and Kir2.3 channels (Coulter et al. 1995; Tsai et al. 1995; Fakler et al. 1996; Choe et al. 1997; McNicholas et al. 1998; Qu et al. 1999).
A novel finding in our current experiments is the opposite effect of protons on Popen and single channel conductance of Kir4.1. Before a complete inhibition of Kir4.1 currents takes place with severe acidification, we have found that Kir4.1 currents are enhanced with modest acidification. The biophysical mechanisms for this biphasic response, that has not been observed in other Kir channels, has been studied in our current experiments at the single channel level. We have shown that Popen is inhibited by intracellular protons, while low pHi enhances the single channel conductance of Kir4.1. Using a simple mathematical equation and Ohm's law, we have calculated the theoretical currents based on percentage changes in Popen and conductance. We have found that the calculated currents are fairly consistent with the experimental currents recorded from the oocytes. Thus, the biophysical basis for the biphasic change in macroscopic currents is explained with our single channel data.
It is worth noting that Kir4.1 has a 53 % identity and 71 % similarity in its amino acid sequence to the ROMK1 channel. A lysine residue that has been demonstrated to be critical in pH sensing in ROMK1 (i.e. Lys80) can be found in Kir4.1 (Lys67). Both these two channels show only a partial recovery from the pH inhibition when the exposure period is long (Schulte et al. 1998). Although these two channels have many such similarities, their pH sensitivity is very different. In contrast to ROMK (pK∼6.8), Kir4.1 shows a pK value of only 6–6.2. These figures strongly suggest that pH sensing is an inherent property of these Kir channels and depends on their amino acid sequences and the tertiary structures of the channel proteins.
Although functions of the paradoxical responses of Popen and conductance to low pH remain to be understood, major changes in K+ currents occur at pH 5.8 to 7.0 through Kir4.1 channels. This, plus the fact that Kir4.1 is predominantly expressed in brainstem (Bredt et al. 1995), seems to allow these channels to be involved in hypercapnia in cells expressing Kir4.1. If these cells indeed have synaptic connections with cardio-respiratory neuronal networks, a change in PCO2 levels in the cerebral spinal fluid or the cerebral circulation may be coupled through these channels to a corresponding change in membrane excitability of cardio-respiratory neurons. Thus, the understanding of Kir4.1 modulation by pH may contribute to the understanding of their potential functions in CO2 and pH sensing in brainstem neurons.
Acknowledgments
This work was supported by the NIH (RO1 HL58410–01) and the Grant-in-Aid Award (9950528 N) from the American Heart Association. The authors would like to thank Dr John Adelman for his generosity in sharing with us the Kir4.1 cDNA. We also thank Dr Zhiqiang Qu and Mr Haoxing Xu for their valuable comments on this work and critical readings of the manuscript.
References
- Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors and Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- Bredt DS, Wang TL, Cohen NA, Guggino WB, Snyder SH. Cloning and expression of two brain-specific inwardly rectifying potassium channels. Proceeding of the National Academy of Sciences of the USA. 1995;92:6753–6757. doi: 10.1073/pnas.92.15.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Zhou H, Palmer LG, Sackin H. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance & gating. American Journal of Physiology. 1997;273:F516–529. doi: 10.1152/ajprenal.1997.273.4.F516. [DOI] [PubMed] [Google Scholar]
- Chuang H, Jan YN, Jan LY. Regulation of IRK3 inward rectifier K+ channel by M1 acetylcholine receptor and intracellular magnesium. Cell. 1997;89:1121–1132. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996a;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Sha Q, Makhina EN, Lopatin AN, Linder ME, Snyder SH, Nichols CG. Inhibition of an inward rectifier potassium channel (Kir2.3) by G-protein βγ subunits. Journal of Biological Chemistry. 1996b;271:32301–32305. doi: 10.1074/jbc.271.50.32301. [DOI] [PubMed] [Google Scholar]
- Coulter KL, Perier F, Radeke CM, Vandenberg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron. 1995;15:1157–1168. doi: 10.1016/0896-6273(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO Journal. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes. The Journal of Physiology. 1996;495:681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Ito H, Vereecke J, Carmeliet E. Intracellular protons inhibit inward rectifier K+ channel of guinea-pig ventricular cell membrane. Pflügers Archiv. 1992;422:280–286. doi: 10.1007/BF00376214. [DOI] [PubMed] [Google Scholar]
- Jiang C, Sigworth FJ, Haddad GG. Oxygen deprivation activates an ATP-inhibitable K+ channel in substantia nigra neurons. Journal of Neuroscience. 1994;14:5590–5602. doi: 10.1523/JNEUROSCI.14-09-05590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. American Journal of Physiology. 1998;275:F972–981. doi: 10.1152/ajprenal.1998.275.6.F972. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annual Review of Physiology. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. The Journal of Physiology. 1999;514:639–653. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO Journal. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Qu Z, Zhu G, Liu CX, Chanchevalap S, Xu H, Jiang C. Identification of a critical motif responsible for gating of Kir2.3 channel by intracellular protons. Journal of Biological Chemistry. 1999;274:13783–13789. doi: 10.1074/jbc.274.20.13783. [DOI] [PubMed] [Google Scholar]
- Schlatter E, Haxelmans S, Hirsch J, Leipziger J. pH dependence of K+ conductances of rat cortical collecting duct principal cells. Pflügers Archiv. 1994;428:631–640. doi: 10.1007/BF00374587. [DOI] [PubMed] [Google Scholar]
- Schulte U, Hahn H, Wiesinger H, Ruppersberg JP, Fakler B. pH-dependent gating of ROMK (Kir1.1) channels involves conformational changes in both N and C termini. Journal of Biological Chemistry. 1998;273:34575–34579. doi: 10.1074/jbc.273.51.34575. [DOI] [PubMed] [Google Scholar]
- Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3) Journal of Biological Chemistry. 1997;272:586–593. doi: 10.1074/jbc.272.1.586. [DOI] [PubMed] [Google Scholar]
- Tsai TD, Shuck ME, Thompson DP, Bienkowski MJ, Lee KS. Intracellular H+ inhibits a cloned rat kidney outer medulla K+ channel expressed in Xenopus oocytes. American Journal of Physiology. 1995;268:C1173–1178. doi: 10.1152/ajpcell.1995.268.5.C1173. [DOI] [PubMed] [Google Scholar]
- Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. American Journal of Physiology. 1990;259:F494–502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wingo CS. Stimulation of total CO2 flux by 10 % CO2 in rabbit CCD: role of an apical Sch-28080- and Ba2+-sensitive mechanism. American Journal of Physiology. 1994;267:F114–120. doi: 10.1152/ajprenal.1994.267.1.F114. [DOI] [PubMed] [Google Scholar]
- Zhu GY, Chanchevalap S, Cui NR, Jiang C. Effects of intra- and extracellular acidification on single channel Kir2.3 currents. The Journal of Physiology. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


