Abstract
The cytoplasmic Ca2+ concentration ([Ca2+]i) was measured in single cells and cell clusters of different sizes prepared from mouse pancreatic islets.
During stimulation with 15 mM glucose, 20 % of isolated cells were inert, whereas 80 % showed [Ca2+]i oscillations of variable amplitude, duration and frequency. Spectral analysis identified a major frequency of 0.14 min−1 and a less prominent one of 0.27 min−1.
In contrast, practically all clusters (2–50 cells) responded to glucose, and no inert cells were identified within the clusters. As compared to single cells, mean [Ca2+]i was more elevated, [Ca2+]i oscillations were more regular and their major frequency was slightly higher (but reached a plateau at ≈0.25 min−1). In some cells and clusters, faster oscillations occurred on top of the slow ones, between them or randomly.
Image analysis revealed that the regular [Ca2+]i oscillations were well synchronized between all cells of the clusters. Even when the Ca2+ response was irregular, slow and fast [Ca2+]i oscillations induced by glucose were also synchronous in all cells.
In contrast, [Ca2+]i oscillations resulting from mobilization of intracellular Ca2+ by acetylcholine were restricted to certain cells only and were not synchronized.
Heptanol and 18α-glycyrrhetinic acid, two agents widely used to block gap junctions, altered glucose-induced Ca2+ oscillations, but control experiments showed that they also exerted effects other than a selective uncoupling of the cells.
The results support theoretical models predicting an increased regularity of glucose-dependent oscillatory events in clusters as compared to isolated islet cells, but contradict the proposal that the frequency of the oscillations increases with the number of coupled cells. Islet cell clusters function better as electrical than biochemical syncytia. This may explain the co-ordination of [Ca2+]i oscillations driven by depolarization-dependent Ca2+ influx during glucose stimulation.
To regulate insulin secretion pancreatic β cells transduce variations in the ambient glucose concentration into changes in cytosolic Ca2+ concentration ([Ca2+]i). Even during stimulation by constant glucose, [Ca2+]i displays oscillations that contribute in a large way to the pulsatility of secretion (Smith et al. 1995; Barbosa et al. 1998; Bergsten et al. 1998; Henquin et al. 1998).
Different types of [Ca2+]i oscillations have been identified in β cells, but their respective mechanisms and significance are only partly understood. Within intact mouse islets, the membrane potential of glucose-stimulated β cells oscillates at a frequency of about 3 min−1, sometimes associated with a slower frequency of about 0.25 min−1 (Henquin et al. 1982; Cook, 1983). These repetitive depolarizations induce intermittent influx of Ca2+ through voltage-dependent Ca2+ channels, which generates synchronous [Ca2+]i oscillations (Valdeolmillos et al. 1989; Santos et al. 1991; Gilon & Henquin, 1992). In isolated β cells, however, [Ca2+]i oscillations are inconsistent, usually slow (0.3 min−1) and often irregular (Herchuelz et al. 1991; Hellman et al. 1992; Wang et al. 1993), probably reflecting the irregularity and heterogeneity of the electrical activity in single cells (Rorsman & Trube, 1986; Hellman et al. 1990; Smith et al. 1990; Satin et al. 1998).
Theoretical models, based on the hypothesis that the electrical activity is poorly organized in isolated β cells because of stochastic fluctuations in ionic channel activity, predict that regular oscillations of the membrane potential emerge when the cells are associated in clusters (Sherman et al. 1988; Sherman & Rinzel, 1991). However, these models have not yet been thoroughly tested experimentally. Thus, most studies of islet cells used single cells or intact islets (thousands of cells), and only exceptionally cell clusters (Rorsman & Trube, 1986; Satin et al. 1998). Since [Ca2+]i oscillations largely depend on membrane potential changes, one might anticipate that they are also influenced by β cell coupling. The single investigation using clusters of (6–14) islet cells only quantified the frequency of glucose-induced [Ca2+]i oscillations and concluded that it is determined by the number of cells (Gylfe et al. 1991). This proposal has, however, been challenged on the basis of theoretical considerations (Smolen et al. 1993). In the present study, therefore, normal mouse islets were dissociated into single cells and clusters of different sizes (2–50 cells). We then evaluated how the characteristics (regularity and frequency) of glucose-induced [Ca2+]i oscillations were affected by the number of cells in the preparation.
METHODS
Solutions
The control medium was a bicarbonate-buffered solution that contained (mM): 120 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2 and 24 NaHCO3. It was gassed with O2-CO2 (94:6) to maintain pH 7.4 and was supplemented with 0.5 mg l−1 bovine serum albumin (fraction V). When the concentration of KCl was increased to 30 mM, that of NaCl was decreased accordingly to maintain isosmolarity. The Ca2+-free solution used to disperse islets in clusters contained (mM): 138 NaCl, 5.6 KCl, 1.2 MgCl2, 5 Hepes and 1 EGTA, with 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin, and the pH was adjusted to 7.35 with NaOH. The medium used for cultures was RPMI 1640 medium containing 10 mM glucose, 10 % heat-inactivated fetal calf serum, 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin.
Preparation
The research project was approved by, and the experiments were conducted in accordance with the guidelines of, the Commission d'Ethique d'Expérimentation Animale of the University of Louvain School of Medicine. Fed female NMRI (Naval Medical Research Institute) mice were killed by decapitation. Islets were isolated by collagenase digestion of the pancreas followed by selection by hand (Jonas et al. 1998). To obtain isolated cells and clusters, the islets were incubated for 5 min in Ca2+-free solution. After brief centrifugation, this solution was replaced by culture medium and the islets were disrupted by gentle pipetting through a siliconized glass pipette. Clusters and isolated cells were then cultured for 1–4 days on 22 mm circular glass coverslips.
To determine the proportion of non-β cells in the preparations, coverslips with cells and clusters cultured for 2 days were fixed in Bouin-Allen's fluid for 6 h at room temperature. They were then processed to immunostain α cells and δ cells with a mixture of anti-glucagon and anti-somatostatin serum, each at a dilution of 1:25 000 (Novo Biolabs, Bagsvaerd, Denmark). Positive cells were identified by a peroxidase method using 3,3′-diaminobenzidine as the substrate for staining. The preparations were then counterstained with hemalun. The method has been described in full elsewhere (Sempoux et al. 1998). Labelled non-β cells were counted and their proportion was determined by counting the number of nuclei.
Measurements of [Ca2+]i
Clusters and cells attached to the coverslips were loaded with fura-2 during 60 min of incubation in control medium containing 10 mM glucose and 1 μM fura-2 acetoxymethylester. In control experiments, the plasma membrane of cells loaded with fura-2 was selectively permeabilized by the α toxin from Staphylococcus aureus (Detimary et al. 1996) to permit exit of the dye located in the cytoplasm. After 10 min of washing, less than 20 % of the dye was retained within organelles. Loading at room temperature did not influence this apparent sequestration, and did not affect glucose-induced [Ca2+]i oscillations, as already reported (Miura et al. 1997).
After the preparation had been loaded with fura-2, the coverslip was transferred into a temperature-controlled perifusion chamber (Intracell, Royston, Herts, UK) of which it formed the bottom. The chamber was placed on the stage of an inverted microscope (× 40 objective) and perfused with control medium containing 3 or 15 mM glucose and the indicated test substances, and maintained at 37°C. The cells in which [Ca2+]i was measured were not identified immunohistochemically. However, single cell measurements were usually performed in large cells, which are more likely to be β than non-β cells (Berts et al. 1995). Clusters used for recordings were selected by size only to obtain a range from 2 to 50 cells (see below). The tissue was successively excited at 340 and 380 nm, and the fluorescence emitted at 510 nm was captured by a CCD camera (Photonic Science Ltd, Tunbridge Wells, Kent, UK). The images obtained at 1.8 s intervals were analysed by the MagiCal system (VisiTech, Sunderland, UK). From the ratio of the fluorescence at 340 and 380 nm, the concentration of intracellular Ca2+ at each pixel was calculated by comparison with an in vitro calibration curve. This curve was based on the equation of Grynkiewiez et al. (1985) and established by filling the chamber with an intracellular-type, K+-rich medium containing less than 1 nM free Ca2+ or 10 mM Ca2+. A dissociation constant (KD) for the fura-2-Ca2+ complex of 224 nM was used. The mean [Ca2+]i in an area of interest (single cell, cluster) was then obtained by averaging the [Ca2+]i of all pixels within this area. At the end of the experiment, the perifusion was stopped and the chamber was filled with control solution containing 1 μM bisbenzimide (Sigma). After 30 min of incubation, the preparation was excited at 365 nm and the number of cells in the studied cluster was determined by counting the fluorescent nuclei.
Analysis of the results
Single cells and clusters of cells from the same islet preparation were cultured for 1, 2 and 4 days before being subjected to stimulation with 15 mM glucose. This experiment was repeated 10 times. Initially, the characteristics of [Ca2+]i oscillations were analysed separately for each period of culture. However, because practically no significant effect of culture duration was observed (see Results), the results obtained after the three culture periods were pooled. Four categories of preparations were analysed separately: single cells, small clusters (2–4 cells), medium clusters (5–15 cells) and large clusters (16–50 cells).
All [Ca2+]i profiles were subjected to spectral analysis to identify the major frequencies of the oscillations. This analysis decomposes the time series data (xi for i = 1 to N) in a sum of cosine waves with periods T, T/2, T/3, …T/N, where T is the length of the experiment and N is the number of samples. To avoid the influence of slow trends of [Ca2+]i on spectral analysis, the best-fit curve (trend) was first calculated for each experiment using a least-squares regression procedure to a third-order polynomial equation (y = a0x3+a1x2+a2x+a3). Detrended series were then calculated by removing the trend from the original series, and spectral analysis was performed by two different approaches. First, the power spectral density (PSD) was estimated by use of a fast Fourier transform (FFT) algorithm allowing analysis of data when N is not a power of 2 (FFT function from MatLab version 4.0). Second, the PSD was estimated, also using MatLab 4.0, by the normalized periodogram method of Lomb (Lomb, 1976), computed according to Press et al. (1992). The highest frequency was set at the level of the Nyquist frequency (0.5/Δ where Δ is the time interval between two consecutive samples). With this method it is possible to test the significance of a peak in the spectrum, the null hypothesis being that the data are independent Gaussian random values. Since the two methods gave similar results, only PSD obtained by FFT analysis is shown. Before averaging, the spectral power was normalized in each experiment by multiplying PSD values by I/J, where I is the area under the curve of squared detrended values and J is the area under the PSD, according to Parseval's theorem (Press et al. 1992).
Presentation of results
The experiments are illustrated by representative recordings, and quantified data are presented as means ±s.e.m. The statistical significance of differences between means was assessed by analysis of variance followed by a Newman-Keuls test, and that of differences between percentages by Fisher's exact test. Differences were considered significant at P < 0.05.
RESULTS
Cellular composition of the preparations
Three preparations cultured for 2 days were immunostained with a mixture of anti-glucagon and anti-somatostatin serum. They were found to contain 11 % (990/8932) non-β cells, which is less than the 20 % non-β cells present in intact mouse islets (Hedeskov, 1980). However, the proportion of non-β cells was different between single cells and clusters. As many as 33 % of single cells were non-β cells, whereas the average proportion of non-β cells was 10 % in small clusters (2–4 cells), 8 % in medium clusters (5–15 cells) and 7 % in large clusters (16–50 cells). Not all clusters contained non-β cells. The probability that at least one non-β cell was present increased with the cluster size: 28 % for small clusters, 51 % for medium clusters and 73 % for large clusters.
Characteristics of [Ca2+]i in single islet cells and clusters
When single islet cells and clusters were perifused with a medium containing a non-stimulatory concentration of glucose (3 mM), [Ca2+]i was low (between 80 and 125 nM) but often slowly increased with time (not shown). In the continuous presence of 15 mM glucose, a concentration that causes half-maximum stimulation of mouse β cells (Detimary et al. 1995), most preparations exhibited repetitive, transient, elevations of [Ca2+]i with peaks generally between 300 and 700 nM, and troughs between 80 and 140 nM. The pattern of these [Ca2+]i responses was variable between clusters or single cells from different or even the same preparation (same coverslip). Note that Fig. 1 illustrates the different patterns of [Ca2+]i response, not their relative incidence, which is quantified in Fig. 2. The oscillations of [Ca2+]i were sometimes regular in single cells after 1 day (Fig. 1A) as well as in large clusters after 4 days of culture (Fig. 1K). In contrast, some single cells and clusters showed [Ca2+]i oscillations that were regular in amplitude but irregular in frequency (Fig. 1B and G), irregular in amplitude but regular in frequency (Fig. 1C and F), or irregular in both (Fig. 1H and J). A minority of single cells did not respond to glucose ([Ca2+]i was stable below 130 mM) (Fig. 1D), whereas certain large clusters showed no oscillations but a sustained elevation of [Ca2+]i (above 300 nM) (Fig. 1L).
Figure 1. Patterns of [Ca2+]i responses in single cells and clusters of cells from mouse pancreatic islets during continuous stimulation with 15 mM glucose.
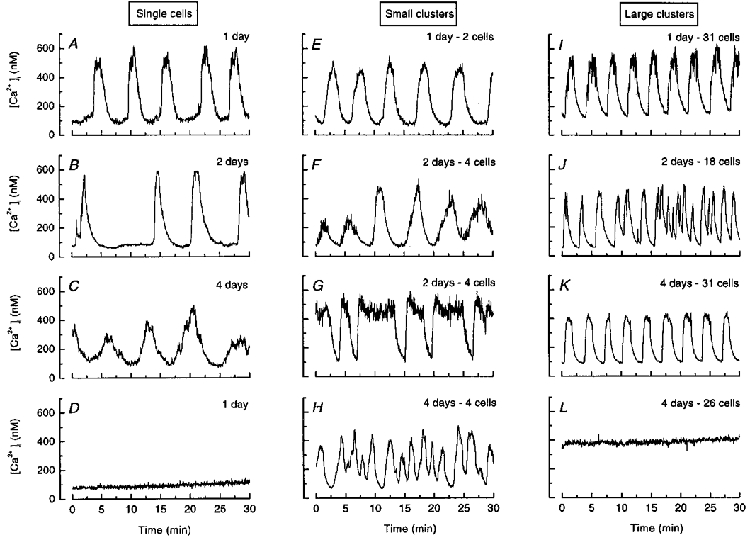
The categories of small and large clusters correspond to 2–4 and 16–50 cells, respectively. The recordings were obtained after 1, 2 or 4 days of culture as indicated. Note that the figure illustrates the different patterns of [Ca2+]i responses but not their relative incidence, which is shown in Fig. 2.
Figure 2. Analysis of [Ca2+]i responses in single cells and clusters of cells from mouse pancreatic islets during continuous stimulation with 15 mM glucose.
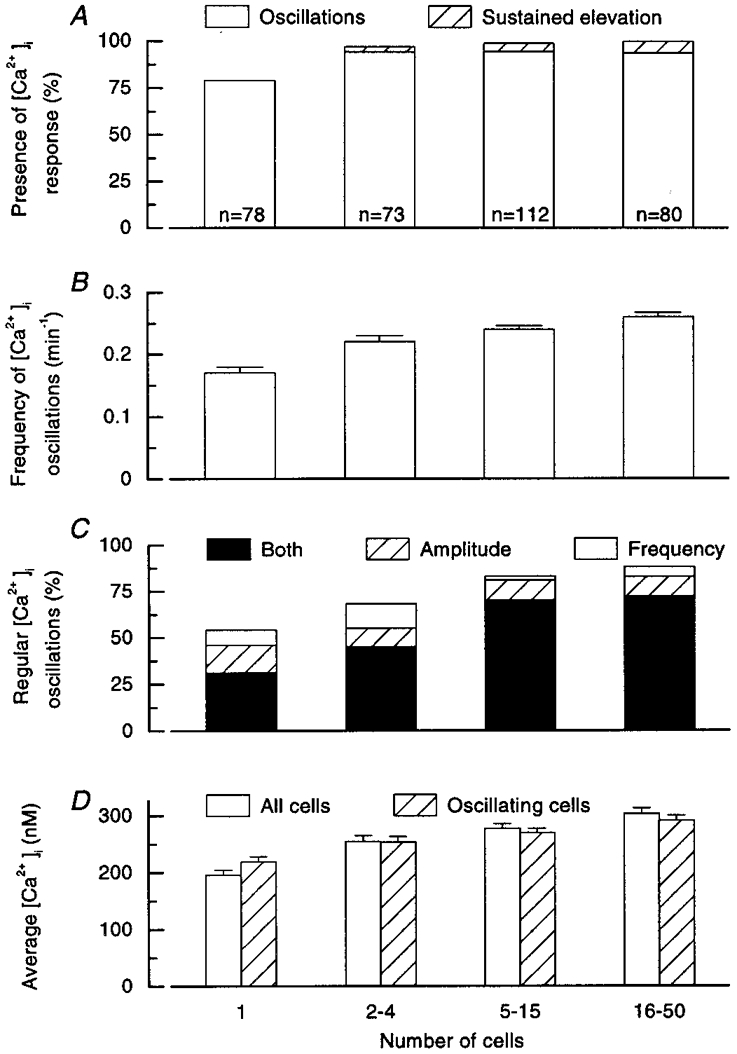
A, percentage of single cells and clusters showing oscillations or a sustained elevation of [Ca2+]i. The total number of preparations studied (n) is given for each column. B, frequency of the major [Ca2+]i oscillation identified by spectral analysis; means and s.e.m.C, percentage of single cells and clusters showing [Ca2+]i oscillations that were regular in amplitude only, frequency only or both amplitude and frequency. D, average [Ca2+]i calculated over the 30 min period of stimulation, for all single cells and clusters, or for only those showing [Ca2+]i oscillations; means and s.e.m.
When intact islets are cultured for 4 days in the presence of 10 mM glucose, the rapid [Ca2+]i oscillations (2–3 min−1) induced by 15 mM glucose progressively disappear and are replaced by a sustained elevation of [Ca2+]i (Gilon et al. 1994). For unknown reasons, the behaviour of the clusters was different. Except for the fact that the sustained elevation of [Ca2+]i in clusters mainly (11/12 clusters) occurred after 4 days, no time-dependent change in the [Ca2+]i responses was observed. In particular, culture duration did not significantly influence the characteristics of [Ca2+]i oscillations. The situation might have been different if the islets had initially been completely dissociated into single cells, and time needed for the clusters to reconstitute. The results obtained after 1, 2 and 4 days of culture were therefore pooled (Fig. 2).
During continuous perifusion with 15 mM glucose, a [Ca2+]i response was observed in 79 % of single cells and 97, 99 and 100 % of the small, medium and large clusters (Fig. 2A). This response was characterized by large, slow oscillations of [Ca2+]i, except in a minority (< 5 %) of the clusters, in which the [Ca2+]i rise was sustained. In some single cells and clusters, faster oscillations were associated with the slower ones, giving the response a mixed pattern. They occurred on top of the slow oscillations, between them or randomly.
Results from all experiments in which [Ca2+]i oscillations were observed were subjected to a spectral analysis, and the results were averaged for the four categories of preparation (Fig. 3). The broad peak obtained in single cells reflects the variability of the response. The major frequency was 0.14 min−1, but a hump on the left of the spectrum indicates that a less prominent frequency of 0.27 min−1 was also present. The distribution was much narrower for clusters, with an increase in the major frequency to 0.20 min−1 in small clusters and to 0.26 min−1 in medium and large clusters. The major frequency of [Ca2+]i oscillations identified by spectral analysis in individual experiments was also averaged for each group (Fig. 2B). It increased from 0.17 min−1 in single cells to 0.26 min−1 in large clusters. The frequency was significantly higher (P < 0.01) in clusters of all sizes than in single cells, and in large clusters than in small clusters, but was not different between medium and large clusters.
Figure 3. Spectral analysis of [Ca2+]i profiles in single cells and clusters of cells from mouse pancreatic islets during continuous stimulation with 15 mM glucose.
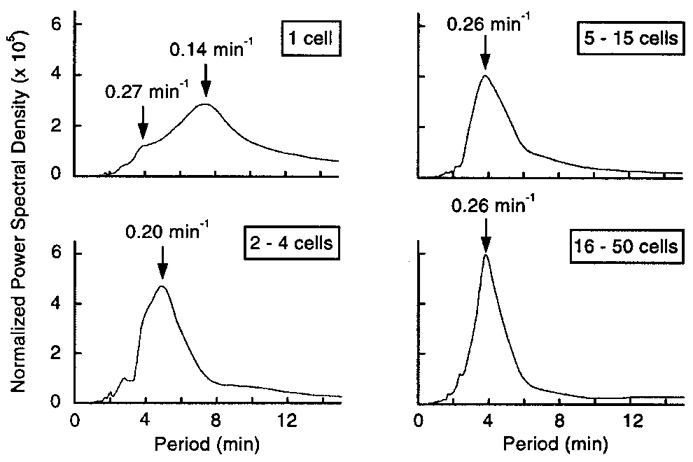
The curves show mean spectra for all responsive preparations in each group.
The association of islet cells in clusters had a marked impact on the regularity of [Ca2+]i oscillations (Fig. 2C). Among responsive single cells, only 30 % showed [Ca2+]i oscillations that were regular in both amplitude and frequency; in 15 % the oscillations were regular in amplitude only and in 8 % they were regular in frequency only; in the remaining 47 % they were irregular in both. The regularity was greater in clusters, and characterized 70–90 % of [Ca2+]i oscillations in medium and large clusters (P < 0.001 vs. single cells) (Fig. 2C). The difference between small clusters and single cells was not significant (P = 0.10).
For each type of preparation, the average [Ca2+]i was calculated for the whole 30 min period of recording (Fig. 2D). It was higher (P < 0.001) in clusters of all sizes than in single cells, and higher (P < 0.01) in large clusters than in small clusters, but not different between medium and small or large clusters. Similar results were obtained after exclusion of unresponsive single cells and clusters with a sustained elevation of [Ca2+]i, i.e. when only those preparations showing [Ca2+]i oscillations were considered (Fig. 2D).
Synchrony of [Ca2+]i changes in clusters of islet cells
The irregularity of the [Ca2+]i changes induced by glucose in certain clusters of islet cells could result from the asynchrony of similar or distinct oscillations in poorly coupled cells. This was evaluated by analysing [Ca2+]i oscillations in different cells of the clusters, with particular attention to those in which the pattern of the response was irregular.
In none of the active clusters that were analysed did we find cells that remained silent. When the global response of the cluster was regular (159/250 preparations), well synchronized, similar oscillations of [Ca2+]i were observed in all regions, as illustrated for five regions each corresponding to 2–3 cells in a cluster of 41 cells (Fig. 4A). Similar findings were obtained in the 37 preparations showing a regular pattern of [Ca2+]i that were examined.
Figure 4. Synchrony of [Ca2+]i oscillations in clusters of cells from mouse pancreatic islets during continuous stimulation with 15 mM glucose.
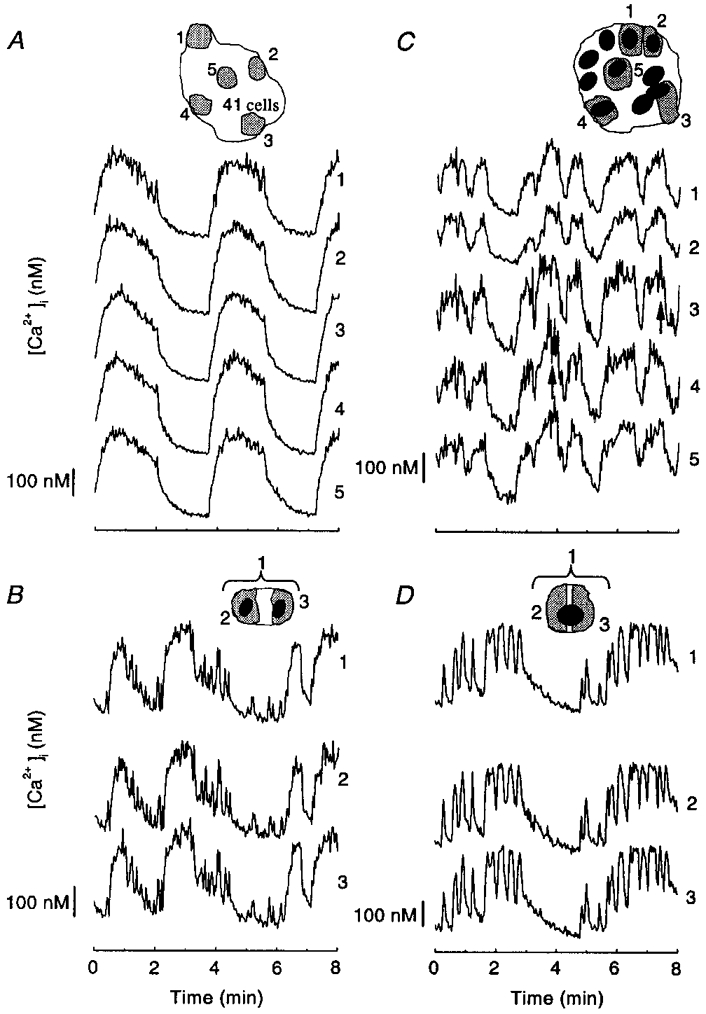
The changes in [Ca2+]i were analysed in the cells or cell regions shown by the numbered shaded areas in the drawings of the whole clusters at the top of each panel. A, cluster of 41 cells (4 days) in which the global response was regular. B and C, cell doublet (2 days), and cluster of 10 cells (2 days) with irregular oscillations. D, single cell (2 days) showing both slow and fast oscillations.
The response was irregular in 61/250 doublets or clusters, and was analysed in 30 of them. In a cell doublet with slow and fast [Ca2+]i oscillations, both types of event were almost perfectly synchronized in the two cells (Fig. 4B). In larger clusters all slow and most rapid events usually remained synchronized. Sometimes, however, brief [Ca2+]i transients (< 10 s) were observed in some but not all cells (Fig. 4C, arrows below traces 3 and 4). The marked variations in the duration of [Ca2+]i oscillations occurring in certain clusters (Fig. 1G) resulted from a similar change in all cells and not from temporary desynchronization of the signal between cells (not shown).
Approximately 10 % of the single cells exhibited both slow and rapid oscillations (Fig. 4D). These were similar in two distinct regions of the cell, but subtle differences might have escaped detection at the magnification used for the present experiments.
The preceding analysis indicates that the mixed or irregular patterns of [Ca2+]i oscillations observed in some clusters do not result from the association of cells showing distinct intrinsic responses or from a poor coupling of the cells. If our interpretation is correct, uncoupling of the cells in a cluster should perturb the global response. This was attempted by treating the clusters with heptanol or 18α-glycyrrhetinic acid, two agents widely used to block gap junctions (Niggli et al. 1989; Goldberg et al. 1996). Both indeed altered the pattern of [Ca2+]i oscillations in glucose-stimulated clusters of islet cells (not shown), but the following control experiments revealed that these drugs do not act selectively by inhibiting gap junctions. In six clusters from three preparations, the amplitude of [Ca2+]i oscillations imposed by repetitive depolarizations with 30 mM K+ was reduced by 1 mM heptanol (from 427 ± 42 to 194 ± 27 nM). A similar, 50 % inhibition of K+-induced [Ca2+]i rise was observed in single cells (not shown). This confirms that heptanol has a direct effect on β cell voltage-dependent Ca2+ channels (Pérez-Armendariz et al. 1991). 18α-Glycyrrhetinic acid rapidly and reversibly raised [Ca2+]i in clusters of cells (not shown), but the same effect also occurred in single cells (Fig. 5), which demonstrates that the drug exerts effects other than those mediated by blockade of gap junctions. The nature of these effects was not investigated.
Figure 5. Effects of a putative blocker of gap junctions on [Ca2+]i in a single islet cell.
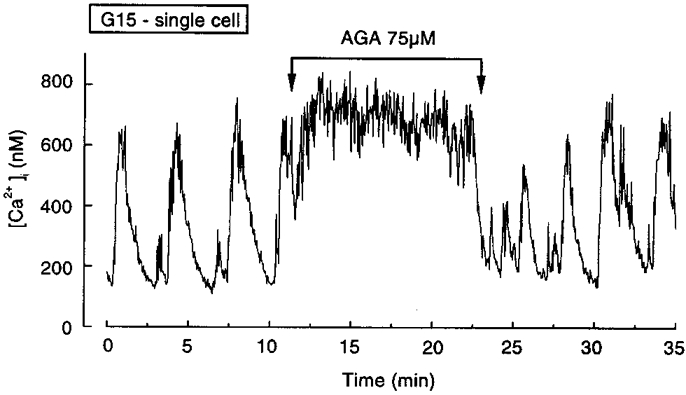
A single islet cell was continuously stimulated with 15 mM glucose (G15), and 75 μM 18α-glycyrrhetinic acid (AGA) was added to the medium for the indicated period. Representative of 6 experiments.
Glucose-induced [Ca2+]i oscillations depend on oscillations of membrane potential and Ca2+ influx. [Ca2+]i oscillations can also result from repetitive mobilization of Ca2+ from intracellular stores. We investigated whether such oscillations are also synchronized in islet cell clusters. When β cells were kept hyperpolarized with diazoxide (Henquin & Meissner, 1982), glucose did not induce [Ca2+]i oscillations (Fig. 6A). Acetylcholine only causes a small (3–4 mV) depolarization without electrical activity under these conditions (Hermans et al. 1987), but induces voltage-independent release of Ca2+ from the endoplasmic reticulum (Gylfe, 1991; Miura et al. 1997).
Figure 6. Asynchrony of [Ca2+]i changes resulting from intracellular Ca2+ mobilization in clusters of cells from mouse pancreatic islets.
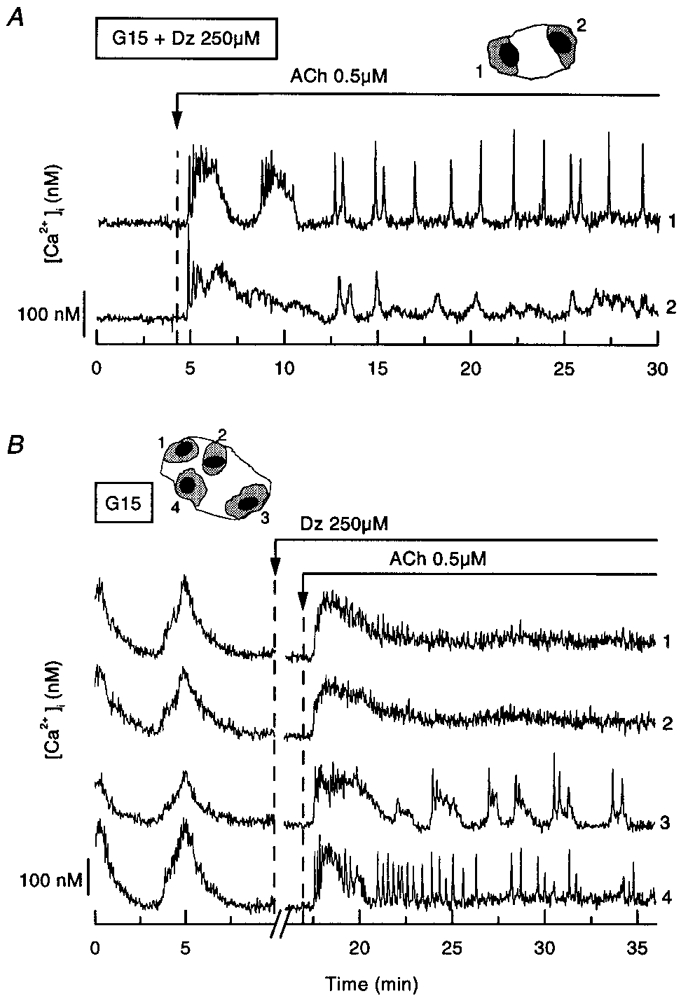
A cell doublet (2 days) and a cluster of 4 cells (2 days) were perifused with a medium containing 15 mM glucose (G15) throughout. A, diazoxide (Dz, 250 μM) was present throughout, and acetylcholine (ACh, 0.5 μM) was added as indicated. B, diazoxide and acetylcholine were added as indicated. The recording was interrupted for 6 min after addition of diazoxide. The changes in [Ca2+]i were analysed in the cells or cell regions shown by the numbered shaded areas in the drawings of the clusters above each panel. Representative of 7 experiments.
In the two cells of the doublet shown in Fig. 6A, acetylcholine (0.5 μM) induced a rapid rise in [Ca2+]i. Thereafter, large [Ca2+]i oscillations appeared in one cell, while asynchronous, smaller [Ca2+]i oscillations appeared in the second cell. In other experiments we first ascertained that the cells were well coupled by adding diazoxide only after recording of the synchronous oscillations of [Ca2+]i induced by glucose. In the cluster of four cells shown in Fig. 6B, the addition of acetylcholine evoked a similar first rise in [Ca2+]i, but subsequent repetitive peaks of [Ca2+]i were observed in only 2/4 cells. Not only were the rapid transients asynchronous, but also the slower [Ca2+]i oscillations, of similar duration to those induced by glucose, were restricted to one cell of the cluster. It should be noted that these repetitive [Ca2+]i transients occurred in only 7/20 clusters stimulated with 0.5 μM acetylcholine. In the others, [Ca2+]i remained steadily elevated slightly above basal levels after the initial large peak. When a higher concentration of acetylcholine was used (10 μM), the proportion of clusters showing repetitive [Ca2+]i transients was lower (4/24) and, again, these transients were asynchronous and occurred in some cells only. Mobilization of intracellular Ca2+ by acetylcholine thus causes asynchronous [Ca2+]i oscillations in clusters of islet cells in which stimulation of Ca2+ influx by glucose causes synchronous [Ca2+]i oscillations. These experiments also indicate that an asynchrony of the Ca2+ responses to glucose would not have been missed with our recording system.
DISCUSSION
Single islet cells exhibit heterogeneous [Ca2+]i responses to glucose (Herchuelz et al. 1991; Hellman et al. 1992; Wang et al. 1993). The present study provides a quantification of this heterogeneity and shows that it decreases upon organization into clusters of coupled cells. About 20 % of the isolated cells did not respond to glucose, 40 % showed irregular [Ca2+]i oscillations, 15 % showed oscillations regular in amplitude or frequency, and only 25 % showed completely regular oscillations. Non-β cells, which made up 33 % of the single cells in our preparations, may contribute to this heterogeneity. However, we are confident that a substantially smaller proportion of non-β cells was studied here because large single cells (mouse β cells are larger than α and δ cells; Berts et al. 1995) were usually selected for [Ca2+]i measurements. It is thus clear that single β cells respond heterogeneously to glucose, but formal identification of the cells would be necessary to determine their proportion among the unresponsive cells. It is unlikely that dead cells were studied because these have apparent high [Ca2+]i and a dense, very fluorescent nucleus after staining with bisbenzimide, two features that did not characterize the unresponsive cells. Moreover, two-thirds of the cells that were unresponsive to glucose alone showed an increase in [Ca2+]i upon stimulation with tolbutamide (F. C. Jonkers & J. C. Henquin, unpublished observations).
In contrast to isolated cells, only a small proportion (1–3 %) of the clusters did not show a Ca2+ response consisting of either oscillations or, much more rarely, a sustained elevation. Since no silent cells were seen within responsive clusters, it appears that cells that are unresponsive when isolated become active when associated with other cells. It also seems that the 7–10 % non-β cells present in the clusters are entrained by the β cells to which they may be coupled (Meda et al. 1982). However, these conclusions are qualified by the fact that some clusters were composed of two layers of cells, and that the signal originating from active cells in one layer may have masked the presence of an inactive cell in the other layer. It is also possible that disruption of the islet architecture alters the genuine behaviour of non-β cells. These have been shown to display [Ca2+]i responses distinct from those of β cells in intact islets (Asada et al. 1998; Nadal et al. 1999).
Mathematical models (Sherman et al. 1988; Sherman & Rinzel, 1991) predict an increase in the regularity of glucose-induced electrical activity in β cells when they associate in coupled clusters of sufficient size. Although these models specifically addressed electrical oscillations faster (3 min−1) than the large [Ca2+]i oscillations occurring in islet cells, we hypothesized that the latter might also be influenced by β cell association because they also result from depolarization-induced Ca2+ influx (Hellman et al. 1992; Miura et al. 1997). Our observation that coupling of just a few cells (not necessarily a whole islet) is sufficient for regularity to appear thus provides the first direct support to the models.
Besides the regularity, the frequency of glucose-induced [Ca2+]i oscillations was also different in isolated β cells and clusters. When a preparation displays only one type of regular oscillation, the frequency is easily determined. This is much less easy when the duration and the amplitude of the oscillations fluctuate. Results from all experiments, therefore, were subjected to spectral analysis to identify the significant frequencies. The major frequency slightly increased with the cluster size but reached a plateau at about 0.25 min−1, which is much less than the increase (0.3–0.6 min−1) found by others who compared a few clusters of 6–14 cells with single cells from ob/ob mouse islets (Gylfe et al. 1991). In agreement with the prediction of mathematical models (Smolen et al. 1993), our data, therefore, do not suggest that the association of increasing numbers of islet cells leads to the emergence of fast oscillations.
There seems to exist a basic oscillatory phenomenon, with a period of ∼4 min, that is detectable in some single cells and becomes much more apparent in clusters. In contrast, assembly of islet cells into clusters did not lead to the appearance of rapid oscillations (several per minute). These were present in certain large clusters but sometimes also in single cells, and may thus depend on factors other than cell association. Fast [Ca2+]i oscillations (∼2 min−1) have also been found to be rare in single rat (Pralong et al. 1994) or ob/ob mouse islet cells (Hellman et al. 1992), except when the cells were studied immediately after isolation of the islets, or when an agent capable of raising cAMP levels was present in the medium. It is possible that paracrine communication between the different types of islet cells is necessary for generation of fast oscillations (Hellman et al. 1992; Nadal et al. 1999). Recently, it has been suggested that the mixed pattern of slow and superimposed faster oscillations is due to separate cell populations with the respective response (Liu et al. 1998). Although this explanation may be correct for whole islets from ob/ob mice, it does not explain the mixed patterns observed here in clusters of islet cells from normal mice; both slow and rapid oscillations could be found in cell doublets and even in certain single cells.
Both regular and irregular oscillations of [Ca2+]i are well synchronized in glucose-stimulated islets (Valdeolmillos et al. 1993; Gilon et al. 1994). Admittedly, the analyses previously performed at the islet level covered zones of several tens or hundreds of cells. Here the area of analysis was cell sized. Yet, even when the pattern of the oscillations was irregular, the synchrony between cells was good. As observed previously between two halves of an islet (Valdeolmillos et al. 1993), partial desynchronization also occurred in small clusters, but was a rare event. Altogether the present and previous data indicate that good electrical coupling of β cells is the rule, resulting in synchronous voltage-dependent Ca2+ influx in all cells.
The contrast with [Ca2+]i oscillations resulting from IP3-induced mobilization of intracellular Ca2+ is striking. Except for a similar initial Ca2+ peak, distinct, asynchronous Ca2+ transients occurred in the different cells of a cluster. Islet cell clusters thus function as electrical syncytia in which co-ordinated changes in membrane potential drive synchronous Ca2+ influx. In contrast, they do not seem to function as biochemical syncytia when cellular Ca2+ is mobilized by IP3.
It has long been known that only a fraction of isolated β cells release insulin upon stimulation by glucose, and that cell contact increases both the number of responsive cells and their individual response (Halban et al. 1982; Pipeleers et al. 1982). Our findings perhaps provide an explanation for these observations. Thus, the proportion of cells failing to show a Ca2+ response was smaller in clusters than in isolated cell preparations, and their mean [Ca2+]i was higher. The triggering signal of exocytosis was therefore present in more cells and was stronger in each cell after formation of the clusters. However, because glucose can also amplify the action of Ca2+ on exocytosis (Gembal et al. 1992), metabolic factors may also participate in the improvement of the secretory response of associated cells. Other specific approaches are needed to distinguish between these possibilities.
In conclusion, our measurements of [Ca2+]i in glucose-stimulated islet cells support theoretical models (Sherman et al. 1988; Sherman & Rinzel, 1991) predicting an increased regularity of the glucose-dependent oscillatory events in clusters as compared with isolated cells. Association of the cells also lowered the proportion of non-responsive cells but only slightly increased the frequency of [Ca2+]i oscillations, leading to the emergence of a major frequency of about 0.25 min−1. The number of β cells in the cluster thus appears to have a greater influence on the regularity than on the frequency of glucose-induced [Ca2+]i oscillations. Even when the Ca2+ response was characterized by a mixed, irregular pattern, both slow and fast events were present in the same cells and, save for a few cases of fast events, were synchronous in all cells of the clusters. In contrast, [Ca2+]i oscillations generated by intracellular Ca2+ mobilization with acetylcholine were restricted to certain cells of each cluster. It appears, therefore, that islet cell clusters function better as electrical than as biochemical syncytia and that this may explain the co-ordination of [Ca2+]i changes driven by membrane potential-dependent Ca2+ influx.
Acknowledgments
F. C. Jonkers is the holder of a research fellowship from the FRIA, Brussels, and P. Gilon and J.-C. Jonas are Chercheurs Qualifiés from the FNRS, Brussels. This work was supported by grant 3.4552.98 from the FRSM, Brussels, by grant ARC 95/00–188 from the General Direction of Scientific Research of the French Community of Belgium, and by the Interuniversity Poles of Attraction Programme (P4/21), Belgian State, Prime Minister's Office. We are very grateful to Dr Y. Guiot and Professor J. Rahier for their help with the immunostainings, and we thank S. Roiseux for editorial assistance.
References
- Asada N, Shibuya I, Iwanaga T, Niwa K, Kanno T. Identification of α- and β-cells in intact isolated islets of Langerhans by their characteristic cytoplasmic Ca2+ concentration dynamics and immunocytochemical staining. Diabetes. 1998;47:751–757. doi: 10.2337/diabetes.47.5.751. [DOI] [PubMed] [Google Scholar]
- Barbosa RM, Silva AM, Tome AR, Stamford JA, Santos RM, Rosario LM. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. The Journal of Physiology. 1998;510:135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten P, Lin J, Westerlund J. Pulsatile insulin release: role of cytoplasmic Ca2+ oscillations. Diabetes and Metabolism. 1998;24:41–45. [PubMed] [Google Scholar]
- Berts A, Gylfe E, Hellman B. Ca2+ oscillations in pancreatic islet cells secreting glucagon and somatostatin. Biochemical and Biophysical Research Communications. 1995;208:644–649. doi: 10.1006/bbrc.1995.1387. [DOI] [PubMed] [Google Scholar]
- Cook DL. Isolated islets of Langerhans have slow oscillations of electrical activity. Metabolism. 1983;32:681–685. doi: 10.1016/0026-0495(83)90124-5. [DOI] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC. Possible links between glucose-induced changes in the energy state of pancreatic B-cells and insulin release: unmasking by decreasing a stable pool of adenine nucleotides in mouse islets. Journal of Clinical Investigation. 1995;96:1738–1745. doi: 10.1172/JCI118219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B-cells. Journal of Clinical Investigation. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. Journal of Biological Chemistry. 1992;267:20713–20720. [PubMed] [Google Scholar]
- Gilon P, Jonas JC, Henquin JC. Culture duration and conditions affect the oscillations of cytoplasmic calcium concentration induced by glucose in mouse pancreatic islets. Diabetologia. 1994;37:1007–1014. doi: 10.1007/BF00400464. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RS, MacPhee DJ, Zhang YC, Naus CCG. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Experimental Cell Research. 1996;222:48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- Grynckiewick G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gylfe E. Carbachol induces sustained glucose-dependent oscillations of cytoplasmic Ca2+ in hyperpolarized pancreatic β cells. Pflügers Archiv. 1991;419:639–643. doi: 10.1007/BF00370308. [DOI] [PubMed] [Google Scholar]
- Gylfe E, Grapengiesser E, Hellman B. Propagation of cytoplasmic Ca2+ oscillations in clusters of pancreatic β-cells exposed to glucose. Cell Calcium. 1991;12:229–240. doi: 10.1016/0143-4160(91)90023-8. [DOI] [PubMed] [Google Scholar]
- Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology. 1982;111:86–94. doi: 10.1210/endo-111-1-86. [DOI] [PubMed] [Google Scholar]
- Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiological Reviews. 1980;60:442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B, Gylfe E, Grapengiesser E, Lund PE, Berts A. Cytoplasmic Ca2+ oscillations in pancreatic β-cells. Biochimica et Biophysica Acta. 1992;1113:295–305. doi: 10.1016/0304-4157(92)90003-s. [DOI] [PubMed] [Google Scholar]
- Hellman B, Gylfe E, Grapengiesser E, Panten U, Schwanstecher C, Heipel C. Glucose induces temperature-dependent oscillations of cytoplasmic Ca2+ in single pancreatic β-cells related to their electrical activity. Cell Calcium. 1990;11:413–418. doi: 10.1016/0143-4160(90)90053-w. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Jonas JC, Gilon P. Functional significance of Ca2+ oscillations in pancreatic β cells. Diabetes and Metabolism. 1998;24:30–36. [PubMed] [Google Scholar]
- Henquin JC, Meissner HP. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B-cells. Biochemical Pharmacology. 1982;31:1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Meissner HP, Schmeer W. Cyclic variations of glucose-induced electrical activity in pancreatic B-cells. Pflügers Archiv. 1982;393:322–327. doi: 10.1007/BF00581418. [DOI] [PubMed] [Google Scholar]
- Herchuelz A, Pochet R, Pastiels Ch, Van Praet A. Heterogeneous changes in [Ca2+]i induced by glucose, tolbutamide and K+ in single rat pancreatic B cells. Cell Calcium. 1991;12:577–586. doi: 10.1016/0143-4160(91)90076-q. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Schmeer W, Henquin JC. Modulation of the effect of acetylcholine on insulin release by the membrane potential of B-cells. Endocrinology. 1987;120:1765–1773. doi: 10.1210/endo-120-5-1765. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Gilon P, Henquin JC. Temporal and quantitative correlations between insulin secretion and stably elevated or oscillatory cytoplasmic Ca2+ in mouse pancreatic β-cells. Diabetes. 1998;47:1266–1273. doi: 10.2337/diab.47.8.1266. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Tengholm A, Grapengiesser E, Hellman B, Gylfe E. Origin of slow and fast oscillations of Ca2+ in mouse pancreatic islets. The Journal of Physiology. 1998;508:471–481. doi: 10.1111/j.1469-7793.1998.471bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomb NR. Least-squares frequency analysis of unequally spaced data. Astrophysics and Space Science. 1976;39:447–462. [Google Scholar]
- Meda P, Kohen E, Kohen C, Rabinovitch A, Orci L. Direct communication of homologous and heterologous endocrine islet cells in culture. Journal of Cell Biology. 1982;92:221–226. doi: 10.1083/jcb.92.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Henquin JC, Gilon P. Emptying of intracellular Ca2+ stores stimulates Ca2+ entry in mouse pancreatic β-cells by both direct and indirect mechanisms. The Journal of Physiology. 1997;503:387–398. doi: 10.1111/j.1469-7793.1997.387bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Quesada I, Soria B. Homologous and heterologous asynchronicity between identified α-, β- and δ-cells within intact islets of Langerhans in the mouse. The Journal of Physiology. 1999;517:85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E, Rüdisüli A, Maurer P, Weingart R. Effects of general anesthetics on current flow across membranes in guinea pig myocytes. American Journal of Physiology. 1989;256:C273–281. doi: 10.1152/ajpcell.1989.256.2.C273. [DOI] [PubMed] [Google Scholar]
- Pérez-Armendariz M, Roy C, Spray DC, Bennett MVL. Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic beta cells. Biophysical Journal. 1991;56:76–92. doi: 10.1016/S0006-3495(91)82200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D, In'T Veld P, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proceedings of the National Academy of Sciences of the USA. 1982;79:7322–7325. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong W-F, Spät A, Wollheim CB. Dynamic pacing of cell metabolism by intracellular Ca2+ transients. Journal of Biological Chemistry. 1994;269:27310–27314. [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C. New York: Cambridge University Press; 1992. [Google Scholar]
- Rorsman P, Trube G. Calcium and delayed potassium currents in mouse pancreatic β-cells under voltage-clamp conditions. The Journal of Physiology. 1986;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Archiv. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Satin L, Kinard T, Devries G, Sherman A. Heterogeneous fast bursting in single mouse β cells. Diabetes. 1998;47(suppl. 1):A264. [Google Scholar]
- Sempoux C, Guiot Y, Dubois D, Nollevaux M-C, Saudubray J-M, Nihoul-Fekete C, Rahier J. Pancreatic β-cell proliferation in persistent hyperinsulinemic hypoglycemia of infancy: an immunohistochemical study of 18 cases. Modern Pathology. 1998;11:444–449. [PubMed] [Google Scholar]
- Sherman A, Rinzel J. Model for synchronization of pancreatic β-cells by gap junction coupling. Biophysical Journal. 1991;59:547–559. doi: 10.1016/S0006-3495(91)82271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Rinzel J, Keizer J. Emergence of organized bursting in clusters of pancreatic β-cells by channel sharing. Biophysical Journal. 1988;54:411–425. doi: 10.1016/S0006-3495(88)82975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Ashcroft FM, Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K+-currents in isolated mouse pancreatic beta-cells. FEBS Letters. 1990;261:187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Smith PA, Duchen MR, Aschroft FM. A fluorimetric and amperometric study of calcium and secretion in isolated mouse pancreatic β-cells. Pflügers Archiv. 1995;430:808–818. doi: 10.1007/BF00386180. [DOI] [PubMed] [Google Scholar]
- Smolen P, Rinzel J, Sherman A. Why pancreatic islets burst but single β cells do not. The heterogeneity hypothesis. Biophysical Journal. 1993;64:1668–1680. doi: 10.1016/S0006-3495(93)81539-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolmillos M, Nadal A, Soria B, Garcia-Sancho J. Fluorescence digital image analysis of glucose-induced [Ca2+]i oscillations in mouse pancreatic islets of Langerhans. Diabetes. 1993;42:1210–1214. doi: 10.2337/diab.42.8.1210. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M, Santos RM, Contreras D, Soria B, Rosario LM. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Letters. 1989;259:19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Wang JL, Corbett JA, Marshall CA, McDaniel ML. Glucose-induced insulin secretion from purified β-cells. Journal of Biological Chemistry. 1993;268:7785–7791. [PubMed] [Google Scholar]


