Abstract
Physical activity is known to increase type I collagen synthesis measured as the concentration of biomarkers in plasma. By the use of microdialysis catheters with a very high molecular mass cut-off value (3000 kDa) we aimed to determine local type I collagen synthesis and degradation in the peritendinous region by measuring interstitial concentrations of a collagen propeptide (PICP; 100 kDa) and a collagen degradation product (ICTP; 9 kDa) as well as an inflammatory mediator (PGE2).
Seven trained human runners were studied before and after (2 and 72 h) 3 h of running (36 km). Two microdialysis catheters were placed in the peritendinous space ventral to the Achilles' tendon under ultrasound guidance and perfused with a Ringer-acetate solution containing 3H-labelled human type IV collagen and [15-3H(N)]PGE2 for in vivo recovery determination. Relative recovery was 37–59% (range of the s.e.m. values) for both radioactively labelled substances.
PICP concentration decreased in both interstitial peritendinous tissue and arterial blood immediately after exercise, but rose 3-fold from basal 72 h after exercise in the peritendinous tissue (55 ± 10 μg l−1, mean ± s.e.m. (rest) to 165 ± 40 μg l−1 (72 h), P < 0·05) and by 25% in circulating blood (160 ± 10 μg l−1 (rest) to 200 ± 12 μg l−1 (72 h), P < 0·05). ICTP concentration did not change in blood, but decreased transiently in tendon-related tissue during early recovery after exercise only. PGE2 concentration increased in blood during running, and returned to baseline in the recovery period, whereas interstitial PGE2 concentration was elevated in the early recovery phase.
The findings of the present study indicate that acute exercise induces increased formation of type I collagen in peritendinous tissue as determined with microdialysis and using dialysate fibre with a very high molecular mass cut-off. This suggests an adaptation to acute physical loading also in non-bone-related collagen in humans.
Exercise is known to improve physical properties, e.g. maximal tensile strength, as well as mass and turnover of collagen in bone, ligaments and tendons (Tipton et al. 1975; Kiiskinen, 1977; Suominen et al. 1980; Woo et al. 1982; Michna & Hartmann, 1989). Nevertheless, the specific mechanisms by which these tissues detect and convert mechanical loading into physical properties of the relevant tissue is still not thoroughly understood (Simonsen et al. 1995), but it has been suggested that local factors such as prostaglandins and cytokines participate in the remodelling process of collagen (Huffer, 1988).
Type I collagen, the dominant connective tissue protein in tendon, ligaments and bone (Risteli et al. 1995), has been considered to have a relatively low tissue turnover (Prockop et al. 1979). However, recent studies have demonstrated that the peritendinous connective tissue exerts more metabolic and inflammatory activity than hitherto thought (Langberg et al. 1999) and is influenced by heredity, nerve supply, physical activity, and systemic factors such as various regulating hormones and local factors like cytokines, prostaglandins and neuropeptides (Huffer, 1988; Goldring & Goldring, 1990; Banes et al. 1995; O'Brien, 1997). During recent years the development of assays for determination of collagen conversion has allowed for a more detailed study of collagen turnover (Melkko et al. 1990, 1996; Eriksen et al. 1995). On this background changes in type I collagen synthesis and degradation have been studied by measuring the carboxy-terminal propeptide of type I collagen (PICP) as a marker for collagen synthesis and the carboxy-terminal telopeptide region of type I collagen (ICTP) as an indicator of collagen breakdown after short and prolonged single bouts of exercise (Takala et al. 1989; Virtanen et al. 1993; Salvesen et al. 1994; Kristoffersson et al. 1995; Thorsen et al. 1996a; Ashizawa et al. 1998) and after weeks of training (Price et al. 1995; Eliakim et al. 1997; Hupli et al. 1997). These studies have shown that a single short bout of exercise does not have the ability to change collagen synthesis and degradation in contrast to prolonged exercise or weeks of training. However, all these studies have measured the levels of PICP and ICTP in serum, making it difficult to detect the location of the specific type of tissue in which changes in synthesis and breakdown are taking place.
It has been suggested that prostaglandin is involved in the conversion of mechanical force into collagen formation in bone (Thorsen et al. 1996b) and thus in the present study PGE2 was measured to investigate any potential increase in interstitial PGE2 concentration and its coupling to changes in collagen synthesis.
The microdialysis technique allows for in vivo monitoring of biochemical substances in local tissue during various forms of intervention (Delgado et al. 1972; Ungerstedt & Pycock, 1974). The method has recently been applied to the peritendinous space around the Achilles' tendon, measuring low molecular mass substances such as glucose, lactate, glycerol, prostaglandin (PGE2) and thromboxane (TXB2) both at rest and during intermittent static exercise (Langberg et al. 1999). It has, however, not been possible so far to use microdialysis for detection of interstitial concentrations of large molecules (> 20 kDa) as the maximal molecular mass cut-off of the microdialysis membranes commercially available was 20 kDa.
In the present study we describe, to our knowledge for the first time, the use of microdialysis probes with a high molecular mass cut-off (3000 kDa), allowing for large molecules involved in type I collagen turnover such as PICP (100 kDa) and ICTP (9 kDa) to be determined in a specific region in situ. The microdialysis method was used to monitor changes in local type I collagen turnover in the connective tissue of the Achilles' peritendinous space after prolonged running in trained individuals.
METHODS
Subjects
Seven volunteers were included in the study (1 woman, 6 men; mean age, 32 years (range, 26–40 years); mean body mass index, 22 (19–26); training period per week, 7 h (4–12 h)). All subjects were experienced marathon runners and had been training for several years, and none of the subjects had any previous history of Achilles' tendon symptoms or injuries. Trained individuals were chosen to minimise the risk of the prolonged intervention being traumatic to the muscular-skeletal system. None of the subjects was on any medication and all were non-smokers. All subjects gave written informed consent, and the study was approved by the Ethical Committee of Copenhagen (KF 01–089/98).
Experimental protocol
All experiments were started at 09.00 h. The subjects were told not to perform any kind of exercise either 24 h prior to the experiment or during the 3 days following (between measurements), except for ordinary daily working activities (students or sedentary office jobs). The experiment consisted of a rest period of 60 min, an exercise period of 180 min and a recovery period of 120 min following the acute exercise, as well as an additional rest period of 120 min 72 h after finishing the exercise bout (Fig. 1). During rest and recovery periods the subjects were prone with the ankle joints in a relaxed neutral position (70–80 deg) at a room temperature of 25°C. The exercise intervention consisted of 36 km of running at a pace of 12 km h−1. The running was performed on a flat premarked route of 12 km, with several checkpoints along the route to ensure that a constant pace was kept for all runners.
Figure 1. The experimental design and sampling.
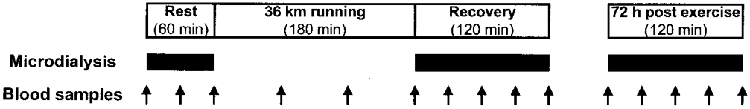
The experiment consisted of a rest period (60 min), an exercise period (180 min) during which the subjects ran 36 km (12 km h−1), a recovery period (120 min) following the acute exercise, and an additional rest period 72 h after the exercise bout (120 min). Microdialysis was performed during rest, recovery immediately after exercise and recovery 72 h following exercise. Sampling was not done till 90 min after insertion of the microdialysis catheters into the tissue in order to minimise the risk of the insertion trauma influencing the results. Blood samples were drawn (arrows) every 30 min during rest, early and late (72 h) recovery, and every 60 min during running.
Blood samples
During local analgesia, an arterial catheter (Ohmeda, Swindon, UK) was inserted percutaneously into the radial artery of the non-dominant arm for blood sampling. The catheter was kept patent by regular flushing with isotonic sodium chloride containing heparin (10 U ml−1). Arterial blood samples were drawn every 30 min during both rest and recovery periods, and every 60 min during running (during a 1 min stop) (Fig. 1). Haematocrit was determined by the microhaematocrit method. The blood samples used for determination of collagen synthesis and degradation were centrifuged at 2000 g for 10 min at 4°C, and the plasma was stored at −70°C for subsequent analysis. The blood samples used for determination of PGE2 concentration had the prostaglandin synthetase inhibitor indomethacin (10 μg ml−1; I-7358, Sigma) and 4·5 mM EDTA added to them, and were centrifuged at 2000 g for 10 min at 4°C. The supernatant was stored at −70°C for subsequent analysis.
Microdialysis
Microdialysis was performed in principle as described by Lönnroth et al. (1987). Two microdialysis catheters, one for determining collagen synthesis and degradation (right Achilles' peritendon), and one for PGE2 determination (left Achilles' peritendon), were placed under ultrasound guidance from the lateral side through the peritendinous space to the medial side just ventral to the Achilles' tendon in each individual. The active part of the membranes covered the area from 30 to 60 mm proximal to the Achilles' tendon insertion on the calcaneus bone.
For determination of collagen synthesis and degradation, microdialysis probes were constructed with a single plasmaphoresis hollow fibre (0·4 mm in diameter, molecular mass cut-off 3000 kDa; Asahi, Japan), glued to a gas-tight nylon inlet tubing and with an inner wire (100 μm stainless steel wire) to improve the mechanical stability of the fibre. The fibre had a membrane of 30 mm available for diffusion. The fibre was sterilised (STERRAD system: low-temperature hydrogen peroxide gas plasma) before use. For sampling of PGE2 (molecular mass, 350 Da) commercially available fibres from CMA were used (20 kDa molecular cut-off, 0·5 mm outer diameter, membrane length 30 mm; CMA 60, CMA/Microdialysis AB, Sweden). Both types of microdialysis catheters were perfused via a high-precision syringe pump (CMA 100) at a rate of 1 μl min−1 with a Ringer-acetate solution (Pharmacia & Upjohn). 3H-labelled human type IV collagen (3 nM; 130 kDa; specific activity, 7·0 TBq mg−1; NEN, Boston, MA, USA) was added to the perfusate used in the microdialysis probes for sampling of collagen metabolism to try to mimic the in vivo recovery of PICP and ICTP using the internal reference method (Scheller & Kolb, 1991). Human type IV collagen was used as radioactively labelled type I procollagen was not commercially available and we decided to use a molecule that was larger rather than smaller than the studied substrates. The perfusate used for measuring PGE2 contained 5 nM [15-3H(N)]PGE2 (specific activity, 3·7 GBq mmol−1; NEN) for determination of the in vivo recovery of PGE2 as previously described (Langberg et al. 1999).
After the microdialysis and arterial catheters had been positioned (as described above) the subjects rested for at least 90 min before starting the experiment to ensure that any reaction from the insertion trauma had minimised (Langberg et al. 1999). This procedure was followed during both rest and recovery and when measuring 72 h after exercise (Fig. 1). After flushing the system, dialysate samples were collected every 30 min, with a delay of 3 min due to a void volume from the probe to the sample collector of 3 μl, providing dialysis samples of 30 μl each. The samples were immediately frozen at −70°C until analyses were done within the following 1–2 weeks. The microdialysis catheters were removed before running and new microdialysis catheters were positioned around the Achilles' tendon after the running bout (Fig. 1).
Calculations
The interstitial concentrations (Ci) were calculated using the internal reference calibration method (Scheller & Kolb, 1991). The relative recovery (RR) was calculated for each microdialysis fibre as (Cp - Cd)/Cp, where Cp is disintegration per minute in the perfusate and Cd is disintegration per minute in the dialysate. It is assumed that RR from interstitial fluid to perfusate of unlabelled metabolite equals relative loss from perfusate to interstitial fluid of labelled metabolite.
Analytical methods
Collagen synthesis and degradation
The concentrations of PICP and ICTP were measured in duplicate samples of plasma and dialysate by equilibration radioimmunoassays (RIA) (Orion Diagnositica, Espoo, Finland). All samples from any individual subject were analysed in the same run. The intra-assay precision (coefficient of variation) was 2·7% at 214 μg l−1 for PICP and 4·9% at 6·1 μg l−1 for ICTP (Orion Diagnositica). As endurance exercise has been shown to influence the plasma volume (Fellmann, 1992), the erythrocyte volume fraction values before, immediately after and 72 h after exercise, measured to estimate the relative changes in plasma volume, and the adjusted serum concentrations of the biochemical markers were calculated in accordance with the formula given by van Beaumont (1972).
Inflammatory mediators
PGE2 was analysed using a commercially available PGE2 RIA kit (NEK-020, Du Pont, Boston, MA, USA) (Langberg et al. 1999). All samples from any individual subject were analysed in the same run. The sensitivity of the assay was 5 pg ml−1.
Statistics
PICP (Fig. 2), ICTP (Fig. 3) and PGE2 (Fig. 4) are given as pooled data for rest, running, recovery and 72 h after exercise. All data are presented as means ±s.e.m., or range. Wilcoxon's non-parametric ranking sum test for paired data was used to detect significant differences between rest and running and between rest and the two recovery periods. P < 0·05 (two-tailed test) was considered significant.
Figure 2. Carboxy-terminal propeptide of type I collagen (PICP) measured as a marker for collagen synthesis.
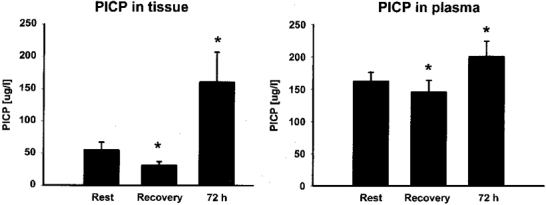
PICP concentration was determined in the tissue around the Achilles' tendon and in plasma during rest, after 36 km of running (Recovery), as well as 72 h after termination of the exercise (means and s.e.m.). * P < 0·05 vs. Rest.
Figure 3. Carboxy-terminal telopeptide region of type I collagen (ICTP) measured as an indicator of collagen breakdown.

ICTP concentration was determined in the tissue around the Achilles' tendon and in plasma at the indicated times (means and s.e.m.). * P < 0·05 vs. Rest.
Figure 4. Effect of exercise on the concentration of PGE2.

PGE2 concentration was measured in the tissue around the Achilles' tendon and in plasma at the indicated times (means and s.e.m.). * P < 0·05 vs. Rest. As microdialysis was not performed during exercise no value for PGE2 was obtained in the tissue during Running.
RESULTS
Recovery of collagen and PGE2
The relative recoveries (RR) of type IV collagen and of PGE2 determined by internal reference calibration are shown in Table 1. No significant differences were found in the RR between rest, recovery and recovery at 72 h for either the collagen metabolite or PGE2. No ultrafiltration was noted as determined from dialysate volume.
Table 1.
Relative recovery of type IV collagen and PGE2 determined by internal reference calibration
| Rest | Recovery 2 h | Recovery 72 h | |
|---|---|---|---|
| Type IV collagen | 46 ± 4% | 59 ± 6% | 37 ± 1% |
| PGE2 | 44 ± 5% | 47 ± 5% | 53 ± 7% |
No significant difference was found between rest, recovery 2 h and recovery 72 h after exercise for either of the two substances measured. Values are means ±s.e.m.
Collagen synthesis and degradation
During the recovery period following running PICP concentration decreased significantly both in the tissue and in plasma (P < 0·05; Fig. 2). However, 72 h after exercise the concentration of PICP both in the peritendinous tissue and in the blood was significantly increased compared to pre-exercise (P < 0·05; Fig. 2). The magnitude of the increase was, however, significantly higher in the tissue (mean, 192% above basal) compared to the rise in plasma (24% above basal) (P < 0·05).
ICTP concentration in the peritendinous tissue decreased significantly immediately after exercise (P < 0·05; Fig. 3), but 72 h after exercise was not significantly different from the concentration measured before exercise. No significant changes in ICTP were observed in plasma.
PGE2
PGE2 concentration increased significantly in plasma during running (P < 0·05), and returned to resting level during recovery (Fig. 4). The concentration measured 72 h after running was not significantly different from the values measured before and after the exercise bout. Measured in the tissue, PGE2 concentration was significantly elevated during the early recovery period immediately after exercise (P < 0·05; Fig. 4), but 72 h after exercise the concentration of PGE2 in the tissue had returned to basal level.
DISCUSSION
In the present study concentrations of PICP and ICTP were measured both in the peritendinous tissue around the Achilles' tendon by microdialysis and in plasma in response to a prolonged bout of exercise. It was possible to quantify changes in the interstitial concentration of high molecular mass substances involved in type I collagen synthesis and degradation in close association with tendinous tissue, and to show that mechanical loading of the calf muscle-tendon unit stimulates type I collagen synthesis in non-bone-associated connective tissue.
Most studies on collagen synthesis and degradation have measured plasma concentrations of PICP and ICTP (Takala et al. 1989; Virtanen et al. 1993; Salvesen et al. 1994; Price et al. 1995; Kristoffersson et al. 1995; Thorsen et al. 1996a; Eliakim et al. 1997; Hupli et al. 1997; Ashizawa et al. 1998), and as type I collagen mainly resides in bone, these results mainly reflect adaptive changes in that tissue, rather than in tendons, ligaments and intramuscular connective tissue (Risteli et al. 1995; Eriksen et al. 1995). However, for in situ measurements of non-bone-related collagen synthesis and degradation methods like for example microdialysis become essential. Previously, studies have been carried out measuring changes in local concentrations of PICP, ICTP and PIIINP (the amino-terminal propeptide of type III procollagen) in human skin (Haukipuro et al. 1991; Oikarainen et al. 1992). In the present study the local synthesis and degradation of type I collagen was measured using microdialysis membranes with a high molecular mass cut-off (3000 kDa), which allows molecules of the size of collagen synthesis and degradation products to diffuse into the dialysate. The relative recovery of these molecules over the membrane was determined by an internal reference method (Scheller & Kolb, 1991) using radioactively labelled type IV collagen molecules in the perfusate. Interestingly, the relative recovery of the radioactively labelled collagen molecules was found to be in the range of, and not significantly different from, the well-accepted recovery determined for glycerol, glucose and prostaglandin (molecular mass of approximately 0·4 kDa) using regular microdialysis membranes with a lower molecular mass cut-off (5–20 kDa) (Langberg et al. 1999) (Table 1). Relative recovery did not vary significantly between sampling times (Table 1); however, some variation is to be expected due to differences in catheter properties and the exact positioning of the catheters within the tissue. In order to account for these differences the relative recovery for each single sample was determined by radioactive substances and used when calculating the interstitial concentration of each sample. Furthermore, the use of microdialysis probes with a larger molecular mass cut-off did not result in any ultrafiltration determined from the dialysate volume. The findings in the present study suggest that the described method would enable in vivo measurements of local tissue concentrations of other substances like hormones or proteins. It is, however, important to stress that the permeability of these membranes for molecules of high molecular mass also allows enzymes to diffuse through the membrane and so react with the substances in the dialysate, and thus potentially can result in degradation or conversion that will lead to an underestimation of the dialysate concentration for the studied substrate.
The concentration of PICP measured in the peritendinous tissue increased significantly, when determined 72 h after prolonged exercise. This indicates an increased synthesis of type I collagen in the peritendinous area in response to increased mechanical loading. In parallel with this, the plasma concentration of PICP was also found to increase 72 h after exercise, which is in accordance with several other studies that have demonstrated similar levels of PICP after various types of exercise (Virtanen et al. 1993; Thorsen et al. 1997; Brahm et al. 1997c). In the present study, interstitial PICP increased 200% in response to exercise when measured in the peritendinous area, whereas plasma PICP only rose 25%. This could indicate that the adaptive and/or reparative process in the connective tissue of the peritendinous region is pronounced after exercise and that its magnitude cannot be deduced from the blood values. To what extent the rise in plasma concentration of PICP reflects an adaptation in tendon vs. bone cannot directly be determined in the present study. However, the findings from our study underline the importance of measuring local collagen synthesis in order to draw conclusions regarding adaptation of specific non-bone-related tissues towards mechanical loading. The fact that the half-life of PICP in the blood of rats is only 5–6 min (Smedsrod et al. 1990) and that no pool of previously formed metabolites exists (Kristoffersson et al. 1995) further supports the view that the concentration of PICP measured most probably reflects the actual status of type I collagen synthesis.
In the present study, physical activity induced a significant decrease in both serum and tissue levels of PICP immediately after exercise (Fig. 2; P < 0·05). This depression in PICP has been demonstrated in several other studies (Virtanen et al. 1993; Thorsen et al. 1996a, 1997; Ashizawa et al. 1998). The explanation for this reduction in PICP immediately after exercise is not known, but it has been hypothesised to be a result of a temporary decrease in type I collagen synthesis induced by intensive mechanical loading (Virtanen et al. 1993) or an increased clearance of PICP by the kidneys (Brahm et al. 1997b). Alternatively, an expansion of plasma volume, which has been shown to occur secondary to acute exercise (Fellmann, 1992), could - at least partly - account for the demonstrated reduction in plasma concentration. However, in the present study no change in haematocrit was observed during the recovery phase after running, which makes this explanation unlikely. Extravasation to the tissue around the Achilles' tendon during running cannot be excluded as a possible explanation for the reduction in tissue PICP.
The concentration of ICTP in the tissue significantly decreased immediately after exercise (Fig. 3; P < 0·05), but normalized after 72 h, whereas the plasma concentration remained unchanged throughout the study (Fig. 3). These findings are in accordance with several other studies on well-trained individuals demonstrating no change in ICTP concentration in response to exercise (Salvesen et al. 1994; Brahm et al. 1996, 1997a,b). However, in studies on physically untrained individuals, ICTP has been found to increase 72 h after exercise (Thorsen et al. 1996a, 1997). These results may indicate that the level of physical fitness could be of importance for the response in collagen metabolism, and that degradation of mainly bone collagen measured as a rise in plasma ICTP occurs only when the load exceeds the strength of the tissue.
It has previously been shown that runners have an increased cross-sectional area of the Achilles' tendon (Engstrom et al. 1985) indicating that training induces a positive protein balance, i.e. synthesis rate of collagen minus degradation rate, compared with untrained individuals. The present study demonstrates an increased PICP concentration in plasma and in the connective tissue around the Achilles' tendon along with an unchanged level of ICTP in the days following acute prolonged exercise, thus supporting the view that exercise induces a positive protein balance in the region around the Achilles' tendon.
The measured concentration of PGE2 in the present study was in accordance with previous findings (Langberg et al. 1999), and it was found to increase in plasma during running, whereas tissue concentration was not determined during exercise, as no microdialysis fibre was present in the tissue during that time period (Fig. 1). As the tissue concentration of PGE2 was 8-fold higher than the plasma concentration during rest, a release of prostaglandins from the tissue to the bloodstream is likely, and it cannot be excluded that the demonstration of an elevated plasma concentration of PGE2 during exercise was due to increased tissue spillover in response to exercise (Fig. 4). The fact that tissue PGE2 concentration was elevated during early recovery and presumably also during exercise together with the demonstrated increase in type I collagen synthesis could indicate that prostaglandins are at least indirectly involved in collagen formation. In support of this, previous in vitro studies of fibroblast cultures obtained from human tendons and animal osteoblast cultures have shown that these types of connective tissue cells release PGE2 when subjected to mechanical load (Almekinders et al. 1993, 1995). Within the physiological range of strain, the release of PGE2 has been found to depend on the magnitude of the applied strain (Murray & Rushton, 1990; Almekinders et al. 1993). In addition animal studies have shown that systemic administration or local intraosseus injection of PGE2 enhances the skeletal response to mechanical loading, leading to stimulation of bone formation, increased metaphyseal bone mass and a marked increased amount of osteoblasts and osteoclasts (Keller et al. 1992; Yang et al. 1993). Taken together, the present findings are in accordance with the hypothesis that PGE2 is involved in the conversion of mechanical stress to type I collagen synthesis and incorporation into connective tissue in humans (Thorsen et al. 1996b). If so the time delay between PGE2 and PICP response seems rather long and may indicate that other factors are involved and that PGE2 is only one part of a cascade coupling loading to tissue adaptation.
In conclusion, the present study describes the use of microdialysis as a reliable method for determination of high molecular mass molecules locally in peritendinous tissue. PICP was found to increase in the peritendinous tissue 72 h after prolonged exercise indicating increased synthesis of type I collagen. The elevated PGE2 levels after strenuous exercise suggest that prostaglandins could be involved in the cascade responsible for conversion of mechanical loading to connective tissue formation.
Acknowledgments
This study was supported by the Team Denmark Research Council, the Danish Sports Science Foundation, the Novo Nordisk Foundation and the Danish National Research Foundation (504–14).
References
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Medicine and Science in Sports and Exercise. 1993;25:603–607. [PubMed] [Google Scholar]
- Almekinders LC, Banes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. American Journal of Sports Medicine. 1995;23:119–123. doi: 10.1177/036354659502300120. [DOI] [PubMed] [Google Scholar]
- Ashizawa N, Ouchi G, Fujimura R, Yoshida Y, Tokuyama K, Suzuki M. Effects of a single bout of resistance exercise on calcium and bone metabolism in untrained young males. Calcified Tissue International. 1998;62:104–108. doi: 10.1007/s002239900402. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Tsuzaki M, Hu P, Brigman B, Brown T, Almekinders L, Lawrence WT, Fischer T. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. Journal of Biomechanics. 1995;28:1505–1513. doi: 10.1016/0021-9290(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Ljunghall S. Biochemical markers of bone metabolism during distance running in healthy, regularly exercising men and women. Scandinavian Journal of Medicine and Science in Sports. 1996;6:26–30. doi: 10.1111/j.1600-0838.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Ljunghall S. Bone metabolism during exercise and recovery: the influence of plasma volume and physical fitness. Calcified Tissue International. 1997a;61:192–198. doi: 10.1007/s002239900322. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Saltin B, Ljunghall S. Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during short lasting dynamic exercise. Calcified Tissue International. 1997b;60:175–180. doi: 10.1007/s002239900210. [DOI] [PubMed] [Google Scholar]
- Brahm H, Strom H, Piehl-Aulin K, Mallmin H, Ljunghall S. Bone metabolism in endurance trained athletes: a comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers. Calcified Tissue International. 1997c;61:448–454. doi: 10.1007/s002239900366. [DOI] [PubMed] [Google Scholar]
- Delgado JM, Defeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Archives of International Pharmacodynamic Therapy. 1972;198:9–21. [PubMed] [Google Scholar]
- Eliakim A, Raisz LG, Brasel JA, Cooper DM. Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males. Journal of Bone and Mineral Research. 1997;12:1708–1713. doi: 10.1359/jbmr.1997.12.10.1708. [DOI] [PubMed] [Google Scholar]
- Engstrom CM, Hampson BA, Williams J, Parker AW. Muscle-tendon relations in runners. In: Oakes BW, editor. Abstracts of the Proceedings of the National Conference of the Australian Sports Medicine Federation, Ballarat. Melbourne, Australia: Australian Sports Medicine Federation; 1985. p. 56. [Google Scholar]
- Eriksen EF, Brixen K, Charles P. New markers of bone metabolism: clinical use in metabolic bone disease. European Journal of Endocrinology. 1995;132:251–263. doi: 10.1530/eje.0.1320251. [DOI] [PubMed] [Google Scholar]
- Fellmann N. Hormonal and plasma volume alterations following endurance exercise. A brief review. Sports Medicine. 1992;13:37–49. doi: 10.2165/00007256-199213010-00004. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Goldring SR. Skeletal tissue response to cytokines. Clinical Orthopaedics. 1990;258:245–278. [PubMed] [Google Scholar]
- Haukipuro K, Melkko J, Ristili L, Kairaluoma M, Risteli J. Synthesis of type I collagen in healing wounds in humans. Annals of Surgery. 1991;213:75–80. doi: 10.1097/00000658-199101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffer WE. Morphology and biochemistry of bone remodelling: possible control by vitamin D, parathyroid hormone, and other substances. Laboratory Investigation. 1988;59:418–442. [PubMed] [Google Scholar]
- Hupli M, Hurri H, Luoto S, Risteli L, Vanharanta H, Risteli J. Low synthesis rate of type I procollagen is normalized during active back rehabilitation. Spine. 1997;22:850–854. doi: 10.1097/00007632-199704150-00004. [DOI] [PubMed] [Google Scholar]
- Keller J, Schumacher B, Lind M. Effect of local prostaglandin E2 on periosteum and muscle in rabbits. Acta Orthopaedica Scandinavica. 1992;63:623–627. doi: 10.1080/17453679209169722. [DOI] [PubMed] [Google Scholar]
- Kiiskinen A. Physical training and connective tissues in young mice - physical properties of Achilles tendons and long bones. Growth. 1977;41:123–137. [PubMed] [Google Scholar]
- Kristoffersson A, Hultdin J, Holmlund I, Thorsen K, Lorentzon R. Effects of short-term maximal work on plasma calcium, parathyroid hormone, osteocalcin and biochemical markers of collagen metabolism. II. International Journal of Sports Medicine. 1995;16:145–149. doi: 10.1055/s-2007-972982. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bülow J, Kjær M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. The Journal of Physiology. 1999;515:919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. American Journal of Physiology. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Melkko J, Kauppila S, Niemi S, Risteli L, Haukipuro K, Jukkola A, Risteli J. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clinical Chemistry. 1996;42:947–954. [PubMed] [Google Scholar]
- Melkko J, Niemi S, Risteli L, Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clinical Chemistry. 1990;36:1328–1332. [PubMed] [Google Scholar]
- Michna H, Hartmann G. Adaptation of tendon collagen to exercise. International Orthopaedics. 1989;13:161–165. doi: 10.1007/BF00268040. [DOI] [PubMed] [Google Scholar]
- Murray DW, Rushton N. The effect of strain on bone cell prostaglandin E2 release: a new experimental method. Calcified Tissue International. 1990;47:35–39. doi: 10.1007/BF02555863. [DOI] [PubMed] [Google Scholar]
- O'Brien M. Structure and metabolism of tendons. Scandinavian Journal of Medicine and Science in Sports. 1997;7:55–61. doi: 10.1111/j.1600-0838.1997.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Oikarinen A, Autio P, Kiistala U, Risteli L, Risteli J. A new method to measure type I and III collagen synthesis in human skin in vivo: demonstration of decreased collagen synthesis after topical glucocorticoid treatment. Journal of Investigative Dermatology. 1992;98:220–223. doi: 10.1111/1523-1747.ep12555884. [DOI] [PubMed] [Google Scholar]
- Price JS, Jackson B, Eastell R, Wilson AM, Russell RG, Lanyon LE, Goodship AE. The response of the skeleton to physical training: a biochemical study in horses. Bone. 1995;17:221–227. doi: 10.1016/8756-3282(95)00221-x. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders. New England Journal of Medicine. 1979;301:13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Risteli J, Niemi S, Kauppila S, Melkko J, Risteli L. Collagen propeptides as indicators of collagen assembly. Acta Orthopaedica Scandinavica Supplement. 1995;266:183–188. [PubMed] [Google Scholar]
- Salvesen H, Piehl-Aulin K, Ljunghall S. Changes in levels of the carboxyterminal propeptide of type I procollagen, the carboxyterminal cross-linked telopeptide of type I collagen and osteocalcin in response to exericse in well-trained men and women. Scandinavian Journal of Medicine and Science in Sports. 1994;4:186–190. [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Simonsen EB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. Journal of Sports Science. 1995;13:291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- Smedsrod B, Melkko J, Risteli L, Risteli J. Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochemical Journal. 1990;271:345–350. doi: 10.1042/bj2710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen H, Kiiskinen A, Heikkinen E. Effects of physical training on metabolism of connective tissues in young mice. Acta Physiologica Scandinavica. 1980;108:17–22. doi: 10.1111/j.1748-1716.1980.tb06495.x. [DOI] [PubMed] [Google Scholar]
- Takala TE, Vuori JJ, Rahkila PJ, Hakala EO, Karpakka JA, Alen MJ, Orava YS, Vaananen HK. Carbonic anhydrase III and collagen markers in serum following cross-country skiing. Medicine and Science in Sports and Exercise. 1989;21:593–597. [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson A, Hultdin J, Lorentzon R. Effects of moderate endurance exercise on calcium, parathyroid hormone, and markers of bone metabolism in young women. Calcified Tissue International. 1997;60:16–20. doi: 10.1007/s002239900179. [DOI] [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson A, Lorentzon R. The effects of brisk walking on markers of bone and calcium metabolism in postmenopausal women. Calcified Tissue International. 1996a;58:221–225. doi: 10.1007/BF02508639. [DOI] [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. Journal of Clinical Investigation. 1996b;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton CM, Matthes RD, Maynard JA, Carey RA. The influence of physical activity on ligaments and tendons. Medicine and Science in Sports. 1975;7:165–175. [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bulletin der Schweizerischen Akademische der Medizinischen Wissenshaften. 1974;30:44–55. [PubMed] [Google Scholar]
- van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. Journal of Applied Physiology. 1972;32:712–713. doi: 10.1152/jappl.1972.32.5.712. [DOI] [PubMed] [Google Scholar]
- Virtanen P, Viitasalo JT, Vuori J, Vaananen K, Takala TE. Effect of concentric exercise on serum muscle and collagen markers. Journal of Applied Physiology. 1993;75:1272–1277. doi: 10.1152/jappl.1993.75.3.1272. [DOI] [PubMed] [Google Scholar]
- Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendon and ligaments. The relationship of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- Yang RS, Liu TK, Lin-Shiau SY. Increased bone growth by local prostaglandin E2 in rats. Calcified Tissue International. 1993;52:57–61. doi: 10.1007/BF00675627. [DOI] [PubMed] [Google Scholar]


