Abstract
Activation of abdominal splanchnic visceral afferents during mesenteric ischaemia induces visceral pain and evokes excitatory cardiovascular responses. Previous studies have shown that interleukin-1β (IL-1β) concentration is increased locally in tissues during ischaemia and reperfusion. Local administration of IL-1β sensitizes somatic afferents to mechanical, thermal and chemical stimulation. Therefore, we hypothesized that IL-1β stimulates or sensitizes splanchnic visceral afferents to ischaemia and to the action of chemical stimuli such as histamine.
The concentration of IL-1β in mesenteric lymph and portal venous plasma in anaesthetized cats was measured with an enzyme-linked immunosorbent assay before, during and after 10 min of abdominal ischaemia. The level of IL-1β was significantly increased during ischaemia in lymph, but not in plasma.
Discharge activity of single-unit abdominal visceral C fibre afferents was measured from the right thoracic sympathetic chain. Ischaemically sensitive C fibre afferents were identified according to their response to 5–10 min of abdominal ischaemia.
Intra-arterial (i.a.) injection of a high dose of IL-1β (500 ng kg−1), but not of a lower dose (i.e. 15, 50 or 150 ng kg−1), stimulated most (six of seven) abdominal visceral afferents.
IL-1β (15 ng kg−1, i.a.) significantly enhanced the increased activity of 11 of 13 C fibre afferents during 10 min of ischaemia. Conversely, an IL-1 type I receptor antagonist (IL-1ra, 1·5 μg kg−1, i.a.) significantly attenuated the increased activity in six of seven other C fibre afferents during ischaemia.
IL-1β (15 ng kg−1, i.a.) significantly augmented the responses of 13 of 16 ischaemically sensitive abdominal afferents to histamine (5–10 μg kg−1, i.a.). Conversely, IL-1ra (1·5 μg kg−1, i.a.) significantly attenuated the responses of five of six other C fibre afferents to histamine.
These data strongly suggest that stimulation of IL-1 type I receptors by IL-1β produced during brief abdominal ischaemia contributes to activation of visceral afferents during ischaemia, at least in part, by sensitizing the afferent nerve endings to ischaemia. Our data also show that exogenous IL-1β sensitizes visceral afferents to histamine.
Brief mesenteric ischaemia stimulates abdominal splanchnic visceral afferents to reflexly evoke excitatory cardiovascular responses (Longhurst, 1995; Rendig et al. 1997). There are a number of potential mediators involved in activation of these afferents. In this regard, our previous studies have demonstrated that lactic acid, bradykinin, cyclo-oxygenase products, histamine, serotonin and reactive oxygen species are produced during abdominal ischaemia and reperfusion (Longhurst, 1995; Fu et al. 1997a). We also have shown that these substances individually contribute to the increase in discharge activity of abdominal splanchnic sensory fibres by activating or sensitizing afferent nerve endings (Longhurst, 1995; Fu et al. 1997b; Fu & Longhurst, 1998). However, it appears that no one of these substances is responsible solely for activation or sensitization of these afferents during ischaemia. We believe, therefore, that other chemicals probably play a role in activation or sensitization of these sensory nerve endings.
Interleukin-1β (IL-1β, a 17 kDa polypeptide), one of the acute reactant cytokines, mediates many cellular responses during inflammation and hyperalgesia (Dinarello, 1996). First, IL-1β is synthesized and released by a variety of cells including monocytes, macrophages, central nervous system microglia, endothelial cells, smooth muscle cells, and intestinal, gingival and cervical epithelia (Dinarello, 1996). Evidence from experimental models of acute ischaemia indicates that ischaemia and reperfusion can increase the production of IL-1β (Entman et al. 1991). For instance, it has been found that ischaemia-reperfusion increases the concentration of IL-1β in skeletal muscle (Ascer et al. 1992), liver (Clavien et al. 1996), and brain (Saito et al. 1996). Second, local administration of IL-1β is capable of stimulating the hepatic branches of vagal afferents and of inducing cutaneous hyperalgesia by activating or sensitizing somatic sensory nerve endings to chemical, mechanical and thermal stimulation (Fukuoka et al. 1994; Perkins & Kelly, 1994; Davis & Perkins, 1995). Finally, Schultzberg et al. (1987) have observed that IL-1 immunoreactive nerve fibre afferents are located in the abdominal visceral organs and the coeliac-superior mesenteric ganglion complex of rat. However, it has not been determined if IL-1β is released in sufficient quantities to activate or sensitize abdominal visceral afferents during ischaemia.
The role of IL-1β in physiology or pathophysiology of disease in vivo is through activation of IL-1 receptors. Recently, two IL-1 receptor subtypes, type I and II, have been identified (Dinarello, 1996). Ek et al. (1998) have found that the IL-1 type I receptor (IL-1RI) is located in the nodose ganglion of rat. The influence of IL-1β on mechanical nociceptors is mediated by IL-1RI (Dinarello, 1996). Also, intravenous injection of IL-1β activates vagal afferents through activation of IL-1RI (Ek et al. 1998). Moreover, it has been found that the interleukin-1 receptor antagonist (IL-1ra) binds to IL-1RI with high affinity, but does not bind to IL-1 type II receptors (Arend, 1991). IL-1ra blocks the action of IL-1β on cutaneous sensory nerves (Oka et al. 1993; Davis & Perkins, 1995). However, there is no information about the role of IL-1 receptors with respect to the action of IL-1β on abdominal visceral afferents.
We have recently documented that activation of ischaemically sensitive abdominal splanchnic afferents by histamine during ischaemia is mediated by histamine H1 receptors (Fu et al. 1997b), which, at least in part, are linked to the production of diacylglycerol (DAG) (Daum et al. 1984; Ruat et al. 1992). There is other evidence showing that activation of IL-1 receptors is coupled to a G-protein that activates phospholipase C which, in turn, hydrolyses phospholipids liberating DAG (Dinarello, 1996). IL-1β stimulates the synthesis and release of histamine in presynaptic terminals located in the hypothalamus through activation of histidine decarboxylase (Niimi et al. 1994). Basophils and mast cells, present in intestinal mucosa, are also capable of releasing histamine when they are stimulated by IL-1β (Subramanian & Bray, 1987). We suggest, therefore, that IL-1β and histamine interact to stimulate ischaemically sensitive abdominal splanchnic afferents.
The purpose of this study was to determine if IL-1β is produced during abdominal ischaemia and whether IL-1β stimulates or sensitizes abdominal splanchnic visceral afferents to ischaemia and histamine. We hypothesized that (i) IL-1β is produced and released into the interstitial space, where afferent nerve endings are located, during brief abdominal ischaemia, (ii) IL-1β contributes to activation of abdominal splanchnic afferents during brief ischaemia by stimulating or sensitizing afferent nerve endings through activation of the IL-1 type 1 receptor, and (iii) IL-1β sensitizes ischaemically sensitive abdominal splanchnic afferents to the action of histamine.
METHODS
Surgical preparation
Experiments were performed on 79 fasted cats of either sex (2·8 ± 0·2 kg). Surgical and experimental protocols used in this study were approved by the Animal Use and Care Committee at the University of California, Davis. The studies conformed to the American Physiological Society Guidelines and Principles Involving Animals. Anaesthesia was induced with ketamine (20–30 mg kg−1, i.m., Phoenix Scientific, Inc., St Joseph, MO, USA) and was maintained with α-chloralose (40–50 mg kg−1, i.v.). Additional injections of α-chloralose (5–10 mg kg−1, i.v.) were given as needed to maintain an adequate depth of anaesthesia that was assessed by observing the absence of the conjunctival reflex. The trachea of the animal was intubated and respiration maintained artificially (Harvard pump, model 661, Ealing, South Natick, MA, USA). Inspired air was supplemented with 100% O2 through the ventilator. A femoral vein was cannulated for administration of drugs and fluid. A femoral arterial catheter was positioned with its tip in the thoracic descending aorta for measurement of pressure and for administration of drugs. Systemic arterial blood pressure was measured by a pressure transducer (Statham P23 ID, Gould) connected to the femoral arterial catheter. Arterial blood gases were frequently assessed using a blood gas analyser (Radiometer ABL-3, Copenhagen, Denmark) and maintained within physiological limits (partial pressures of oxygen (PO2) > 100 mmHg and CO2 (PCO2) 28–35 mmHg, pH 7·35-7·45) by adjusting the respirator rate or tidal volume, or by intravenously administering 2–3 ml of 1 M NaHCO3 (8·4% (w/v)). Body temperature was monitored using a rectal thermistor and was maintained at 36–38°C with a circulating water heating pad and heat lamp. At the end of the experiment, the animals were killed by administration of saturated potassium chloride (i.v.).
IL-1β measurement
The abdomen of the animal was opened via a mid-line incision to expose abdominal viscera. The descending thoracic aorta was exposed through the diaphragm to allow placement of an inflatable occlusion cuff. A catheter (3·0 Fr; 1 mm diameter) was inserted into a splenic vein and the tip was advanced into the portal vein for withdrawal of venous blood samples. Portal venous blood samples (1 ml) were withdrawn into heparinized syringes and placed into iced polypropylene microcentrifuge tubes containing 200 units of heparin. After each blood sample was withdrawn an equal volume of physiological saline was injected into the femoral vein. A major postnodal lymphatic duct draining the intestine was identified and cannulated with a catheter (PE-90, 7–8 cm, Clay Adams, Parsippany, NJ, USA). A surgical microscope was frequently used to facilitate lymph duct isolation and catheter insertion. The distal tip of the catheter was externalized through the abdominal wall and was positioned level with or below the lymph vessel. Lymph fluid was collected directly into a 0·5 ml polypropylene microcentrifuge tube (Sarstedt, Germany) containing 100 U of heparin. The viscera were covered with gauze pads that were kept moist with warm Ringer solution.
Assays
Lymph and plasma IL-1β levels were measured by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's directions (Heney & Whicher, 1995). ELISA kits were purchased from Amersham International (Amersham Place, Little Chalfont, Bucks, UK). The sensitivity of this assay for IL-1β is 0·1 pg ml−1. The specificity of this assay recognizes both natural and recombinant IL-1β. It does not cross react with IL-1α, IL-1ra, IL-2, IL-3, IL-4, IL-6, IL-7, IL-8, tumour necrosis factor α, γ-interferon or α-interferon.
Afferent recording
Single-unit activity of abdominal visceral C fibre afferents was recorded as described previously (Fu et al. 1997b; Fu & Longhurst, 1998). In brief, a mid-line sternotomy was performed. The third to eleventh right ribs and the middle and caudal lobes of the right lung were removed. Both phrenic nerves were isolated and cut. An inflatable occlusion cuff was placed around the descending thoracic aorta just above the diaphragm. The right paravertebral sympathetic chain was isolated and then draped over a plexiglass platform and covered with warm mineral oil. Small nerve filaments were dissected gently from the chain between T6 and T10 under an operating microscope (Zeiss, Germany) and the caudal ends were placed across one pole of the recording electrode. The other pole of the recording electrode was grounded with cotton thread to the animal. The recording electrode was attached to a high impedance probe (model HIP511, Grass Instruments, Quincy, MA, USA), and the signal was amplified (model P511 preamplifier, Grass, USA) and processed through an audioamplifier (AM8B, Audiomonitor, Grass, USA), an oscilloscope (model 2201, Tektronix, Beavertown, OR, USA), and then recorded on a chart recorder (TA 4000B, Gould, Cleveland, OH, USA). The neurogram also was fed into an IBM compatible Pentium computer through an analog-to-digital interface card (RC Electronics Inc., Santa Barbara, CA, USA) for subsequent off-line analysis. The discharge frequency of afferents was analysed by using a data acquisition and analysis software (EGAA, version 3.02, RC Electronics Inc.).
We exposed abdominal visceral organs through a ventral mid-line incision. Receptive fields of afferents were located precisely using a fine-tipped glass rod and a stimulating electrode to evoke an action potential. Conduction time of the afferent was determined by measuring the time interval from the signal of electrical stimulation to recording of the action potential. Conduction distance was estimated with a thread placed from the receptive field along the supposed afferent pathway through the prevertebral ganglion along the course of the major splanchnic nerve to the splanchnic chain and the recording electrode. Conduction velocity (CV) of each afferent fibre was calculated by dividing the conduction distance by the conduction time. C fibre afferents were classified as those with a conduction velocity of < 2·5 m s−1. The CV of afferents included in this study was 0·45 ± 0·02 m s−1 (mean ±s.e.m.); and each afferent had a single receptive field that could be located precisely. Afferents were considered to be ischaemically sensitive if their discharge activity during 5–10 min of abdominal ischaemia was increased at least twofold above baseline activity (Fu et al. 1997b; Fu & Longhurst, 1998). We closed the abdominal incision with towel clamps and covered the viscera with warm saline-soaked gauze to prevent fluid and heat losses.
Experimental protocols
IL-1β concentration during abdominal ischaemia
Each animal was stabilized for at least 30 min following instrumentation. Samples of intestinal lymph fluid (∼0·4 ml) and portal venous blood (1 ml) were then collected and placed on ice. Ten minutes of abdominal ischaemia was initiated by inflation of the aortic occlusion cuff and additional samples of lymph fluid were collected either throughout the 10 min period of ischaemia or throughout the first 10 min of reperfusion, because intestinal lymph flow averages 44 ± 3·7 μl ml−1 (Fu et al. 1997a). Portal venous blood was collected at 530–590 s during the 10 min period of ischaemia and immediately after release of the occlusion. Only one ischaemic period was conducted in each cat. The IL-1β concentration of samples was measured by ELISA in each of these 10 cats.
To determine the effects of surgical manipulation, a time control group (10 cats) was treated in an identical manner but was not subjected to ischaemia. IL-1β concentrations were measured.
Effect of IL-1β directly on afferent discharge activity
Thirteen animals were subjected to 5–10 min of abdominal ischaemia followed by 3–5 min of reperfusion. After identification of an ischaemically sensitive unit, we opened the abdomen, located the receptive field of the nerve ending and measured the conduction velocity, as described previously. Warm, moist gauze sponges were placed over the viscera and the abdomen was closed with towel clamps. Thirty minutes later, afferent activity was measured in response to injection of one of several doses of IL-1β (15 or 50 ng kg−1, n = 9 afferents; 150 or 500 ng kg−1, n = 7 afferents) through the catheter into the descending thoracic aorta (i.a.) in a randomized fashion. Recombinant human IL-1β was purchased from Sigma Chemical Company (Sigma, St Louis, MO, USA) and dissolved in sterile phosphate-buffered saline (PBS, pH 7·6) containing 0·1% bovine serum albumin (BSA) to a concentration of 1 μg ml−1, and was stored at −70°C (prepared freshly each month). Afferent activity was recorded for a 45 min period (5 min before and 40 min after injection of IL-1β). At least 25 min of recovery time was allowed to elapse between the interventions to avoid tachyphylaxis. The chosen doses of IL-1β have been shown to effectively stimulate or sensitize vagal and cutaneous afferents (Fukuoka et al. 1994; Niijima, 1996). Forty-five minutes after application of IL-1β, we injected histamine (10 μg kg−1, i.a.) to establish that the afferent nerve ending was accessible. In the same group, activity of afferents was recorded in response to the appropriate volume of vehicle (i.e. PBS containing 0·1% BSA).
Effect of IL-1β on the response of afferents to ischaemia
The effect of IL-1β (15 ng kg−1, i.a.) on the response of abdominal afferents to ischaemia was investigated in 11 cats. After identification of a C fibre, the discharge activity of the fibre was measured during 10 min of abdominal ischaemia followed by 3–5 min of reperfusion. If the afferent was ischaemically sensitive, a second bout of ischaemia was repeated 45 min later in the presence of IL-1β (15 ng kg−1, i.a.).
To estabilish reproducibility, the discharge activities of five additional C fibre afferents in four cats (2 C fibre afferents were recorded in 1 of the 4 cats) were measured in response to repeated periods of ischaemia. After identification, each fibre was treated identically except that the same volume of vehicle (PBS with 0·1% BSA) was used in place of IL-1β.
Effect of IL-1ra on the response of afferents to ischaemia
In this protocol, after identifying C fibre afferents, five cats were subjected to 10 min abdominal ischaemia followed by 3–5 min reperfusion. If the afferent responded to ischaemia, we repeated ischaemia 35–45 min after the first period, including 25–30 min after treatment with IL-1ra (1·5 μg kg−1, i.a.). This dose of IL-1ra effectively blocks the action of IL-1β on cutaneous afferents (Oka et al. 1993; Davis & Perkins, 1994). Recombinant human IL-1ra (R&D Systems, Minneapolis, MN, USA) was dissolved in sterile PBS containing 0·1% BSA to a concentration of 10 μg ml−1, and stored at −70°C (freshly prepared each month).
Effect of IL-1β on the response of afferents to histamine
In 12 cats, we examined the effect of IL-1β on the response of abdominal C fibre afferents to histamine. After identifying an ischaemically sensitive unit with a receptive field in the abdominal region, we measured the response of afferents to injection of histamine (5–10 μg kg−1, i.a.) in the absence or presence (45 min later) of IL-1β (15 ng kg−1, i.a.). Afferents were re-challenged with 10 μg histamine administered intra-arterially if they did not respond to 5 μg histamine. Afferents were not included in the present study if they did not respond to 5 or 10 μg of histamine.
To determine the reproducibility of afferent response to histamine, nine additional cats were studied as time controls. In this protocol, after identification of ischaemically sensitive afferents, each fibre was treated identically but was not subjected to IL-1β.
Effects of IL-1ra and IL-1β on the response of afferents to histamine
This protocol consisted of five cats subjected to 5–10 min of abdominal ischaemia followed by 3–5 min of reperfusion. After identifying an ischaemically sensitive C fibre, we measured the response of afferents to injection of histamine (5–10 μg kg−1, i.a.) in the absence or the presence (45 min later) of IL-1ra (1·5 μg kg−1, i.a.) and IL-1β (15 ng kg−1, i.a.).
Data analysis
Peak discharge rates of ischaemically sensitive afferents were averaged over 60 s during 3–5 min of control and 10 min of ischaemia, when the greatest number of spikes occurred (Longhurst et al. 1991; Fu et al. 1997). We measured the response of afferent nerve endings to histamine, IL-1β and IL-1ra by averaging discharge rates of afferents during the entire period of response. We assessed the latency of afferent responses to ischaemia and each chemical stimulus from the time of arterial occlusion or intra-arterial injection of the chemicals to the point when sustained discharge activity of afferents exceeded a 50% increase over baseline activity.
Data are expressed as means ±s.e.m. We compared the effects of histamine, IL-1β, IL-1ra and vehicle on the activity of afferents using Student's paired t test. The effects of IL-1β, IL-1β with IL-1ra, and IL-1ra on histamine- or ischaemia-induced increases in activity of the afferents were compared using one-way repeated-measures analysis of variance (ANOVA) with a Tukey post hoc test. The same test also was used to compare the effects of repeated injections of histamine, IL-1β (i.e. 15, 50, 150 and 500 ng kg−1) and repeated ischaemia on the activity of the afferents, and ischaemia on the concentration of IL-1β in lymph and in plasma. If the data were not normally distributed, as determined by the Kolmogorov-Smirnov test, they were compared with the Friedman repeated-measures analysis of variance on ranks with a Dunnett's post hoc test. All statistical calculations were performed with Sigmastat software (Jandel scientific Software, San Rafael, CA, USA). Values were considered to be significantly different when P < 0·05.
Part of this work was presented as a preliminary communication at the Experimental Biology Meeting in San Francisco in 1998 (Yu et al. 1998).
RESULTS
IL-1β concentration in mesenteric lymph during abdominal ischaemia
The concentration of IL-1β in mesenteric lymph fluid during the entire 10 min period of ischaemia increased significantly from pre-ischaemia levels of 0·90 ± 0·07 to 1·96 ± 0·18 pg ml−1 (Fig. 1). Conversely, the concentration of IL-1β in lymph during sham ischaemia remained unchanged from pre-ischaemia levels in the time control animals (0·90 ± 0·12 vs. 0·91 ± 0·07 pg ml−1, respectively, P > 0·05). Compared with the corresponding time control cats, the concentration of IL-1β in mesenteric lymph was elevated significantly during ischaemia. The increase in concentration of IL-1β in lymph during the first 10 min period of reperfusion was insignificant compared with pre-ischaemia levels. The concentration of IL-1β in portal venous plasma before and during ischaemia was undetectable.
Figure 1. Effect of abdominal ischaemia on production of IL-1β in mesenteric lymph.
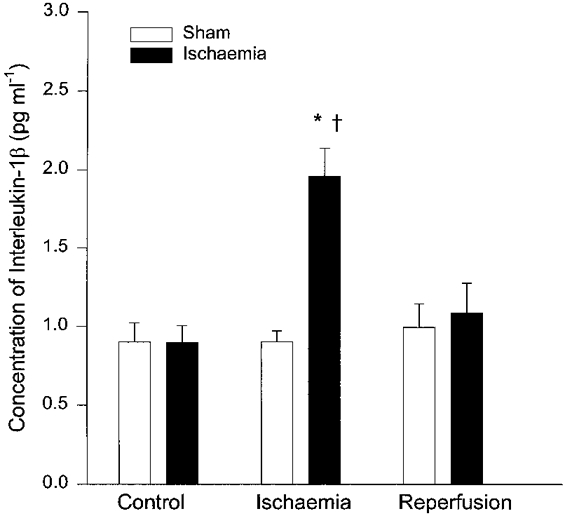
Concentration of IL-1β was significantly increased in mesenteric lymph in 10 cats during ischaemia (▪) compared with pre-ischaemia and corresponding time controls of 10 sham cats (□). Data are means ±s.e.m.*P < 0·01vs. pre-ischaemia, †P < 0·05vs. corresponding time controls. There was no change in IL-1β during reperfusion compared with pre-ischaemia and sham groups.
Response of abdominal afferents to IL-1β
The effect of the different doses of IL-1β on the discharge activity of 16 ischaemically sensitive C fibre afferents is summarized in Fig. 2. Intra-arterial injections of the three lower doses of IL-1β (15 or 50 ng kg−1, n = 9 (2 C fibres were recorded in 2 of the 7 animals), and 150 ng kg−1, n = 7) did not alter activity of afferents although each afferent was stimulated subsequently by histamine (5–10 μg kg−1, i.a.). However, the highest dose of IL-1β (500 ng kg−1, i.a.) stimulated six of seven (2 C fibres were recorded in 1 of the 6 animals) ischaemically sensitive abdominal afferents (P < 0·05) after a latency of 148 ± 43 s. Injection of the vehicle (PBS containing 0·1% BSA) did not stimulate any of the 16 C fibre afferents tested.
Figure 2. Responses of ischaemically sensitive C fibre afferents to different doses of IL-1β.
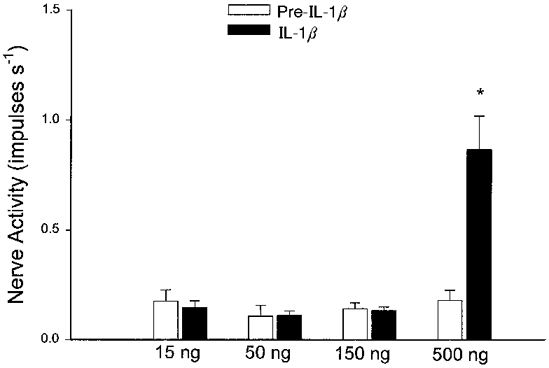
Bar graph showing peak impulse activity of ischaemically sensitive abdominal C fibre afferents before (□) and after (▪) intra-arterial injection of IL-1β (15 and 50 ng kg−1, n = 9; 150 and 500 ng kg−1, n = 7). Columns and error bars are means ±s.e.m.*P < 0·05 compared with pre-IL-1β.
Effect of IL-1β on the response of abdominal visceral afferents to ischaemia
Representative tracings of an ischaemically sensitive C fibre innervating the portal hepatis with a conduction velocity of 0·34 m s−1 are shown in Fig. 3. Treatment with IL-1β (15 ng kg−1, i.a.) augmented the increase in discharge activity of this afferent and decreased the onset latency during repeat ischaemia compared with the initial period of ischaemia.
Figure 3. Effect of IL-1β on the response of a C fibre afferent during abdominal ischaemia.
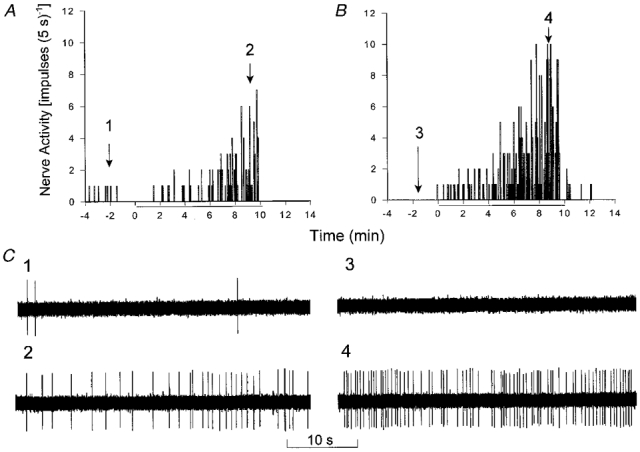
A and B, histograms showing response of an abdominal visceral C fibre (CV, 0·34 m s−1) innervating the portal hepatis to 10 min of ischaemia (indicated by the bars beneath the x-axes). Ischaemia increased baseline activity from 0·07 impulses s−1 to a peak activity of 0·63 impulses s−1 during ischaemia, after an onset latency of 265 s (A). Treatment with IL-1β (15 ng kg−1, i.a.) enhanced the increase in discharge activity of this afferent from 0 to 1·35 impulses s−1 during repeated ischaemia, after an onset latency of 105 s (B). C, neurograms 1–4 are tracings of C fibre discharge activity at the times indicated above the histograms in A and B.
Figure 4A summarizes the effect of treatment with IL-1β on the peak 60 s impulse activity of 11 of 13 (2 C fibre afferents were recorded in 2 of the 11 animals) ischaemically sensitive C fibre afferents during ischaemia. Two of the 13 afferents were unresponsive to IL-1β. Aortic occlusion significantly decreased mean arterial pressure from 86 ± 10 to 12 ± 2 mmHg (P < 0·05). Ischaemia significantly increased the discharge activity of these afferents from 0·12 ± 0·05 to 0·74 ± 0·09 impulses s−1 after an onset latency of 232 ± 17 s. The afferent nerve endings were located in the bile duct, duodenum, gallbladder, mesentery, pancreas or porta hepatis (Table 1). IL-1β treatment did not alter distal arterial pressure during ischaemia (12 ± 2 vs. 13 ± 2 mmHg, before vs. after) or the pre-occlusion mean arterial pressure (86 ± 10 vs. 88 ± 9 mmHg). However, IL-1β significantly augmented the response to ischaemia in the 11 responsive C fibre afferents (Fig. 4A), and decreased the onset latency (232 ± 17 vs. 147 ± 11 s, P < 0·05). This increase in activity of afferents during the second period of ischaemia was not due to a general increase in reactivity over time since five additional ischaemically sensitive afferents were consistently responsive to repeated 10 min periods of abdominal ischaemia (0·04 ± 0·02 to 0·84 ± 0·16 vs. 0·08 ± 0·05 to 0·85 ± 0·16 impulses s−1, initial vs. repeat ischaemia), an observation that was consistent with our previous study (Fu et al. 1997b).
Figure 4. Influence of IL-1β on group responses of C fibre afferents during abdominal ischaemia.
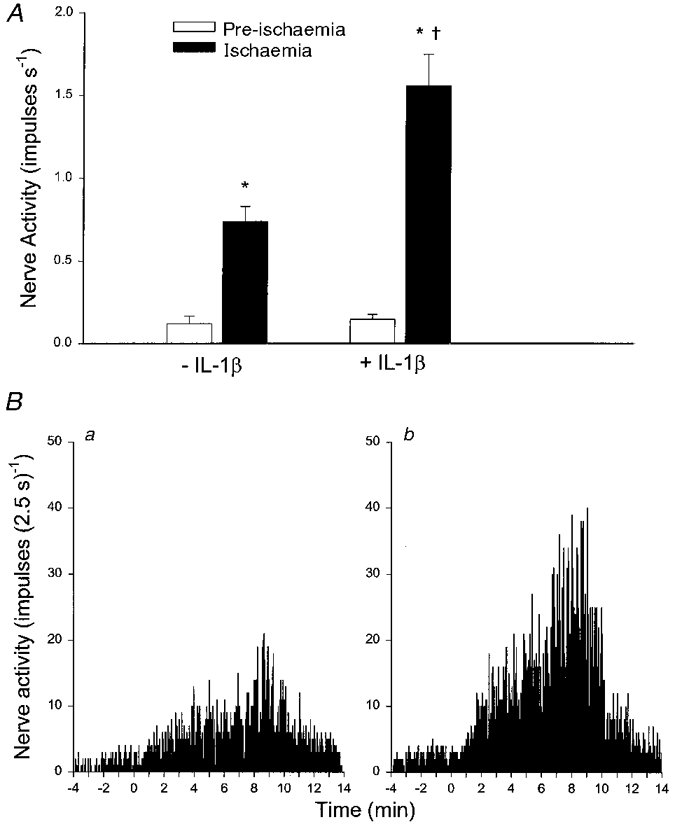
A, bar graph summarizing the effect of IL-1β (15 ng kg−1, i.a.) on peak (60 s) impulse activity of 11 abdominal visceral C fibre afferents before (□) and during (▪) 10 min of ischaemia. -IL-1β and +IL-1β, responses before and after treatment with IL-1β, respectively. B, neurohistograms show summed 2·5 s discharge activity of 11 ischaemically sensitive C fibre afferents during 10 min of ischaemia before (a) and after (b) treatment with IL-1β (15 ng kg−1, i.a.). Data are presented as means ±s.e.m.*P < 0·05 compared with pre-ischaemia; †P < 0·05, post-IL-1βvs. pre-IL-1β controls.
Table 1.
Location of ischaemically sensitive abdominal visceral C fibre afferent endings
| Location | IL-1β | Ischaemia +IL-1β | Repeat ischaemia | Ischaemia +IL-1ra | Histamine +IL-1β | Repeat histamine | Histamine +IL-1ra +IL-1β |
|---|---|---|---|---|---|---|---|
| Bile duct | 3 | 1 | — | 1 | 3 | 2 | — |
| Duodenum | 3 | 1 | 1 | 2 | 4 | 3 | 2 |
| Gallbladder | 2 | 2 | — | — | — | — | 1 |
| Mesentery | 3 | 4 | 1 | 1 | 3 | 3 | 1 |
| Pancreas | 3 | 2 | 2 | 2 | 3 | 2 | 1 |
| Porta hepatic | 2 | 3 | 1 | 1 | 3 | 1 | 1 |
| Total | 16 | 13 | 5 | 7 | 16 | 11 | 6 |
Values reflect numbers of afferent endings. IL-1β, interleukin-1β; IL-1ra, IL-1 type I receptor antagonist.
Figure 4A shows neurohistograms displaying summed 2·5 s discharge activity of afferents during 10 min of ischaemia in the 11 C fibre afferents in the absence (Fig. 4Ba) and presence (Fig. 4Bb) of IL-1β. Similar to the change in peak 60 s discharge activity, summed activity during the entire 10 min of ischaemia was augmented by 116% after administration of IL-1β.
Effect of IL-1ra on response of abdominal visceral afferents to ischaemia
Figure 5 summarizes the effect of treatment with IL-1ra on the peak 60 s impulse activity during ischaemia in six of seven (2 C fibre afferents were recorded in 2 of 5 animals) ischaemically sensitive C fibre afferents. Ischaemia significantly increased discharge activity of these afferents from 0·12 ± 0·04 to 0·90 ± 0·16 impulses s−1, after an onset latency of 212 ± 36 s. After treatment of IL-1ra, however, the ischaemia-induced increase in the peak discharge activity of afferents was significantly attenuated (0·08 ± 0·04 to 0·30 ± 0·07 impulses s−1) in six of seven fibre afferents and their onset latency of response was significantly increased to 373 ± 51 s, compared with the initial period of ischaemia. Aortic occlusion to induce ischaemia similarly decreased mean arterial pressure in the absence (85 ± 10 to 11 ± 3 mmHg) and presence of IL-1ra (87 ± 11 to 10 ± 2 mmHg, P < 0·05). An original tracing of an ischaemically sensitive C fibre (CV, 0·38 m s−1) innervating the pancreas, before and after blockade of IL-1 receptors with IL-1ra, is shown in Fig. 6. The afferent nerve endings studied in this protocol were located in the bile duct, duodenum, mesentery or pancreas (Table 1).
Figure 5. Effect of IL-1 receptor blockade on the response of C fibre afferents to ischaemia.
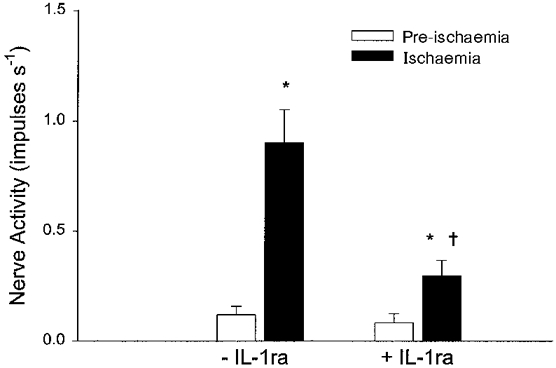
Bar graph summarizing the effect of IL-1ra (1·5 μg kg−1, i.a.) on peak (60 s) impulse activity of six abdominal visceral C fibre afferents during 10 min of ischaemia. Columns and error bars represent means ±s.e.m.*P < 0·05 compared with pre-ischaemia; †P < 0·05, post-IL-1ra vs. pre-IL-1ra controls.
Figure 6. Effect of IL-1ra on the response of a C fibre afferent to abdominal ischaemia.
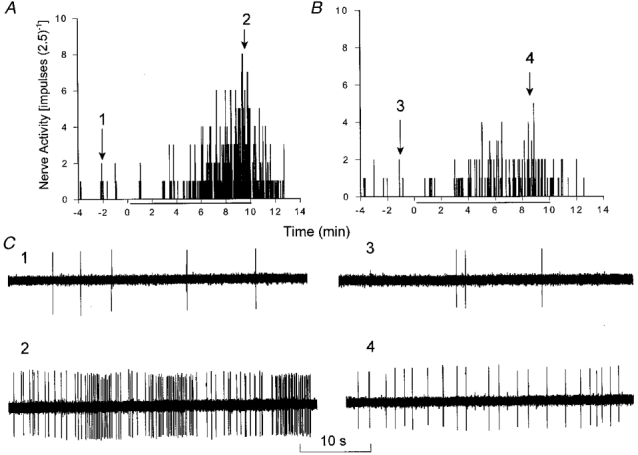
Histograms displaying the response of a C fibre (CV, 0·38 m s−1) innervating the pancreas to 10 min of ischaemia (indicated by bars beneath the x-axes). Ischaemia increased baseline activity from 0·10 impulses s−1 to a peak activity of 1·58 impulses s−1 during ischaemia (A). Treatment with IL-1ra (1·5 μg kg−1, i.a.) attenuated the increase in discharge activity of this afferent from 0·07 to 0·51 impulses s−1 during repeated ischaemia (B). C, neurograms 1–4 represent original tracings of C fibre discharge activity at the times indicated above the histograms in A and B.
Effect of IL-1β on the response of abdominal visceral afferents to histamine
In 12 animals, we found that intra-arterial IL-1β (15 ng kg−1) augmented the response of 13 of 16 ischaemically sensitive abdominal afferents to histamine (5–10 μg kg−1, i.a.) (Fig. 7A). Furthermore, the onset latency of discharge activity of these 13 afferents to histamine was decreased after treatment with IL-1β (16·8 ± 2·0 vs. 10·1 ± 1·1 s, P < 0·05). The afferent nerve endings studied in this protocol innervated the bile duct, duodenum, mesentery, pancreas or porta hepatis (Table 1). Figure 7A displays an original tracing of an ischaemically sensitive C fibre (CV, 0·26 m s−1) innervating the duodenum during injection of histamine (5 μg kg−1, i.a.) in the absence and the presence of IL-1β.
Figure 7. Influence of IL-1β on the response of ischaemically sensitive afferents to histamine.
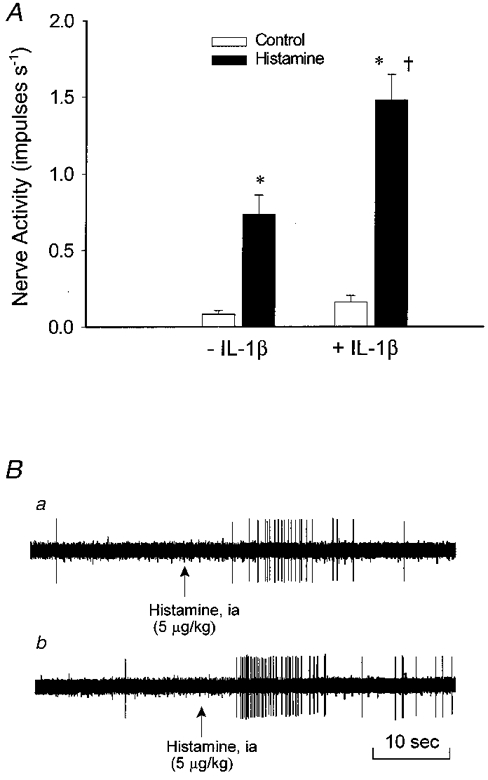
A, effect of injection of histamine (5–10 μg kg−1) on mean activity of 13 ischaemically sensitive C fibre afferents before (-IL-1β) and after (+IL-1β) treatment with IL-1β (15 ng kg−1, i.a.). B, neurogram of discharge activity of an ischaemically sensitive abdominal afferent (CV, 0·26 m s−1) innervating the duodenum during injection of histamine (5 μg kg−1, i.a.) before (a) and after (b) treatment with IL-1β. Columns and error bars are means ±s.e.m.*P < 0·05 compared with control; †P < 0·05, post-IL-1βvs. pre-IL-1β controls.
Effect of co-administration of IL-1ra and IL-1β on response of abdominal afferents to histamine
Co-administration of IL-1ra (1·5 μg kg−1, i.a.) and IL-1β (15 ng kg−1, i.a.) attenuated the response of five of six C fibre afferents to histamine (5–10 μg kg−1, i.a.) (Fig. 8A). The onset latency of response was insignificantly increased from 12·6 ± 1·0 (initial) to 19·2 ± 4·3 s (repeat). These afferent nerve endings were located in the duodenum, gallbladder, mesentery, pancreas or porta hepatis (Table 1). The mean conduction velocity was 0·50 ± 0·06 m s−1.
Figure 8. Interaction between histamine, IL-1ra and IL-1β on ischaemically sensitive C fibre afferents.
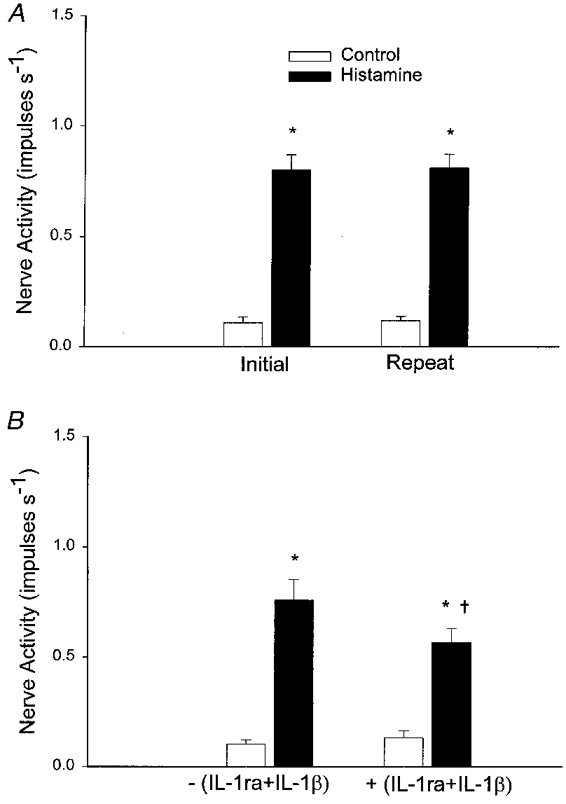
A, bar graph of peak impulse activity of 11 ischaemically sensitive C fibre afferents during repeated injection of histamine (5–10 μg kg−1). B, effect of injection of histamine (5–10 μg kg−1) on impulse activity of five ischaemically sensitive C fibre afferents before and after treatment with IL-1β (15 ng kg−1, i.a.) plus IL-1ra (1·5 μg kg−1, i.a.). Data are presented as means ±s.e.m.*P < 0·05 compared with respective control values. †P < 0·05, post-IL-1ra+IL-1βvs. pre-IL-1ra+IL-1β controls.
Changes in responsiveness of afferents to histamine after IL-1β or IL-1ra with IL-1β were not due to a general alteration in activities over time because 11 other ischaemically sensitive abdominal afferents responded in a consistent manner to repeated injections of histamine (5–10 μg kg−1) over the same time frame (Fig. 8A). Afferent nerve endings studied in this protocol were located in the duodenum, gallbladder, mesentery, pancreas or porta hepatis (Table 1).
DISCUSSION
Four important observations were made in this study. First, the concentration of IL-1β in mesenteric lymph was increased during a brief period (10 min) of abdominal ischaemia. Second, exogenous IL-1β at a high dose (500 ng kg−1), but not at lower doses (i.e. 15, 50 and 150 ng kg−1), is capable of directly stimulating ischaemically sensitive abdominal visceral afferents. Third, endogenously produced IL-1β contributes to activation of abdominal splanchnic visceral C fibre afferents during brief ischaemia through the activation of IL-1 receptors. In this regard, intra-arterial injection of IL-1β (15 ng kg−1) augmented the response of ischaemically sensitive abdominal visceral afferents to ischaemia. Furthermore, the response of these afferents to ischaemia was attenuated significantly after treatment with IL-1ra, an IL-1 type I receptor antagonist. Fourth, IL-1β sensitizes ischaemically sensitive abdominal splanchnic afferents to histamine. We observed that the response of ischaemically sensitive abdominal afferents to histamine was augmented by 116% in the presence of IL-1β compared with that in the absence of IL-1β, while administration of IL-1ra attenuated the response of these afferents to histamine. Thus, data from the present study strongly suggest that IL-1β produced during ischaemia sensitizes ischaemically sensitive abdominal splanchnic visceral C fibre afferents to ischaemia. In addition, our data indicate that IL-1β also sensitizes ischaemically sensitive abdominal visceral afferents to histamine through activation of IL-1 type I receptors.
There are a number of potential mediators involved in ischaemia-induced activation of abdominal visceral afferents. Our previous studies have shown that lactic acid, prostaglandins, bradykinin, histamine, serotonin and reactive oxygen species (Longhurst, 1995; Fu et al. 1997a) are produced during abdominal ischaemia and reperfusion. These substances can individually stimulate or sensitize ischaemically sensitive splanchnic afferent nerve endings (Longhurst et al. 1991, 1995; Fu et al. 1997b; Fu & Longhurst, 1998). Many cell types including monocytes, macrophages, neurons, endothelial cells, smooth muscle cells, and intestinal epithelia can synthesize and release IL-1β (Dinarello, 1996). Recently, others have found that IL-1β is increased in the venous effluent from canine skeletal muscle during ischaemia and reperfusion (Ascer et al. 1992). The concentration of IL-1β in the brain also is increased during the early reperfusion period after transient global ischaemia induced by bilateral occlusion of the carotid arteries (Saito et al. 1996). In the abdominal region, the level of IL-1β in plasma from the liver has been shown to be markedly increased after 5 min of reperfusion following prolonged (90 min) ischaemia in rats (Clavien et al. 1996). Additionally, the number of IL-1β immunoreactive cells has been observed to increase in the ischaemic region within 15 min of occlusion of the middle cerebral artery in mice (Zhang et al. 1998). However, until the present study there has been no information about the influence of brief abdominal ischaemia on the concentration of IL-1β in the abdominal region. This study is the first to document an increase of IL-1β in mesenteric lymph during brief (10 min) abdominal ischaemia.
IL-1 immunoreactive afferent nerve fibres are located in the abdominal visceral organs and the coeliac-superior mesenteric ganglion complex of the rat (Schultzberg et al. 1987). Local administration of IL-1β is capable of inducing mechanical and thermal hyperalgesia (Ferreira et al. 1988; Fukuoka et al. 1994) and can stimulate vagal afferents (Ek et al. 1998). Although our previous studies have demonstrated that activation of splanchnic visceral afferents during ischaemia is multifactorial, with various ischaemic metabolites acting in concert to stimulate or sensitize sensory nerve endings (Longhurst, 1995; Fu et al. 1997b; Fu & Longhurst, 1998), we found that brief ischaemia increases the concentration of IL-1β in mesenteric lymph. Because lymph provides a measure of endogenous mediators in the interstitial compartment where abdominal visceral C fibre nerve endings are located (Mei, 1985), there was a substantial rationale for hypothesizing that IL-1β stimulates or sensitizes ischaemically sensitive abdominal visceral afferents.
To assess the potential for IL-1β to stimulate ischaemically sensitive abdominal splanchnic C fibre afferents, we examined the influence of exogenous IL-1β on these afferents. We found that 500 ng kg−1 IL-1β administered intra-arterially increased the discharge activity of ischaemically sensitive abdominal splanchnic afferents. This finding is similar to the observations by Fukuoka et al. (1994) who noted that large doses of IL-1β (1175 ± 2740 pg rat−1, plantar injection) increased the discharge activities of somatic afferent fibres. In addition, Watkins et al. (1994) have observed that the tail-flick reflex is enhanced after intraperitoneal injection of 10 μg kg−1 IL-1β and that this enhancement is mediated by vagal afferents. Ek et al. (1998) reported that intravenous injection of 2 μg kg−1 IL-1β stimulates gastric vagal afferents. However, these observations raise questions about the amount of IL-1β required to stimulate or sensitize sensory nerve fibres.
In the first protocol of this study, although we observed that the concentration of IL-1β in mesenteric lymph during ischaemia was increased only to 1·8-2·1 pg ml−1, we believe that the concentration of IL-1β present at sites of release was considerably higher during ischaemia, since the ELISA of IL-1β detects the mature form of IL-1β but fails to detect over 90% of the pro-IL-1β (Herzyk et al. 1992). However, both pro-IL-1β and mature IL-1β are synthesized and released in a disease process, or in response to stimulation. In this regard, de Kleijn et al. (1997) reported that the concentration of blood IL-1β increases up to 8·4 ng ml−1 in patients with fever of unknown origin by using radioimmunoassay of IL-1β that detects both mature as well as native pro-IL-1β (Numerof et al. 1990). IL-1β is diluted during the process of diffusion into the interstitium. In this regard, we have shown previously that aortic flow in cat is 200–230 ml min−1 (Huang et al. 1995). It took approximately 5 s (equivalent to 18–20 ml of blood) to inject 1 ml IL-1β (∼42 ng ml−1, i.e. 15 ng kg−1× 2·8 kg (mean body weight) = 42 ng) into the thoracic descending aorta in the present study. Thus, the concentration of IL-1β in coeliac or superior mesenteric arterial blood was in the range 2·1-2·3 ng ml−1 since the IL-1β concentration was diluted with aortic blood during the period of injection (i.e. 42 ng diluted by 18–20 ml = 2·1-2·3 ng ml−1). In addition, serum contains a number of specific and non-specific IL-1 inhibitors, which are able to bind IL-1β (Symons et al. 1991; Heney & Whicher, 1995). Therefore, the concentration of unbound IL-1β arriving at the afferent endings would be far less than that administered. In addition, previous studies have shown that both pro-IL-1β and mature IL-1β are biologically active molecules (Jobling et al. 1988; Dinarello 1996). Based on this information, we chose the smaller dose (i.e. 15 ng kg−1) of IL-1β to investigate the possibility that IL-1β may sensitize abdominal visceral afferents to ischaemia. We believe that this dose of IL-1β is relevant to the concentration of IL-1β produced during pathophysiological conditions. We observed that the response of abdominal visceral afferents to ischaemia was significantly enhanced after intra-arterial injection of this dose of IL-1β, an observation consistent with previous studies. In this regard, Fukuoka et al. (1994) also observed that the response of somatic afferents to mechanical and thermal stimulation was increased by 143% and 200–392%, respectively, after plantar injection of IL-1β (100–500 pg rat−1) into the hind-paw skin. Niijima (1996) also found that intraportal injection of 10 and 100 pg rat−1 IL-1β increased the activity of vagal afferents in a dose-dependent fashion. Therefore, it is likely that, within the pathophysiological concentration range, IL-1β is capable of sensitizing abdominal visceral afferents.
We evaluated whether endogenous IL-1β produced during abdominal ischaemia contributes to activation of ischaemically sensitive abdominal splanchnic afferents. The biological role of IL-1β is mediated through its action on IL-1 receptors. IL-1ra is a pure receptor antagonist, as it only binds to IL-1 type I receptors of target cells and does not trigger a signal (Arend, 1991). IL-1ra can block multiple biological responses to IL-1β. For example, intracerebro-ventricular injection of IL-1β induces hyperalgesia that is completely blocked by simultaneous injections of IL-1ra (Arend, 1991; Oka et al. 1993). Davis & Perkins (1994) have found that local administration of IL-1ra into the knee joint blocks the hyperalgesia induced by IL-1β. In the present study, we observed that ischaemia-induced increases in abdominal visceral afferent activity were attenuated by IL-1ra. Conversely, our time control studies yielded similar responses to repeated periods of abdominal ischaemia, in a manner consistent with our previous studies (Fu et al. 1997b; Fu & Longhurst, 1998). Thus, our data demonstrate, for the first time, that endogenous IL-1β contributes to activation of abdominal visceral afferents during ischaemia by sensitizing nerve endings through stimulation of IL-1 type I receptors.
Previously, we demonstrated that histamine stimulates ischaemically sensitive abdominal splanchnic afferents through activation of histamine H1 receptors (Fu et al. 1997b), which are linked to production of DAG (Daum et al. 1984; Ruat et al. 1992). IL-1β induces histamine release from basophils and mast cells (Subramanian et al. 1987). Activation of IL-1 receptors is also coupled to the production of DAG (Entman et al. 1991). We have shown that DAG triggers the activation of protein kinase C, which contributes to activation of abdominal visceral afferents during ischaemia (Guo et al. 1998). It has been uncertain, however, if an interaction exists between IL-1β and histamine on ischaemically sensitive abdominal afferents. In the present study, we confirmed our previous observation (Fu et al. 1997b) that repeated administration of histamine consistently stimulates ischaemically sensitive abdominal afferents. More importantly, we observed that the response of afferents to histamine was significantly enhanced in the presence of IL-1β. Furthermore, we observed that administration of IL-1ra not only eliminated the action of exogenous IL-1β on afferents to histamine, but also that the response of afferents to histamine was further attenuated by administration of IL-1ra (Figs 8B and 7A). In this regard, it is possible that endogenously released IL-1β contributes to the sensitizing action of IL-1β on afferents to histamine since a low concentration of IL-1β present in the abdominal region in the absence of ischaemia was observed in our initial protocol. Taken together, these results strongly suggest that IL-1β sensitizes ischaemically sensitive splanchnic visceral afferents to histamine through activation of IL-1 type I receptors.
One important consideration arising from our study relates to our inability to detect IL-1β in portal venous plasma during ischaemia. Previous studies have demonstrated that monoclonal antibody-based ELISA assays, as well as immunoradiometric assays, measure only unbound IL-1β in circulating blood (Heney & Whicher, 1995). Serum contains a number of non-specific IL-1 inhibitors, such as lipoproteins, lipid and α2-macroglobulin, which are able to bind IL-1β (Heney & Whicher, 1995). Serum also contains soluble IL-1 receptors, acting as specific IL-1 inhibitors, which are capable of binding to IL-1β thereby preventing the measurement of IL-1β by ELISA (Symons et al. 1991). Consistent with our results are those of Airaghi et al. (1995) who reported that IL-1β was undetectable in plasma of patients with acute myocardial infarction and unstable angina pectoris. Thus, it is likely that IL-1β, once produced by intestinal epithelia, smooth muscles, monocytes, and macrophages etc, is exported to the interstitial and then to the intravascular space where IL-1β is bound by these specific and non-specific inhibitors reducing the concentration of unbound IL-1β in portal venous plasma to undetectable levels.
In summary, the results of this study have demonstrated that abdominal ischaemia increases the concentration of IL-1β in mesenteric lymph fluid. A high dose of exogenous IL-1β is capable of stimulating ischaemically sensitive abdominal splanchnic afferents. A low dose of IL-1β is capable of enhancing the responses of these afferents to ischaemia and histamine. Conversely, blockade of IL-1 type I receptors with IL-1ra attenuates the response of these afferents to ischaemia and histamine. Thus, these data clearly indicate that IL-1β contributes to the activation of abdominal splanchnic visceral afferents by ischaemia and histamine by sensitizing these afferent nerve endings through the activation of IL-1 type I receptors.
References
- Airaghi L, Lettino M, Manfredi MG, Lipton JM, Catania A. Endogenous cytokine antagonists during myocardial ischemia and thrombolytic therapy. American Heart Journal. 1995;130:204–211. doi: 10.1016/0002-8703(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. Journal of Clinical Investigation. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascer E, Mohan C, Gennaro M, Cupo S. Interleukin-1 and thromboxane release after skeletal muscle ischemia and reperfusion. Annals of Vascular Surgery. 1992;6:69–73. doi: 10.1007/BF02000671. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Camargo CJ, Gorczynski R, Washington MK, Levy GA, Langer B, Greig PD. Acute reactant cytokines and neutrophil adhesion after warm ischemia in cirrhotic and noncirrhotic human livers. Hepatology. 1996;23:1456–1463. doi: 10.1002/hep.510230623. [DOI] [PubMed] [Google Scholar]
- Daum PR, Downes CP, Young JM. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. Journal of Neurochemistry. 1984;43:25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Perkins MN. The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. British Journal of Pharmacology. 1994;113:63–68. doi: 10.1111/j.1476-5381.1994.tb16174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleijn EM, Drenth JP, Pesman GJ, van Druten H, Demacker PN, van der Meer JW. Circulating and ex vivo production of pyrogenic cytokines and interleukin-1 receptor antagonist in 123 patients with fever of unknown origin. The Netherlands Fever of Unknown Origin Study Group. Journal of Infectious Diseases. 1997;175:191–195. doi: 10.1093/infdis/175.1.191. Published erratum Journal of Infectious Diseases175, 1027. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. Journal of Neuroscience. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, Smith CW. Inflammation in the course of early myocardial ischemia. FASEB Journal. 1991;5:2529–2537. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Fu LW, Longhurst JC. Role of 5-HT3 receptors in activation of abdominal sympathetic C fibre afferents during ischaemia in cats. The Journal of Physiology. 1998;509:729–740. doi: 10.1111/j.1469-7793.1998.729bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LW, O'Neill CA, Longhurst JC. Increased histamine and 5-HT in portal vein plasma and mesenteric lymph during brief ischemia and reperfusion. American Journal of Physiology. 1997a;273:H1135–1141. doi: 10.1152/ajpheart.1997.273.3.H1135. [DOI] [PubMed] [Google Scholar]
- Fu LW, Pan HL, Longhurst JC. Endogenous histamine stimulates ischemically sensitive abdominal visceral afferents through H1 receptors. American Journal of Physiology. 1997b;273:H2726–2737. doi: 10.1152/ajpheart.1997.273.6.H2726. [DOI] [PubMed] [Google Scholar]
- Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1β in the rat. Brain Research. 1994;657:133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- Guo ZL, Fu LW, Symons JD, Longhurst JC. Signal transduction in activation of ischemically sensitive abdominal visceral afferents: role of PKC. American Journal of Physiology. 1998;275:H1024–1031. doi: 10.1152/ajpheart.1998.275.3.H1024. [DOI] [PubMed] [Google Scholar]
- Heney D, Whicher JT. Factors affecting the measurement of cytokines in biological fluids: implications for their clinical measurement. Annals of Clinical Biochemistry. 1995;32:358–368. doi: 10.1177/000456329503200402. [DOI] [PubMed] [Google Scholar]
- Herzyk DJ, Berger AE, Allen JN, Wewers MD. Sandwich ELISA formats designed to detect 17 kDa IL-1β significantly underestimate 35 kDa IL-1β (see comments) Journal of Immunological Methods. 1992;148:243–254. doi: 10.1016/0022-1759(92)90178-v. [DOI] [PubMed] [Google Scholar]
- Huang HS, Stahl GL, Longhurst JC. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. American Journal of Physiology. 1995;268:H2114–2124. doi: 10.1152/ajpheart.1995.268.5.H2114. [DOI] [PubMed] [Google Scholar]
- Jobling SA, Auron PE, Gurka G, Webb AC, Mcdonald B, Rosenwasser LJ, Gehrke L. Biological activity and receptor binding of human prointerleukin-1β and subpeptides. Journal of Biological Chemistry. 1988;263:16372–16378. [PubMed] [Google Scholar]
- Longhurst JC. Chemosensitive abdominal visceral afferents. In: Gebhart GF, editor. Visceral Pain, Progress in Pain Research and Management. Seattle, WA, USA: The International Association for the Study of Pain (IASP) press; 1995. pp. 99–132. [Google Scholar]
- Longhurst JC, Rotto DM, Kaufman MP, Stahl GL. Ischemically sensitive abdominal visceral afferents: response to cyclooxygenase blockade. American Journal of Physiology. 1991;261:H2075–2081. doi: 10.1152/ajpheart.1991.261.6.H2075. [DOI] [PubMed] [Google Scholar]
- Mei N. Intestinal chemosensitivity. Physiological Reviews. 1985;65:211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- Niijima A. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. Journal of the Autonomic Nervous System. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- Niimi M, Mochizuki T, Yamamoto Y, Yamatodani A. Interleukin-1β induces histamine release in the rat hypothalamus in vivo. Neuroscience Letters. 1994;181:87–90. doi: 10.1016/0304-3940(94)90566-5. [DOI] [PubMed] [Google Scholar]
- Numerof RP, Kotik AN, Dinarello CA, Mier JW. Pro-interleukin-1β production by a subpopulation of human T cells, but not NK cells, in response to interleukin-2. Cellular Immunology. 1990;130:118–128. doi: 10.1016/0008-8749(90)90166-o. [DOI] [PubMed] [Google Scholar]
- Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1β induces hyperalgesia in rats. Brain Research. 1993;624:61–68. doi: 10.1016/0006-8993(93)90060-z. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Kelly D. Interleukin-1β induced-desArg9bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Rendig SV, Chahal PS, Longhurst JC. Cardiovascular reflex responses to ischemia during occlusion of celiac and/or superior mesenteric arteries. American Journal of Physiology. 1997;272:H791–796. doi: 10.1152/ajpheart.1997.272.2.H791. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Schwartz JC. Biochemical properties of histamine receptors. In: Schwartz JC, Haas HL, editors. The Histamine Receptor. New York, USA: Wiley-Liss Press; 1992. pp. 97–107. [Google Scholar]
- Saito K, Suyama K, Nishida K, Sei Y, Basile AS. Early increases in TNF-α, IL-6 and IL-1β levels following transient cerebral ischemia in gerbil brain. Neuroscience Letters. 1996;206:149–152. doi: 10.1016/s0304-3940(96)12460-5. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Svenson SB, Unden A, Bartfai T. Interleukin-1-like immunoreactivity in peripheral tissues. Journal of Neuroscience Research. 1987;18:184–189. doi: 10.1002/jnr.490180126. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Bray MA. Interleukin 1 releases histamine from human basophils and mast cells in vitro. Journal of Immunology. 1987;138:271–275. [PubMed] [Google Scholar]
- Symons JA, Eastgate JA, Duff GW. Purification and characterization of a novel soluble receptor for interleukin 1. Journal of Experimental Medicine. 1991;174:1251–1254. doi: 10.1084/jem.174.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Research. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Yu Q, Fu L-W, Longhurst JC. Concentration of interleukin 1β is increased in intestinal lymph during ischemia. FASEB Journal. 1998;12:A34. [Google Scholar]
- Zhang Z, Chopp M, Goussev A, Powers C. Cerebral vessels express interleukin 1beta after focal cerebral ischemia. Brain Research. 1998;784:210–217. doi: 10.1016/s0006-8993(97)01317-6. [DOI] [PubMed] [Google Scholar]


