Abstract
We have investigated the physiological and structural changes that occur in skeletal muscle vasculature during acclimation to chronic hypoxia in rats exposed to 12% O2 in a hypoxic chamber for 7 or 18 days (7CH and 18CH rats, respectively) and in age-matched normoxic (7N and 18N) rats.
Under anaesthesia and breathing 12% O2, 7CH and 18CH rats had lower arterial blood pressure (ABP) than 7N and 18N rats breathing air, but the haematocrit of the CH rats was increased so that their arterial O2 content equalled that of N rats. Blood flow recorded from the iliac or femoral artery and used to compute muscle vascular conductance (MVC: blood flow/ABP) showed that, in 18CH rats, MVC was comparable with that of 18N rats.
Maximal MVC induced by infusion of sodium nitroprusside (SNP) was used as an index of structural vascular conductance and compared with the MVC evoked by acute hypoxia (breathing 8% O2). Hypoxia induced similar increases in MVC in 7N and 7CH rats and in 18N and 18CH rats, even though N rats were switched from air to 8% O2 and CH rats were switched from 12 to 8% O2. The MVCs attained with 8% O2 and SNP were similar in 7N and 18N rats. However, the MVCs attained with 8% O2 in 7CH and 18CH rats were only ≈60% of those evoked by SNP, while the MVC attained with SNP was greater in 18CH than in 18N rats.
Vascular casts of the spinotrapezius muscle analysed ex vivo showed that interbranch intervals along primary, secondary and terminal arterioles (22–50, 13–18 and 7–13 μm diameter, respectively) were 30–50% shorter in 7CH and 18CH rats than in 7N and 18N rats. Further, the proportions of branches that were of the secondary and terminal arteriolar categories were increased such that the mean diameter of the branches was lower in 7CH than in 7N rats and lower in 18CH than in 18N rats.
These results indicate that arteriolar remodelling and angiogenesis occurs in skeletal muscle during acclimation to chronic hypoxia, beginning by the 7th day and progressing at least until the 18th day, so that the number of small arterioles and the functional size of the vascular bed is increased. We propose that these structural and functional changes enhance the ability of skeletal muscle to respond to acute hypoxia by facilitating the increase in vascular conductance, blood flow and thereby the O2 that can be delivered to muscle.
It is generally accepted that chronic systemic hypoxia induces a number of changes that improve the distribution of oxygen (O2) to the tissues. For example, respiratory minute volume increases, while raised erythropoietin levels stimulate the production of new red blood cells, so increasing arterial O2 content (Ca,O2) at any given arterial partial pressure of O2 (Pa,O2) (Olson & Dempsey, 1978; Ou et al. 1985, 1992). Studies on human subjects who climb to high altitude, and on rats exposed to chronic hypoxia in a hypobaric or hypoxic chamber, indicate that these changes begin within the first few days of the onset of hypoxia, but develop gradually over the following 3–4 weeks (Olson & Dempsey, 1975; Ou et al. 1985, 1992). In brain tissue, hypobaric hypoxia at 0·5 atm has been shown to produce capillary budding within 1 week (La Manna et al. 1994) and by 3 weeks, capillary volume was found to be greatly increased by a combination of capillary sprouting, growth of new capillaries, elongation of capillaries and an increase in capillary diameter (La Manna et al. 1994; Harik et al. 1995; Lauro & La Manna, 1997). Application of an analytical model to these experimental findings indicated that this vascular remodelling together with the raised haematocrit improves O2 diffusion to the tissue such that mean tissue PO2 within the brain is maintained at the level present under normoxic conditions (Lauro & La Manna, 1997).
By contrast, the question of whether chronic hypoxia induces angiogenesis within skeletal muscle has been controversial. Although chronic hypoxia lasting 3–12 weeks has been shown to increase capillary density in skeletal muscle in some studies (Cassin et al. 1971; Sillau & Banchero, 1977; Snyder et al. 1985), it has been argued that this largely reflects a decrease in muscle fibre size. Thus, when the number of capillaries was expressed as the numerical ratio of capillaries to muscle fibres, most studies apparently showed that chronic hypoxia caused no change in capillary fibre ratio in skeletal muscle (Sillau & Banchero, 1977; Snyder et al. 1985; Abdelmalki et al. 1996; for reviews see Banchero, 1987; Adair et al. 1990).
However, the conclusion that chronic hypoxia does not induce angiogenesis in skeletal muscle is surprising in view of the substantial evidence that prolonged exposure to hypoxia, or to a situation in which O2 supply is inadequate for tissue metabolic requirements, can increase the growth of new blood vessels (Adair et al. 1990). For example, in studies on chick embryo, incubation in a hypoxic environment of 15% O2 produced a 50% increase in the density of arterioles and venules in the chorioallantoic membrane. Furthermore, incubation in various levels of hypoxia from 21–12% O2 produced graded decreases in the structural vascular resistance of the whole body and of hindlimb muscles: structural vascular resistance was defined as the minimum vascular resistance recorded when the vasculature was maximally dilated, and was taken as an index of the numbers and maximum diameters of the vascular tree (Adair et al. 1987, 1988; Dusseau & Hutchins, 1989). It was also found that intermittent exposure to 12% O2, such that average O2 concentration was 19·5%, was as effective as continuous exposure to 12% O2 in decreasing the structural vascular resistance and four times more effective than continuous exposure to 19·5% O2 (Adair et al. 1988). This suggested that the vascularity of the tissues adapts to the minimum O2 levels within the tissue, rather than to the average tissue O2 levels. In view of this, and the evidence that growth of new arterioles can sometimes be dissociated from capillary angiogenesis in skeletal muscle during exercise training (Lash & Bohlen, 1992), we hypothesised that chronic hypoxia does induce angiogenesis at the arteriolar level in skeletal muscle and that this is most likely to begin soon after the onset of hypoxia, before the increase in ventilation and erythropoiesis are fully developed. It seemed likely that over this time period, hypoxia per se accentuated by any muscular activity would reduce tissue O2 levels below that required to stimulate angiogenesis.
As a first step towards testing this hypothesis, the present study was performed on rats that were exposed to hypoxia (12% O2) for 7 and 18 days and on age-matched controls. We analysed structural aspects of the dimensions of the vascular tree in vascular casts of the spinotrapezius muscle and assessed the functional size of the vasculature tree of hindlimb muscle at maximal dilatation. Since we were interested in how any structural changes might correlate with the other physiological changes associated with chronic hypoxia we also followed the haematocrit, Ca,O2, lung and heart weight and the systemic cardiovascular responses and responses evoked in hindlimb muscle by a further acute hypoxic challenge. The spinotrapezius muscle is a flat muscle that is particularly suitable for analysis of vascular casts and is the muscle we have used for studies of microvascular responses to acute and chronic systemic hypoxia (e.g. Mian & Marshall, 1991, 1996), while we have used the hindlimb muscles for many previous studies of gross muscle vascular conductance in systemic hypoxia (e.g. Thomas & Marshall, 1997; Marshall & Davies, 1999).
Since we began our studies, Price & Skalak (1998) reported, in agreement with our hypothesis, that rats chronically exposed to 10% O2 for 18 days show an increase in the number of arcade arterioles within skeletal muscle, probably resulting from the anastomosing of terminal arterioles across existing capillary connections. They did not attempt to establish when such changes begin, nor did they correlate these structural changes with physiological changes. Some of our results have already been reported in brief (Smith & Marshall, 1997).
METHODS
All experiments were performed on male Wistar rats supplied by Charles River. There were four experimental groups: rats that were chronically exposed to 12% O2 in a hypoxic chamber for 7 days and 18 days (7CH and 18CH rats, respectively) and age-matched normoxic rats (7N and 18N rats, respectively). The hypoxic normobaric chamber has been described before (Thomas & Marshall, 1995). Briefly, O2 concentration was maintained at 11·75-12·25% by a servo-controlled system, CO2 was maintained at ∼0·03% by circulating the chamber air through soda lime, ammonia was removed by a molecular sieve and humidity was maintained at ∼60% by condensation and by using silica gel. The chamber was opened to the atmosphere for 20–30 min every 3–4 days to allow routine cleaning and maintenance. The normoxic animals were kept under comparable environmental conditions in the University Biomedical Services Unit, but the concentration of O2 was that of atmospheric air (20–21% O2).
For the acute experiments on six 7CH rats and seven each of 18CH, 7N and 18N rats, the animal was anaesthetised with 3·5% halothane in O2 so that a jugular vein could be cannulated. Gaseous anaesthesia was gradually withdrawn and anaesthesia was maintained by continuous i.v. infusion of Saffan (P. K. Morgan, Gillingham, Kent, UK) delivered at 7–12 mg kg−1 h−1 during surgery and at 5–7 mg kg−1 h−1 during the experimental period (see Thomas & Marshall, 1995 and references therein for further details). Thereafter, the trachea was cannulated and connected to a spirometer flow head so that respiratory frequency (RF) could be continuously recorded. An air pump was used to blow air or a hypoxic gas mixture through tubing connected across the end of the flow head. Routinely, 7CH and 18CH rats breathed 12% O2, whereas 7N and 18N rats breathed air, until the inspirates were changed as part of the experimental protocol (see below). Arterial blood pressure (ABP) was recorded from a cannula placed in the right femoral artery, mean arterial blood pressure (MABP) being derived from the ABP recording. Blood flow to the muscles of the left leg was recorded via an electromagnetic transducer placed on the femoral artery in 18N, 18CH and 7N rats and on the iliac artery in 7CH rats (FBF and IBF, respectively); unfortunately, the femoral artery of 7CH rats proved too small for the smallest diameter flow probe available. In each case, branches of the artery that did not supply muscle were ligated. Vascular conductances (VCs) in femoral and iliac beds (FVC and IVC, respectively) were computed on-line as FBF or IBF divided by ABP. The equipment used for recording these variables has been described before. All variables were collected via a MacLab/8s to a PowerMac at a sampling rate of 40 Hz (Bryan & Marshall, 1999).
In addition, a cannula was placed in the left brachial artery to allow samples to be taken for analysis of blood gases and haematocrit. Another cannula was placed in the tail artery, with the tip pushed up to the bifurcation of the iliac arteries: this allowed blood flow to the tail to be prevented without performing the surgery necessary to ligate the tail artery. A cannula was inserted in the right femoral vein to allow infusion (see below).
Experimental protocol
When surgery had been completed, each animal was allowed to stabilise at the experimental level of anaesthesia (see above) for at least 30 min until all recorded variables had stabilised. Recordings were then made of respiratory and cardiovascular responses evoked by changing the O2 concentration in the inspirate. In 7N and 18N rats the inspirate was changed from air to 8% O2 for 5 min. Following this, the animal was returned to air breathing for at least 10 min, to allow variables to stabilise. Similarly, in 7CH and 18CH rats, the inspirate was changed from 12% O2 to 8% O2 for 5 min, the animal then being returned to 12% O2 to allow variables to stabilise. Arterial blood samples were removed at the end of the control period and in the 5th minute of each 5 minute period in which the inspirate was changed, for analysis of haematocrit and blood gases. The haematocrit was measured in 80 μl samples after they had been centrifuged at 11 800 r.p.m. for 5 min. Blood gas analysis was performed on 130 μl samples by a NovaStat Profile analyser (Stat 3, V. A. Howe, MA, USA), which provided Pa,O2, arterial partial pressure of CO2 (Pa,CO2) and pH, together with haemoglobin O2 saturation (Sa,O2). Ca,O2 was then calculated for each sample from the haematocrit and Sa,O2 values, as described previously (Marshall & Davies, 1999).
When responses to the changed inspirates had been recorded, and variables had again stabilised, sodium nitroprusside (SNP) was infused via the femoral vein in a concentration of 0·0325 mg ml−1 at 3 ml h−1 for 10 min. This concentration and infusion rate was chosen from preliminary experiments as one that would produce maximal dilatation of the femoral vasculature and therefore indicate the structural vascular conductance (SVC) of hindlimb muscle. Recordings were made throughout this period, the maximal FVC or IVC recorded at the 10th minute of SNP infusion being taken for analysis (see below).
At the end of the acute experiments, one each of the 7N, 7CH and 18CH rats described above were used for the preparation of vascular casts (see below). The remaining rats were killed by an overdose of Saffan. The heart and lungs were then quickly removed and weighed (wet weight). These tissues were then dried in an oven over 2 days until there was no further weight loss, the final weight being recorded as dry weight.
Vascular casts
These were prepared on one each of the 7N, 7CH and 18CH rats that were used in the acute experiments and on a further two 7N, 7CH and 18CH and three 18N rats that were prepared exactly as described above for the acute experiments, so as to allow recordings of ABP, limb blood flow and VC and infusion via the femoral vein. In the animals that received the SNP infusion in the acute experiments (see above), the level of anaesthesia was again increased to the infusion rate used for surgery (see above). In the remaining animals the level of anaesthesia was maintained at the level used for surgery. The right carotid artery was then cannulated to allow infusion of the vascular cast material as described by Williams & Segal (1992). Briefly, maximal dilatation was first achieved with SNP infusion as described above. Then the rat was perfused with microfil silicon rubber injection compound (Canton Bio-Medical Products Inc., Boulder, CO, USA) made up at 5 ml diluent for every 4 ml compound and with 5% by volume of the curing agent, this being delivered from a 50 ml syringe. Immediately perfusion was begun, the right atrium was cut to allow fluid to flow out. At the end of the perfusion, the rat was placed in a refrigerator for at least 24 h to allow the cast to polymerise. Both spinotrapezius muscles were then carefully removed and dehydrated progressively for 24 h periods in 25, 50, 75, 95 and 100% ethyl alcohol and finally cleared by immersion in the methylsalicylate, in which they were then stored.
For analysis, the spinotrapezius muscle was placed ventral surface uppermost on the stage of a Zeiss ACM microscope and viewed through eye pieces of × 10 magnification and objectives of ×∼32 or × 50 magnification. Main arteries (of 40–90 μm diameter) that supply the spinotrapezius muscle, primary and secondary arterioles (22–50 μm and 13–18 μm, respectively) of the primary and secondary anastomosing networks and terminal arterioles (7–13 μm) that show a terminal branching pattern that gives rise to the capillaries, were readily identified as previously described in vivo (see Marshall, 1982; Mian & Marshall, 1996) by their morphological relationships to one another and by their diameters. Although the cast material did not generally pass through the entire capillary network to the venous vessels, the arterial and arteriolar tree was well filled and we could not detect gaps between the cast material and the inner wall of the vessels. To measure interbranch interval along the arteriolar network, the main artery was first identified at the rostral end of the muscle. This was followed into the muscle until it branched, usually at the first bifurcation, to form arterioles whose diameters placed them in the primary arteriolar category. By using an eye-piece graticule that was calibrated with a stage micrometer, interbranch intervals were then measured along these primary arterioles and their secondary and terminal arteriolar branches as the distance between the mid-points of successive branches. Ten to twenty measurements were made of interbranch interval for each category of vessel in each of the right and left spinotrapezius muscles of each animal. In addition, the diameters of each of these vessels that branched from each category of vessel were measured as soon as the walls became parallel, and on the basis of their diameter (see above) they were assigned to one of the three categories, primary, secondary and terminal arterioles.
Statistical analysis
All data are expressed as means ±s.e.m. unless otherwise stated. For analysis of acute responses to a change in the inspirate, measurements were made at each minute of the 5 min stimulus and were compacted within each animal to give a single value. SVC was taken as the maximum vascular conductance at the end of the 10th minute of SNP infusion. Comparisons within groups between responses evoked by hypoxia and SNP were made by Student's paired t test. Differences between groups for baselines and responses were compared by unpaired t tests. For analysis of vascular casts, the data collected from each muscle were combined: since the right and left spinotrapezius muscles were used, the n value (6 for each variable) was twice the number of animals. Comparisons between groups of animals were made by unpaired t test. The fact that individual muscles were treated as units did not artificially reduce the sample variance and did not alter the significant effects we have discussed below. We had no reason at the outset of these experiments to expect that vascular cast data collected from the 7N and 18N rats would be different. Thus ANOVA did not seem the appropriate analysis to use, since ANOVA is suited to data in which each factor can be considered as a main effect and every combination of factors can produce interaction effects (Abacus Concepts, 1996). However, accepting that our premise might be wrong, the vascular cast data for all four groups were also analysed by ANOVA followed, when a significant F ratio was obtained, by post hoc Fisher's PLSD (Protected Least Significant Difference) tests in order to make comparisons between groups. In all cases P < 0·05 was considered significant.
RESULTS
Baseline values
At the time of the acute experiments, the body weights of the 7N and 7CH rats were 265·4 ± 7·8 and 207·7 ± 4·6 g, respectively, while those of the 18N and 18CH rats were 308 ± 6·7 and 277·3 ± 2·5 g, respectively. The 7CH rats were significantly lighter than the 7N rats (P < 0·05), but the difference between the 18N and 18CH rats did not reach statistical significance. The weights of the heart and lungs in each group are shown in Table 1. When expressed per body weight, the dry heart weights and dry lung weights were greater in the 18CH than in the 18N rats. Assuming that the wet weight minus the dry weight indicates the water content, the amount of water expressed per gram wet tissue weight (% water) was not different between 7N and 7CH rats nor between 18N and 18CH rats, indicating that oedema was not present in either tissue (Table 1).
Table 1.
Heart and lung weights and water content in 7N, 7CH, 18N and 18CH rats
| Heart weight (mg g−1) | Heart water content (% wet tissue wt) | Lung weight (mg g−1) | Lung water content (% wet tissue wt) | |
|---|---|---|---|---|
| 7N | 0.62 ± 0.04 | 76.7 ± 2.5 | 0.88 ± 0.06 | 78.5 ± 2.0 |
| 7CH | 0.63 ± 0.03 | 78.5 ± 1.1 | 1.13 ± 0.07* | 78.8 ± 3.1 |
| 18N | 0.55 ± 0.02 | 76.3 ± 2.5 | 0.74 ± 0.04 | 77.4 ± 0.8 |
| 18CH | 0.85 ± 0.08** | 74.7 ± 0.8 | 1.09 ± 0.05** | 76.2 ± 1.0 |
Heart and lung weights are shown as dry tissue wt (body wt)−1, while the water content of each tissue is shown as % wet tissue wt. Each value is shown as the mean ± s.e.m.
P < 0.01
P < 0.05, significant difference between 7N and 7CH or 18N and 18CH.
As expected, the Pa,O2 values in 7CH and 18CH rats breathing 12% O2 were lower than in their respective controls breathing air (Table 2). However, the haematocrit values were greater in 7CH and 18CH rats (49·7 ± 1·1 and 49·4 ± 2·1%, respectively) than in their controls (43·2 ± 0·9 and 44·7 ± 0·3%, respectively; P < 0·05 in each case), which meant that Ca,O2 values were comparable in 7N and 7CH rats and were slightly higher in 18N rats than in 18CH rats (Table 2). The Pa,CO2 values of 7CH and 18CH rats breathing 12% O2 were lower than in 7N and 18N rats breathing air, indicating hyperventilation in the CH rats: the levels of Pa,CO2 were comparable in 7CH and 18CH rats. The difference between RF values in 7CH and 7N rats did not reach significance (131·4 ± 4·9 vs. 116·1 ± 6·8 breaths min−1), but RF was significantly greater in 18CH than in 18N rats (131·9 ± 5·1 vs. 109·9 ± 3·9 breaths min−1; Figs 1 and 2).
Table 2.
Blood gas values and arterial O2 content recorded in 7N, 7CH, 18N and 18CH rats
| Pa,O2 (mmHg) | Ca,O2 (ml(100 ml)−1) | Pa,CO2 (mmHg) | ||
|---|---|---|---|---|
| 7N | Air | 82.4 ± 1.3 | 21.6 ± 0.27 | 45.7 ± 2.7 |
| 8% O2 | 31.6 ± 0.1 | 14.5 ± 0.56 | 31.3 ± 3.8 | |
| 7CH | 12% O2 | 39.5 ± 2.2*** | 22.08 ± 0.47 | 34.0 ± 1.0** |
| 8% O2 | 32.6 ± 1.5 | 18.28 ± 0.86* | 29.0 ± 0.8 | |
| 18N | Air | 85.7 ± 1.8 | 22.36 ± 0.25 | 45.4 ± 2.5 |
| 8% O2 | 32.4 ± 0.9 | 15.4 ± 0.8 | 32.6 ± 2.2 | |
| 18CH | 12% O2 | 40.6 ± 2.6** | 19.96 ± 0.6* | 34.5 ± 1.5** |
| 8% O2 | 30.7 ± 1.8 | 16.19 ± 1.12 | 29.6 ± 1.8 |
7N and 18N rats were switched from breathing air to breathing 8% O2 for 5 min, while 7CH and 18CH were switched from breathing 12% O2 to breathing 8% O2 for 5 min. Blood samples were taken under the respective control conditions at the 5th minute of breathing 8% O2. Values are given as means ± s.e.m.
P < 0.001
P < 0.01
P < 0.05, significant difference between N and CH rats for values recorded in N rats breathing air and CH rats breathing 12% O2, or in N and CH rats breathing 8% O2.
Figure 1. Responses evoked by breathing 8% O2 in 7N and 7CH rats.
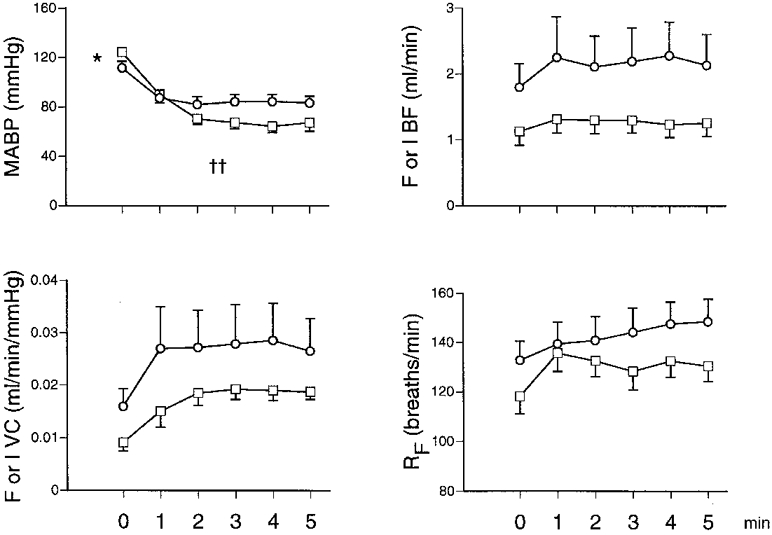
MABP, mean arterial blood pressure; F or I BF, femoral or iliac blood flow (ml min−1); F or I VC, femoral or iliac vascular conductance (ml min−1 mmHg−1); RF, respiratory frequency. □ and ○ indicate 7N and 7CH rats, respectively; each value is shown as the mean ±s.e.m. Horizontal axes show time before and during a 5 min period of 8% O2. †† Significant difference between 7N and 7CH rats for change evoked from baseline (P < 0·01). * Significant difference between baseline values of 7N and 7CH rats (P < 0·05). Note that 7N rats were switched from air to 8% O2 for 5 min, while 7CH rats were switched from 12 to 8% O2 for 5 min.
Figure 2. Responses evoked by breathing 8% O2 in 18N and 18CH rats.
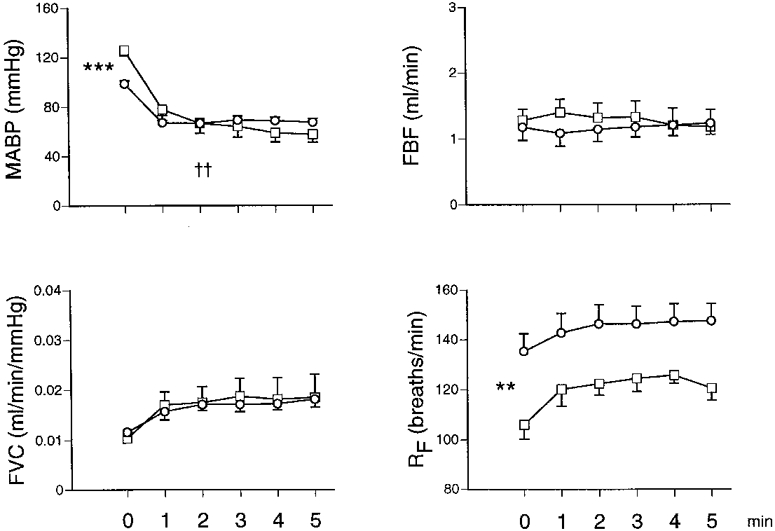
Abbreviations as in Fig. 1. □ and ○ indicate 18N and 18CH rats, respectively; each value is shown as the mean ±s.e.m. Horizontal axes show time before and during the 5 min period of 8% O2. †† Significant difference between 18N and 18CH for change evoked from baseline (P < 0·01). ***P < 0·001, **P < 0·01, significant difference between baseline values of 18N and 18CH rats. Note that 18N rats were switched from air to 8% O2 for 5 min, while 18CH rats were switched from 12 to 8% O2 for 5 min.
Baseline MABP values were significantly lower in 7CH and 18CH rats breathing 12% O2 (111·0 ± 5·2 and 99·6 ± 2·2 mmHg, respectively) than in their controls, 7N and 18N rats breathing air (122·6 ± 3·1 and 123·8 ± 3·7 mmHg; Figs 1 and 2). The absolute values of hindlimb VC cannot be directly compared in 7N and 7CH rats because flow was recorded from the femoral artery in the former and from the iliac artery in the latter (see Methods). The FVC values of the 18CH and 18N rats were not significantly different from one another (0·013 ± 0·001 vs. 0·010 ± 0·002 ml min−1 mmHg−1, respectively; Fig. 2).
Responses to 8% O2 and to SNP
When the inspirate of 7CH rats was changed to 8% O2 for 5 min they showed a fall in ABP, as did 7N rats when their inspirate was changed from air to 8% O2 (Fig. 1). The magnitude of the fall in ABP was greater in 7N than in 7CH rats. Although the absolute changes in limb VC cannot be compared in the two groups (see above), when the increases in IVC and FVC are considered as a percentage change from the respective baseline value, they were not significantly different in 7N and 7CH rats (+86 ± 9 and +72 ± 8%, respectively). Not surprisingly, switching the inspirate to 8% O2 caused a fall in Pa,O2 in both groups and in both groups this was accompanied by a fall in Pa,CO2 (Table 2) and an increase in RF (Fig. 1). The greater haematocrit in the 7CH rats meant that Ca,O2 when breathing 8% O2 was greater in 7CH than in 7N rats.
Similarly, both the 18CH and 18N rats showed a fall in ABP when they were switched to 8% O2 for 5 min and the magnitude of the fall was smaller in the 18CH rats (Fig. 2). This was accompanied by an increase in FVC that was fully comparable in 18CH and 18N rats. Further, Pa,O2 and Pa,CO2 fell in both 18CH and 18N rats and in both groups, RF increased. Any difference between Ca,O2 values in 18CH and 18N rats breathing 8% O2 did not reach significance.
Finally, when SNP was infused so as to give an index of SVC, it induced substantial dilatation in the hindlimb muscle in both groups (Fig. 3). In 7N and 18N rats, the values of FVC attained during 8% O2 and SNP were similar (Fig. 3). In 7CH rats, the IVC attained during SNP infusion was not significantly different from that induced during 8% O2, but in 18CH rats, the FVC attained during SNP was significantly greater than that attained during 8% O2. Further, the FVC attained during SNP was significantly greater in absolute terms in 18CH rats than in 18N rats (see Fig. 3). Similarly, when considered as a percentage change from baseline, the increase in VC induced by SNP was significantly greater in 7CH rats than in 7N rats (+130 ± 12 vs.+68 ± 8%, respectively; P < 0·05).
Figure 3. Muscle vascular conductance values recorded when breathing 8% O2 and during infusion of SNP in 7N, 7CH, 18N and 18CH rats.
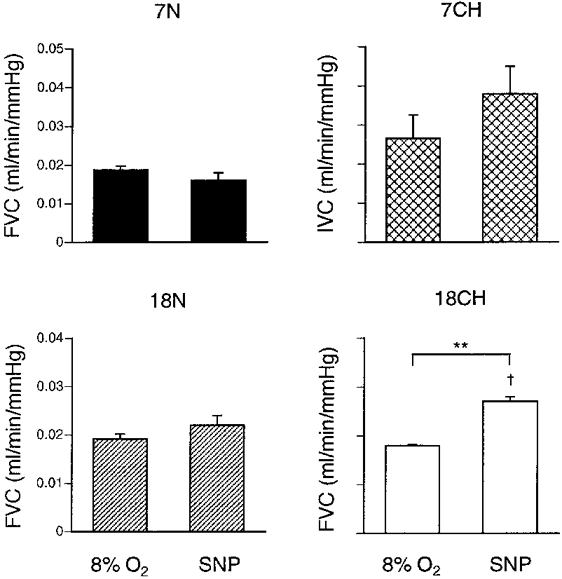
Values are shown as means ±s.e.m.▪, 7N;  , 7CH;
, 7CH;  , 18N; and □, 18CH. Note that in 7CH rats vascular conductance was recorded from the iliac artery (IVC) whereas in all other groups it was recorded from the femoral artery (FVC). ** Significant difference between values recorded during 8% O2 and SNP infusion in 18CH rats (P < 0·01). † Significant difference between 18N and 18CH rats for FVC recorded during SNP infusion (P < 0·05).
, 18N; and □, 18CH. Note that in 7CH rats vascular conductance was recorded from the iliac artery (IVC) whereas in all other groups it was recorded from the femoral artery (FVC). ** Significant difference between values recorded during 8% O2 and SNP infusion in 18CH rats (P < 0·01). † Significant difference between 18N and 18CH rats for FVC recorded during SNP infusion (P < 0·05).
Vascular casts
Examination of the vascular casts of the spinotrapezius muscle showed that the interbranch interval decreased progressively from the primary arterioles to the secondary arterioles and terminal arterioles in all four groups of animals (Fig. 4). There was no significant difference between 7N and 18N rats for interbranch interval in any category of arteriole. However, in each category of arteriole, the interbranch interval was shorter in 7CH than in 7N rats and in 18CH than in 18N rats. Further, the interbranch interval was shorter in 18CH than in 7CH rats for each category of arteriole, suggesting that the change was progressive from the 7th to the 18th day of chronic hypoxia. These changes meant that the interbranch interval was reduced by ∼33% in each category of arteriole in 7CH relative to 7N rats and by almost 50% in 18CH relative to 18N rats.
Figure 4. Mean interbranch interval along primary, secondary and terminal arterioles in 7N, 18N, 7CH and 18CH rats.
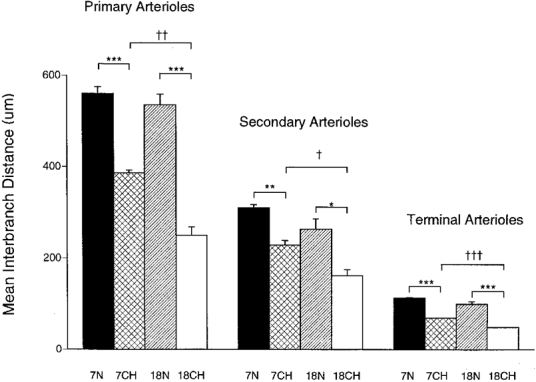
Each column shows the mean ±s.e.m. of interbranch interval along arteriole, the category of arteriole being indicated above the columns, the group of rats being indicated below individual columns and by shading as in Fig. 3. ***P < 0·001, **P < 0·01, *P < 0·05, significant difference between 7N and 7CH or 18N and 18CH rats. †††P < 0·001, ††P < 0·01, †P < 0·05, significant difference between 7CH and 18CH rats. n = 6 for each group, each value having been generated from the means of 10–20 measurements made in each of the right and left muscles of each animal.
Since these data implied that additional branches were being formed along each category of arteriole, further analyses were performed to establish the nature of these vessels. When the branches that arose from primary and secondary arterioles were allocated to each of the categories of arterioles, it became apparent that there were higher proportions of smaller arterioles in the CH rats than in N rats (Fig. 5). Thus, in the 7N rats, most of the branches from primary arterioles were, in fact, primary arterioles on the basis of their internal diameter, whereas in the 7CH rats, the larger proportions of branches were secondary and terminal arterioles. As far as the branches from secondary arterioles are concerned, in the 7N rats they were mainly equally divided between secondary arterioles and terminal arterioles, whereas in the 7CH rats, by far the greatest proportion were terminal arterioles. The trends were similar in 18CH relative to 18N rats (see Fig. 5).
Figure 5. Categories of vessels that branched from primary arterioles and secondary arterioles in 7N, 18N, 7CH and 18CH rats.
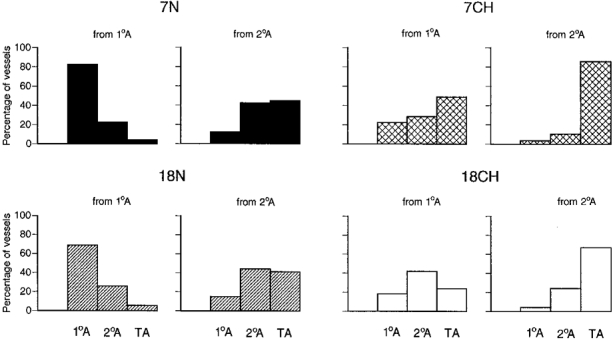
Each set of histograms shows the percentage of branches from primary arterioles (1°A, lefthand set) or secondary arterioles (2°A, righthand set) that were identified as 1°A, 2°A and terminal arterioles (TA) on the basis of internal diameter. The total number of branches considered was 60–80 for both primary and secondary arterioles in the 6 spinotrapezius muscles of each group of rats. Shading of columns for each group of rats is as in Fig. 3.
These general comments on the arteriolar branches are fully consistent with the analyses performed on the average diameters of the arterioles that arose as branches from the primary and secondary arterioles (Fig. 6). The branches of the primary arterioles and of the secondary arterioles were significantly smaller in 7CH than in 7N rats, such that the average diameter of the branches of the primary arterioles changed from that of the primary arteriolar category (22–50 μm) in 7N rats to that of the border between the secondary and terminal arteriolar category (13–18 and 7–13 μm, respectively) in 7CH rats, while the average diameter of the branches of the secondary arterioles changed from that of the secondary/terminal arteriolar border to that of the terminal arteriolar category. The difference between the average diameters of the branches of primary arterioles in 18CH and 18N rats did not reach significance, but the average diameter of the branches of secondary arterioles was significantly smaller in 18CH than 18N rats, shifting from the secondary to the terminal arteriolar category (Fig. 6).
Figure 6. Diameters of arterioles that branched from primary and secondary arterioles in 7N, 18N, 7CH and 18CH rats.
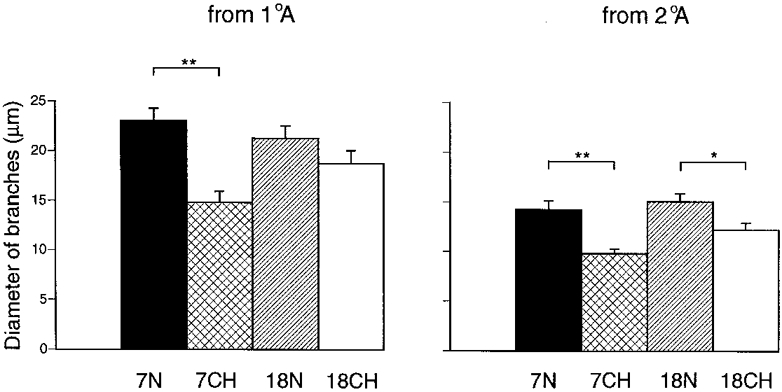
Each column shows the mean ±s.e.m. Left- and righthand sets of columns show diameters of vessels that branched from primary arterioles (1°A) and secondary arterioles (2°A), respectively, the group of rats being indicated below the columns and by shading as in Fig. 3. **P < 0·01, significant difference between 7N and 7CH rats; *P < 0·05, significant difference between 18N and 18CH rats. n = 6 for each group, each value having been generated from the means of 10–20 measurements made in each of the right and left muscles of each animal.
We did not formally analyse the branches of the terminal arterioles in any of the groups of animals; they were either small terminal arterioles or capillaries, but we did not feel able to judge in which category they should be placed.
As indicated under ‘Statistical analysis’, the vascular cast data were analysed both by unpaired t tests and ANOVA with post hoc tests. The description given above, and the symbols indicating levels of statistical significance shown in Figs 4–6, relate to the outcome of the t tests. The outcome of ANOVA and post hoc tests were essentially comparable; there were no differences between 7N and 18N rats, but there were significant differences between 7N and 7CH, 18N and 18CH and between 7CH and 18CH for the mean interbranch interval on primary, secondary and terminal arterioles (P < 0·01 or P < 0·05 in each case) and between the diameters of branches of primary arterioles in 7N and 7CH and between those of secondary arterioles in 7N and 7CH and in 18N and 18CH rats (P < 0·05).
DISCUSSION
The present study has provided the first evidence that substantial physiological and structural changes occur in the vasculature of skeletal muscle within 7 days of the onset of chronic systemic hypoxia and that these changes progress from the 7th to the 18th day.
Effects on body weight, tissue weight and blood gases
The body weight of the 7CH rats was significantly less than that of their age-matched control (7N) rats. This is consistent with much previous evidence that chronic hypoxia causes weight loss, or impairs normal weight gain (e.g. Ishihara et al. 1995). Nevertheless, in both the 7CH and 18CH rats, dry lung weights were greater when expressed relative to body weight than in their matched controls. This probably reflects pulmonary vascular remodelling, including the extension of vascular smooth muscle along the terminal arterioles towards the capillaries and an increase in the number and size of alveoli (Rabinovitch et al. 1979; Sobin et al. 1983; Roos et al. 1996). The fact that there was no increase in the water content of lungs when expressed relative to tissue weight indicates there was no oedema.
On the other hand, dry heart weight relative to body weight was similar in the 7N and 7CH rats, but was substantially greater in the 18CH rats relative to their controls. The latter can probably be explained by hypertrophy of the right ventricle in response to the pulmonary remodelling and raised pulmonary resistance (see Roos et al. 1996). This change apparently lagged behind the change in the pulmonary vasculature.
The haematocrit was raised in both 7CH and 18CH rats relative to their own controls, but it was the same in the 7CH and 18CH rats (49–50%), showing there was no further increase between the 7th and 18th day of chronic hypoxia. Since the haematocrit is substantially higher (56%) in this same strain of rats when they are exposed to 12% O2 for up to 4 weeks (Marshall & Davies, 1999), this implies a secondary, or later change in haematocrit between 18 and 28 days. This is consistent with previous findings that the haematocrit shows a biphasic change. Both phases seem to be associated with an increase in erythropoietin levels and the consequent generation of new red blood cells, as well as with a reduction in plasma volume. It is not clear why the haematocrit stabilises and erythropoietin levels fall for a short period from ∼7 days after the onset of hypoxia, nor why they rise again (see Ou et al. 1992). Since there was no evidence of pulmonary oedema nor of excessive polycythaemia or right ventricular hypertrophy, it seems the strain of Wistar rats we used acclimates relatively well to exposure to 12% O2: they showed no tendency to develop the equivalent of high altitude or acute mountain sickness which is seen in some strains of rats and in other species (see Ou et al. 1992).
As Pa,CO2 values in the 7CH and 18CH rats breathing 12% O2 were similar to one another, but lower than those in 7N and 18N rats breathing air, we can deduce that both 7CH and 18CH rats were hyperventilating, as indicated by their high RF values relative to the matched controls, but that the ventilatory adaptations did not progress further between the 7th and 18th day of chronic hypoxia. This is fully consistent with previous evidence that, in the rat, the ventilatory response to hypoxia is well established within 4 days of chronic exposure to hypoxia and develops little over the following 1–2 weeks (Olson & Dempsey, 1978). The consequence of these changes in haematocrit and ventilation meant that the Pa,O2 values of 7CH and 18CH rats breathing 12% O2were similar and their Ca,O2 values were comparable to those of 7N and 18N rats breathing air. It appears therefore that the changes required to normalise oxygen levels in the systemic arterial blood are well developed by the 7th day of chronic hypoxia.
Significance of changes in baseline vascular conductance in skeletal muscle
Although the systemic Ca,O2 was normalised by the 7th day of chronic hypoxia, this does not necessarily mean that there was no tissue hypoxia by this stage. In the rat, acute hypoxia evokes a fall in arterial pressure, which is mainly attributable to vasodilatation in skeletal muscle caused by local tissue hypoxia and predominantly by the action of adenosine (see Mian & Marshall, 1991; Bryan & Marshall, 1999). Thus, the fact that baseline ABP was reduced in the 7CH and 18CH rats breathing 12% O2 relative to their matched 7N and 18N controls breathing air, even though the Ca,O2 values were similar in all groups, would be consistent with increased peripheral VC in the 7CH and 18CH rats, secondary to muscle vasodilatation. However, the interpretation is not straightforward for the reduced ABP in the 7CH and 18CH rats might also reflect an increase in the SVC of peripheral vasculature in the CH rats, caused by vascular remodelling.
Unfortunately, we cannot make direct comparisons between the absolute VCs of skeletal muscle in 7N and 7CH rats, because the flow probe was placed on the femoral artery in 7N and on the iliac artery in 7CH rats. However, in a recent study using a different recording technique we showed that baseline FVC was substantially higher in 7CH rats breathing 12% O2 than in 7N rats breathing air (Walsh & Marshall, 1999). By contrast, in the 18CH and 18N rats, the values for FVC when the former were breathing 12% O2 and the latter air, were very similar. Thus, it seems that the effects on baseline VC of an increase in SVC and/or of the dilator influence of tissue hypoxia is present at the 7th day of chronic hypoxia, but has been compensated for by the 18th day by a loss of this dilator influence, an increase in myogenic tone, or by the action of vasoconstrictor substances. In fact, in pilot experiments, the adenosine receptor antagonist 8-phenyl theophylline (8-PT) reduced baseline IVC in four out of five of the 7CH rats when they were breathing 12% O2 (K. Smith & J. M. Marshall, unpublished observations) whereas it had no effect on N rats breathing air, nor on 3–4 week CH rats breathing 12% O2 (Thomas & Marshall, 1997). This suggests that tissue hypoxia sufficient to generate adenosine and cause tonic dilatation is present in the skeletal muscle of 7CH rats, but has disappeared at least by 3–4 weeks of chronic hypoxia. This possibility requires further investigation and is relevant when considering the factor(s) that may trigger vascular remodelling (see below).
Responses evoked by acute hypoxia
Both the 7CH and 18CH rats showed smaller falls in ABP when they were switched to breathing 8% O2 than their matched controls. However, the increases in muscle VC evoked by 8% O2, considered as percentage increases in FVC and IVC since direct comparisons of absolute values cannot be made, were similar in 7CH and 7N rats, as were the absolute increases in FVC in 18CH and 18N rats. This is particularly remarkable because when the 7CH and 18CH rats were acutely switched from 12% to 8% O2, they were subjected to a much smaller fall in the systemic arterial O2 level, whether considered as a change in Pa,O2 or Ca,O2, than the 7N and 18N rats when they were acutely switched from air to 8% O2. This alone suggests either that vasodilator substances such as adenosine are more readily released in the skeletal muscle of animals that are acclimating to chronic hypoxia and/or that the vasculature is more sensitive to these vasodilator influences. However, the full effect on vascular sensitivity to acute hypoxia may be even more dramatic, given the accompanying increase in the structural size of the muscle vascular bed.
Thus, the fact that the percentage increase in muscle VC (IVC/FVC) achieved with SNP was increased in 7CH relative to 7N rats, while the absolute FVC was increased in 18CH relative to 18N rats, indicates that chronic hypoxia produced an increase in SVC which had begun by the 7th day and progressed between the 7th and the 18th day. As discussed below, this was attributable to an increase in the number of branches of the arteriolar tree and probably to an increase in the density of the capillary network (Deveci et al. 1998, 1999). With a larger number of arteriolar branches, it is likely that arterioles at any level of the arteriolar tree would have to dilate more in order to produce a given change in the gross VC. Thus, simple consideration of the change in gross VC induced by acute hypoxia, without recognition of the increase in size of the vascular bed, almost certainly underestimates the extent to which the responsiveness of the vasculature to acute hypoxia is accentuated. We demonstrated by intravital microscopy that spinotrapezius arterioles that had the same baseline diameter in N rats and in 3–4 week CH rats showed similar increases in diameter when the inspirate of the N rats was changed from 21 to 8% O2 and that of the CH rats was changed only from 12 to 8% O2 (Mian & Marshall, 1996). The present study provides the first evidence that an increase in arteriolar responsiveness may be present as early as 7 days after the onset of chronic hypoxia.
A further important point is that whereas in 7N and 18N rats the FVC values attained during 8% O2 and SNP infusion were similar, indicating that 8% O2 induced maximal vasodilatation in muscle, in 7CH and 18CH rats, the IVC, or FVC, values attained with SNP tended to be, or were, greater, respectively, than those achieved during 8% O2. This demonstrates that possibly at 7 days and certainly within 18 days of the onset of chronic hypoxia, acute exposure to 8% O2 no longer evoked maximal muscle vasodilatation. The muscle dilator response evoked by acute hypoxia is a form of metabolic feedback mediated by adenosine and other locally released dilator factors (Mian & Marshall, 1991, 1996) such that in N rats and 3–4 week CH rats, the vasodilatation that occurs during 8% O2 is sufficient to allow muscle O2 consumption (V̇O2) to remain at that pertaining under their respective control conditions (breathing air or 12% O2; Marshall & Davies, 1999). The present results therefore suggest that the structural and functional changes induced by chronic hypoxia puts skeletal muscle in a better position to deal with systemic, or local hypoxia more severe than that induced by breathing 8% O2: potentiating the increase in muscle VC, blood flow and O2 delivery.
Effects of chronic hypoxia on the structure of the muscle vascular network
The vascular casts of spinotrapezius revealed that the distance between successive branches along the arteriolar tree was reduced by ∼33% in 7CH relative to 7N rats and by ∼50% in 18CH relative to 18N rats. Comparison of the 7N and 18N rats showed this change was over and above any change that occurred during normal maturation in the N rats. These findings are consistent with the report of Price & Skalak (1998) that the number of arcade arterioles and terminal arterioles was greatly increased in the gracilis muscle of rats exposed to 10% O2 for ∼18 days, but indicate that such changes are already well under way by the 7th day of hypoxia. Further, as the gracilis muscle is a leg muscle, the results of Price & Skalak (1998) give us confidence that the structural changes we have seen in the spinotrapezius muscle can be extrapolated to the leg muscles from which we have made our physiological recordings of VC.
From our analysis of the diameters of the branches, the reduction in the branching interval along the primary and secondary arterioles might be attributed to an increase in the number of terminal arterioles and arcades formed from pre-existing capillaries, as proposed by Price & Skalak (1998). However, if this is the case, it would imply that in N rats, capillaries arise as branches from primary and secondary arterioles. This is not impossible, but is not something we have regularly observed, at least not along primary arterioles; capillaries typically arise from the terminal arterioles and occasionally from secondary arterioles (see Marshall, 1982). Alternative explanations are as follows: (i) new terminal arterioles may have arisen as such, from both the primary and secondary arterioles; (ii) new capillaries may have arisen from these arteriolar categories and then muscularised so that we identified them as terminal arterioles in both the 7CH and 18CH rats; and (iii) some of the arterioles we identified as primary arterioles in CH rats and that were used for assessment of interbranch interval may have been what we would have identified as secondary arterioles in N rats, while some of the arterioles we identified as secondary arterioles may have been what we would have identified as terminal arterioles in N rats. This could have happened if the maximum diameter of existing secondary and terminal arterioles was increased by chronic hypoxia as was shown for arterioles of the spinotrapezius in rats that underwent exercise training (Lash & Bohlen, 1992). It should be noted that under the latter circumstances also, the increase in maximal arteriolar diameter was associated with an increase in the branching density of the spinotrapezius arteriolar tree and with an increase in the sensitivity to the dilator influences of acute muscle contraction (Lash & Bohlen, 1992), suggesting parallels between the effects of chronic hypoxia and training.
Clearly, we cannot distinguish between these alternative explanations. We simply emphasise our new finding that substantial remodelling, so as to functionally increase the size of the arteriolar tree, had occurred by the 7th day of chronic hypoxia and had progressed further by the 18th day. We also note that rats exposed to chronic hypoxia (12% O2) for 3 weeks, showed a substantial increase in the size of the capillary bed of a range of leg muscles as indicated by an increase in the capillary:fibre ratio with no change in muscle fibre size (Deveci et al. 1998, 1999) in contrast to many reports in the literature that chronic systemic hypoxia does not induce angiogenesis at the capillary level in skeletal muscle (see Introduction). We do not yet know when the increase in the size of the capillary network begins. However, in view of the evidence that the V̇O2 of the vasculature represents a substantial portion of the total V̇O2 of resting skeletal muscle (see Ye et al. 1990a,b; Marshall & Davies, 1999), our findings that the size of the arteriolar and capillary networks is greatly increased during acclimation to chronic hypoxia are fully compatible with the demonstration that the V̇O2 of resting skeletal muscle in 3–4 week CH rats is ∼2·5-fold that of N rats (Marshall & Davies, 1999).
The stimulus for angiogenesis and remodelling
Given that acute systemic hypoxia induces a fall in systemic arterial pressure and vasodilatation in skeletal muscle with no significant change in muscle blood flow (see Bryan & Marshall, 1999) and given that this pattern is evident in the baseline values of rats exposed to chronic hypoxia (12% O2) for just 1 day and is still evident in 7CH rats (Walsh & Marshall, 1999), it seems that the stimulus for angiogenesis or remodelling at the arteriolar level in systemic hypoxia is not an increase in wall tension as has been suggested by Price & Skalak (1998): a reduced perfusion pressure is likely to result in a decrease in wall tension. It is possible that an increase in shear stress is important, as has been suggested by others (Hudlicka et al. 1992): a modest increase in haematocrit was already present in 7CH rats and probably results in an increase in shear stress in the arterioles and capillaries. However, an attractive possibility is that hypoxia per se is the major initiator. Hypoxic conditions were shown to cause angiogenesis, an increase in arteriolar branching and in the diameter of arterial vessels in chick allantoic membranes and in skeletal muscle of chick embryos in vivo (Adair et al. 1988; Dusseau & Hutchins, 1989), and hypoxia caused proliferation of endothelial cells in vitro (Meninger et al. 1988). Further, adenosine, which we know is a major contributor to the muscle vasodilatation of acute hypoxia both in spinotrapezius and hindlimb muscles of N rats and in CH rats (Mian & Marshall, 1996; Thomas & Marshall, 1997), can induce angiogenesis, at least in part, by increasing the expression and action of vascular endothelial growth factor (VEGF; Meninger et al. 1988; Hashimoto et al. 1994; Fischer et al. 1997). Increased expression of VEGF mRNA and VEGF protein have already been implicated in the angiogenesis that occurs in the brain in chronic hypoxia (Xu & Severinghaus, 1998; Kuo et al. 1999).
In summary, the present study has shown that within the first 7–18 days from the onset of chronic hypoxia (12% O2), the well-known hyperventilation, pulmonary vascular remodelling, right ventricular hypertrophy and increase in haematocrit are accompanied by normalisation of Ca,O2 and progressive, structural remodelling of skeletal muscle vasculature so that the extent of arteriolar branching, the number of small arterioles and the functional size of the vascular bed at maximal vasodilatation are greatly increased. This study has also shown that the acute hypoxic challenge of breathing 8% O2 evoked maximal muscle vasodilatation in N rats, but only ∼60% of maximal vasodilatation in 18CH rats even though Pa,O2 and Ca,O2 fell to approximately the same levels in the two groups. We propose that the structural and functional changes induced by chronic hypoxia enable skeletal muscle to cope with local hypoxia even more severe than that evoked by 8% O2, by facilitating the increase in muscle VC and thereby the blood flow and O2 that can be delivered to muscle.
Acknowledgments
We are grateful to The Physiological Society for the award of a Vacation Scholarship to K. Smith.
References
- Abacus Concepts. Stat View Reference. Berkeley, CA, USA: Abacus Concepts, Inc.; 1996. [Google Scholar]
- Abdelmalki A, Fimbel S, Mayet-Sornay MH, Sempore B, Favier R. Aerobic capacity and skeletal muscle properties of normoxic and hypoxic rats in response to training. Pflügers Archiv. 1996;431:671–679. doi: 10.1007/BF02253829. [DOI] [PubMed] [Google Scholar]
- Adair TH, Gay WJ, Montani J-P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. American Journal of Physiology. 1990;259:R393–404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- Adair TH, Guyton AC, Montani J-P, Lindsay HL, Stanek KA. Whole body structural vascular adaptation to prolonged hypoxia in chick embryos. American Journal of Physiology. 1987;252:H1228–1234. doi: 10.1152/ajpheart.1987.252.6.H1228. [DOI] [PubMed] [Google Scholar]
- Adair TH, Montani J-P, Guyton AC. Effects of intermittent hypoxia on structural vascular adaptation in chick embryos. American Journal of Physiology. 1988;254:H1194–1199. doi: 10.1152/ajpheart.1988.254.6.H1194. [DOI] [PubMed] [Google Scholar]
- Banchero N. Cardiovascular responses to chronic hypoxia. Annual Review of Physiology. 1987;49:465–476. doi: 10.1146/annurev.ph.49.030187.002341. [DOI] [PubMed] [Google Scholar]
- Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. The Journal of Physiology. 1999;514:151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassin S, Gilbert RD, Bunnell CE, Johnson EM. Capillary development during exposure to chronic hypoxia. American Journal of Physiology. 1971;220:448–451. doi: 10.1152/ajplegacy.1971.220.2.448. [DOI] [PubMed] [Google Scholar]
- Deveci D, Bryan P, Egginton S, Marshall JM. Chronic hypoxia induces angiogenesis in striated muscle of the rat. The Journal of Physiology. 1998;513.P:86. P. [Google Scholar]
- Deveci D, Egginton S, Marshall JM. Relationship between capillary angiogenesis and muscle fibre size in response to chronic hypoxia in rats. The Journal of Physiology. 1999;515.P:145–146. P. [Google Scholar]
- Dusseau JW, Hutchins PM. Microvascular responses to chronic hypoxia by the chick chorioallantoic membrane: a morphometric analysis. Microvascular Research. 1989;37:138–147. doi: 10.1016/0026-2862(89)90033-2. [DOI] [PubMed] [Google Scholar]
- Fischer S, Knoll R, Renz D, Karliczek GF, Schaper W. The role of adenosine in the hypoxic induction of vascular endothelial growth factor in porcine brain derived microvascular endothelial cells. Endothelium. 1997;5:155–165. doi: 10.3109/10623329709053395. [DOI] [PubMed] [Google Scholar]
- Harik SI, Hritz MA, LaManna JC. Hypoxia-induced brain angiogenesis in the adult rat. The Journal of Physiology. 1995;485:525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto E, Kage K, Ogita T, Nakaoka T, Matsuoka R, Kira Y. Adenosine as an endogenous mediator of hypoxia for induction of vascular endothelial growth factor messenger RNA in U-937 cells. Biochemical and Biophysical Research Communications. 1994;204:318–324. doi: 10.1006/bbrc.1994.2462. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown MD, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiological Reviews. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Itoh K, Orshi Y, Itoh M, Hirofuji I, Hayashi H. Effects of hypobaric hypoxia on histochemical fibre type composition and myosin heavy chain isoform component in the rat soleus muscle. Pflügers Archiv. 1995;429:601–606. doi: 10.1007/BF00373980. [DOI] [PubMed] [Google Scholar]
- Kuo NT, Benhayan D, Przybylski RJ, Martin AJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. Journal of Applied Physiology. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Cordisco BR, Kneuse DE, Hudetz AG. Increased capillary segment length in cerebral cortical microvessels of rats exposed to three weeks of hypobaric hypoxia. Advances in Experimental Biology. 1994;345:627–631. doi: 10.1007/978-1-4615-2468-7_83. [DOI] [PubMed] [Google Scholar]
- Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. Journal of Applied Physiology. 1992;72:2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- Lauro KL, LaManna JC. Adequacy of cerebral vascular remodeling following three weeks of hypobaric hypoxia. Examined by an integrated composite analytical model. Advances in Experimental Medicine and Biology. 1997;411:369–376. doi: 10.1007/978-1-4615-5865-1_47. [DOI] [PubMed] [Google Scholar]
- Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. The Journal of Physiology. 1982;332:160–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Davies WR. The effects of acute and chronic systemic hypoxia on muscle oxygen supply and muscle oxygen consumption in the rat. Experimental Physiology. 1999;84:57–68. doi: 10.1111/j.1469-445x.1999.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Meninger CJ, Schelling ME, Granger HJ. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. American Journal of Physiology. 1988;255:H554–562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- Mian R, Marshall JM. The role of adenosine in dilator responses induced in arterioles and venules of rat skeletal muscle in systemic hypoxia. The Journal of Physiology. 1991;443:499–511. doi: 10.1113/jphysiol.1991.sp018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R, Marshall JM. The behaviour of muscle microcirculation in chronically hypoxic rats: the role of adenosine. The Journal of Physiology. 1996;491:489–498. doi: 10.1113/jphysiol.1996.sp021233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Dempsey JA. Rat as a model for human-like ventilatory adaptation to chronic hypoxia. Journal of Applied Physiology. 1978;44:763–769. doi: 10.1152/jappl.1978.44.5.763. [DOI] [PubMed] [Google Scholar]
- Ou LC, Cai YN, Tenney SM. Responses of blood volume and red cell mass in two strains of rats acclimatized to high altitude. Respiration Physiology. 1985;62:85–94. doi: 10.1016/0034-5687(85)90052-0. [DOI] [PubMed] [Google Scholar]
- Ou LC, Chen J, Fiore E, Leiter JC, Brinck-Johnsen T, Birchard GF, Clemans G, Smith RP. Ventilatory and hematopoietic responses to chronic hypoxia in two rat strains. Journal of Applied Physiology. 1992;72:2354–2363. doi: 10.1152/jappl.1992.72.6.2354. [DOI] [PubMed] [Google Scholar]
- Price RJ, Skalak TC. Arteriolar remodelling in skeletal muscle of rats exposed to chronic hypoxia. Journal of Vascular Research. 1998;35:238–244. doi: 10.1159/000025589. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble W, Nadas AS, Miettinen DS, Reid L. Rat pulmonary circulation after chronic hypoxia: Haemodynamic and structural features. American Journal of Physiology. 1979;236:H818–827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Roos CM, Frank DU, Xue C, Johns RA, Rich GF. Chronic inhaled nitric oxide: Effects on pulmonary vasomotor endothelial function and pathology in rats. Journal of Applied Physiology. 1996;80:252–260. doi: 10.1152/jappl.1996.80.1.252. [DOI] [PubMed] [Google Scholar]
- Sillau AH, Banchero N. Effects of hypoxia on capillary density and fiber composition in rat skeletal muscle. Pflügers Archiv. 1977;370:227–232. doi: 10.1007/BF00585531. [DOI] [PubMed] [Google Scholar]
- Smith KA, Marshall JM. Does chronic hypoxia induce angiogenesis in skeletal muscle of the rat? The Journal of Physiology. 1997;499:118–119. P. [Google Scholar]
- Snyder GK, Wilcox EE, Burnham EW. Effects of hypoxia on muscle capillarity in rats. Respiratory Physiology. 1985;62:135–140. doi: 10.1016/0034-5687(85)90057-x. [DOI] [PubMed] [Google Scholar]
- Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. Journal of Applied Physiology. 1983;55:1445–1455. doi: 10.1152/jappl.1983.55.5.1445. [DOI] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. The Journal of Physiology. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Marshall JM. The roles of adenosine in regulating the respiratory and cardiovascular systems in chronically hypoxic, adult rats. The Journal of Physiology. 1997;501:439–447. doi: 10.1111/j.1469-7793.1997.439bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Marshall JM. Early effects of chronic systemic hypoxia upon muscle circulation in the rat. The Journal of Physiology. 1999;515.P:114. P. [Google Scholar]
- Williams DA, Segal SS. Microvascular architecture in rat soleus and extensor digitorum longus muscle. Microvascular Research. 1992;43:192–204. doi: 10.1016/0026-2862(92)90016-i. [DOI] [PubMed] [Google Scholar]
- Xu F, Severinghaus JW. Rat brain VEGF expression in alveolar hypoxia: possible role in high-altitude cerebral oedema. Journal of Applied Physiology. 1998;85:53–57. doi: 10.1152/jappl.1998.85.1.53. [DOI] [PubMed] [Google Scholar]
- Ye JM, Colquhoun EQ, Clark MG. A comparison of vasopressin and noradrenaline on oxygen uptake by perfused rat hindlimb, kidney, intestine and mesenteric arcade suggests that it is in part due to contractile work by blood vessels. General Pharmacology. 1990a;21:805–810. doi: 10.1016/0306-3623(90)91037-r. [DOI] [PubMed] [Google Scholar]
- Ye JM, Colquhoun EQ, Hettiarachchi M, Clark MG. Flow-induced oxygen uptake by the perfused rat hindlimb is inhibited by vasodilators and augmented by norepinephrine: a possible role of the microvasculature in hindlimb thermogenesis. Canadian Journal of Physiology and Pharmacology. 1990b;68:119–125. doi: 10.1139/y90-018. [DOI] [PubMed] [Google Scholar]


