Abstract
Cellular mechanisms of the actions of angiotensin II (ANGII) within the nucleus of the solitary tract (NTS) were studied using rat brain slices in 78 neurones recorded in the whole-cell configuration. Twenty-nine per cent of cells had an on-going activity and with only one exception these cells responded to tractus solitarii (TS) stimulation with a monophasic excitatory postsynaptic potential (EPSP). In approximately half of the silent cells, TS stimulation evoked an EPSP-inhibitory postsynaptic potential (IPSP) complex.
The ANGII (200 or 1000 nM) effect on TS-evoked EPSPs depended on the cell subpopulation. In cells with on-going activity, ANGII (1000 nM) increased evoked EPSP amplitude by +70 ± 13% (means ± s.e.m., n = 5) but reduced it (200 and 1000 nM) in silent cells where both evoked EPSPs and IPSPs were present. ANGII either increased TS-evoked IPSP conductances in cells where they were detectable or revealed an evoked IPSP (200 nM ANGII: IPSP conductance increased from 70 ± 29 to 241 ± 34 pS; n = 11). All ANGII effects were prevented by the ANGII type 1 (AT1) receptor blocker losartan. Since 200 nM ANGII did not increase responses to iontophoretically applied GABA, the effect of ANGII on TS-evoked IPSPs may occur presynaptically.
The neurokinin type 1 (NK1) receptor antagonist CP-99,994 (5 μM) blocked the ANGII-induced increase in EPSPs but had no effect on TS-evoked IPSP potentiation by ANGII.
Thus, ANGII can potentiate both inhibitory and excitatory synaptic transmission within different subpopulations of NTS neurones. Potentiation of evoked EPSPs, but not of IPSPs, involves activation of NK1 receptors. The balance of these actions of ANGII could be reflex specific: for the baroreflex circuitry the inhibitory action might predominate while the peripheral chemoreceptor reflex may be facilitated due to enhanced excitatory transmission.
Angiotensin II (ANGII) may be synthesised by brain tissue independently of peripheral sources (Richards et al. 1989; reviewed in Phillips et al. 1993; Sernia et al. 1997) and is currently viewed as a peptidergic neurotransmitter in certain brain pathways (Ferguson & Washburn, 1998). Within the nucleus tractus solitarii (NTS), which contains predominantly AT1-type receptors (Allen et al. 1991), ANGII is likely to play an important role in cardiovascular homeostasis. In particular, ANGII attenuates the baroreceptor reflex as demonstrated by NTS microinjection studies in rats (Casto & Phillips, 1986; Michelini & Bonagamba, 1990; Luoh & Chan, 1998; Paton & Kasparov, 1999). Further, blockade of AT1 receptors within the NTS can increase the baroreceptor reflex gain (Campagnole-Santos et al. 1988; Kasparov et al. 1998a). All told the data support an inhibitory role for ANGII in baroreceptive transmission in the NTS.
In the accompanying paper (Paton & Kasparov, 1999) we demonstrate that this inhibitory action of ANGII co-exists with another effect, namely potentiation of the peripheral chemoreceptor reflex. The latter, but not the former, results from activation of neurokinin 1 (NK1) receptors since the ANGII-induced potentiation of the chemoreceptor reflex was blocked by an NK1 antagonist - CP-99,994. The likeliest scenario for this finding is that ANGII causes release of substance P within NTS and this affects the circuitry mediating the chemoreceptor reflex. This is substantiated by the finding that micromolar concentrations of ANGII caused release of substance P from rat medullary slices of both normotensive and (mRen2)27 transgenic hypertensive rats (Barnes et al. 1991; Diz et al. 1998).
Information about the actions of ANGII on NTS neurones at the intracellular level is lacking. Extracellular recordings from NTS both in vivo (Hegarty et al. 1996) and in vitro (Barnes et al. 1988; Qu et al. 1996) described an excitatory effect of ANGII on some NTS neurones. These excitatory effects were comparable with responses induced by substance P in a population of NTS cells recorded in vitro (Barnes et al. 1991). In many NTS neurones blockade of synaptic transmission prevented the excitatory action of ANGII (Qu et al. 1996). These authors proposed that ANGII excited NTS neurones via a presynaptic release of substance P. Further, activation of NK1 receptors in the NTS led to a depolarisation in vitro (Maubach & Jones, 1997). ANGII-induced increases in evoked EPSPs in some intracellularly recorded NTS neurones were reported in two abstracts (Yang & Andresen, 1991; Barnes & DeWeese, 1996). To our knowledge these are the only published source of information about NTS actions of ANGII. However, exclusively excitatory effects of ANGII in the NTS cannot explain the attenuation of the baroreceptor reflex seen in vivo or in the working heart-brainstem preparation.
In the present study we have used brain slices to investigate the actions of ANGII on NTS neurones at the intracellular level. We were specifically interested in any effects that could provide a plausible explanation for the ANGII-induced inhibition of the baroreceptor reflex. We also attempted to identify a neuronal mechanism that could account for the ANGII-induced potentiation of other reflexes such as the chemoreceptor reflex described in our accompanying paper and whether this was dependent upon the integrity of NK1 receptors.
Preliminary reports of parts of this study have been published in abstract form (Kasparov & Paton, 1998; Kasparov et al. 1998b).
METHODS
Slice preparation
Sprague-Dawley rats of either sex aged between postnatal day 9 and 28 (modal value, 15 days; mean, 15 ± 1·3 days) were deeply anaesthetised by halothane and decapitated. Transverse slices of brainstem were cut (350 μm thick) using a vibroslice (Campden Instruments Ltd) in chilled, carbogen (95% O2-5% CO2)-gassed artificial cerebrospinal fluid (ACSF). The ACSF contained the following (mM): 10 dextrose, 125 NaCl, 24 NaHCO3, 5 KCl, 2·5 CaCl2, 1·25 MgSO4 and 1·25 KH2PO4. The porous matter of the area postrema was removed with fine forceps and transverse slices (+300 μm to −700 μm relative to obex) were cut. Subsequently the slices were stored in carbogen-gassed ACSF. In five animals NTS-containing slices were cut in the horizontal plane. As the results obtained with ANGII depended neither on the age of the animals nor on the orientation of the slice all data were pooled. For recording, slices were transferred into a submerged-type chamber (volume, 1·5 ml) and superfused with warm (31 ± 0·5°C) ACSF at a controlled and continuously monitored rate of 2 ml min−1.
Recording techniques
Intracellular recordings were made in the whole-cell mode from subnuclei of the NTS known to be the sites of termination of baroreceptor afferents (i.e. dorsomedial and medial to the tractus solitarii (TS), see Fig. 1A) as well as pulmonary, cardiac and other visceral afferents (reviewed in Loewy, 1990; Ciriello et al. 1994; Blessing, 1997). Patch pipettes (3–4 MΩ) were filled with the following intracellular solution (mM): 130 potassium gluconate, 10 Hepes, 11 EGTA, 4 NaCl, 2 MgCl2, 1 CaCl2, 2 ATP, 0·5 GTP and 5 glucose. Recorded signals were amplified (SEC 05L, NPI, Tamm, Germany) and stored on a hard disk using Spike2 software (Cambridge Electronic Design; sampling frequency, 3·3 kHz) for subsequent off-line analysis. Recordings were made in bridge mode unless indicated otherwise. Positions of the recorded cells within the slice were documented on pre-drawn sections from Paxinos & Watson (1986).
Figure 1. Features of the NTS neurones included in the sample.
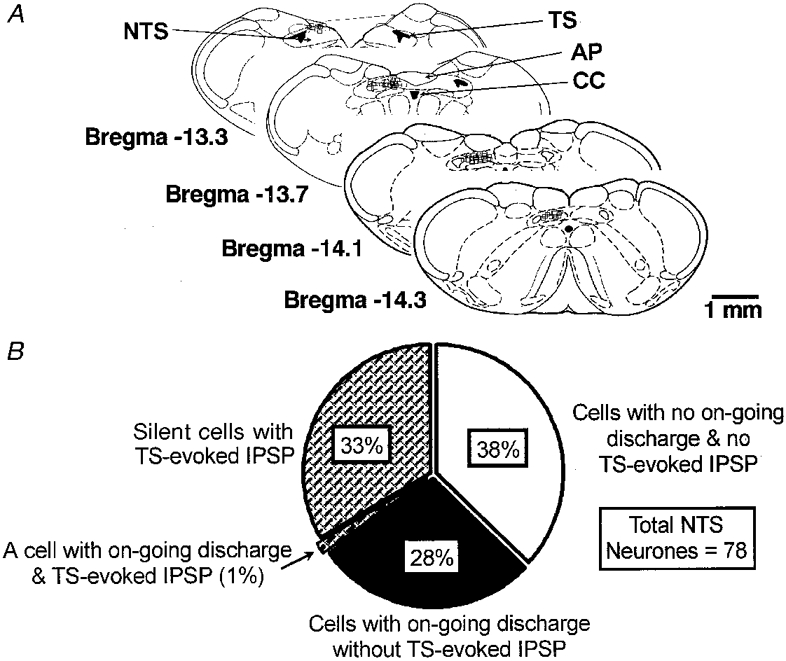
A, position of the recording sites of most experiments in transverse slices (open boxes). In some cases the sites overlapped completely. The drawings are modified from Paxinos & Watson (1986). TS, tractus solitarii; CC, central canal; AP, area postrema. (Note that AP was actually removed in our slices.) B, functional distribution of the cells (n = 78) according to two criteria: presence of on-going discharge before establishing whole-cell configuration; presence of IPSPs either from control or after administration of ANGII.
Experimental protocols and analysis
The experimental protocol began at least 5 min after whole-cell configuration was established. Passive membrane properties were assessed by a series of hyperpolarising and depolarising current pulses (1000–2000 ms) delivered via the recording electrode. Synaptic potentials were evoked by electrically stimulating within the ipsilateral TS (Neurodata PG 1000; 0·2 ms pulse-width; 0·5–5 V, 0·2-0·3 Hz) via a bipolar concentric electrode (120 μm tip diameter). Synaptic stimuli were combined with intracellular current pulses (with 400–800 ms delay) and postsynaptic potentials measured at various levels of membrane potential. The intensity of the synaptic stimulus was first varied to estimate the orthodromic action potential threshold at resting membrane potential (RMP) and then lowered by approximately 20% so as not to evoke spikes when combined with small positive current injections and allow detection of hyperpolarising IPSPs. Once set the stimulus intensity remained unchanged throughout the experiment.
TS-evoked EPSPs (average of 2 or 4 EPSPs) were measured at holding potentials of −75 to −70 mV, below the calculated chloride equilibrium potential for our solutions, in order to minimise contamination from inhibitory, Cl−-mediated events. Evoked IPSPs were measured during current injections (a mean of 2 sweeps per current step) and their conductance was estimated from the slope of the linear regression line of the IPSP amplitude-current plots (see Results, Fig. 4A) similar to the methods used in previous studies (Kasparov et al. 1994). In cases when under control conditions no negative-going IPSPs could be detected, their conductance was taken as zero. The evoked IPSPs in the present experiments always reversed at around −60 mV, close to the estimated reversal potential for Cl− based on our solutions, and to that reported for GABAA-mediated IPSPs in the NTS in other studies (Andresen & Mendelowitz, 1996). In our previous work, evoked IPSPs recorded from NTS neurones were all sensitive to the GABAA receptor blocker bicuculline (Butcher et al. 1999). Therefore, although in this study we did not systematically apply GABAA receptor blockers, we assume that IPSPs were mediated by GABAA receptors. This is consistent with the notion of a paucity of glycinergic inhibition in the NTS (Jordan et al. 1988).
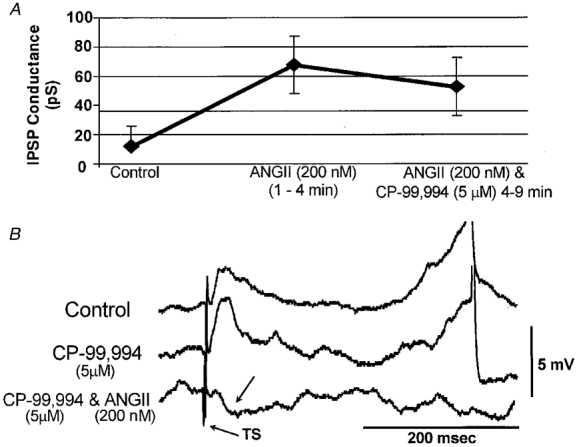
Figure 4. ANGII induced an increase in the conductance of an evoked IPSP in an NTS neurone.
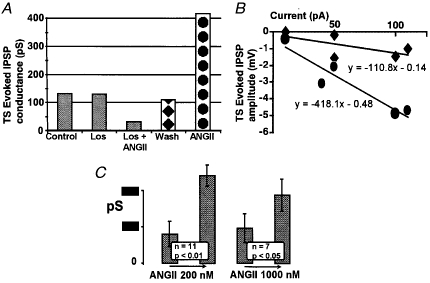
A, an example of an NTS neurone where ANGII (200 nM) increased the conductance of the TS-evoked IPSPs. This action could be prevented by losartan (Los; 20 μM). IPSP attenuation in losartan is a single observation. B, an example to illustrate TS-evoked IPSP conductance measurement in this neurone. Evoked IPSP peak amplitudes were plotted against the injected current and a slope of the linear regression was used to estimate the conductance. ♦, values taken during wash (see A);•, values obtained after administration of ANGII. Previous measurements are not plotted for clarity. C, pooled data on IPSP conductance changes evoked by ANGII in NTS neurones. One cell of 12 tested with 200 nM ANGII had an unusually high evoked IPSP conductance in control (over 600 pS) which was decreased by ANGII; this cell is not included in the graph. In cells where no negative-going IPSPs could be detected at depolarised levels of membrane potential, IPSP conductance was taken as zero (such as the cells in Figs 2 and 3, 5C and 7B).
Ca2+-dependent K+ conductances are present in many NTS neurones (Butcher et al. 1999), and in other tissues, such as the adrenal glomerulosa, ANGII may inhibit these channels (Payet et al. 1995). As Ca2+-dependent K+ currents are likely to affect firing properties of the neurones, trains of action potentials were evoked by depolarising current pulses of increasing intensity (range, 0·05-0·8 pA) and current-spike frequency plots constructed. As a measure of spike frequency adaptation, a ratio between the number of action potentials in the first and second halves of a train (duration, 1·5 s; frequency, 10–20 Hz) evoked by a positive current pulse was calculated.
In seven neurones a possible effect of ANGII on postsynaptic GABAA receptors was studied. After establishing the whole-cell configuration a multibarrel iontophoretic pipette was positioned blindly in close proximity to the recorded cell using continuous application of L-glutamate (-80 to −100 nA) as a search stimulus. Once the position of the iontophoretic pipette had been optimised, GABA was applied in regular cycles (1–2 s with 15–20 s interval). Current neutralisation was used via a 1·5 M NaCl-filled barrel. Of the seven neurones studied, four were voltage clamped at −80 mV so that GABAA receptor-mediated Cl− currents were inward. The other three cells were recorded in bridge mode with continuous negative current injections to observe GABA-evoked changes in input resistance. Recording in bridge mode allowed postsynaptic potentials to be studied in parallel with monitoring their responses to iontophoretically applied GABA.
In a subset of 30 neurones the effect of an NK1 receptor blocker (CP-99,994) on ANGII-induced changes was studied. In these experiments either ANGII was applied for 3 min and then in combination with CP-99,994 for a further 5 min, or, alternatively, CP-99,994 was administered for 3 min and then ANGII and CP-999,94 were co-applied for an additional 5 min.
Unpaired or paired data were analysed using Student's two-tailed t tests as appropriate. All values quoted are the mean ± standard error of mean (s.e.m.) while n is the number of observations. Differences were taken as significant at the 95% confidence limit.
Application of drugs
ANGII (200 or 1000 nM), losartan (20 μM) and CP-99,994 (5 μM) were all dissolved in ACSF and bath applied. In most cases ANGII was applied for 6–7 min and effects measured between 3 and 6 min. Equilibration of the concentration of the drugs in our chamber occurred within 2 min. For microiontophoresis a 0·5 M solution of L-glutamate (pH 8) and a 200 mM solution of GABA (pH 5) were used. Losartan potassium was kindly supplied by DuPont (USA) and CP-99,994 by Pfizer (UK). Other chemicals were obtained from Sigma-Aldrich (UK).
RESULTS
In this study ANGII was applied to 78 medial NTS neurones recorded using whole-cell patch clamp. Figure 1A shows the positions of the recording sites. All cells in this study received a synaptic input following electrical stimulation via a bipolar electrode placed within the TS. In 48 of these cells the effects of ANGII alone were studied. In the other 30 cells we sought to reveal interactions between ANGII and CP-99,994. As the protocols applied in these two parts of the study were different the data are presented separately.
Properties of the cells included in the sample
For the sake of presentation we have subdivided the cells as shown in Fig. 1B. First, if the cells generated action potentials before the whole-cell configuration was established they qualified as neurones with on-going activity. Such cells constituted 29% of the total sample. Note, that this value incorporates both cells with (1%) and without (28%) TS-evoked IPSPs (see below). Usually after 3–4 min in the whole-cell configuration these cells hyperpolarised by 3–4 mV and on-going discharge ceased although their RMP remained slightly more positive (-56 ± 2 mV; n = 23) than that of silent cells (-59 ± 1 mV; n = 55). However, in our slices the majority of cells were without on-going spiking activity and action potential discharge could only be elicited by either injecting depolarising current or strong synaptic stimulation. Second, further subdivision could be made according to the type of postsynaptic potential evoked by electrical stimulation within the TS. If a cell responded with an EPSP-IPSP sequence under control conditions or with an IPSP after administration of ANGII (see below), it qualified as having a TS-evoked IPSP (34%; 33% silent + 1% with on-going activity). All other neurones had no TS-evoked IPSPs (Fig. 1A). Only one neurone with on-going activity exhibited an evoked IPSP following synaptic stimulation in contrast to the ‘silent’ cells where evoked IPSPs were either present in control or revealed by ANGII in nearly half of the cases (see below).
Effects of ANGII on NTS neurones (48 neurones)
Effects of ANGII on ‘intrinsic’ membrane properties
ANGII caused no consistent change in membrane resistance either in the whole population of the recorded neurones (97 ± 2·4% of control with 200 nM, n = 26, and 98 ± 2% with 1000 nM, n = 22) or in any of the subgroups (Fig. 1A). A slight reversible depolarisation (range, 1–5 mV) occurred in four neurones tested with 200 nM ANGII and in five neurones tested with 1000 nM ANGII. An effect of ANGII on firing properties was studied in 19 and 11 neurones with 200 and 1000 nM ANGII, respectively. The current-spike frequency plots were not affected by ANGII at either concentration in any consistent way. Any minor changes observed in a few individual cells were related to fluctuations in either RMP or input resistance. For example, 200 nM ANGII reversibly shifted these plots to the right in six cells (decreased excitability), to the left in five cells (increased excitability) and caused no change in eight neurones. Spike frequency adaptation ratio (see Methods) was measured in 12 cells tested with 200 nM ANGII. Six of these neurones featured adaptation under control conditions (ratio < 1; 0·8 ± 0·04). Again, no systematic change could be detected: of these six cells, three increased their adaptation ratio (mean decrease of −0·24) whereas this was decreased in two cells (+0·34 and +0·14) and did not change in one. We also measured the amplitude of rebound depolarisation following release from a 1·5 s hyperpolarising pulse to −78 or −80 mV. In two of nine cells this reversibly increased with exposure to 200 nM ANGII.
Effect of ANGII on TS-evoked EPSPs in NTS neurones
The latency of EPSPs evoked by stimulation within the TS varied between cells and both putative mono- and polysynaptic connections were activated in all cell groups (Table 1). To study the effect of ANGII on evoked EPSPs, measurements were taken at hyperpolarised levels of membrane potential (see Methods). The effect of ANGII on evoked EPSPs in different groups of neurones, subdivided according to Fig. 1A, is shown in Table 2.
Table 1.
Mono- vs. polysynaptic EPSPs evoked by stimulation within the TS in different subpopulations of NTS neurones
| Putative monosynaptic (latency ≤ 2 ms; 1.4 ± 0.1 ms) | Putative polysynaptic (latency ≥ 2 ms; 3.9 ± 0.36 ms) | |
|---|---|---|
| Silent, no IPSPs | 40% | 60% |
| On-going activity | 57% | 43% |
| Cells with IPSPs | 38% | 62% |
The table depicts proportions of cells with short latency (≤ 2 ms; putative monosynaptic) and long latency (≥ 2 ms; putative polysynaptic) EPSPs in 48 NTS neurones treated with ANGII only, as indicated in the text. As current spread in the slice is unknown, the presynaptic elements could be both primary afferents and local interneurones.
Table 2.
Effects of ANGII on TS-evoked EPSP amplitude in different subpopulations of NTS neurones
| 200 nm ANGII (% change) | 1000 nm ANGII (% change) | Washout of 1000 nm ANGII (% change) | |
|---|---|---|---|
| Silent, no IPSPs | −9 ± 12 | −2 ± 14 | +3 ± 14 |
| (n = 9) | (n = 8) | (n = 6) | |
| On-going activity | +7.7 ± 11.2 | +70 ± 13*† | +18 ± 17 |
| (n = 4) | (n = 5) | (n = 4) | |
| Cells with IPSPs | −18 ± 6* | −16 ± 4* | +2 ± 14 |
| (n = 9) | (n = 8) | (n = 6) |
The data refer to the sample of NTS cells treated with ANGII only.
In one neurone with on-going activity, 1000 nm ANGII did not change the TS-evoked EPSP. This cell is not included in the data presented here.
P < 0.05.
In silent cells with no detectable TS-evoked IPSPs (i.e. where the EPSPs should not have been affected by concomitant Cl− fluxes) neither concentration of ANGII (200 or 1000 nM) caused a significant change in EPSP amplitude (Table 2). However, in five of six cells with on-going activity, and which also lacked TS-evoked IPSPs, 1000 nM ANGII reversibly increased EPSP amplitude by +70 ± 13% (n = 5, P < 0·05). In contrast, in silent cells exhibiting TS-evoked IPSPs, administration of 200 and 1000 nM ANGII resulted in a reversible decrease in EPSP amplitude (Table 2). Note, however, that as TS-evoked IPSPs also increased in these cells (see below), the decrease in EPSP amplitude could reflect a shunting action by Cl−-mediated conductances.
Effect of ANGII on TS-evoked IPSPs in NTS neurones
Eighteen NTS neurones exhibited IPSPs following stimulation within the TS either in control or after bath application of ANGII (all but one were silent). Twelve of these cells were treated with 200 nM ANGII, seven with 1000 nM ANGII, and one cell with both concentrations.
ANGII either increased evoked IPSPs in cells where they were detectable under control conditions, or revealed an evoked IPSP. The latter occurred in neurones where TS stimulation evoked only a depolarising monophasic EPSP under control conditions (Figs 2, 3, 5C and 7B). After administration of ANGII, TS stimulation could shunt a spike induced by injection of depolarising current (Fig. 2A). In three cells treated with 1000 nM ANGII, we observed barrages of spontaneous IPSPs (Fig. 3A) which reversed around the same membrane potential (-60 to −65 mV) as the TS-evoked IPSPs. These barrages lasted 20–60 s and usually occurred shortly after the arrival of ANGII in the chamber. An example of the effect of ANGII on TS-evoked IPSP conductance and the pooled data on TS-evoked IPSPs is presented in Fig. 4. This figure indicates that both concentrations of ANGII caused significant (P < 0·01 for 200 nM, n = 11, and P < 0·05 for 1000 nM, n = 7) increases in IPSP conductance. Although concentration-response characteristics were not studied in detail, Fig. 4A suggests that the effect of ANGII on evoked IPSPs is fully developed at 200 nM concentration. The time of ANGII application in this study never exceeded 7 min during which period ANGII-induced changes in TS-evoked PSPs normally persisted.
Figure 2. ANGII reveals a TS-evoked IPSP in an NTS neurone.
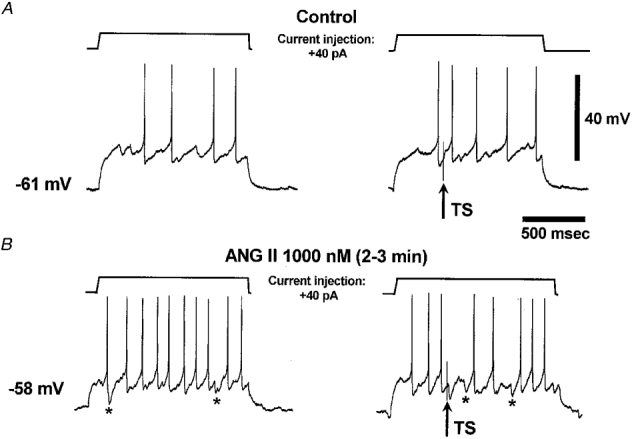
The traces are single sweeps obtained when positive current injections were combined with electrical stimulation via a bipolar concentric electrode (120 μm diameter) placed within the TS (5 V, 400 ms delay). In control (A) the stimulus (arrow) evoked an EPSP and a spike (right panel) when combined with current injection. After administration of ANGII the cell was depolarised by 3 mV and therefore the same current injection evoked more action potentials (B, left panel). However, stimulation now evoked an IPSP, which was capable of shunting an induced spike (B, right panel). After ANGII some spontaneous IPSPs were visible (marked by asterisks) at depolarised potentials (B, left and right panels). This was the second application of ANGII to this cell (see Fig. 3).
Figure 3. Losartan prevents transformation of an evoked EPSP into an EPSP-IPSP complex by ANGII.
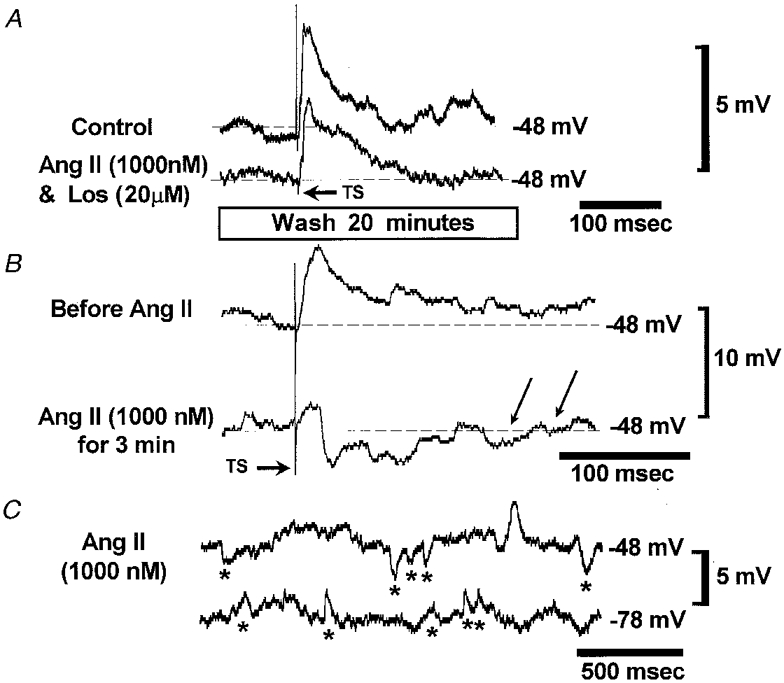
A, initially a combination of ANGII and an AT1 blocker, losartan (Los), was administered. The traces are means of two sequential EPSPs evoked at a depolarised potential by stimulation of the TS. Note that a combination of ANGII and losartan did not change the evoked EPSP. B, after a 20 min wash, ANGII was re-applied alone. Within 3 min the evoked monophasic EPSP was transformed into an EPSP-IPSP complex (single sweeps). Note two spontaneous IPSPs indicated by arrows which appeared during application of ANGII. C, spontaneous IPSP barrages (marked by asterisks) triggered in this neurone by ANGII were recorded at depolarised and hyperpolarised levels of membrane potential. At −78 mV all PSPs were positive going indicating that the IPSPs reversed and were mediated by Cl−. These data are from the same neurone as depicted in Fig. 2.
Figure 5. ANGII does not potentiate responses of NTS neurones to iontophoretically applied GABA.
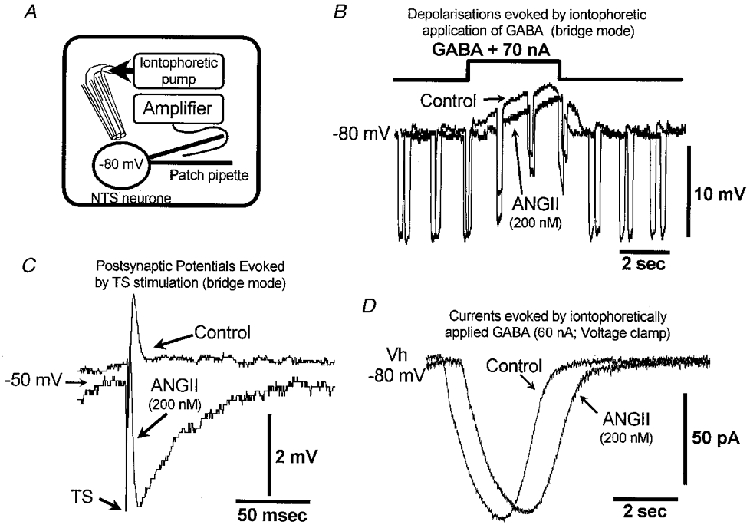
A, schematic diagram to illustrate the method employed. GABA was applied in pulses from a four-barrel pipette positioned in close proximity to the recorded cell. Three neurones were studied in bridge mode and another four in voltage clamp at −80 mV. B and C show a cell recorded in bridge mode where TS-evoked IPSPs were measured in combination with GABA iontophoresis. B, the responses of this neurone to application of GABA in control and after administration of 200 nM ANGII. The cell was hyperpolarised to −80 mV by continuous negative current injection combined with short negative pulses (200 ms, −30 pA) to estimate GABA-evoked drops in input resistance. Note that in ANGII, neither the potential shift nor the GABA-evoked resistance decrease was potentiated. C, averaged TS-evoked PSP (2 sweeps) in control and in 200 nM ANGII taken at a depolarised level of membrane potential. Note reversal of the polarity of the synaptic response to an IPSP in the presence of ANGII. D, currents (means of 5) evoked by 2 s GABA pulses in a voltage-clamped NTS neurone. Currents are inward because the cell was held below the Cl− reversal potential. ANGII did not potentiate postsynaptic responses to GABA.
Figure 7. CP-99,994 does not antagonise TS-evoked IPSP potentiation by ANGII.
A, in three cells where ANGII revealed TS-evoked IPSPs they persisted when ANGII was co-applied with 5 μM CP-99,994. B, CP-99,994 did not prevent ANGII-induced potentiation of TS-evoked IPSPs in this NTS neurone. In this case CP-99,994 was applied first and no major changes in the TS-evoked EPSPs occurred. After 3 min in 200 nM ANGII the TS-evoked PSP transformed into an IPSP (arrow) in which the EPSP was presumably shunted. The traces are means of two sweeps taken at −47 mV.
Receptor specificity of the effect of ANGII on evoked PSPs
NTS contains primarily AT1 receptors which can be blocked by losartan. As many effects of ANGII are known to desensitise and receptors internalise we sought to compare the effect of a combination of ANGII and losartan (20 μM) followed by ANGII alone. Following application of ANGII and losartan, cells were washed for 20 min before ANGII was applied alone. Of seven cells tested three displayed TS-evoked IPSPs and in all cases these remained unchanged during combined application of ANGII and losartan but were potentiated by subsequent administration of ANGII alone (1 cell with 200 nM and 2 with 1000 nM ANGII; Figs 3A and B, and 4A). The remaining four neurones did not exhibit TS-evoked IPSPs but one exhibited on-going activity and in this neurone TS-evoked EPSPs were not affected by 1000 nM ANGII plus 20 μM losartan but increased when 1000 nM ANGII was washed in alone. Thus, in all four cells where ANGII effects could be detected they could be prevented by losartan. In three other silent cells application of either ANGII plus losartan or ANGII alone did not result in any change in TS-evoked PSPs.
Mechanism of action of ANGII on IPSPs: post- or presynaptic?
Fast IPSPs in the NTS are mediated predominantly via GABAA receptors (McWilliam & Shepheard, 1988; Andresen & Mendelowitz, 1996; Butcher et al. 1999). It is therefore possible that ANGII increased the efficacy of inhibition by potentiating GABAA receptor function at the level of the postsynaptic membrane. To test this hypothesis, GABA was applied to seven cells using microiontophoresis while bath applying 200 nM ANGII (Fig. 5A). In all three neurones recorded in bridge mode, the decreases in membrane resistance induced by GABA pulses remained within ±10% of their control levels during bath administration of ANGII (Fig. 5A). In two of these neurones, TS-evoked IPSPs increased profoundly (Fig. 5A). Four additional cells were recorded in voltage clamp mode and held at −80 mV. These cells were all silent and are not included in the overall statistics (Fig. 1A) since their properties could not be studied in the same way as those of the other neurones. However, in all cases, there was no increase in GABAA-mediated currents (Fig. 5A) and the mean GABA-evoked current integral 3–4 min after ANGII was applied was 89 ± 3% (n = 4) of the control level.
Interactions between ANGII and CP-99,994 (30 neurones)
These neurones were not different from those in the first part of the study in terms of their resting membrane potential, input resistance (P > 0·1) or the age of the animals used. Forty-three per cent of them exhibited on-going activity; 30% displayed TS-evoked IPSPs and were all silent. We sought to determine whether an NK1 receptor blocker, CP-99,994, would antagonise the ANGII-induced potentiation of TS-evoked IPSPs (in silent cells) and evoked EPSPs in cells with on-going activity (see above).
In four cells with on-going activity, CP-99,994 (5 μM) was added to the perfusate and then co-applied with 1000 nM ANGII for a further 5 min. The mean amplitude of evoked EPSPs after administration of CP-99,994 increased slightly (+18 ± 2%; P < 0·05, n = 4). However, co-application of CP-99,994 with ANGII (1000 nM) did not potentiate EPSPs in any of these cells (+3 ± 1% relative to the CP-99,994 level). In five other neurones with on-going firing, 1000 nM ANGII was applied alone and enhanced the size of the TS-evoked EPSPs in four neurones (+59 ± 20%) but a combination of ANGII (1000 nM) and CP-99,994 (5 μM) decreased evoked EPSPs to −22 ± 16% of control within 2–3 min (Fig. 6; P < 0·05). Thus, CP-99,994 antagonism of NK1 receptors blocked the ANGII-induced potentiation of TS-evoked EPSPs in the NTS.
Figure 6. ANGII-induced potentiation of TS-evoked EPSPs in active NTS cells can be abolished by an NK1 receptor blocker, CP-99,994.
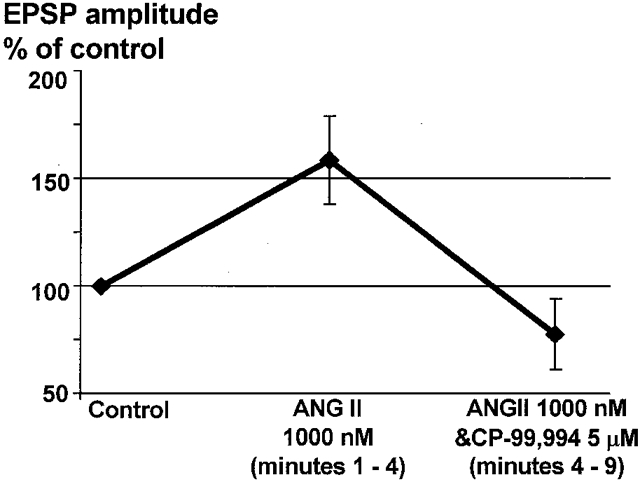
In all four cells with on-going discharge and where TS-evoked EPSPs were potentiated by 1000 nM ANGII, a combination of CP-99,994 (5 μM) and ANGII (1000 nM) resulted in an immediate and significant (P < 0·05) decrease in EPSP amplitude to below the initial level.
To reveal any role for substance P in mediating the potentiation of TS-evoked IPSPs by ANGII, CP-99,994 (5 μM) was co-applied with ANGII (200 nM) after a 3 min exposure to ANGII (200 nM) alone in three cells. As shown in Fig. 7A, TS-evoked IPSPs persisted in the presence of CP-99,994. An additional five silent NTS neurones were tested in reverse mode whereby CP-99,994 (5 μM) was applied alone for 3 min and then in combination with ANGII (200 nM). In two neurones, ANGII revealed an evoked IPSP, which was not detected in control (Fig. 7A). Therefore, the integrity of NK1 receptors is not a pre-requisite for ANGII-induced increases in inhibition in the NTS.
DISCUSSION
The present study describes the effects of ANGII on NTS neurones recorded using the whole-cell patch technique. Specifically we sought to find clues to the neuronal mechanisms that could potentially account for the differential actions of this peptide on cardiorespiratory reflexes described in the accompanying paper (Paton & Kasparov, 1999). These effects were an inhibition of the baroreceptor reflex and potentiation of the chemoreceptor reflex. In the present study, we found that in a subpopulation of NTS neurones ANGII (200 and 1000 nM), acting presynaptically, enhanced IPSPs evoked by stimulation within the TS. This mechanism could potentially account for the ANGII-mediated depression of the baroreceptor reflex. We also found that the potentiation by ANGII of evoked IPSPs persisted in the presence of NK1 receptor blockade suggesting that this was not due to the release of substance P. In a different subpopulation of cells, which tended to exhibit on-going activity, 1000 nM ANGII potentiated TS-evoked EPSPs, and this was sensitive to NK1 receptor blockade and may account for the potentiation of the chemoreceptor reflex. We do not claim that all cells in the first group are baroreceptive and in the second chemoreceptive. In fact, both reflexes can be depressed by low concentrations of ANGII (Paton & Kasparov, 1999) and there are other reflex pathways in this region of the NTS (for references see Introduction) that could also be modulated by this peptide.
The previously obtained information on the neuronal mechanisms of action of ANGII in the NTS did not provide an explanation for its differential actions on cardiorespiratory reflexes (see Paton & Kasparov, 1999). Excitatory effects of ANGII in the NTS related to substance P have been documented by extracellular recordings in brain slices (Qu et al. 1996). In these experiments application of 1 μM ANGII increased the spontaneous activity in some NTS neurones. In certain cells this was prevented by blockade of synaptic transmission suggesting a presynaptic site of action. In the present study, an increase in TS-evoked EPSP amplitude with 1 μM ANGII was also observed and this was sensitive to CP-99,994. Taken together these results show that 1 μM ANGII may induce release of substance P in NTS and, via NK1 receptors, potentiate synaptic transmission. It should be stressed that we only consistently observed ANGII-induced enhancement of EPSPs in the cells with on-going discharge. This is important because the extracellular electrodes used in previous studies (Barnes et al. 1988; Qu et al. 1996) would have preferentially selected active neurones. Thus, both the latter studies and the present findings are consistent in that ANGII produces a ‘stimulatory’ effect on a population of NTS neurones, which for some reason tend to generate on-going action potentials in vitro. We tentatively suggest that these neurones are not involved in mediating the baroreceptor reflex. Interestingly, the NTS neurones in which Yang & Andresen (1991) observed ANGII-induced increases in evoked EPSPs also demonstrated on-going firing.
In our slices the majority of NTS neurones were silent (71%) and did not generate action potentials unless made to do so by depolarising current injection. These neurones would be ‘invisible’ to an extracellular electrode, as used in previous studies (Barnes et al. 1988; Hegarty et al. 1996), but it is this population where ANGII enhanced the efficacy of evoked synaptic inhibition. On the other hand, TS-evoked inhibitory responses were rarely found in the active NTS neurones of our present study (see Fig. 1A). It is likely that these and most other NTS cells do have inhibitory inputs. However, the potency or density of these inhibitory connections may strongly vary between different neurones.
ANGII increased TS-evoked IPSPs in approximately 25% of caudal NTS neurones (based on the sample of cells treated with ANGII only). This effect was fully manifested with a 200 nM concentration of ANGII. The increase in evoked IPSP conductances could be prevented by losartan and therefore was mediated via AT1 receptors. This is in agreement with the information indicating that the AT1 receptor is the predominant ANGII receptor in the NTS (Millan et al. 1991; Song et al. 1991) for mediating cardiovascular actions (Fow et al. 1994; Luoh & Chan, 1998). ANGII-induced increases in IPSPs could be due to enhanced release of GABA as ANGII is known to enhance release of neurotransmitter substances in several types of synapses. The other possibility is that the potentiation of evoked IPSPs might reflect a sensitisation of the GABAA receptors by ANGII. However, in our experiments ANGII never potentiated the effects of GABA applied directly onto the recorded neurone in all seven NTS neurones tested yet TS-evoked IPSPs were potentiated in some of these neurones. Therefore, we suggest that the increase in synaptic inhibition occurred at the level of the terminals of inhibitory interneurones. The short-lasting barrages of spontaneous IPSPs induced by ANGII in some cells (see Fig. 3A) are consistent with this idea. The effect of ANGII on inhibitory synaptic transmission was functionally effective: in cells where TS stimulation previously evoked a spike, the ANGII-induced evoked IPSP could shunt an on-going spike instead.
The sources of synaptic drive to NTS inhibitory interneurones are not fully documented, although it is known that in anaesthetised animals these interneurones provide certain tonic inhibitory drive to baroreceptive cells (Suzuki et al. 1993). These interneurones have been implicated in baroreceptor reflex inhibition during stimulation of, for example, the cerebellar uvula (Paton, 1997) or hypothalamic defence area (Jordan et al. 1988). Interestingly, the hypothalamic defence area in the rat contains a high number of ANGII-positive cells (Lind et al. 1985). Thus, there is a possibility that ANGII-containing fibres from the hypothalamus descend to the NTS and may act to potentiate local inhibitory interneurones. However, the NTS itself also contains many ANGII-positive cells (Lind et al. 1985) which might equally well be activated by hypothalamic or cerebellar uvula stimulation.
Earlier, Cai et al. (1994) using extracellular recording in a slice preparation from rabbit found that application of ANGII onto the area postrema inhibited synaptic responses evoked from the TS in 37% of NTS neurones which were without on-going activity. They proposed that the area postrema neurones may be activated by blood-borne ANGII leading to an inhibition of NTS neurones which could result in an inhibition of baroreflexes. However, the anatomical boundaries between NTS and the area postrema are somewhat vague and it is very difficult to exclude the possibility of some diffusion of ANGII into NTS. In our experiments, the porous matter of the area postrema was always removed during the preparation of the slices and some of the cells in which ANGII increased IPSPs were located up to 300 μm rostral to the obex, a level where the area postrema is absent. Nevertheless, it is possible that the increase in TS-evoked IPSPs observed in the present study and the findings of Cai et al. (1994) reflect the same action of ANGII, namely potentiation of inhibition within the NTS. In the preceding paper (Paton & Kasparov, 1999) we also demonstrate that removal of the area postrema did not affect the gain of the baroreceptor reflex per se or the ability of ANGII to suppress this reflex.
In the present studies although the inhibitory synaptic response to afferent stimulation was potentiated by ANGII there was no effect on input resistance. We suggest that ANGII did not evoke a tonic release of large quantities of GABA from the GABAergic terminals in most cases. Further, we speculate that this effect might lead to selective ‘deafferentation’ of some afferent inputs to NTS neurones. The resultant effect will depend on the site of contact of afferents and the relative position of GABAA receptors on an NTS neurone. If this is dendritic it may explain the absence of input resistance changes given the limited space clamp capacity of the recording technique we used and the relatively long thin dendrites of some NTS neurones (Deuchars et al. 1998). Indeed, it may be that the excitability of the soma is only slightly affected. Such a mode of action would explain why inhibition of the baroreceptor reflex with ANGII may occur without an expected tachycardia (Michelini & Bonagamba, 1990; Paton & Kasparov, 1999) or why a microinjection of ANGII antagonists into NTS evokes little or no bradycardia although potentiating the baroreceptor reflex (Campagnole-Santos et al. 1988; Michelini & Bonagamba, 1990; Kasparov et al. 1998a).
Another significant effect of ANGII on NTS neurones was a 20% reduction in TS-evoked EPSPs in some NTS cells. However, this effect was restricted to the same ‘silent’ cells in which evoked IPSPs were also enhanced (Table 2). In these cells after administration of ANGII, evoked postsynaptic potentials appeared as EPSP-IPSP complexes. Although the evoked EPSP measurements were taken at −75 to −70 mV, which is near or below the reversal potential for CI−-mediated conductances, it cannot be excluded that evoked EPSPs were partially shunted by the increased IPSPs. Therefore the possibility that the release of an excitatory transmitter could be reduced by ANGII is also acknowledged. This would be consistent with the reported inhibition by ANGII of Ca2+ currents in a subpopulation of cultured nodose ganglion cells (Bacal & Kunze, 1994).
Finally, ANGII evoked inconsistent changes in resting membrane potential, input resistance and firing behaviour evoked by injection of positive current. In this respect, ANGII effects were very different from those seen in the rostral ventrolateral medulla where 1 μM ANGII typically evoked a strong depolarisation and an increase in input resistance due to a reduction in a K+ conductance (Li & Guyenet, 1996).
We conclude that ANGII can potentiate both inhibitory and excitatory synaptic transmission within the NTS. Based on data in our preceding paper, these actions of ANGII could be reflex specific: while the baroreceptor input is shunted (presumably due to activation of inhibitory circuits) others including the peripheral chemoreceptor reflex may be potentiated. The latter effect is due to an ANGII-mediated increase in the release of substance P. Future experiments on physiologically identified subsets of NTS neurones are necessary to provide more detailed information about the actions of ANGII on defined reflex pathways integrated within the NTS. These experiments require intracellular recordings in a system which allows physiological characterisation of NTS neurones and lacks peripheral ANGII sources such as the working heart- brainstem preparation.
Acknowledgments
These studies were supported by the Wellcome Trust (044994), the British Heart Foundation (BS BS/93003) and the Royal Society.
References
- Allen A M, Paxinos G, McKinley M J, Chai S Y, Mendelsohn F A O. Localization and characterization of angiotensin II receptor binding sites in the human basal ganglia, thalamus, midbrain pons, and cerebellum. Journal of Comparative Neurology. 1991;312:291–298. doi: 10.1002/cne.903120211. [DOI] [PubMed] [Google Scholar]
- Andresen M C, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius - Common denominators. Chemical Senses. 1996;21:387–395. doi: 10.1093/chemse/21.3.387. [DOI] [PubMed] [Google Scholar]
- Bacal K, Kunze D L. Dual effects of angiotensin II on calcium currents in neonatal rat nodose neurons. Journal of Neuroscience. 1994;14:7159–7167. doi: 10.1523/JNEUROSCI.14-11-07159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K L, DeWeese D M. Angiotensin potentiates solitary tract afferent responses in medial nucleus tractus solitarii (mnTS) neurons responsive to glutamate but not to substance P. Society for Neuroscience Abstracts. 1996;22:632. [Google Scholar]
- Barnes K L, Diz D I, Ferrario C M. Functional interactions between angiotensin II and substance P in the dorsal medulla. Hypertension. 1991;17:1121–1126. doi: 10.1161/01.hyp.17.6.1121. [DOI] [PubMed] [Google Scholar]
- Barnes K L, Knowles W D, Ferrario M C. Neuronal responses to angiotensin II in the in vitro slice from the canine medulla. Hypertension. 1988;11:680–684. doi: 10.1161/01.hyp.11.6.680. [DOI] [PubMed] [Google Scholar]
- Blessing W W. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. Anatomy of the lower brainstem; pp. 29–99. [Google Scholar]
- Butcher J W, Kasparov S, Paton J F R. Differential effects of apamin on neuronal excitability in the nucleus tractus solitarii studied in vitro. Journal of the Autonomic Nervous System. 1999;77:90–97. [PubMed] [Google Scholar]
- Cai Y, Hay M, Bishop V S. Stimulation of area postrema by vasopressin and angiotensin II modulates neuronal activity in the nucleus tractus solitarius. Brain Research. 1994;647:242–248. doi: 10.1016/0006-8993(94)91323-4. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos M J, Diz D I, Ferrario C M. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension. 1988;11(suppl. I):I167–171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- Casto R, Phillips M I. Angiotensin II attenuates baroreflexes at nucleus tractus solitarius of rats. American Journal of Physiology. 1986;250:R193–198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Hochstenbach S L, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Barraco I R A, editor. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press; 1994. pp. 35–50. [Google Scholar]
- Deuchars J, Li Y W, Kasparov S, Paton J F R. Morphology of physiologically characterized baroreceptive neurones in the nucleus tractus solitarius (NTS) of the rat. Society for Neurocsience Abstracts. 1998;24:625. [Google Scholar]
- Diz D I, Westwood B, Bosch S M, Ganten D, Ferrario C. NK1 receptor antagonist blocks angiotensin II responses in renin transgenic rat medulla oblongata. Hypertension. 1998;31:473–479. doi: 10.1161/01.hyp.31.1.473. [DOI] [PubMed] [Google Scholar]
- Ferguson A V, Washburn D S. Angiotensin II: A peptidergic neurotransmitter in central autonomic pathways. Progress in Neurobiology. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Fow J E, Averill D B, Barnes K L. Mechanisms of angiotensin-induced hypotension and bradycardia in the medial solitary tract nucleus. American Journal of Physiology. 1994;267:H259–266. doi: 10.1152/ajpheart.1994.267.1.H259. [DOI] [PubMed] [Google Scholar]
- Hegarty A A, Hayward L F, Felder R B. Influence of circulating angiotensin II and vasopressin on neurons of the nucleus of the solitary tract. American Journal of Physiology. 1996;270:R675–681. doi: 10.1152/ajpregu.1996.270.3.R675. [DOI] [PubMed] [Google Scholar]
- Jordan D, Mifflin S W, Spyer K M. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is GABA mediated. The Journal of Physiology. 1988;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Butcher J W, Paton J F R. Angiotensin II receptors within the nucleus of the solitary tract mediate the developmental attenuation of baroreceptor vagal reflex in pre-weaned rats. Journal of the Autonomic Nervous System. 1998a;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Jones R S G, Paton J F R. Angiotensin II modulates inhibitory and excitatory neurotransmission in the solitary tract nucleus (NTS) in vitro. Society for Neuroscience Abstracts. 1998b;24:625. [Google Scholar]
- Kasparov S, Paton J F R. Effects of angiotensin II on neurones in the nucleus of the solitary tract (NTS) of the developing rat in vitro. The Journal of Physiology. 1998;509:128–129. P. [Google Scholar]
- Kasparov S, Pawelzik H, Zieglgänsberger W. Thyrotropin-releasing hormone enhances excitatory postsynaptic potentials in neocortical neurons of the rat in vitro. Brain Research. 1994;656:229–235. doi: 10.1016/0006-8993(94)91465-6. [DOI] [PubMed] [Google Scholar]
- Li Y W, Guyenet P G. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circulation Research. 1996;78:274–282. doi: 10.1161/01.res.78.2.274. [DOI] [PubMed] [Google Scholar]
- Lind R W, Swanson L W, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. An immunohistochemical study. [DOI] [PubMed] [Google Scholar]
- Loewy A D. Central autonomic pathways. In: Loewy A D, Spyer K M, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press Inc.; 1990. pp. 88–103. [Google Scholar]
- Luoh H F, Chan S H H. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Research. 1998;782:73–82. doi: 10.1016/s0006-8993(97)01198-0. [DOI] [PubMed] [Google Scholar]
- McWilliam P N, Shepheard S L. A GABA-mediated inhibition of neurones in the nucleus tractus solitarius of the cat that respond to electrical stimulation of the carotid sinus nerve. Neuroscience Letters. 1988;94:321–326. doi: 10.1016/0304-3940(88)90038-9. [DOI] [PubMed] [Google Scholar]
- Maubach K A, Jones R S G. Electrophysiological characterisation of tachykinin receptors in the rat nucleus of the solitary tract and dorsal motor nucleus of the vagus in vitro. British Journal of Pharmacology. 1997;122:1151–1159. doi: 10.1038/sj.bjp.0701482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini L C, Bonagamba L H. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension. 1990;15:I45–50. doi: 10.1161/01.hyp.15.2_suppl.i45. [DOI] [PubMed] [Google Scholar]
- Millan M A, Jacobowitz D M, Aguilera G, Catt K J. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proceedings of the National Academy of Sciences of the USA. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J F R. Cerebellar control of cardiovascular and respiratory activity. In: Jordan D, editor. Central Nervous Control of Autonomic Function. Amsterdam: Harwood Academic Publishers; 1997. pp. 225–258. [Google Scholar]
- Paton J F R, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii - a microinjection study in the rat. The Journal of Physiology. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rats Brain in Stereotaxic Coordinates. London: Academic Press Ltd; 1986. [Google Scholar]
- Payet M D, Bilodeau L, Drolet P, Ibarrondo J, Guillon G, Gallo-Payet N. Modulation of a Ca2+-activated K+ channel by angiotensin II in rat adrenal glomerulosa cells: Involvement of a G protein. Molecular Endocrinology. 1995;9:935–947. doi: 10.1210/mend.9.8.7476991. [DOI] [PubMed] [Google Scholar]
- Phillips M I, Speakman E A, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regulatory Peptides. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- Qu L, McQueeney A J, Barnes K L. Presynaptic or postsynaptic location of receptors for angiotensin II and substance P in the medial solitary tract nucleus. Journal of Neurophysiology. 1996;75:2220–2228. doi: 10.1152/jn.1996.75.6.2220. [DOI] [PubMed] [Google Scholar]
- Richards E M, Hermann K, Sumners C, Raizada M K, Phillips M A. Release of immunoreactive angiotensin II from neuronal cultures: Adrenergic influences. American Journal of Physiology. 1989;257:C588–595. doi: 10.1152/ajpcell.1989.257.3.C588. [DOI] [PubMed] [Google Scholar]
- Sernia C, Zeng T, Kerr D, Wyse B. Novel perspectives on pituitary and brain angiotensinogen. Frontiers in Neuroendocrinology. 1997;18:174–208. doi: 10.1006/frne.1997.0150. [DOI] [PubMed] [Google Scholar]
- Song K, Zhuo J, Allen A M, Paxinos G, Mendelsohn F A O. Angiotensin II receptor subtypes in rat brain and peripheral tissues. Cardiology. 1991;79:45–54. doi: 10.1159/000174906. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kuramochi T, Suga T. GABA receptor subtypes involved in the neuronal mechanisms of baroreceptor reflex in the nucleus-tractus-solitarii of rabbits. Journal of the Autonomic Nervous System. 1993;43:27–36. doi: 10.1016/0165-1838(93)90318-o. [DOI] [PubMed] [Google Scholar]
- Yang M, Andresen M C. Angiotensin enhances sensory afferent synaptic transmission in neurons of rat medial nucleus tractus solitarius (mNTS) in vitro. FASEB Journal. 1991;5:A677. [Google Scholar]


