Abstract
The effect of microinjecting angiotensin II (ANGII) into the nucleus of the solitary tract (NTS) on both baroreceptor and peripheral chemoreceptor reflexes was compared.
Experiments were performed in a working heart-brainstem preparation of rat. Baroreceptors were stimulated by raising perfusion pressure and chemoreceptors were activated with aortic injections of sodium cyanide (0·025%, 25–75 μl). Reflex changes in phrenic nerve activity and heart rate were measured after bilateral NTS microinjection (50 nl) of ANGII (0·5–5000 fmol).
NTS microinjection of 5 fmol ANGII elicited a transient (28·2 ± 6 s; mean ± s.e.m.) bradycardia (-18 ± 3 beats min−1), and decreased phrenic nerve activity cycle length and amplitude (P < 0·05). At higher doses of ANGII a similar respiratory response was seen but heart rate changes were inconsistent.
The baroreceptor reflex bradycardia was depressed significantly by NTS microinjections of ANGII (5–5000 fmol) in a dose-dependent manner with the reflex gain decreasing from 1·7 ± 0·16 to 0·66 ± 0·1 beats min−1 mmHg−1 (P < 0·01) at 5000 fmol. Although the chemoreceptor reflex bradycardia was depressed at a low dose of ANGII (5 fmol), all higher doses (50–5000 fmol) produced a dose-dependent potentiation of the reflex bradycardia (maximally +64 ± 8%). The respiratory component was unaffected. The effects of ANGII on both reflexes were blocked by an ANGII type 1 (AT1) receptor antagonist, losartan (20 μM).
The potentiating action of ANGII on the chemoreceptor reflex cardiac response was abolished by a neurokinin type 1 (NK1) receptor blocker (CP-99,994, 5 μM) but this had no effect on the baroreceptor reflex.
AT1 receptors in the NTS can depress the baroreceptor reflex bradycardia which is independent of NK1 receptors. The ANGII effect on the cardiac component of the chemoreceptor reflex is bi-directional being inhibited at low concentrations and potentiated at higher concentrations; the latter involves NK1 receptors and presumably results from release of substance P.
The nucleus tractus solitarii (NTS), the central site of termination of baroreceptive afferents, is intimately involved in arterial pressure control. This nucleus contains a high density of angiotensin II (ANGII) AT1 receptors located both presynaptically, on vagal and carotid sinus afferents, and on interneurones (Healy et al. 1989). It is well known that systemically injected ANGII has the unique ability to induce a pressor response with little or no reflex bradycardia. This is in striking contrast to virtually all other known pressor substances (reviewed in Reid, 1992; Bishop et al. 1995). One explanation of this phenomenon is that ANGII may attenuate the baroreceptor reflex by acting at the level of the NTS. In this regard, microinjection of ANGII into the NTS attenuated the baroreceptor reflex in both the anaesthetised normotensive adult rat (Casto & Phillips, 1986; Luoh & Chan, 1998) and the unanaesthetised rat (Michelini & Bonagamba, 1990). These data are consistent with results obtained following intracerebroventricular administration of ANGII (Hayashi et al. 1988). In our recent experiments, losartan blockade of AT1 receptors in the NTS increased baroreceptor reflex efficacy in the 10–20 day old rat (Kasparov et al. 1998) - an age when the baroreceptor reflex is depressed (Kasparov & Paton, 1997). In addition, AT1 receptor blockade in the NTS of mature rats increased the baroreceptor reflex (Campagnole-Santos et al. 1988). Taken together these results imply that ANGII acting on AT1 receptors depresses transmission of baroreceptive reflex pathways in the NTS. Indeed, this would explain the pressor response following ANGII microinjection into the NTS of both dogs (Averill et al. 1987) and rats (Casto & Phillips, 1984).
Several other communications, however, describe hypotension and bradycardia following a microinjection of low doses of ANGII into the NTS (Rettig et al. 1986; Kubo & Kihara, 1990; Mosqueda-Garcia et al. 1990; Fow et al. 1994; Tseng et al. 1994). This pattern of response is the same as that seen during glutamate stimulation of NTS neurones. Thus, one might expect that NTS neurones mediating the baroreceptor reflex would also be excited by ANGII. However, it is possible that the ANGII-induced hypotension/bradycardia is due to activation of neurones belonging to other reflex pathways within the NTS. Indeed, such pathways include peripheral chemoreceptor, cardiac and pulmonary vagal C-fibre-mediated reflexes which evoke falls in heart rate and/or arterial pressure and terminate in neighbouring regions of the NTS to those that mediate the baroreceptor reflex. In contrast, high doses of ANGII microinjected into the NTS caused an opposite pattern of response - hypertension and tachycardia (Rettig et al. 1986; Mosqueda-Garcia et al. 1990; Tseng et al. 1994), suggesting that ANGII may activate multiple mechanisms in the NTS.
One such mechanism is ANGII-induced release of substance P from primary afferents or other NTS sources (Barnes et al. 1991). Indeed, an action of substance P on NK1 receptors was proposed as a major mechanism of ANGII action on NTS circuitry controlling the baroreceptor reflex (Diz et al. 1997).
All previous studies have left a number of ambiguities and unanswered questions. First, how does an NTS microinjection of ANGII both depress the baroreceptor reflex (Casto & Phillips, 1986; Averill et al. 1987; Campagnole-Santos et al. 1988; Michelini & Bonagamba, 1990; Luoh & Chan, 1998; Kasparov et al. 1998) and induce a bradycardia and hypotension (Rettig et al. 1986; Fow et al. 1994; Tseng et al. 1994; Diz et al. 1997)? Is NTS baroreceptor circuitry the only target for ANGII or may other pathways be affected? Second, what are the neuronal mechanisms that could account for the inhibitory effects of ANGII on the arterial baroreceptor reflex at the level of the NTS? Third, what is the functional significance of ANGII-induced release of substance P and how does this affect NTS circuitry mediating the baroreceptor reflex?
The purpose of our experiments was, therefore, threefold. First, we re-evaluated the effect of microinjecting ANGII into the NTS on the arterial baroreceptor reflex and compared the response with that seen for the peripheral chemoreceptor reflex in a working heart-brainstem preparation (WHBP; Paton, 1996). The WHBP offers a valuable advantage for addressing this particular question as virtually all anaesthetics routinely used in microinjection studies cause a massive release of renin (reviewed in Keeton & Campbell, 1980; Timmermans et al. 1993; see also Lee & Shin, 1994). Thus, it is possible that circulating ANGII might affect the NTS via the area postrema (reviewed in Reid, 1992) and bias the results. The WHBP is decerebrated and unanaesthetised and lacks both the liver (the source of peripheral angiotensinogen) and the kidney - a major source of renin. Second, to our knowledge no intracellular data have been published on the effects of ANGII in the NTS. Therefore we have investigated the effects of ANGII on NTS neurones in vitro and searched specifically for synaptic mechanisms which could explain its inhibitory effects on the baroreceptor reflex (see accompanying paper by Kasparov & Paton, 1999). Third, we sought to determine the physiological significance of the ANGII-induced substance P release in the NTS using an NK1 receptor blocker, CP-99,994.
METHODS
Working heart-brainstem preparation (WHBP)
A WHBP of young Sprague-Dawley rats (30–35 days old; 90–130 g) was used to perform microinjections. Animals were initially deeply anaesthetised with halothane, bisected below the diaphragm, decerebrated precollicularly, and cerebellectomised to expose the fourth ventricle (Paton, 1996). The thorax and head were perfused using artificial cerebrospinal fluid (ACSF) containing dextran (2·2%) or ficoll (1·25%) at 31°C which was gassed with carbogen (95% O2-5% CO2). Perfusate was pumped retrogradely into the descending aorta via a double lumen catheter; the second lumen was connected to a pressure transducer. Phrenic nerve activity was recorded via a suction electrode and monitored continuously with the electrocardiogram (ECG). Using a window discriminator, the R-wave of the ECG was used to generate transistor-transistor logic (TTL) pulses, which together with the digitized arterial pressure signal were acquired by a CED 1401plus interface, displayed on a computer using Spike2 software (CED, UK) and stored on a hard drive. The instantaneous frequency of the TTL pulses was computed to give heart rate in beats per minute (beats min−1). The ACSF contained the following (mM): 10 dextrose, 125 NaCl, 24 NaHCO3, 5 KCl, 2·5 CaCl2, 1·25 MgSO4 and 1·25 KH2PO4.
Reflex measurements
At least three stable reflex control responses were obtained before an NTS microinjection. The baroreceptor reflex bradycardia was evoked by raising perfusion pressure for 3–6 s (increases of up to +55%). This stimulus evoked a reflex baroreceptor vagal bradycardia which could be blocked either by localised cooling of the NTS or by administration of atropine (2 μM) to the perfusate. The peak changes in perfusion pressure and heart rate were measured on line and baroreceptor reflex gain calculated as the ratio of the change in heart rate to the change in perfusion pressure (Δ heart rate/Δ perfusion pressure). The rate of rise of the pressure ramps was constant within a given preparation. At the beginning of an experiment a set of pressure ramps of different magnitudes was performed in each WHBP to determine pressure levels necessary to obtain a baroreceptor reflex input-output curve. Intervals of at least 5 min between the tests were necessary to obtain reproducible gain values. As in many cases complete recovery from the effect of both low and intermediate doses of ANGII occurred within 15 min (see Results) we could not study the effect of ANGII on such curves in all experiments. Instead, in the majority of experiments we chose one magnitude of the pressure ramp for all tests (typically between 15 and 20 mmHg) which fell within the linear part of the input-output curve and used it for all tests. The effect of ANGII on the baroreflex input-output curve was studied in detail in an additional group of four WHBP using 500 fmol dose (see Results).
Sodium cyanide (NaCN; 0·05% solution, 50 μl) was injected into the perfusion cannula to activate peripheral chemoreceptors. Our previous studies have shown that this dose gives consistently repeatable submaximal responses (Paton & Butcher, 1998). In this case, as with the baroreceptor reflex, intervals of 4–5 min between NaCN injections were required to obtain reproducible reflex responses. As lung stretch receptors are not activated in the WHBP, the chemoreceptor reflex consisted of a powerful bradycardia accompanied by an increase in central inspiratory drive as revealed in the phrenic nerve. The respiratory response was measured from integrals of rectified nerve discharges and data were expressed as percentage change.
Microinjections
In the WHBP the surface landmarks of the dorsal medulla are clearly seen with the cerebellum removed. Calamus scriptorius was used as a landmark for orientation of micropipettes into sites known to contain baroreceptive NTS neurones in the rat (Deuchars et al. 1998) and baroreceptor afferent terminals (see Ciriello et al. 1994 for review). Drugs were applied bilaterally using pressure injection from a 4-barrelled micropipette (tip diameter about 35 μm) driven by a micromanipulator to 400–450 μm ventral to the dorsal surface, ±0·5 mm rostro-caudal relative to calamus scriptorius and between 250 and 800 μm from midline with the most medial injections being the most caudal. The injected volume (50 nl) was measured by observing the movement of the meniscus through a binocular microscope fitted with a calibrated eye-piece graticule. In three preparations the area postrema was surgically removed by cutting it away from the edges of the IVth ventricle using superfine spring-bow scissors under visual control using a binocular microscope after control reflex testing. Histological verification of the removal of area postrema was performed subsequently (see below).
ANGII was dissolved in 0·9% NaCl (5 μM to 5 mM). Bilateral NTS microinjections of 0·9% NaCl alone did not cause any measurable effects on resting cardiorespiratory parameters or reflexes. Approximately 1 min after a bilateral microinjection of ANGII into the NTS, the baro- and chemoreceptor reflexes were both re-tested in random order and further tests performed at 5 min intervals. In most cases the effects of ANGII were reversible and washed out over a period of 15–20 min depending on the dose. The exception was a 5000 fmol dose where recovery took > 30 min. Once reflexes had recovered to control levels, a subsequent bilateral microinjection of ANGII (same dose or higher) was given. Up to five bilateral microinjections of ANGII per preparation were made. At the end of the experiment the position of the micropipette was marked by injection of 50 nl of 2% Pontamine Sky Blue. The brainstem was removed, fixed and subsequently sectioned (50 μm) and counterstained with Neutral Red. Microinjection sites were documented on pre-drawn sections from Paxinos & Watson (1986).
The receptors mediating the effects of ANGII in NTS were assessed using an AT1 receptor blocker, losartan potassium (20 μM), and/or CP-99,994, an NK1 receptor blocker (5 μM), which were added to the perfusate 6–10 min after microinjection of the highest dose of ANGII (5000 fmol). In a few experiments an NTS microinjection of ANGII was repeated 5 min after CP-99,994 and/or losartan were added to the perfusate. We also compared the effects of microinjecting bilaterally losartan (50 nl, 20 μM) and CP-99,994 (50 nl, 5 μM) into the NTS with those we obtained by adding these drugs into the perfusate.
Drugs
Losartan was kindly supplied by Merck & Co. (USA) and CP-99,994 by Pfizer (UK). Other chemicals were obtained from Sigma-Aldrich (UK). Doses of losartan and CP-99,994 were based on previous studies in vivo (Chizh et al. 1995; Kasparov et al. 1998), in the WHBP (Paton, 1998) and in vitro (Maubach & Jones, 1997).
Statistical analysis
All data were analysed using Spike2 software with custom-written scripts. To estimate the effects of ANGII on the phrenic nerve activity and respiratory component of the reflexes, the integral of phrenic nerve discharge was computed for a period covering at least three control cycles. This was compared with the phrenic nerve integral during the reflex response using an identical time window. To standardise data across preparations phrenic nerve integrals were expressed as percentages. The significance of the ANGII-induced changes was assessed by applying Student's paired two-tailed t tests to the raw data. Other unpaired or paired data were analysed using Student's two-tailed t tests or ANOVA as appropriate. All values quoted are means ± standard error of mean (s.e.m.) and n is the number of observations unless stated otherwise. Differences were taken as significant at the 95% confidence limit. To obtain the baroreceptor and chemoreceptor reflex input-output curves fits were made using Excel (Microsoft) and Prizm (GraphPad).
RESULTS
Baseline cardiorespiratory variables and reflex responses
The following results refer to experiments performed in 21 preparations. At a perfusion pressure of 90·5 ± 3 mmHg, heart rate was 358 ± 5·6 beats min−1 and phrenic nerve activity occurred at a cycle length of 3·0 ± 0·2 s and duration of 0·74 ± 0·04 s (Figs 1, 2 and 5–7). Heart rate was modulated by central respiratory activity (i.e. respiratory sinus arrhythmia was present; Fig. 1, for example) and increased spontaneously during the experiment at a rate of 9 ± 1 beats min−1 h−1 (Fig. 2). In addition, phrenic nerve activity cycle length was reduced by 0·2 ± 0·01 s h−1 (Fig. 5).
Figure 1. Cardiorespiratory response following microinjections of ANGII into the NTS.
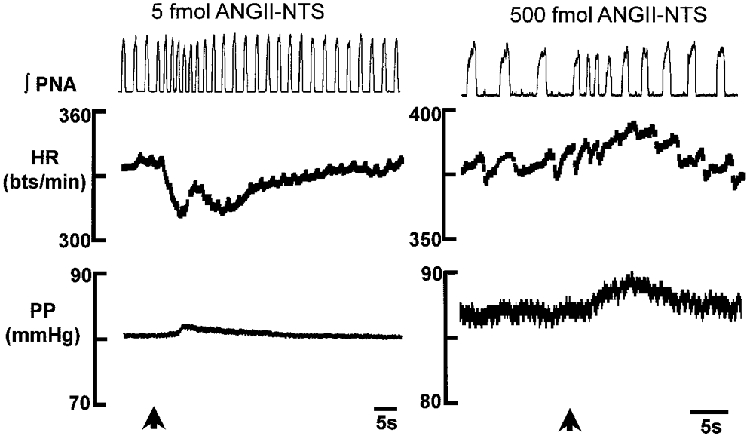
An NTS microinjection of a low dose of ANGII (5 fmol) produced a significant bradycardia consistently whereas higher doses of ANGII (5–5000 fmol) produced inconsistent effects, the most common being a tachycardia. At all doses of ANGII tested there was a significant decrease in both phrenic nerve activity cycle length and amplitude. The time of the microinjection is indicated by the arrows. The data are taken from two different preparations. Changes in perfusion pressure were very small and not consistent with all doses of ANGII. Note the ongoing respiratory sinus arrhythmia in each preparation. Abbreviations∫PNA, integrated phrenic nerve activity; HR, heart rate; bts/min, beats per minute; PP, perfusion pressure.
Figure 2. Dose-dependent depression of the baroreceptor reflex-mediated bradycardia induced by NTS microinjections of ANGII.
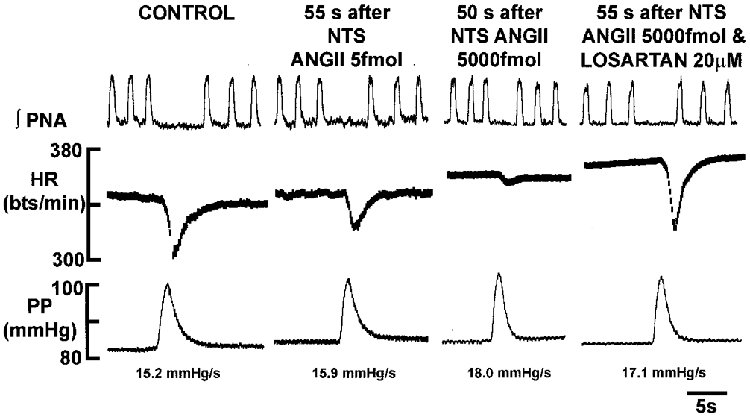
In the WHBP, the baroreceptor reflex was stimulated by increasing perfusion pressure. Pressure ramps generated were within the linear part of the baroreceptor reflex sensitivity curve. Note that the values for the rate of rise of the pressure ramp (dP/dt) are comparable. Microinjection of ANGII produced a dose-dependent suppression of the reflex bradycardia (P < 0·01) whereas phrenic nerve activity responses were not affected significantly. The ANGII-induced depression of the baroreceptor-evoked bradycardia was mediated by AT1 receptors since it could be reversed by addition of losartan to the perfusate. Abbreviations as in Fig. 1.
Figure 5. Differential, dose-dependent effect of ANGII microinjected into the NTS on the peripheral chemoreceptor reflex-mediated bradycardia.
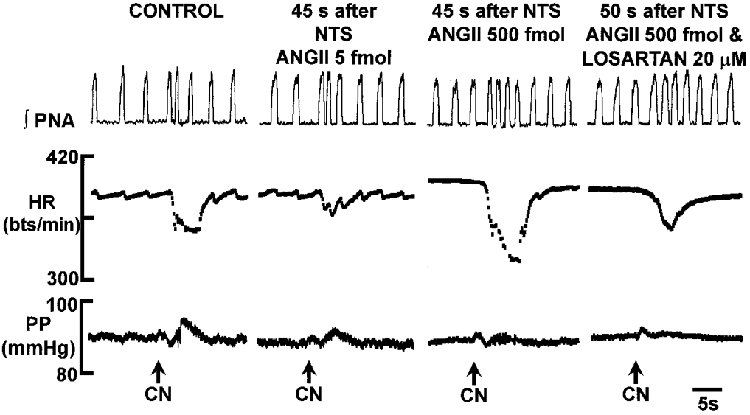
NaCN stimulation of peripheral chemoreceptors evoked a bradycardia and decrease in phrenic nerve activity cycle length. The reflex bradycardia was depressed significantly at low doses of ANGII (5 fmol; P < 0·01) but potentiated at higher doses (i.e. > = 50 fmol; P < 0·01). The potentiated response was mediated by AT1 receptors as revealed by addition of losartan to the perfusate. There were no significant changes in the respiratory component of this reflex. Abbreviations as in Fig. 1. NaCN (0·05%; 50 μl) was injected into the aorta.
Figure 7. Potentiation of the chemoreceptor reflex bradycardia by ANGII in the NTS is mediated by NK1 receptors.
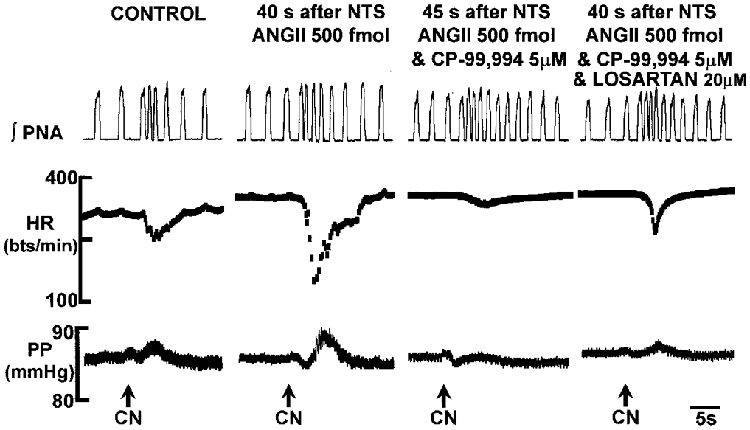
A microinjection of 500 fmol ANGII into the NTS enhanced significantly the bradycardic component of the chemoreceptor reflex. This potentiation was dependent upon the integrity of NK1 receptors since their blockade with CP-99,994 revealed an attenuation of the reflex bradycardia compared to the control response. This attenuation was reversed by losartan indicating that AT1 receptors also caused an inhibitory effect on NTS circuitry mediating the chemoreceptor reflex. Abbreviations as in Fig. 1. NaCN (0·05%; 50 μl) was injected into the aorta.
Stimulation of the baroreceptors by raising perfusion pressure to a level on the linear part of the baroreceptor reflex input-output curve (see below) resulted in a mean gain of 1·7 ± 0·16 beats min−1 mmHg−1 (Fig. 3). Concurrent with the reflex bradycardia was an increase in the cycle length of phrenic nerve activity (Fig. 2). This computed as a reduction of −35·3 ± 3·6% in the integral of phrenic nerve inspiratory activity. Stimulation of peripheral chemoreceptors with NaCN (25 μg) produced a bradycardia of −27·6 + 1·6% relative to control and increased the integral of phrenic nerve discharge by +36·6 ± 4·2% (e.g. Fig. 5). Overall there was no significant change in perfusion pressure.
Figure 3. Dose-dependent effects of NTS microinjections of ANGII on baroreceptor and chemoreceptor reflexes.
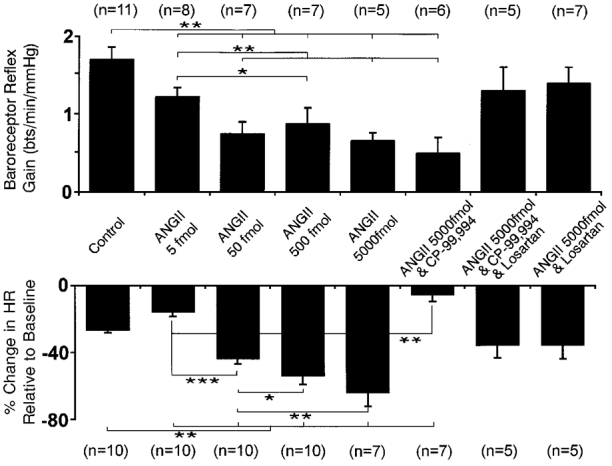
The cardiac response of the baroreceptor reflex was expressed as the gain (in beats min−1 mmHg−1, i.e. Δ heart rate/Δ perfusion pressure; upper graph). The percentage change in heart rate from baseline is shown for the chemoreceptor reflex (lower graph). The baroreceptor reflex gain showed a dose-dependent depression following microinjection of ANGII into the NTS. This reflex gain depression was insensitive to substance P receptor blockade with CP-99,994 (5 μM), but reversed by losartan (20 μM), indicative of a role for AT1 receptors. The chemoreceptor reflex-mediated bradycardia was also depressed at the lowest dose of ANGII (5 fmol) microinjected into the NTS. In contrast to the baroreceptor reflex, the chemoreceptor reflex bradycardia was potentiated after NTS microinjection of higher doses of ANGII in a dose-dependent manner. Potentiation was reversed by CP-99,994 (5 μM) into a depression compared to control. This reflex depression was abolished by losartan (20 μM) administration into the perfusate. Following losartan the reflex bradycardia was not different to the control response. n, number of preparations; * P < 0·05, ** P < 0·01, *** P < 0·001.
Effects of a bilateral microinjection of ANGII into the NTS
Baseline cardiorespiratory variables
A wide range of doses of ANGII (0·5–5000 fmol) was microinjected into the NTS. The lowest dose (0·5 fmol) had no effect on either baseline variables or cardiorespiratory reflexes studied (n = 4, not shown). Doses of 5–500 fmol ANGII evoked a short-lasting (< 30 s) but significant decrease in phrenic nerve activity cycle length and a transient decrease in phrenic nerve activity amplitude of approximately 20% (Table 1; Fig. 1). However, these effects were not dose dependent. In contrast, only at the lowest dose of ANGII (5 fmol) did heart rate show a transient, but significant bradycardia of −16 ± 2 beats min−1 consistently (P < 0·05; Table 1; Fig. 1). At higher doses (50–500 fmol) the effect of ANGII on heart rate was inconsistent (Table 1) with a tachycardia being the most frequent response. However, overall heart rate changes at the higher doses were not significant (Table 1).
Table 1.
Effect of microinjecting ANGII into the NTS on cardiorespiratory variables in the WHBP
| PNA cycle length | Heart rate | ||||||
|---|---|---|---|---|---|---|---|
| ANGII dose | n | Control (s) | Change (%) | PNA amplitude (% change) | Control (beats min−1) | Change (%) | Response duration (s) |
| 5 fmol | 6 | 3.1 ± 0.4 | −26.5 ± 7* | −18.2 ± 3.6* | 366 ± 6 | −5.1 ± 0.9* | 28.2 ± 6 |
| 50 fmol | 6 | 3.0 ± 0.5 | −38.3 ± 7* | −9.0 ± 1.1* | 344 ± 15 | −1.2 ± 1.9 | 18.0 ± 1 |
| 500 fmol | 6 | 3.5 ± 0.2 | −33.6 ± 7* | −19.0 ± 3* | 356 ± 9 | +0.1 ± 2.4 | 21.2 ± 1 |
PNA, phrenic nerve activity.
P < 0.05 relative to control (paired t test).
ANGII produced a highly significant (P < 0·01) depression of the baroreceptor reflex bradycardia throughout the whole range of doses between 5 and 5000 fmol (Figs 2–4; n≥5). The effect reached a plateau between 500 and 5000 fmol. Although there was a trend towards an increase in the baroreceptor reflex-evoked respiratory depression this did not quite reach significance (50 fmol; P = 0·06; n = 11). There was no significant change in the rate of rise of the arterial pressure ramps after ANGII microinjections for a given preparation (see Figs 2 and 6B).
Figure 4. Effect of ANGII on input-output functions of the baroreceptor and chemoreceptor reflexes.
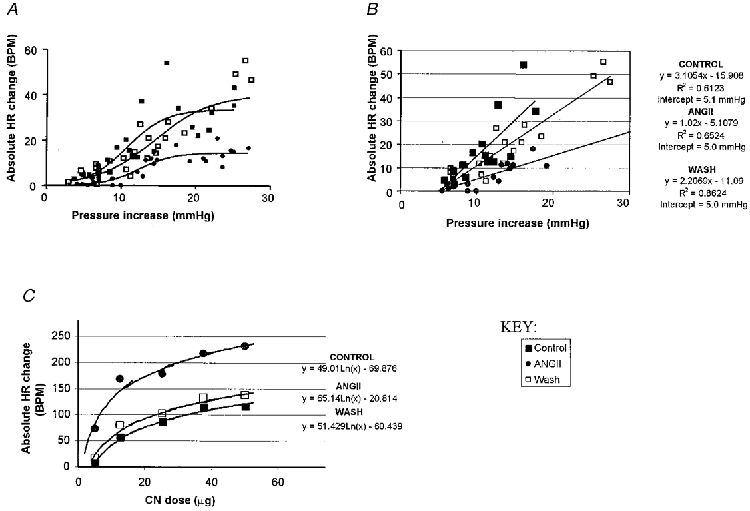
In a separate group of four WHBP a series of pressure ramps of different amplitude but constant dP/dt was performed before and after bilateral NTS microinjection of 500 fmol ANGII. The effect of this dose persisted for 15 min or more which allowed several tests to be performed. In the same experiments several doses of NaCN were used to activate chemoreceptors in control and after an ANGII microinjection. A, baroreceptor reflex input-output curves: changes in heart rate (BPM, beats min−1) evoked by pressure ramps are plotted against the stimulus amplitude and fitted with a sigmoid line (see text for details). Note that ANGII reversibly decreased the peak response. B, data points from the linear part of the input-output curves (A) fitted with linear regression (equations on the right). Note that the slope (the first parameter) was reversibly decreased by ANGII while the intercept (used as an index of the threshold of response) remained unchanged. C, chemoreflex input-output curves: changes in heart rate were plotted against the NaCN dose and fitted with a logarithmic function (equations on the right). Note that ANGII reversibly increased the maximal response (also reflected by the last parameter in the equations). The dose of 25 μg NaCN used throughout the study was therefore submaximal and coincides with the middle part of the stimulus-response relationship.
Figure 6. Removal of area postrema did not affect the depression of the baroreceptor reflex bradycardia caused by a microinjection of ANGII into the NTS.
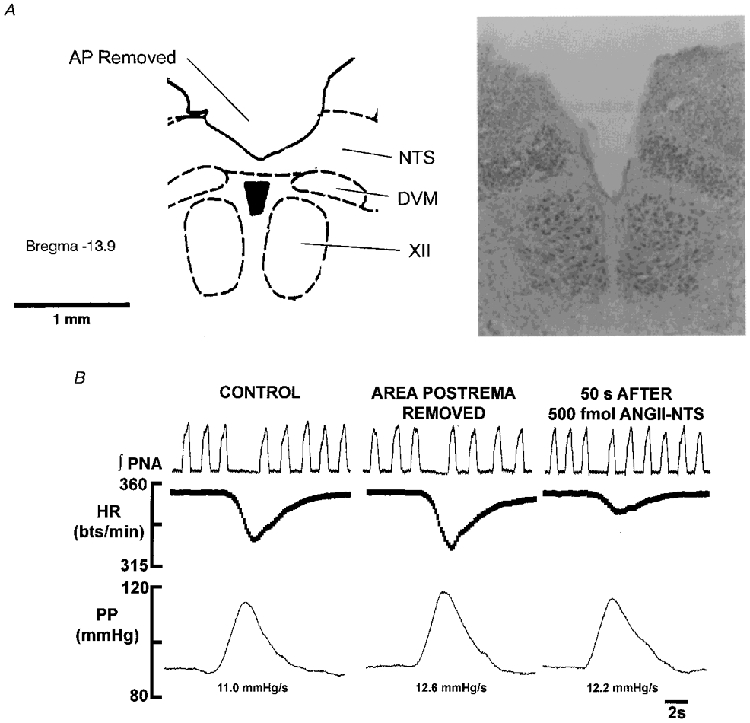
The data shown in A and B are from the same WHBP. A, a representative section (based on Paxinos & Watson, 1986) and a photomicrograph of the medulla to illustrate the almost complete removal of area postrema. The photomicrograph is not identical to the section due to cutting angle differences and distortion caused by fixation. Abbreviations: AP, area postrema; DVM, dorsal vagal motor nucleus; XII, hypoglossal motor nucleus. B, a baroreceptor reflex is shown under control conditions, after surgical removal of the area postrema (see A) and after a bilateral microinjection of 500 fmol ANGII into the NTS. Removal of the area postrema did not cause any effect on either baroreceptor reflex performance or the depressant effect of ANGII on the cardiac component in the WHBP.
In an additional set of four WHBP we performed a series of experiments to study the effects of ANGII on the input-output function of the baroreceptor reflex in detail (Fig. 4A and B). The perfusion pressure was ramped up at the same rate to different levels and the corresponding peak heart rate changes measured before and after an NTS microinjection of 500 fmol ANGII. This dose was chosen because the effect of ANGII persisted for at least 15 min, a time sufficient to perform several pressure ramps. The resultant changes in heart rate were plotted against the pressure changes and fitted with a commonly used equation for sigmoid curves: y =xmin+ (xmax- xmin)/(1 + exp((Midpoint - x)(Sigmoid slope coefficient))), where x is the change in perfusion pressure and y is the change in heart rate (Fig. 4A). In our case the minimal change in pressure (xmin) was zero and therefore was set to zero for the fit. In all cases the goodness of fit (R2) was ≥0·6. To estimate the slope of the linear parts of the input-output curves the corresponding data points were fitted with a straight line using linear regression analysis (Fig. 4A).
Table 2 and Fig. 4A and B both indicate that ANGII (500 fmol) reversibly decreased both the slope and ‘peak response’ of the baroreceptor reflex input-output curve. A threshold for the baroreceptor reflex-evoked bradycardia was estimated from the intercept of the linear regression line with the x-axis (pressure, see Fig. 4A). In contrast to the gain and peak response, ANGII did not affect the apparent pressure threshold (Table 2, Fig. 4A). Therefore, single point measurements taken from the linear part of the input-output curve for all other comparisons gave an adequate description of the effect of ANGII on the baroreceptor reflex gain.
Table 2.
Effect of microinjecting ANGII into the NTS on cardiorespiratory variables in the WHBP
| Control | ANGII | Wash | |
|---|---|---|---|
| ymax (peak response) (beats min−1) | 33.59 | 14.48 | 39.9 |
| Midpoint (beats min−1) | 10.77 | 12.42 | 14.45 |
| Sigmoid slope coefficient | 0.38 | 0.44 | 0.26 |
| ΔPthreshold (mmHg) | 5.1 | 5.0 | 5.0 |
| Slope of the linear part (gain of the reflex) | 3.10 | 1.02 | 2.2 |
The peak response (ymax), midpoint and sigmoid slope coefficients were obtained using Prizm software. The values for the change in perfusion pressure threshold (ΔPthreshold) were obtained from the intercepts derived from a linear regression analysis (Excel). See text and Fig. 4 for further details.
In contrast to the baroreceptor reflex, a microinjection of ANGII into the NTS had a bi-directional effect on the chemoreceptor reflex bradycardia which was dose dependent (Figs 3 and 5). At the lowest effective dose (5 fmol) ANGII depressed the reflex bradycardia (Figs 3 and 5; P < 0·01; n = 10; t test, ANOVA) but at all higher doses of ANGII (50–5000 fmol) the chemoreceptor reflex-evoked bradycardia was powerfully potentiated (Figs 3, 5 and 7; P < 0·01; n≤7; t test, ANOVA). The chemoreceptor reflex increase in phrenic nerve discharge was not altered significantly at any dose of ANGII (P > 0·1 in all cases; n≥7).
To study the effects of ANGII on the chemoreceptor reflex in more detail, in four WHBP a range of doses of NaCN (5–50 μg) was first used before and after an NTS microinjection of 500 fmol of ANGII. Figure 4A illustrates that NaCN-evoked bradycardia was fitted with a simple logarithmic function (goodness of fit > 0·9). ANGII reversibly shifted the input-output curves to the left and strongly increased the peak of the response. Figure 4A also illustrates that the stimulus provided by 25 μg NaCN, which was used in all other experiments in this study, lay approximately in the middle of the linear part of the chemoreflex input-output curve which allowed both an increase and a decrease in reflex performance to be revealed.
Removing area postrema
Surgical removal of area postrema (Fig. 6A) affected neither baseline parameters nor the baro- or chemoreceptor reflexes. Furthermore, the ANGII-induced transient changes in phrenic nerve activity and heart rate as well as the effects of ANGII on both reflexes were unaffected following removal of the area postrema (n = 3; Fig. 6A). Thus, the data from these preparations were pooled together with results obtained from intact preparations.
Effect of losartan and CP-99,994 on baseline cardiorespiratory variables and reflex responses
In naive preparations neither losartan (n = 4) nor CP-99,994 (n = 4) altered baseline cardiorespiratory variables (P > 0·1 in all cases). Further, losartan and CP-99,994 administered either separately (n = 4) or together (n = 3) failed to change the baroreceptor reflex-evoked bradycardia from its control value of 1·50 ± 0·3 beats min−1 mmHg−1 (after losartan: 1·51 ± 0·2 beats min−1 mmHg−1; after CP-99,994: 1·47 ± 0·3 beats min−1 mmHg−1; after losartan and CP-99,994: 1·4 ± 0·4 beats min−1 mmHg−1; P > 0·1) or the chemoreceptor-mediated bradycardia (control: −30·7 ± 6·0%; after losartan: −30·9 ± 8%; after CP-99,994: −33·4 ± 4%; after losartan and CP-99,994: −36·9 ± 7%; P > 0·1). In addition, the reflex phrenic nerve activity cycle length responses following stimulation of both baro- and chemoreceptors remained unchanged (P > 0·1).
Losartan abolishes the effects of NTS microinjection of ANGII on cardiorespiratory reflex cardiac responses
Following microinjection of 5000 fmol ANGII into the NTS, losartan was added to the perfusate (20 μM; n = 5). In all cases, losartan blocked the ANGII-induced depression of the baroreceptor reflex cardiac gain such that it was increased to a level not different (P > 0·1) to control (Figs 2 and 3). Losartan also blocked the potentiating effect of an NTS microinjection of ANGII on the chemoreceptor reflex bradycardia (Figs 3 and 5; n = 5). Similar data were obtained when losartan or CP-99,994 was microinjected into the NTS (n = 3).
Effect of CP-99,994 following bilateral NTS microinjection of ANGII on cardiorespiratory reflex responses
The ANGII-induced depression of the baroreceptor gain was consistently resistant to systemic administration of 5 μM CP-99,994 (n = 6; Fig. 3). In contrast, blockade of NK1 receptors after an NTS microinjection of ANGII reversed its potentiating effect on chemoreceptor reflex-evoked bradycardia which was now strongly attenuated relative to both the ANGII-alone state and the original control level (Figs 3 and 7; n = 7; P < 0·01). However, subsequent administration of losartan restored the reflex bradycardia back to control levels (Figs 3 and 7; n = 5).
Location of NTS microinjection sites
Throughout the experiments microinjection pipettes were positioned consistently at co-ordinates relative to surface landmarks of the dorsal medulla. Post hoc histological analysis was performed to reveal the exact site of microinjection. NTS microinjection sites, as revealed by Pontamine staining, were successfully recovered in 11 of the 21 preparations (Fig. 8) and found to be dorsomedial and medial relative to the solitary tract at levels corresponding to the area postrema and extending up to 0·6 mm caudal. We believe these sites are representative of all other microinjection loci which used identical co-ordinates.
Figure 8. Effective ANGII microinjection sites in the NTS.
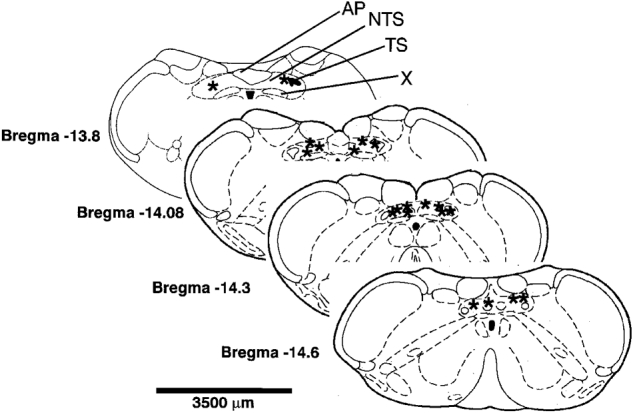
Representative transverse sections depict the NTS sites (asterisks) where ANGII microinjections modulated the cardiac components of the baroreceptor and chemoreceptor reflexes in the WHBP. The sites are depicted for 11 preparations where bilateral microinjections were performed. Sites were typically medial to the solitary tract and extended from the level of area postrema to −0·6 mm caudal to it. Abbreviations: AP, area postrema; NTS, nucleus of the solitary tract; TS, solitary tract; X, dorsal vagal motonucleus.
DISCUSSION
A number of studies have related the development of genetically pre-programmed hypertension in animal models to an increase in brain ANGII concentration (Phillips et al. 1977; Des Senanayake et al. 1994; Campbell et al. 1995; Morishita et al. 1995; Teruya et al. 1995). NTS levels of AT1 receptors are elevated in spontaneously hypertensive rats (Gutkind et al. 1988). One of the mechanisms by which increases in central ANGII activity could lead to hypertension is if ANGII could disable central negative feedback mechanisms by suppressing baroreceptive afferent input at the level of the NTS. Consistent with this view are the data from the present work and previous studies demonstrating that NTS microinjection of ANGII inhibits the baroreceptor reflex (Casto & Phillips, 1986; Michelini & Bonagamba, 1990; Luoh & Chan, 1998) whereas ANGII antagonists increase the baroreceptor reflex gain (Campagnole-Santos et al. 1988; Kasparov et al. 1998). These data all support an inhibitory action of ANGII on NTS circuitry mediating the baroreceptor reflex. However, it is difficult to comprehend how these findings relate to those describing hypotension and bradycardia after microinjections of low doses of ANGII into the NTS (Rettig et al. 1986; Mosqueda-Garcia et al. 1990; Fow et al. 1994; Tseng et al. 1994). The latter findings require that ANGII excites NTS neurones to produce a depressor/bradycardic response. Alternatively, the NTS neurones mediating this response may not be involved in mediating the baroreceptor reflex.
We believe that the present results support this last suggestion as the same dose of ANGII microinjected into the NTS evoked opposite changes in the sensitivity of two different reflex pathways. For example, ≥50 fmol of ANGII microinjected into the NTS attenuated the baroreceptor reflex bradycardia whereas it potentiated the cardiac component of the peripheral chemoreceptor reflex. Thus, ANGII can exert both inhibitory and excitatory effects in the NTS mediated by two opposing neuronal mechanisms. Both of these effects were reversible and losartan sensitive, the latter indicative of a role for AT1 receptors. Thus, the bradycardia and hypotension effects observed after NTS microinjections of ANGII (Rettig et al. 1986; Fow et al. 1994; Tseng et al. 1994) are likely to be mediated by activation of non-baroreceptive NTS circuits or neurones such as those relaying the bradycardia elicited by stimulation of the peripheral chemoreceptors.
In our experiments a significant bradycardia could only be evoked by 5 fmol ANGII and this was very short lasting (< 30 s) compared with that reported by Diz et al. (1997) using 250 fmol dose or Rettig et al. (1986) using ∼1000 fmol. In the present study ANGII doses of > 50 fmol evoked inconsistent heart rate changes with a tachycardia as the most frequent response (see Fig. 1). Qualitatively this is similar to the data of Rettig et al. (1986) and Casto & Phillips (1985) although the dose range varies widely and in our experiments seemed to be shifted towards the low dose end. As shown by Diz et al. (1997) the depressor/ bradycardic response to ANGII microinjections into the NTS is due to release of substance P. We propose that the direction of the baseline heart rate response may reflect a combination of excitatory versus inhibitory effects within the NTS and depends on the reflex circuitry exposed to ANGII and its dose. Another possibility is that the tachycardia is secondary to the increased central inspiratory drive. It is also worth stating that anaesthesia might significantly influence the results of many previous studies because of the inevitable rise in circulating ANGII levels. For example, urethane/chloralose anaesthesia in rats increased plasma renin activity approximately 5 times (Lee & Shin, 1994). Interestingly, microinjections of ANGII in unanaesthetised rats caused minimal changes in baseline heart rate and arterial pressure (Michelini & Bonagamba, 1990).
The duration of the effect of the lower doses of ANGII was not sufficient to perform a detailed test of baroreflex input- output curves. Thus we studied this with one intermediate dose of 500 fmol in a separate subset of experiments. It was demonstrated previously in anaesthetised rats that NTS microinjections of ANGII only affect the pressor-evoked bradycardia but not the tachycardic response to nitroprusside-induced depressor effects (Michelini & Bonagamba, 1990). Similarly the ANGII antagonist saralasin only affected the baroreflex response to hypertensive stimuli (Campagnole-Santos et al. 1988). Therefore, in this study we did not investigate the effects of ANGII on heart rate responses during decreases in perfusion pressure. The relationship between the heart rate response and the amplitude of the pressure ramp could be described with sigmoidal curve (see Fig. 4A). These input-output curves and the corresponding parameter ymax (Table 2) show that ANGII reversibly decreased the maximal amplitude of the baroreceptor reflex response. The meaning of the midpoint parameter cannot be compared to the ‘set point’ as normally done when reflex tachycardia data are also included. Because of this, a linear regression analysis was performed to obtain an index of the baroreceptor reflex bradycardia threshold. This resulted in a similar intercept value before and after ANGII (all around 5 mmHg). The slope of the linear regression line calculated in this way was reversibly reduced by ANGII (Fig. 4A and Table 2). Thus, ANGII depresses both the reflex gain and peak response without altering the threshold. We therefore believe that single point measurements taken from the linear part of the reflex curve provided an adequate measure of the action of ANGII on the baroreceptor reflex sensitivity allowing comparisons to be made between the whole range of doses used.
Our present results show that a mechanism involving NK1 receptors appears to underlie the potentiation effect of ANGII on the chemoreceptor reflex bradycardia but not the depressant action on the cardiac component of the baroreceptor reflex. Blockade of NK1 receptors with CP-99,994 had no affect on the baroreceptor reflex bradycardia either in control conditions or when it was attenuated by ANGII. This is consistent with our previous data on NK1 knockout mice where the baroreceptor reflex was not compromised (Butcher et al. 1998). Thus, the present data suggest that: (i) substance P is not released during baroreceptor stimulation, and (ii) NTS circuitry mediating this reflex is not affected by substance P released by higher doses of ANGII, whatever the source of substance P might be. In contrast, both microinjections (Chan et al. 1990) and microdialysis (Chan et al. 1995) of substance P into the NTS have been reported to potentiate the baroreceptor reflex in anaesthetised rats. It is therefore possible that although substance P is not directly involved in transmission of baroreceptor information, it may affect this reflex pathway when released by some other presently undefined mechanism/source.
Although there was no effect of blocking substance P receptors on the chemoreceptor reflex in control conditions, indicating that substance P is not normally involved in transmission of this reflex pathway within the NTS, the ANGII-induced potentiation of the chemoreceptor reflex bradycardia was blocked by CP-99,994. This implies that ANGII can cause release of substance P from some source within the NTS. This may be from neuronal elements mediating the chemoreceptor reflex pathway itself. In this context there is an intimate relationship between glutamate and substance P at the level of NTS (Hall et al. 1993). Moreover, release of substance P by ANGII was reported earlier from an in vitro slice preparation containing the NTS (Barnes et al. 1991; Diz & Pirro, 1992). However, a vagally mediated bradycardia evoked by an NTS microinjection of substance P (Hall et al. 1989) or a selective NK1 receptor agonist (Feldman, 1995) was not associated with changes in the sensitivity of the baroreceptor reflex. The conclusion was made that substance P is not integral to circuitry subserving the baroreflex (Feldman, 1995). Based on the present data, under certain circumstances (such as increase in ANGII concentration) release of substance P may result in modulation of the chemoreceptor reflex.
For both baro- and chemoreceptor reflexes the changes in phrenic nerve activity were unaffected by NTS microinjection of ANGII. One possibility is that ANGII did not reach the NTS sites where respiratory neurones receiving these afferents are located. Alternatively, ANGII acts selectively within the NTS by altering the sensitivity of circuitry mediating the cardiac but not the respiratory component of these reflexes. As to the transient effects of ANGII on phrenic nerve discharge frequency, this action was not dose dependent and a plausible explanation is that delivery of ANGII into the NTS from a microelectrode first produces a localised spot of relatively high concentration of the peptide. This might release substance P in a small volume of NTS and transient activation of a few cells involved in pacing the respiratory network. From our data we presume that the relatively high concentrations of ANGII were more effective in releasing substance P. Within a short time the concentration of microinjected ANGII equilibrates within a larger volume of the NTS and the stimulatory effect gets balanced out by numerous other ANGII-induced effects and interactions such as enhancing inhibitory transmission (as we report in the accompanying paper).
Although in this ‘quasi-equilibrated’ state ANGII did not cause a shift in baseline cardiorespiratory variables, clearly it was effective in depressing both reflexes at the lowest dose we used (5 fmol). Presumably the higher concentrations of ANGII increase release of substance P and this over-rides the inhibitory effect on the chemoreceptor reflex seen at the low dose (5 fmol). This is substantiated by the observation that blockade of substance P receptors reveals a powerful depressant action on this reflex even when high doses (i.e. 500 fmol) of ANGII are used (see Figs 3 and 7). This indicates that although ANGII stimulates both substance P release and an inhibitory mechanism simultaneously, the overall effect on the chemoreceptor-evoked bradycardia reflects a balance between these opposing forces.
In conclusion, the present study demonstrates that at the level of NTS ANGII has opposite effects on the sensitivity of different cardiorespiratory reflex pathways. Throughout a wide range of doses ANGII depresses the baroreceptor reflex circuitry mediating the cardiac vagal response but all except the lowest dose of ANGII potentiate the chemoreceptor reflex-mediated bradycardia. The effects of ANGII are mediated via AT1 receptors. Therefore, the end-organ responses (heart rate and/or arterial blood pressure) reflect a product of several actions of ANGII in the NTS. Potentiation of the peripheral chemoreceptor reflex-evoked bradycardia (but not the inhibition of the baroreceptor cardiac reflex) is due to release of substance P as it may be blocked by an NK1 antagonist, CP-99,994. Plausible cellular mechanisms responsible for these multiple effects are addressed in the accompanying paper (Kasparov & Paton, 1999).
Acknowledgments
We thank Professor I. Zucker for his valuable suggestions concerning the methodology of the baroreflex measurements. We are grateful to Mrs D. Martin for her excellent assistance with processing of the histological material. These studies were performed with the support of the British Heart Foundation (BS BS/93003), the Wellcome Trust (044994) and the Royal Society.
References
- Averill DB, Diz DI, Barnes KL, Ferrario CM. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Research. 1987;414:294–300. doi: 10.1016/0006-8993(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Barnes KL, Diz DI, Ferrario CM. Functional interactions between angiotensin II and substance P in the dorsal medulla. Hypertension. 1991;17:1121–1126. doi: 10.1161/01.hyp.17.6.1121. [DOI] [PubMed] [Google Scholar]
- Bishop VS, Ryuzaki M, Cai Y, Nishida Y, Cox BF. Angiotensin II-dependent hypertension and the arterial baroreflex. Clinical and Experimental Hypertension. 1995;17:29–38. doi: 10.3109/10641969509087052. [DOI] [PubMed] [Google Scholar]
- Butcher JW, de Felipe C, Smith A, Hunt SP, Paton JFR. Comparison of cardiorespiratory reflexes in NK1 receptor knockout, heterozygous and wild type mice in vivo. Journal of the Autonomic Nervous System. 1998;69:89–95. doi: 10.1016/s0165-1838(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension. 1988;11(suppl. I):I167–171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- Casto R, Phillips MI. Cardiovascular actions of microinjections of angiotensin II in the brain stem of rats. American Journal of Physiology. 1984;246:R811–816. doi: 10.1152/ajpregu.1984.246.5.R811. [DOI] [PubMed] [Google Scholar]
- Casto R, Phillips MI. Neuropeptide action in nucleus tractus solitarius: angiotensin specificity and hypertensive rats. American Journal of Physiology. 1985;249:R341–347. doi: 10.1152/ajpregu.1985.249.3.R341. [DOI] [PubMed] [Google Scholar]
- Casto R, Phillips MI. Angiotensin II attenuates baroreflexes at nucleus tractus solitarius of rats. American Journal of Physiology. 1986;250:R193–198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- Chan JYH, Barnes CD, Chan SHH. Tonic enhancement of the sensitivity of baroreceptor reflex response by endogenous substance P in the rat. Regulatory Peptides. 1990;29:199–213. doi: 10.1016/0167-0115(90)90083-9. [DOI] [PubMed] [Google Scholar]
- Chan JYH, Tsou M-Y, Len W-B, Lee T-Y, Chan SHH. Participation of noradrenergic neurotransmission in the enhancement of baroreceptor reflex response by substance P at the nucleus tractus solitarii of the rat: A reverse microdialysis study. Journal of Neurochemistry. 1995;64:2644–2652. doi: 10.1046/j.1471-4159.1995.64062644.x. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Cumberbatch MJ, Birch PJ, Headley PM. Endogenous modulation of excitatory amino acid responsiveness by tachykinin NK1 and NK2 receptors in the rat spinal cord. British Journal of Pharmacology. 1995;115:1013–1019. doi: 10.1111/j.1476-5381.1995.tb15912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton, FL, USA: CRC Press; 1994. pp. 35–50. [Google Scholar]
- Des Senanayake P, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Li Y-W, Kasparov S, Paton JFR. Morphology of neurones in the nucleus tractus solitarius (NTS) of the working heart-brainstem preparation (WHBP) of the rat receiving afferent input from baroreceptors. The Journal of Physiology. 1998;513.P:83. P. [Google Scholar]
- Diz DI, Fantz DL, Benter IF, Bosch SM. Acute depressor actions of angiotensin II in the nucleus of the solitary tract are mediated by substance P. American Journal of Physiology. 1997;273:R28–34. doi: 10.1152/ajpregu.1997.273.1.R28. [DOI] [PubMed] [Google Scholar]
- Diz DI, Pirro NT. Differential actions of angiotensin II and angiotensin-(1–7) on transmitter release. Hypertension. 1992;19:II41–48. doi: 10.1161/01.hyp.19.2_suppl.ii41. [DOI] [PubMed] [Google Scholar]
- Feldman PD. Neurokinin1 receptor mediation of the vasodepressor effects of substance P in the nucleus of the tractus solitarius. Journal of Pharmacology and Experimental Therapeutics. 1995;273:617–623. [PubMed] [Google Scholar]
- Fow JE, Averill DB, Barnes KL. Mechanisms of angiotensin-induced hypotension and bradycardia in the medial solitary tract nucleus. American Journal of Physiology. 1994;267:H259–266. doi: 10.1152/ajpheart.1994.267.1.H259. [DOI] [PubMed] [Google Scholar]
- Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. Journal of Hypertension. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- Hall ME, Greer RA, Stewart JM. Effects of L-glutamate, substance P and substance P (17) on cardiovascular regulation in the nucleus tractus solitarius. Regulatory Peptides. 1993;46:102–109. doi: 10.1016/0167-0115(93)90019-5. [DOI] [PubMed] [Google Scholar]
- Hall ME, Miley FB, Stewart JM. Cardiovascular effects of substance P peptides in the nucleus of the solitary tract. Brain Research. 1989;497:280–290. doi: 10.1016/0006-8993(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Hayashi J, Takeda K, Kawasaki S, Nakamura Y, Oguro M, Nakata T, Tanabe S, Lee LC, Sasaki S, Nakagawa M. Central attenuation of baroreflex by angiotensin II in normotensive and spontaneously hypertensive rats. American Journal of Hypertension. 1988;1:15–22. doi: 10.1093/ajh/1.3.15s. S S. [DOI] [PubMed] [Google Scholar]
- Healy DP, Rettig R, Nguyen T, Printz MP. Quantitative autoradiography of angiotensin II receptors in the rat solitary-vagal area: effects of nodose ganglionectomy or sinoaortic denervation. Brain Research. 1989;489:1–12. doi: 10.1016/0006-8993(89)90343-0. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Butcher JW, Paton JFR. Angiotensin II receptors within the nucleus of the solitary tract mediate the developmental attenuation of baroreceptor vagal reflex in pre-weaned rats. Journal of the Autonomic Nervous System. 1998;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JFR. Changes in baroreceptor vagal reflex performance in the developing rat. Pflügers Archiv. 1997;434:438–444. doi: 10.1007/s004240050418. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JFR. Differential effects of angiotensin II in the nucleus tractus solitarii of the rat - plausible neuronal mechanisms. The Journal of Physiology. 1999;521:227–238. doi: 10.1111/j.1469-7793.1999.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacological Reviews. 1980;31:81–227. [PubMed] [Google Scholar]
- Kubo T, Kihara M. Modulation of the aortic baroreceptor reflex by neuropeptide Y, neurotensin and vasopressin microinjected into the nucleus tractus solitarii of the rat. Naunyn-Schmiedeberg's Archives of Pharmacology. 1990;342:182–188. doi: 10.1007/BF00166962. [DOI] [PubMed] [Google Scholar]
- Lee BH, Shin HS. In vivo pharmacological evaluation of newly synthesized nonpeptidic AT1 receptor antagonists in rats. Archives of Pharmacological Research. 1994;17:263–268. [Google Scholar]
- Luoh HF, Chan SHH. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Research. 1998;782:73–82. doi: 10.1016/s0006-8993(97)01198-0. [DOI] [PubMed] [Google Scholar]
- Maubach KA, Jones RSG. Electrophysiological characterisation of tachykinin receptors in the rat nucleus of the solitary tract and dorsal motor nucleus of the vagus in vitro. British Journal of Pharmacology. 1997;122:1151–1159. doi: 10.1038/sj.bjp.0701482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini LC, Bonagamba LH. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension. 1990;15:I45–50. doi: 10.1161/01.hyp.15.2_suppl.i45. [DOI] [PubMed] [Google Scholar]
- Morishita R, Higaki J, Nakamura Y, Aoki M, Yamada K, Moriguchi A, Rakugi H, Tomita N, Tomita S, Yu H, Nakamura F, Mikami H, Ogihara T. Effect of an antihypertensive drug on brain angiotensin II levels in renal and spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology. 1995;22:665–669. doi: 10.1111/j.1440-1681.1995.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Robertson D. Cardiovascular effects of microinjection of angiotensin II in the brainstem of renal hypertensive rats. Journal of Pharmacology and Experimental Therapeutics. 1990;255:374–381. [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. Journal of Neuroscience Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Importance of neurokinin-1 receptors in the nucleus tractus solitarii of mice for the integration of cardiac vagal inputs. European Journal of Neuroscience. 1998;10:2261–2275. doi: 10.1046/j.1460-9568.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Butcher JW. Cardiorespiratory reflexes in mice. Journal of the Autonomic Nervous System. 1998;68:115–124. doi: 10.1016/s0165-1838(97)00125-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rats Brain in Stereotaxic Coordinates. London: Academic Press Ltd; 1986. [Google Scholar]
- Phillips MI, Mann JFE, Hideyuki H, Hoffman WE, Dietz R, Schelling P, Ganten D. Lowering of hypertension by central saralasin in the absence of plasma renin. Nature. 1977;270:445–447. doi: 10.1038/270445a0. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. American Journal of Physiology. 1992;262:E763–778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Rettig R, Healy DP, Printz MP. Cardiovascular effects of microinjections of angiotensin II into the nucleus tractus solitarii. Brain Research. 1986;364:233–240. doi: 10.1016/0006-8993(86)90835-8. [DOI] [PubMed] [Google Scholar]
- Teruya H, Muratani H, Takishita S, Sesoko S, Matayoshi R, Fukiyama K. Brain angiotensin II contributes to the development of hypertension in Dahl-Iwai salt-sensitive rats. Journal of Hypertension. 1995;13:883–890. doi: 10.1097/00004872-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Timmermans PBMWM, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JAM, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacological Reviews. 1993;45:205–251. [PubMed] [Google Scholar]
- Tseng CJ, Chou LL, Ger LP, Tung CS. Cardiovascular effects of angiotensin III in brainstem nuclei of normotensive and hypertensive rats. Journal of Pharmacology and Experimental Therapeutics. 1994;268:558–564. [PubMed] [Google Scholar]


