Abstract
Intracellular and extracellular electrophysiological recording techniques were employed to examine the mechanisms involved in adaptation of guinea-pig airway sensory neurones to suprathreshold mechanical stimulation in vitro. Extracellular recordings performed using an in vitro airway preparation revealed two unambiguously distinct subsets of mechanically sensitive nerve endings in the trachea/bronchus. In one group of fibres, the mechanical stimulus caused a brief burst of action potentials, after which the nerve rapidly adapted. In the other group of fibres, repetitive action potentials were evoked as long as the stimulus was maintained above threshold.
The adaptation response strictly correlated with ganglionic origin of the soma. Those fibres derived from the nodose ganglion adapted rapidly, whereas those derived from the jugular ganglion were slowly or non-adapting.
Intracellular recordings from airway-identified neurones in isolated intact ganglia revealed that the majority of neurones within either the nodose or jugular ganglion adapted rapidly to prolonged suprathreshold depolarizing current injections.
The electrophysiological adaptation of nodose ganglion-derived neurones following prolonged suprathreshold current steps was greatly reduced by 4-aminopyridine. However, 4-aminopyridine did not affect the adaptation of rapidly adapting nodose ganglion-derived nerve endings in response to mechanical stimuli.
The data suggest that ganglionic origin dictates adaptive characteristics of guinea-pig tracheal and mainstem bronchial afferent neurones in response to mechanical stimulation. Also, the rapid adaptation of nodose nerve endings in the trachea observed during a mechanical stimulus does not appear to be related to the adaptation observed at the soma during prolonged suprathreshold depolarizing current injections.
The sensory nerve fibres supplying the guinea-pig trachea and main-stem bronchi are mainly vagal fibres with somata located within the nodose and jugular ganglia (Kummer et al. 1992; Riccio et al. 1996b). While subpopulations of these nerve endings can be excited by chemical and non-iso-osmotic stimuli, the majority of them respond to mechanical stimuli (Riccio et al. 1996a; Pedersen et al. 1998). Of the fibres that respond to a von Frey fibre mechanical stimulus, there are both low threshold and high threshold mechanoreceptors. Previous studies from our laboratory have shown that the low threshold mechanoreceptors originate from somata within the nodose ganglion and the high threshold mechanoreceptors originate from somata within the jugular ganglion (Riccio et al. 1996a).
Two types of mechanoreceptors have been characterized based on their response pattern to the mechanical forces imposed upon lung inflation, namely slowly adapting receptors (SARs) and rapidly adapting receptors (RARs). RARs, which discharge during lung inflation and then become refractory during maintained inflation, were first discovered in the airways by Knowlton & Larrabee (1946). Later, Widdicombe (1954) described RARs as irritant receptors that were largely located in the conducting airways and SARs as stretch receptors located primarily within the lung. Although this dichotomy has been established for some time, relatively little is known regarding the mechanism of adaptation to mechanical stimuli.
A central question regarding the adaptation of sensory nerves to mechanical stimulation is whether the electrophysiological properties of the neurone per se can support the adaptive response, or whether the adaptation is secondary to the manner in which the nerve ending interacts with the airway tissue. Harper (1991) observed that the adaptation of somata within the dorsal root ganglia to prolonged suprathreshold depolarizing current steps mimicked the adaptation of the nerve endings arising from these somata to mechanical stimulation in the rat paw. In other words, the adaptation to a prolonged suprathreshold stimulus appeared to be an intrinsic property of the neurones and not a function of the nerve ending-tissue interaction. Also supporting this concept is the observation that a neuroma created by nerve crush experiments, therefore removed from the environment it normally innervates, maintains its activation-response characteristics to mechanical stimulation (Koschorke et al. 1991). In contrast in the airways, experiments using antidromic electrical stimulation have led to the suggestion that the adaptation of sensory nerves to mechanical stimulation is not a function of the neurone per se, but a function of visco-elastic elements within the airway wall (Davenport et al. 1981).
The in vitro preparation used in the present study allows for the study of controlled mechanical force displacement steps and of specific ionic mechanisms at the level of the airway nerve ending, independent of such variables as haemodynamics and lung mechanics. The results reveal two distinct populations of nerve endings in the trachea and main-stem bronchus based on adaptive properties, one slowly adapting, the other rapidly adapting. The data obtained are consistent with the hypothesis that the ganglionic location of the cell body and elements of the nerve-airway tissue interaction play critical roles in determining whether the afferent fibre adapts slowly or rapidly in response to a mechanical stimulus.
METHODS
Tissue preparation
Male Hartley guinea-pigs (100–400 g) were killed by exposure to a rising concentration of CO2 gas followed by exsanguination. The airways with intact right-side extrinsic innervation (including nodose and jugular ganglia) were removed and placed in a dissecting dish containing Krebs bicarbonate buffer solution gassed with 95% O2-5% CO2 and composed of (mM): NaCl, 118; KCl, 5·4; NaH2PO4, 1·0; MgSO4, 1·2; CaCl2, 1·9; NaHCO3, 25·0; dextrose, 11·1 (pH 7·4). Connective tissue was trimmed away, leaving the trachea, larynx and right bronchus with intact nerves (vagus, superior laryngeal, and recurrent), including nodose and jugular ganglia. A transverse cut was made along the ventral surface to open the larynx, trachea and bronchus. Airways were then pinned to a Sylgard-lined Perspex chamber. The right nodose and jugular ganglia, along with the rostralmost vagus and superior laryngeal nerves, were gently pulled through a small hole into an adjacent compartment of the same chamber for recording of single fibre activity. Both compartments were superfused with the Krebs bicarbonate buffer solution. The temperature of the buffer was maintained at 37°C with a flow rate of 6–8 ml min−1. Studies using blue dye revealed that the buffer solutions perfusing each compartment remained separate. This method was adapted from previous studies (Bradley & Scheurmier, 1977; Fox et al. 1993) and has been described previously (Riccio et al. 1996b). The experiments were approved by the Johns Hopkins Animal Care and Use committee.
Extracellular recording of action potentials
Extracellular recordings were performed by manipulating a fine aluminosilicate glass electrode near cell bodies in either the jugular or nodose ganglion. The microelectrodes were pulled using a Flaming-Brown micropipette puller (Sutter Instrument Company, Novato, CA, USA) and filled with 3 M sodium chloride. The filled electrode was placed into an electrode holder connected directly to a headstage (A-M Systems, Everett, WA, USA). A return electrode of silver-silver chloride wire and an earthed silver-silver chloride pellet were placed in the perfusion fluid of the recording chamber and attached to the headstage. The recorded signal was amplified (A-M Systems) and filtered (low cut off, 0·3 kHz; high cut off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and a model TA240S chart recorder (Gould, Valley View, OH, USA). The data were stored on digital tape (DT-120RA, Sony Corporation, Tokyo, Japan) for off-line waveform analysis on a Macintosh computer using the software program The NerveOfIt (PHOCIS, Baltimore, MD, USA). The frequency and number of action potentials per 1 s bins were imported into a spreadsheet (Microsoft Excel 4.0) for analysis.
Discrimination of single fibre activity, location of receptive fields and determination of conduction velocities
Single fibre activity was discriminated by placing a concentric electrical stimulating electrode on the recurrent laryngeal nerve, through which the majority of fibres enter the trachea (Riccio et al. 1996a). The recording electrode was placed within the ganglion and manipulated until single unit activity was detected. When electrically evoked action potentials were seen, the stimulator was switched off and the trachea and bronchi were gently probed with a von Frey filament. Mechanically sensitive receptive fields were revealed when a burst of action potentials was elicited in response to von Frey filament stimulation. Conduction velocity and amplitude of the action potential were then compared with responses elicited by electrical stimulation of either the superior laryngeal, recurrent laryngeal, or vagus nerve trunks in order to determine the trunk that supplied the fibre.
Conduction velocities were calculated by electrically stimulating the receptive field and measuring the distance travelled along the nerve pathway divided by the time between the shock artifact and the recorded action potential. Fibres were classified as C fibres if their action potentials travelled slower than 1·5 m s−1. Fibres were classified as Aδ fibres if their action potentials travelled at speeds greater than 2 m s−1. The few fibres falling in between these two values were not studied further.
Mechanical stimulation
A blunt cylindrical Plexiglas probe connected to a Grass model FT03C force transducer (Astra-Med, Inc., Warwick, RI, USA) was attached to a motorized micromanipulator (MS 314, DC3001R, WPI, Sarasota FL, USA). The force transducer was connected to the second channel of the Gould chart recorder, so that the degree of force applied to the tissue could be monitored on-line. The probe was lowered onto the receptive field until action potential discharge was noted. The threshold for stimulation averaged about 1·0 g for nodose ganglion-derived fibres and 1·5 g for jugular ganglion-derived fibres. Due to the large diameter (∼3 mm) of the probe, the threshold force was greater than that previously noted with von Frey filaments (Riccio et al. 1996a). After an individual fibre's threshold for action potential discharge was determined, multiples of threshold force were applied and held for 10 s in a ramp-and-hold protocol. The rate at which the motor lowered the probe could be adjusted as necessary.
In a subset of seven studies the mechanical stimulus used to activate the afferent fibres was an increase in intralumenal tracheal pressure. The trachea was isolated with the vagus nerves and sensory ganglia intact, as described above. The trachea was left as a tube. The caudal and rostral ends were cannulated and perfused (5 ml min−1) with warmed (37°C) Krebs buffer solution. A pressure transducer (Statham Model P23, Gould, Inc. Oxnard, CA, USA) was attached to a side port of the cannula and the pressure was monitored on a separate channel of the chart recorder used to monitor single unit activity. After finding a single unit in the recurrent laryngeal nerve, perfusion was stopped via 3-way stopcocks. The pressure in the buffer-filled trachea was increased by pushing down on a syringe attached to the cannula at the rostral end of the trachea. The pressure was rapidly increased until action potentials were evoked from the mechanosensitive fibre arising from either the nodose or jugular ganglion. The threshold pressure was not determined, but exceeded 100 cmH2O. The pressure was held above threshold value for 10 s and then returned to baseline. The adaptation index of the nerve was then calculated as described below.
Calculation of adaptation index
The number and frequency of action potentials discharged during the mechanical stimuli were collected. A modification of the adaptation indices previously described (Knowlton & Larrabee, 1946; Widdicombe, 1954) was used to classify fibres as slowly or rapidly adapting. The number of action potentials recorded during the dynamic phase of force application was divided by the total number of action potentials elicited and this number was multiplied by 100. A score of 90 or above was considered to be rapidly adapting, whereas scores of 60 or below were considered slowly adapting.
Pharmacological manipulation of ion channels
For local delivery of pharmacological agents, Krebs bicarbonate buffer solution containing the appropriate agent was superfused through the tracheal compartment of the recording chamber for at least 15 min prior to mechanical stimulation.
Injection of fluorescent tracer into airways and tissue isolation
Male Hartley guinea-pigs (100–300 g) were anaesthetized with intramuscular injections of 50 mg kg−1 ketamine hydrochloride and 2·5 mg kg−1 xylazine hydrochloride. A medial ventral skin incision was made, the trachea was exposed and a small slit was cut between two tracheal cartilage rings at midcervical level, with topical 1% lidocaine (lignocaine) used as analgesia. A Hamilton syringe was inserted through this slit, for 30 μl injection of tracer dye (tetramethylrhodamine dextran, 1%, Molecular Probes, Eugene, OR, USA). The incision was sutured closed and the animals were allowed to recover. After 7–14 days the animals were killed by exposure to a rising concentration of CO2 gas followed by exsanguination, the right side nodose and jugular ganglia were then immediately removed, connective tissue was dissected away and the ganglia were placed in a recording chamber perfused with the same Krebs bicarbonate buffer listed above. This technique is a modification of that described by Christian et al. (1993).
Labelled neurone visualization and intracellular recordings
Nodose and jugular ganglia preparations were pinned to a Sylgard-coated recording chamber (100 μl volume) and superfused with Krebs solution (35–37°C, 6–8 ml min−1), as previously described (Undem & Weinreich, 1993). Labelled neurones were visualized with epifluorescent light under a fixed-stage upright microscope (BX50WI, Olympus America Inc., Melville, NY, USA) with a reflected light fluorescence attachment (BX-FLA, Olympus America Inc.), with care taken to minimize the time the cells were exposed (Christian et al. 1993). Following the visualization, a borosilicate microelectrode (40–70 MΩ resistance) fabricated from thick-walled capillary stock (0·5 mm i.d., 1·0 mm o.d., WPI, Inc.) by a Brown-Flaming microelectrode puller (Model P-87)) filled with 3 M KCl solution (pH 7·4) was used to impale the neurone. The electrolyte in the micropipette was connected by a silver-silver chloride wire in an electrode holder (Axon Instruments, Foster City, CA, USA) by a headstage to an electrometer (Axoclamp 2A, Axon Instruments). A silver-silver chloride pellet was placed in the bathing solution as an earth. Impalement of the neurones was aided by a 20 ms overcompensation (i.e. ‘buzz’) of the capacitance neutralization circuit of the Axoclamp amplifier. Input impedance of the neurone was determined by applying 100 pA current steps and calculating the impedance based on Ohm's law. In order to determine whether the neuronal soma adapted rapidly (phasic pattern) or slowly (tonic pattern) to electrophysiological stimuli, 500 ms cathodal current steps of 1–4 nA were delivered through the electrode.
Drugs and solutions
4-Aminopyridine was purchased from Sigma (USA) and dissolved in deionized H2O (dH2O) at a concentration of 100 mM for subsequent dilution into physiological buffer solution. Glybenclamide was purchased from Sigma and dissolved in DMSO at 10 mM. Tetraethylammonium (TEA) chloride and apamin were purchased from Sigma and dissolved in deionized H2O at concentrations of 1 M and 100 μM, respectively.
Ketamine hydrochloride and xylazine hydrochloride were purchased from Sigma and were dissolved in 0·9% saline at 1 mg ml−1. Lidocaine hydrochloride was purchased from Sigma and dissolved in 70:30 0·9% saline: ethanol. Iberiotoxin was purchased from Biomol (Plymouth Meeting, MA, USA) and dissolved in dH2O at a concentration of 100 μM. Tetramethylrhodamine dextran was purchased from Molecular Probes, Inc. (Eugene, OR, USA) and dissolved in dH2O.
Data analysis
Data are presented as means ±s.e.m. The peak frequency in response to mechanical stimuli represents the largest number of action potentials in a 1 s bin. Electrophysiological data were compared using a one-way analysis of variance (ANOVA), followed by Student's non-paired t test to locate any differences detected with ANOVA.
RESULTS
Extracellular recordings were made from 48 mechanically sensitive receptive fields within the trachea and right bronchus of 44 animals. Of these, 24 were found to have their somata located within the nodose ganglion, whereas 24 of the mechanically sensitive fibres had their somata in the jugular ganglion. The nodose ganglion-derived fibres conducted in the Aδ range (5·1 ± 0·3 m s−1), whereas 14 of the jugular ganglion-derived fibres conducted in the Aδ range (5·0 ± 0·6 m s−1) and 10 conducted in the C range (0·9 ± 0·1 m s−1). These fibres were carried through the vagus, recurrent laryngeal, or superior laryngeal nerves.
The location of mechanical receptive fields was not noticeably different between the jugular and nodose neurones. The trachea can be divided into cartilaginous and the non-cartilaginous portions, with the latter containing the trachealis smooth muscle. The vast majority of the receptive fields were found within the cartilaginous portion of the airway, with relatively few receptive fields localized to the smooth muscle portion (Fig. 1). Receptive fields of nodose and jugular fibres were noted both over and between the cartilage rings. Parenthetically, it should be noted that although it appears from the receptive fields denoted in Fig. 1 that the rostral trachea is preferentially innervated by nodose fibres, this does not hold true with a larger sample size (obtained by combining data from other studies).
Figure 1. Diagram of the experimental method used to study mechanically sensitive afferent endings in the guinea-pig trachea/bronchus.
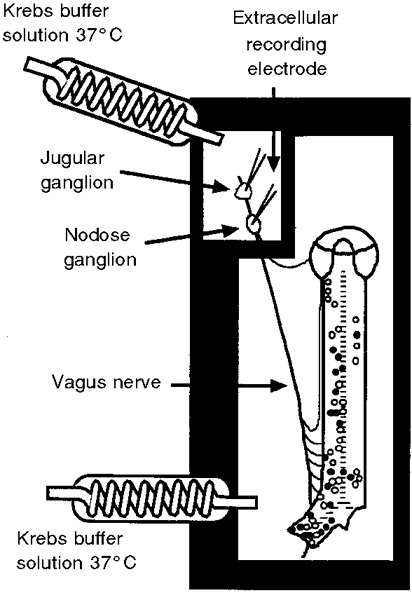
The trachea/bronchus was opened with a cut along the ventral surface and pinned, epithelial-side up, to a recording chamber. The airway and ganglion compartments were separately superfused with Krebs buffer solution (37 °C). Glass microelectrodes positioned near neuronal cell bodies in either the jugular or nodose ganglion were used to record extracellular action potentials arising from mechanically receptive fields in the trachea and right bronchus. The approximate location of the receptive field for the nodose (○) and jugular (•) ganglion-derived nerve endings in the airway. In all cases only one receptive field was found to be associated with a given nerve fibre.
Out of 24 nodose-derived nerve endings, 22 fibres adapted rapidly (i.e. adaptation index > 90, Fig. 2) in response to a mechanical stimulus equal to approximately three times the fibre's mechanical threshold, whereas only two fibres adapted slowly (i.e. adaptation index of < 60). The mean adaptation index of these fibres was 94·9 ± 2·1. The rapidly adapting nodose endings also typically had an ‘off’ response, in that the fibre responded upon removal of the stimulus (Fig. 2). In contrast, 18 of 24 jugular fibres were slowly adapting, one was rapidly adapting and five were in between. C and Aδ-fibres from the jugular ganglion were grouped together since their adaptation indices were not statistically different (45·6 ± 7·5, n = 14 for Aδ fibres; 41·7 ± 7·1, n = 10 for C fibres).
Figure 2. Adaptation of airway afferent nerve endings in vitro.
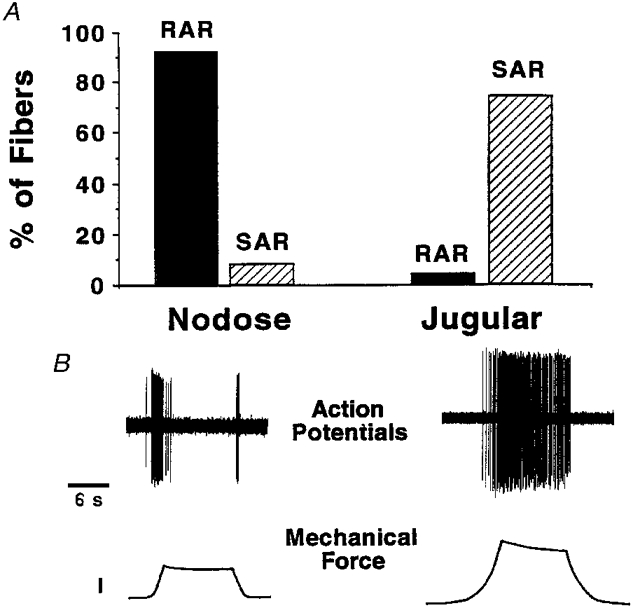
A, histogram representing percentages of fibres within either of the two vagal sensory ganglia that fell into the rapid adaptation receptor (RAR) or slowly adaptation receptor (SAR) class. A fibre with an adaptation index of < 60 was considered SAR, whereas an index of > 90 was used to define RAR (n = 24 for nodose fibres, and n = 24 for jugular fibres). Note, 6 of 24 jugular fibres were not characterized as their adaptation index fell between 60 and 90. B, typical tracings of the response of a nodose (left) and jugular (right) nerve ending to a 10 s mechanical displacement to approximately ×3 threshold. In the force trace, the calibration = 2 g of force. The single unit potentials recorded from the jugular fibre in this experiment are off the chart-recorder scale.
As we previously noted (Riccio et al. 1996a) the mechanical threshold was smaller for nodose nerve endings than jugular nerve endings. It is unlikely that this accounts for the adaptation pattern, because in four experiments we increased the mechanical stimulation of nodose fibres to ×8 threshold, and in 4 of 4 fibres the response was still rapidly adapting in nature.
We next addressed the concern that the adaptation pattern was dependent upon the method of mechanical stimulation. In seven experiments we used increases in intralumenal pressure as the stimulus. The intralumenal pressure of the trachea was rapidly increased to beyond action potential threshold, then held constant for 10 s, as described in Methods. The observed response was similar to that observed with the blunt probe technique. In 4 of 4 nodose Aδ nerve endings, increasing intralumenal pressure caused a rapid brief discharge of action potentials then very rapidly adapted. The adaptation index was greater than 95 for each fibre. In contrast, 3 of 3 jugular fibre endings (1 C fibre and 2 Aδ fibres) responded to an increase in intralumenal pressure with a slowly or non-adapting pattern. The adaptation index of the three jugular fibres was 7·5, 10 and 15, which was significantly different from that obtained with the nodose endings (P < 0·05). In all subsequent studies lowering a blunt probe onto the receptive field was the method used for mechanical stimulation.
Intracellular recordings were obtained from somata in the nodose ganglion which projected fibres to the trachea/bronchus (i.e. retrogradely labelled with tetramethylrhodamine dextran, see Methods). Resting membrane potentials and input impedance in these neurones were −58·9 ± 5·1 mV and 29 ± 6·0 MΩ, respectively (n = 11). Of the labelled somata, 8 of 11 responded to a prolonged (175–500 ms) injection of suprathreshold depolarizing current in a rapidly adapting (phasic) fashion. These neurones responded with one or two action potentials then adapted to the suprathreshold depolarizing stimulus. The remaining 3 of 11 neurones responded in a tonic or non-adapting manner with action potentials evoked throughout the duration of the current step (Fig. 3). The tetramethylrhodamine dextran did not appear to influence the passive or active properties of the neurones, as the resting membrane potential and input impedance of the labelled neurones were similar to values obtained in four unlabelled nodose ganglion neurones (n = 4, P > 0·05). Also, all four of the unlabelled neurones responded in a phasic fashion in response to the prolonged suprathreshold current step (data not shown).
Figure 3. Intracellular recordings of membrane potentials of airway-specific (tetramethylrhodamine dextran-labelled) neurones within the nodose ganglion.
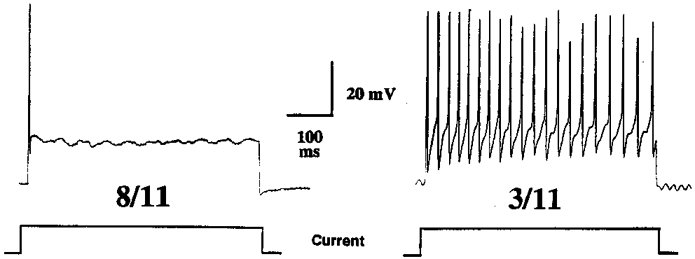
As represented below the traces, injection of prolonged (500 ms) 2 nA depolarizing current steps caused action potential generation in all fibres studied. Over 70% (8 of 11) nodose ganglion neurones responded to this stimulus as indicated in the left trace, with a brief action potential discharge (phasic pattern) that ceased despite continued application of depolarization current. The remaining three nodose ganglion neurones responded as indicated in the right trace, with a continuous burst of action potentials throughout the current step (tonic pattern).
Jugular neurones also responded to a prolonged suprathreshold current step in a fashion similar to nodose neurones. Thus, 4 of 6 airway-specific (tetramethylrhodamine dextran-labelled) jugular neurone somata responded to 500 ms suprathreshold current steps with a phasic discharge, while 2 of 6 responded with a tonic pattern. The membrane potential of jugular neurones was −57·8 ± 2·2 mV and the input impedance was 21·7 ± 4·7 MΩ.
In 3 of 4 unlabelled-nodose neurones, and 2 of 2 tetramethylrhodamine dextran-labelled nodose neurones, the rapid electrophysiological adaptation was blocked or inhibited by 10 min application of 4-AP (10 μM, Fig. 4). Based on the observations that relatively low concentrations of 4-AP reduced adaptation to electrophysiological stimuli in the somata of nodose ganglion neurones, 10 μM 4-AP was applied to mechanically sensitive receptive fields of rapidly adapting, nodose ganglion-derived fibres. In contrast to intracellular recordings from somata within the nodose ganglion, 4-AP was without affect on adaptation at the level of the nerve ending (n = 4, Fig. 4). It should be noted that no effect on adaptation index was noted, even in one experiment in which the concentration of 4-AP was increased to 100 μM, a concentration that actually caused a low frequency discharge, confirming the ability of 4-AP to reach effective concentrations at the level of the nerve ending. In an attempt to discern whether other potassium channels might be involved in the rapid adaptation, several other potassium channel blocking agents were used. TEA (10 mM, n = 3), glybenclamide, (10 μM, n = 3), apamin (100 nM, n = 3), and iberiotoxin (100 nM, n = 3) all had no effect on the adaptation rate of rapidly adapting nerve endings (data not shown).
Figure 4. Effect of 4-AP on adaptation.
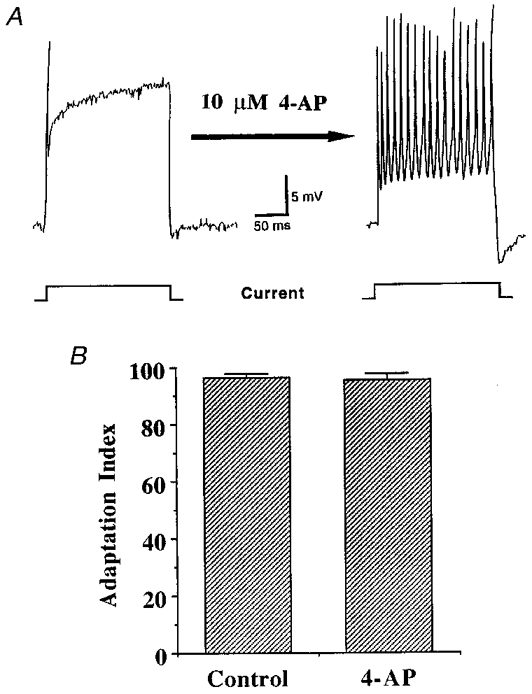
A, a representative intracellular recording of the membrane potential of a neurone within the nodose ganglion. Injection of prolonged depolarizing current steps (denoted at the bottom of the figure) through an intracellular electrode produced one action potential followed by subsequent adaptation to the stimulus. This adaptation was inhibited by superfusion of the nodose ganglion with Krebs solution containing 4-AP (10 μM, 10 min), as delivery of the same stimulus following 4-AP application yielded several action potentials. B, histogram demonstrating the means ±s.e.m. of the adaptation index (calculated as shown in Methods) of five nodose ganglion-derived, rapidly adapting nerve endings both before and after superfusion of the isolated airway with 4-AP (10 μM, 15 min). In contrast to its marked inhibition of adaptation to current steps delivered to nodose ganglion somata, 4-AP had no effect on the adaptation of nodose ganglion-derived fibres in response to mechanical ramp and hold stimuli.
Electrical stimulation of the nodose ganglion-derived nerve fibres in the trachea with square pulses of suprathreshold voltages caused action potential discharge that faithfully followed the stimulus up to frequencies as high as 50 Hz (highest frequency studied). Stimulating 200 action potentials over a 4 s period had no effect on the pattern of action potential discharge in response to the mechanical stimulation delivered immediately upon cessation of the electrically induced impulses (Fig. 5).
Figure 5. Effect of electrical stimulation on adaptation.
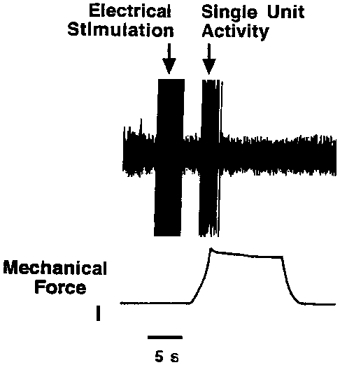
Single unit recording showing the effect of electrical stimulation of a nodose ganglion-derived rapidly adapting nerve ending in the trachea on the subsequent response to a mechanical ramp-and-hold mechanical stimulus (calibration, 1 g force). The mechanical stimulus is depicted in the lower trace and the action potential discharge is depicted in the upper trace. The electrical stimulation of the nerve ending was achieved by placing a stimulating electrode in juxtaposition to the mechanically sensitive receptive field. The stimulus was a 0·5 ms square pulse of suprathreshold voltage delivered at 50 Hz for 4 s. This resulted in 200 shock artifacts each followed successfully by an action potential. This could be readily observed on the oscilloscope screen, although the chart recording could not resolve the individual shock artifacts or the action potentials caused by the electrical stimulus. The figure demonstrates that 200 impulses of the sensory nerve caused by electrical stimulation did not prevent the nerve from responding to mechanical stimulation applied within 3 s of cessation of the electrical stimulation.
We next attempted to evoke action potential discharge from nodose nerve endings during the period in which they had adapted to mechanical stimulation. As described above, mechanical stimulation caused an initial burst of action potentials which then ceased. Immediately upon cessation of action potential discharge, the fibre could once again be activated by applying additional force to the receptive field (Fig. 6).
Figure 6. Single unit recording of a rapidly adapting (RAR) nodose ganglion-derived fibre.
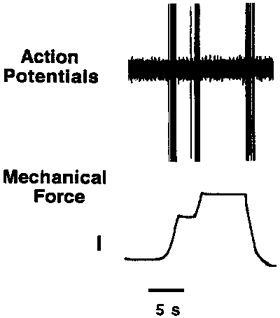
Application of mechanical force equal to ×3 the threshold value was initially applied, once the fibre adapted (stopped discharging) the probe was lowered further, as demonstrated in the lower trace (calibration, 1 g force). This manipulation caused action potential discharge during the time period when the fibre appeared to have been rendered refractory. This fibre, as with most nodose fibres, also discharged action potentials when the probe was withdrawn from the tissue.
Applying mechanical force to nodose nerve endings at a slower rate produced prolonged action potential discharge, as long as the stimulus was increasing (Fig. 7A). Nodose ganglion-derived fibres, but not jugular ganglion-derived fibres, were sensitive to the change in the rate of force application. In nodose ganglion-derived fibres, the peak frequency of action potential discharge was significantly reduced from 25·7 ± 4·8 to 9·3 ± 2·9 Hz when the rate at which the motor driving the mechanical probe was reduced to one quarter speed (Fig. 7; n = 4). In contrast, jugular ganglion-derived nerve endings responded to a maximal rate of force application with a peak action potential discharge frequency of 22·9 ± 2·8 Hz, which was no different from the peak frequency noted when the motor driving the probe was slowed to one quarter speed (18·1 ± 3·7 Hz; n = 7). Both jugular and nodose nerve endings were sensitive to the amount of force applied, as peak frequency of action potential discharge increased in response to ×2, ×4 and ×8 threshold force applications (Fig. 8).
Figure 7. Effect of rate of mechanical stimulation on action potential discharge from nodose and jugular nerve endings.
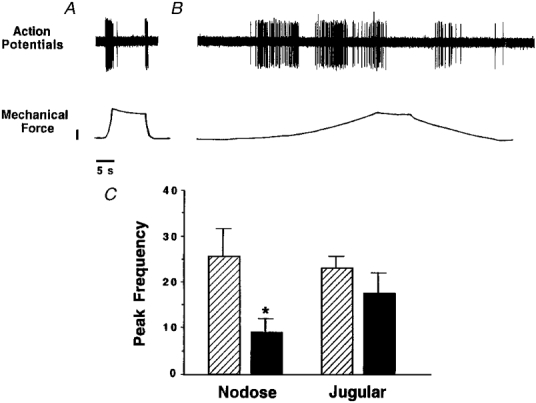
A, a single unit recording from a nodose ganglion-derived fibre. The response to a mechanical stimulus delivered, as seen in the bottom trace, with the motor lowering the probe at full speed (calibration, 2 g force). B, the same fibre as A was stimulated with the motor lowering the mechanical probe at 1/4 speed. Note that in both A and B the fibre responded continuously as long as the mechanical stimulus was changing. C, histogram showing means ±s.e.m. peak frequency of action potential discharge of nodose and jugular nerve fibres during a 1 s period in response to mechanical stimulation to ×3 threshold with the motor set at full ( ) or one quarter (▪) of maximum speed; n = 4. * Statistically significant difference in the values obtained within each fibre type between the two stimuli.
) or one quarter (▪) of maximum speed; n = 4. * Statistically significant difference in the values obtained within each fibre type between the two stimuli.
Figure 8. Effect of graded increases in force on activation of tracheobronchial afferent fibres.
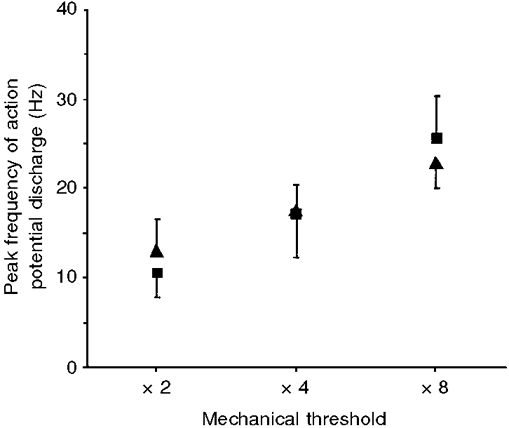
Graph of means ±s.e.m. peak action potential discharge frequency during a 1 s period for nodose ganglion-derived (▪) and jugular ganglion-derived (▴) fibres versus amount of force applied by a blunt probe. After the mechanical threshold of the fibre was determined, successive ramp-and-hold mechanical stimulations of ×2, × 4 and ×8 the value of the fibre's mechanical threshold value were performed. Peak frequencies of action potential discharge in fibres derived from both the nodose ganglion (n = 4) and jugular ganglion (n = 7) increased with the amount of force applied to the receptive field. There were no statistical differences in peak frequencies of action potential discharges seen between nodose ganglion-derived and jugular ganglion-derived fibres.
DISCUSSION
The adaptation of sensory nerve endings to a mechanical stimulus in guinea-pig trachea and bronchus was investigated in vitro. As previously observed in pulmonary afferent nerve studies using lung inflation strategies (Knowlton & Larrabee, 1946), the response of tracheobronchial nerve endings in vitro to a sustained suprathreshold mechanical force could be segregated unambiguously into rapidly and slowly adapting categories. Regardless of the biophysical mechanisms underlying the process of adaptation, our results support the conclusion that in the guinea-pig trachea, the adaptation of sensory fibres to mechanical stimulation is neither correlated with conduction velocity nor peak discharge frequency, but rather with the ganglionic origin of their soma. The rapidly adapting fibres were derived almost exclusively from somata located within the nodose ganglion, while the overwhelming majority of the slowly adapting fibres were derived from cell bodies within the jugular ganglion. This conclusion is in agreement with preliminary findings using hand-held von Frey filaments (Riccio et al. 1996a).
Previous studies have suggested that nodose and jugular neurones have distinct embryological origins (D'Amico-Martel & Noden, 1983). Consistent with this, sensory nerves projecting into the airways that are derived from jugular neurones are phenotypically distinct from those derived from nodose neurones. Nodose afferent fibres in the guinea-pig trachea/bronchus are relatively unresponsive to capsaicin, hypertonic saline or bradykinin, but are very sensitive to mechanical stimuli. Jugular afferent fibres, regardless of their conduction velocity, respond vigorously to capsaicin, bradykinin, and hypertonic saline, but are relatively less sensitive to mechanical stimulation (Ricco et al. 1996a; Pedersen et al. 1998; Kajekar et al. 1999). These findings support the hypothesis that the adaptation pattern of guinea-pig airway afferent nerve endings studied in vitro correlates with whether the nerve is a jugular ganglion-derived nociceptive-like sensor (slowly adapts) or a nodose ganglion-derived physiological sensor (rapidly adapts).
The classically defined SARs are characterized according to their response to lung inflation. The most studied SARs are fast-conducting (Aβ range) stretch receptors (Knowlton & Larrabee, 1946; Widdicombe, 1954). It should therefore be noted that the slowly adapting jugular Aδ and C fibres in the isolated trachea/bronchus studied here do not correspond with traditional SAR fibres which are defined on the basis of in vivo experiments. The receptive fields for both the jugular and nodose fibres in the present study were rarely over the smooth muscle layer of the trachea, and thus are also unlikely to represent the stretch receptors studied by Bartlett et al. (1976), in the in situ canine trachea preparation.
Studies by Harper (1991) in the somato-sensory system in the rat led to the conclusion that: ‘the adaptation properties of a mechanoreceptor may be due to the presence of a particular type of ionic channel in the terminals of the nerve which is also present in the membrane of its soma’. This provocative conclusion was based on a set of elegant electrophysiological experiments that demonstrated that a nerve ending's adaptation to mechanical stimuli directly correlated with the adaptation of the soma of the same nerve to a prolonged suprathreshold depolarizing current injection. This hypothesis would predict that a compound that blocks the ion channels responsible for the adaptation observed in the soma should, when applied to the nerve endings, inhibit the adaptation to mechanical stimulation.
In the present study, intracellular recordings from identified airway-specific neurones within the nodose ganglion showed electrophysiological adaptation in response to prolonged suprathreshold depolarizing current steps. This correlated with rapid adaptation seen in the vast majority of nodose ganglion-derived nerve endings in the extrathoracic airways. This would seem to support Harper's hypothesis that a certain type of ion channel, present in both the somata and nerve endings, is responsible for the adaptation of action potential discharge in response to a mechanical stimulus. Two other observations, however, lead to the conclusion that these two phenomena (electrophysiological adaptation at the soma and mechanical adaptation at the nerve ending) may not be causally related in airway sensory fibres. First, the majority of the airway-specific jugular ganglion neurones were similar to nodose ganglion neurones in that they possessed a strong electrophysiological adaptation during a prolonged suprathreshold current step. This is consistent with prior findings in unlabelled jugular ganglion neurones (Christian & Togo, 1995). This does not correlate with the nerve endings of jugular ganglion-derived neurones, which were nearly uniformly slowly or non-adapting to a sustained mechanical stimulus. Secondly, the electrophysiological adaptation observed in the somata of nodose ganglion neurones could be effectively inhibited by low concentrations of 4-AP, consistent with previous findings in unlabelled rat nodose ganglion neurones (Stansfield et al. 1986), whereas mechanical adaptation was not affected by application of 4-AP to the airway. A variety of selective and non-selective potassium channel blockers also had no affect on adaptation of the nerve endings to mechanical stimuli.
It is possible that an ion channel or channels, not affected by the pharmacological agents used in the present study and located in the nerve ending of rapidly adapting nodose ganglion neurones, was activated during the initial burst of action potentials that then rendered the nerve refractory to subsequent stimulation. An attractive candidate for such a channel is the channel which supports the so-called afterspike slow hyperpolarizing current (AHPslow). This current, which is present in subpopulations of guinea-pig nodose ganglion neurones (Undem & Weinreich, 1993), is not inhibited by known ion channel blockers and serves to limit neuronal excitability by causing a long-lasting hyperpolarization of the membrane following a burst of action potentials. The AHPslow is caused by a potassium channel that is activated by an intracellular rise in calcium ions. Since there are no known blockers of this current, it is difficult to critically address its involvement in the adaptation of nodose ganglion-derived tracheobronchial fibres to mechanical stimulation. The finding that electrical stimulation of the rapidly adapting nodose fibre at frequencies up to 50 Hz for 4 s (Fig. 5) did not inhibit the subsequent action potential discharge induced by mechanical stimulation suggests that adaptation to mechanical stimulation is not causally linked to inhibitory ionic currents activated by an initial discharge of action potentials. This is consistent with similar findings from in vivo studies of sensory fibres in the canine trachea (Davenport et al. 1981).
The hypothesis that the rapid adaptation of the nodose ganglion-derived nerve endings to a ‘ramp-and-hold’ type mechanical stimulation is not an active consequence of the initial burst of action potentials is also supported by the observations made when the rate of force application to the nodose ganglion-derived nerve endings was decreased. This stimulus caused prolonged and continuous (non-adapting) action potential discharge which only ended when the dynamic phase of the stimulus ceased. This concept is also supported by the data in Fig. 6 which show that following adaptation to mechanical stimulation, a further increase in force, or removal and immediate reapplication of the force, successfully elicited action potential generation.
Not only did the ‘rapidly adapting’ nodose ganglion-derived fibres continue to discharge during the dynamic phase of mechanical stimulation, but, in contrast to jugular slowly adapting fibres, their peak frequency of action potential discharge was graded in accordance with the rate at which the stimulus was applied. This is consistent with in vivo studies in the dog that demonstrated that RAR discharge increases with expiratory flow rate (Pack et al. 1981).
Considered together, the data suggest that the nodose ganglion-derived fibres are not rapidly adapting fibres that discharge and then become refractory due to an ionic mechanism caused by the initial burst of action potentials. Rather, they are situated within the tissue in such a fashion that their adequate stimulus for activation is the dynamic phase of a mechanical stimulus. This conclusion has implications when considering mechanisms by which action potential pattern in sensory nerves are modulated by disease processes. In chronic inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease there is substantial remodelling of the airway wall. The morphological changes associated with this process may potentially have significant impact on action potential discharge pattern. How the tissue environment surrounding the nerve ending could lead to the nodose ganglion-derived fibres only, responding during a dynamic stimulus, is not known. There are relatively few microscopic studies of afferent nerves in guinea-pig airways, and these were not correlated with physiological data (Baluk & Gabella, 1991). The finding that the adaptation pattern of guinea-pig tracheal afferent nerves to mechanical stimulation can be predicted based on their somal location should prove useful to those using orthograde labelling strategies to investigate such structure-function relationships.
Acknowledgments
We acknowledge Ms Sonya Meeker for her outstanding technical assistance. This work was supported by the National Institutes of Health, Bethesda, MO, USA.
References
- Baluk P, Gabella G. Afferent nerve endings in the tracheal muscle of guinea pigs and rats. Anatomical Embryology. 1991;183:81–87. doi: 10.1007/BF00185838. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Jr, Sant'Ambrogio G, Wise JCM. Transduction properties of tracheal stretch receptors. The Journal of Physiology. 1976;258:421–432. doi: 10.1113/jphysiol.1976.sp011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley GW, Scheurmier N. The transduction properties of tracheal stretch receptors in vitro. Respiration Physiology. 1977;31:365–375. doi: 10.1016/0034-5687(77)90079-2. [DOI] [PubMed] [Google Scholar]
- Christian EP, Togo JA. Excitable properties and underlying Na+ and K+ currents in neurons from the guinea-pig jugular ganglion. Journal of the Autonomic Nervous System. 1995;56:75–86. doi: 10.1016/0165-1838(95)00058-0. [DOI] [PubMed] [Google Scholar]
- Christian EP, Togo JA, Naper KE, Koschorke G, Taylor GA, Weinreich D. A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. Journal of Neuroscience Methods. 1993;47:147–160. doi: 10.1016/0165-0270(93)90031-l. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. American Journal of Anatomy. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Sant'Ambrogio FB, Sant'Ambrogio G. Adaptation of tracheal stretch receptors. Respiration Physiology. 1981;44:339–349. doi: 10.1016/0034-5687(81)90028-1. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Barnes PJ, Urban L, Dray A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. The Journal of Physiology. 1993;469:21–35. doi: 10.1113/jphysiol.1993.sp019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA. Similarities between some properties of the soma and sensory receptors of primary afferent neurones. Experimental Physiology. 1991;76:369–377. doi: 10.1113/expphysiol.1991.sp003504. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Proud D, Myers A, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. Journal of Pharmacology and Experimental Therapeutics. 1999;289:682–687. [PubMed] [Google Scholar]
- Knowlton GC, Larrabee MG. A unitary analysis of pulmonary volume receptors. American Journal of Physiology. 1946;147:100–114. doi: 10.1152/ajplegacy.1946.147.1.100. [DOI] [PubMed] [Google Scholar]
- Koschorke GM, Meyer RA, Tillman DB, Campbell JN. Ectopic excitability of injured nerves in monkey: entrained responses to vibratory stimuli. Journal of Neurophysiology. 1991;65:693–701. doi: 10.1152/jn.1991.65.3.693. [DOI] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Pack AI, DeLaney RG, Fishman AP. Augmentation of phrenic neural activity by increased rates of lung inflation. Journal of Applied Physiology. 1981;50:149–161. doi: 10.1152/jappl.1981.50.1.149. [DOI] [PubMed] [Google Scholar]
- Pedersen KE, Meeker SN, Riccio MM, Undem BJ. Selective stimulation of jugular ganglion afferent neurons in guinea pig airways by hypertonic saline. Journal of Applied Physiology. 1998;84:499–506. doi: 10.1152/jappl.1998.84.2.499. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. The Journal of Physiology. 1996a;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. The Journal of Physiology. 1996b;491:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield CE, Marsh SJ, Halliwell JV, Brown DA. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neuroscience Letters. 1986;64:299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. Journal of the Autonomic Nervous System. 1993;44:17–34. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Receptors in the trachea and bronchi of the cat. The Journal of Physiology. 1954;29:125–142. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]


