Abstract
The discharges of two motor units were identified in an intrinsic hand muscle (first dorsal interosseous, FDI) or an axial muscle (lumbar paraspinals, PSP) in ten healthy subjects. Each motor unit was situated in the homologous muscle on either side of the body (bilateral condition) or in the same muscle (ipsilateral condition). The relationship between the times of discharge of the two units was determined using coherence analysis.
Motor unit pairs in the ipsilateral FDI showed significant coherence over the frequency bands 1-10 Hz and 12-40 Hz. Motor units in the ipsilateral PSP were significantly coherent below 5 Hz. In contrast there was no significant coherence at any frequency up to 100 Hz in the bilateral FDI condition and only a small but significant band of coherence below 2 Hz in the bilateral PSP condition.
Common drive to motor units at frequencies of < 4 Hz was assessed by cross-correlation of the instantaneous frequencies of the motor units. A significantly higher coefficient was found in the ipsilateral FDI, ipsi- and bilateral PSP compared with shifted, unrelated data sets. This was not the case for the bilateral FDI condition.
The presence of higher frequency coherence (> 10 Hz) in the ipsilateral FDI condition and its absence in ipsilateral PSP is consistent with a more direct and influential cortical supply to the intrinsic hand muscles compared with the axial musculature. The presence of low frequency drives (< 4 Hz) in the bilateral PSP condition and its absence in the bilateral FDI condition is consistent with a bilateral drive to axial, but not distal, musculature by the motor pathways responsible for this oscillatory input.
Hand and axial muscles differ in their functional use, principally participating in prehension or postural control and locomotion. These functional differences are mirrored by differences in their neural control. Direct corticomotoneurone projections, for example, seem to be important for manual dexterity (Lemon, 1993). Differences in the response of hand muscles and proximal and axial muscles to transcranial magnetic stimulation of the motor cortex support the importance of these projections in the control of hand muscles in humans (Rothwell et al. 1991). In contrast, there may be more significant control of axial muscles from brainstem and spinal centres (Kuypers, 1981). The neural control of hand and axial muscles also differs in the degree of lateralisation, with hand muscles being predominantly controlled by the contralateral motor cortex and axial muscles receiving bilateral input from many sites along the neuraxis (Kuypers, 1981). Branching of presynaptic axons to numerous motoneurones means that motor units both within the same muscle and between different muscles are not controlled in isolation (Shinoda et al. 1981). A degree of common control may be important in both simplifying the recruitment of motor units (De Luca & Erim, 1994) and in the formation of functional synergies between muscles (Lemon, 1993). One consequence of a common input to motoneurones is the synchronisation of discharges above that expected by chance (Sears & Stagg, 1976; Datta & Stephens, 1990). Motor unit synchronisation has been extensively investigated within and between hand muscles (Datta et al. 1991; Bremner et al. 1991a; Farmer et al. 1993a,b). The abolition of motor unit synchronisation following stroke (Datta et al. 1991; Farmer et al. 1993b) or spinal cord lesions (Davey et al. 1990) and its preservation in the presence of peripheral deafferentation (Farmer et al. 1993a) suggest that synchronisation is caused by central, probably corticospinal, sources.
Two or more motoneurones may also show an increase in the degree of synchrony if the inputs they receive are themselves synchronised, rather than simply branched. The degree of motor unit synchronisation in the hand is correlated with the presence of a common rhythmic drive in the frequency range 15-30 Hz, as determined by the presence of coherence between the firing of unit potentials over this frequency band. This 15-30 Hz oscillatory influence is also abolished by stroke (Farmer et al. 1993b). In recent years other methods of investigation such as extracellular recordings in animals, electro-encephalogram (EEG) and magneto-encephalogram (MEG) have supported the hypothesis that higher frequency oscillatory drive to muscle involves circuits within the motor cortex (Sanes & Donoghue, 1993; Murthy & Fetz, 1996a,b; Baker et al. 1997; Salenius et al. 1997; Halliday et al. 1998; Donoghue et al. 1998; Brown et al. 1998). Therefore, the presence of high frequency coherence between two motor units within a given muscle may be an indicator of the cortical drive to that muscle.
Other oscillations of central origin at lower frequencies can also influence motor unit control, namely oscillations between 8 and 12 Hz which underlie the neurogenic component of physiological tremor (Elble & Randall, 1976) and a low frequency (< 4 Hz) common modulation of the instantaneous firing rate of motor units termed the ‘common drive’ (De Luca et al. 1982, 1993; De Luca & Mambrito, 1987; Kamen & De Luca, 1992). The neural circuits which generate these lower frequency drives remain unclear, although the poor correlation of measures of common drive and motor unit synchronisation (Semmler et al. 1997) and the preservation of low frequency coherence between motor units following stroke (Farmer et al. 1993b) suggest that they may not be mediated by the corticospinal tract.
Thus common presynaptic inputs to motor units may arise from different neural circuits and may ultimately fashion motor unit behaviour in different ways. We therefore compared the influence of common presynaptic inputs on motor unit pairs in muscles of the hand and back, assessing the degree of synchronisation and coherence between motor unit pairs and the correlation between their instantaneous frequencies.
METHODS
Ten healthy male subjects (aged 36.5 ± 6.1 years, mean ±s.d.) participated with informed, written consent according to the Declaration of Helsinki and the approval of the local ethics committee. Unilateral or bilateral activity in the first dorsal interosseous (IPSIFDI and BIFDI) and lumbar paraspinal (IPSIPSP and BIPSP) muscles were investigated. Data from all four conditions were collected from six subjects. The remaining four subjects had data collected and accepted from the IPSIPSP and IPSIFDI (3 subjects) or from BIPSP, IPSIPSP and IPSIFDI (1 subject).
When examining the FDI, the subjects’ right forearm was supported pronated on a table and the subject abducted the index finger against a firm stop. The PSP muscles were assessed whilst the subject sat astride a chair, the subjects partly supporting their weight with their arms on a table placed in front to minimise discomfort. Subjects were asked to produce a weak contraction of the relevant muscle. Auditory feedback of the firing of individual motor units was given, the subject was asked to keep the motor unit firing as regular as possible, although they were not asked to keep the units firing at any prespecified rate.
Individual motor units were recorded using two concentric fine needle electrodes. When recording from unilateral muscles the electrodes were placed 1-1.5 cm apart in the muscle belly. When recording from bilateral PSP the electrodes were at least 5 cm apart. The needles inserted into the paraspinals were medial to the lateral raphe at the level of L4, in the lumbar iliocostalis and lumbar longissimus. The signals were band pass filtered (53 Hz to 1 kHz) and amplified. Individual motor units were identified on an oscilloscope. Motor unit potentials that fell within an adjustable window were registered as transistor-transistor logic (TTL) pulses of 1 ms duration (Fig. 1A). To isolate units using window discrimination either a neural pulse sorter was used in conjunction with a spike processor (model D130, Digitimer Ltd) or two spike processors were used. The neural pulse sorter, which was built in-house, imposed a 2 ms delay on the recording of TTL pulses and this was corrected for during analysis.
Figure 1. Individual data from one subject and summary of methodology used in the present study.
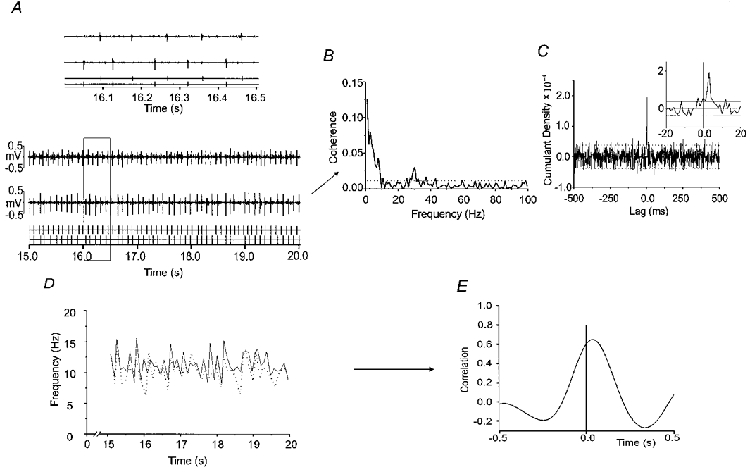
A, motor unit potentials recorded from 2 motor units within FDI and their associated TTL pulses. The area inside the box is shown expanded above. B and C, coherence and associated cumulant density estimates. The cumulant density peak is shown expanded as an inset in C. D and E, instantaneous frequency for the 2 motor units over the period indicated (D) and the cross-correlogram (E) between the Hanned waveforms in D.
In six subjects, surface electromyography (EMG) electrodes (Ag-AgCl) were attached onto the muscle belly each side of the needle electrode(s). Prior to the insertion of the needle electrodes three maximal voluntary contractions (MVC) were recorded. The surface electrodes remained in place during the experiment. Surface EMG was filtered (0.53-300 Hz) and digitally rectified off-line. For the period over which motor unit data were collected the ratio of the mean amplitude of rectified EMG activity to the mean amplitude during MVC was calculated. This gave an estimate of the percentage of MVC produced during the experiment.
All data were recorded on-line onto a personal computer using a 1401 laboratory interface (Cambridge Electronic Design, Cambridge, UK). Motor unit data were sampled at either 2 or 5 kHz and the TTL pulses and surface EMG recordings sampled at 1 kHz with a 12-bit resolution. Two types of analysis were then performed off-line (Fig. 1).
Spectral and coherence analysis
Motor unit data were only accepted for spectral analysis after the following checks. (1) Motor units were assessed by eye to determine that the TTL pulses coincided with motor units of constant shape. (2) Motor units had to fire for over 60 s without ceasing activity for longer than 2 s. (3) Interval histograms of 1 ms resolution and a maximum interval of 3 s were constructed from each TTL pulse channel. Data were accepted if less than 1 % of the total counts had an interval of less than 20 ms and less than 3 % had an interval of less than 50 ms. Further, only data in which less than 1 % of the counts were at intervals greater than 500 ms were accepted.
The times of occurrence of the motor unit spikes, registered by the leading edge of the TTL pulses, were assumed to be realisations of stochastic point processes. The data were analysed using a frequency domain method previously described (Rosenberg et al. 1989; Farmer et al. 1993a,b; Halliday et al. 1995). A Fourier transform up to a frequency of 100 Hz was performed on disjoint segments of data of equal length (1024 data points). The data from each of the segments were then averaged and the autospectra, cross-spectra, coherence and associated phase were calculated. The coherence between motor unit a and b is defined by:
where faa(λ) and fbb(λ) are the autospectra of the 2 point processes and fab(λ) is the cross-spectrum of the processes. Coherence is a normalised, unitless measure of linear association which ranges from 0 (independence) to 1 (complete linear independence). In the present study we use it to infer the frequency components present in common inputs to motoneurones during maintained voluntary contractions. The frequency resolution was 0.96 Hz for the coherence and phase data shown.
The coherence data were then pooled using a mean of the individual coherences weighted according to record length. Data contributing to the pooled coherence were analysed to determine whether the values of the individual coherence data differed significantly from each other at each frequency and if so whether the pooled coherence was a true representation of the contributing data. This test was done using the extended difference of coherence analysis introduced by Amjad et al. (1997). This technique allows an arbitrary number of coherence estimates to be compared and combined, and is based on the use of Fisher's transformed coherency estimates to test the hypothesis of equal coherence values at each frequency in the original records. The complex valued function representing the square root of the coherence is called the coherency; see Amjad et al. (1997) for details.
To obtain a measure of association in the time domain the cumulant density was estimated via the inverse Fourier transform of the cross-spectrum. Cumulant density functions have an interpretation similar to a cross-correlation histogram. They have the advantage that, as with the coherence spectrum, the 95 % confidence interval may be readily estimated (Halliday et al. 1995). The pooled cumulant density was also calculated using a weighted mean of the contributing data.
Common drive coefficient
We also used the technique described by De Luca and others to investigate common modulation of motor unit discharge at very low frequency (De Luca et al. 1982). This technique depends on the cross-correlation of heavily smoothed time varying instantaneous motor unit discharge frequencies (see Fig. 1D and E). For each data set, two 5 s epochs of data were chosen and the time varying instantaneous frequency calculated. The data were then smoothed with a 400 ms wide symmetric Hanning filter and high-pass filtered to remove DC bias. The two records were then cross-correlated for lags of ±0.5 s and the highest positive peak within ±50 ms of time zero was calcuated. This has previously been termed the common drive coefficient (ρ) (Semmler et al. 1997), a term we shall use throughout.
Differences in the percentage of maximal voluntary contraction and the mean interstimulus interval were assessed using a repeated measures general linear model. Statistical significance was taken as P < 0.05.
RESULTS
The number of motor unit pairs recorded in each condition, their mean interspike interval and the percentage of MVC are shown in Table 1. There was a significant difference in the mean interspike interval of motor units located in the paraspinals (either IPSIPSP or BIPSP) and those in the first dorsal interosseous (either BIFDI or IPSIFDI) (P < 0.001), with PSP units having longer interspike intervals. During motor unit recording the contraction produced was a significantly greater percentage of the maximum (MVC) in the PSP, compared with FDI. There were no detectable differences between the left and the right sides (Table 1).
Table 1. Motor unit parameters.
| Condition | Subject no. | No. of motor unit pairs | Total duration of data (min) | Mean interspike interval (s) (±s.d.) | Mean percentage MVC (±s.d.) |
|---|---|---|---|---|---|
| IPSIFDI | 10 | 25 | 61 | 0.12 ± 0.05 | 11.2 ± 9.4 |
| BIFDI | 6 | 23 | 47 | 0.12 ± 0.04 | — |
| IPSIPSP | 9 | 26 | 71 | 0.15 ± 0.04 | 36.6 ± 27.7 |
| BIPSP | 7 | 22 | 61 | 0.16 ± 0.04 | — |
The motor unit parameters for the 4 task conditions IPSIFDI (unilateral FDI), BIFDI (bilateral FDI), IPSIPSP (unilateral PSP) and BIPSP (bilateral PSP).
Coherence analysis
Unilateral FDI
The pooled coherence was significant below 10 Hz and between 12-40 Hz (Fig. 2A). The incidence of peaks of significant coherence within three frequency bands (< 4 Hz, 6-12 Hz and 12-40 Hz) and significant peaks in the cumulant density estimate is indicated in Table 2. Pooled phase and cumulant density estimates showed that the motor units were synchronised up to 40 Hz (Fig. 2B and C).
Figure 2. Coherence between motor units within IPSIFDI.
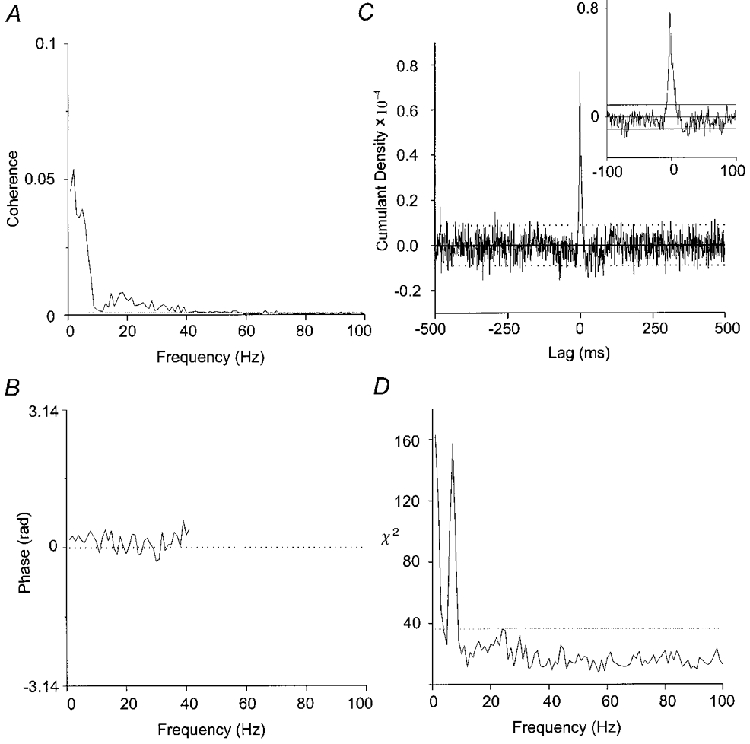
Pooled coherence (A), phase (B) and cumulant density (C) for 25 motor unit pairs from the right first dorsal interosseous (IPSIFDI). Pooled phase is only indicated over those periods in which the pooled coherence was significant. The results of the χ2 test are illustrated in D, which is significant at < 3.5 Hz and from 5-9 Hz. Horizontal dotted lines in this and subsequent figures indicate the 95 % confidence limits.
Table 2. Incidence of peaks in motor units (MU)in the coherence and cumulant density estimates above the 95% confidence limit and mean common drive coefficient.
| Condition | %MU pairs with coherence >4Hz | %MU pairs with coherence 6–12 Hz | %MU pairs with coherence 15–40 Hz | % significant peaks ± 5 ms in cumulant density | Common drive coefficient (ρ) (±s.e.m.) |
|---|---|---|---|---|---|
| IPSIFDI | 60 | 36 | 24 | 60 | 40 ± 0.05* |
| BIFDI | 0 | 0 | 0 | 0 | 0.09 ± 0.05 |
| IPSIPSP | 57 | 12 | 4 | 12 | 0.58 ± 0.04* |
| BIPSP | 14 | 0 | 0 | 14 | 0.25 ± 0.05* |
A peak was defined as being at least 3 consecutive data points (2.8 Hz or 3 ms wide for the coherence and cumulant density estimates, respectively).
Significantly different from the unrelated data (P < 0.001, one-way ANOVA). Coefficients for the unrelated data were calculated from the time-varying instantaneous firing frequency of 2 motor units taken from different subjects. A total of 50 pairs were assessed.
Above 10 Hz the magnitude of coherence in individual records was not statistically different as indicated by a non-significant χ2 test. However, below 10 Hz the χ2 test showed that the coherence of the lower frequency peaks varied between individual recordings (Fig. 2D). Assessment of the individual data revealed that all ten subjects demonstrated a peak below 4 Hz and nine out of ten showed a peak between 6-12 Hz in at least one unit pair. However, the size of the peaks in these frequency bands varied between subjects and from record to record in each subject.
Bilateral FDI
The pooled coherence between two motor units located in the FDI on opposite sides of the body showed no significant coherence at any frequency (Fig. 3A). In accord with this the motor units showed no consistent phase relationship (not shown) and no significant peak in the pooled cumulant density (Fig. 3B) or in any of the individual data (Table 2). The pooled data were representative of the sample as there were no significant differences between the individual coherence values at any frequency (Fig. 3C).
Figure 3. Coherence between motor units within BIFDI.

Pooled coherence (A), cumulant density (B) and χ2 test (C) for 23 motor unit pairs. One motor unit was situated in the right and the other in the left first dorsal interosseous (BIFDI). Pooled phase is not indicated as the pooled coherence showed no sections of significant coherence (see Fig. 2A).
Unilateral PSP
The pooled coherence between two motor units located in the paraspinal muscles on the same side was significant below 5 Hz (Fig. 4A). The cumulant density estimate (Fig. 4C) and phase relationship (Fig. 4B) suggested that the motor unit pairs were synchronised. As with the unilateral FDI data, the χ2 test indicated that there were significant differences in the incidence and size of coherence between different records at these frequencies (Fig. 4D).
Figure 4. Coherence between motor units within IPSIPSP.

Pooled coherence (A), phase (B), cumulant density (C) and χ2 test (D) for 26 motor unit pairs from the right paraspinal muscles (IPSIPSP).
Bilateral PSP
The pooled coherence indicated a small but significant peak at below 2 Hz (Fig. 5A). Only three subjects showed small significant peaks in the cumulant density (Table 2) and this was reflected in the pooled cumulant density which was not significant (Fig. 5C).
Figure 5. Coherence between motor units with BIPSP.
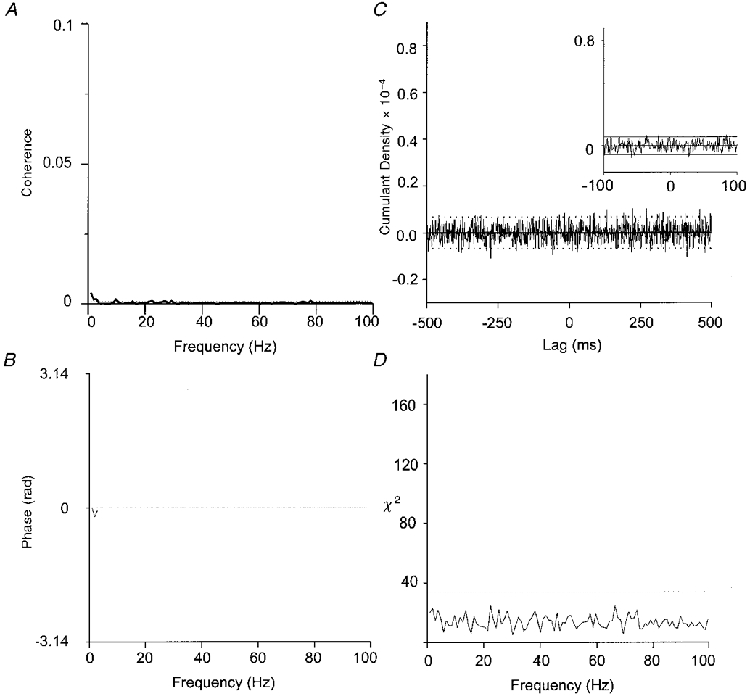
Pooled coherence (A), phase (B), cumulant density (C) and χ2 test (D) for 22 motor unit pairs. One motor unit was situated in the right and the other in the left paraspinals (BIPSP). Note the low coherence values at the lower frequencies compared with the IPSIPSP condition.
Common drive coefficient
Although the ipsilateral FDI and PSP and bilateral PSP conditions demonstrated coherence below 4 Hz, coherence was estimated over long periods. Some of this low frequency coherence might therefore represent small voluntary fluctuations in the level of tonic contraction. We tried to limit the effect of such variations by excluding those data in which more than 1 % of the TTL pulses had an interval greater than 500 ms, thus excluding regular gaps in the data that would have affected the frequency analysis. The similar variance in interspike interval, total number of motor unit pairs recorded and total length of recording would also indicate that the observed differences in coherence at low frequency were not greatly influenced by voluntary waxing and waning of the contraction force or fatigue. In addition, we calculated the common drive coefficient (ρ) from the instantaneous firing frequency of motor units over short (5 s) data sections, in which the firing rate of motor units remained stable and unaffected by voluntary modulation (see Methods and Fig. 1). Individual examples from each condition are illustrated in Fig 6 and Fig 7. The common drive coefficient varied greatly for a given motor unit pair depending on which epochs were chosen, which is to be expected for a statistical parameter estimated from only 5 s of data. The common drive coefficients for each condition were compared with those obtained by correlating sets of unrelated data. The mean maximum coefficients are given in Table 2 and the mean correlations are plotted in Fig. 8. A one-way ANOVA revealed a significant difference between group effect (P < 0.001). Post hoc tests (Scheffé‘s test) indicated that there was no significant difference between the BIFDI and data obtained from unrelated data sets. Differences were significant between all the remaining conditions and unrelated data (P < 0.001).
Figure 6. Instantaneous firing frequency and common drive coefficient for motor units situated within FDI.

Individual motor unit potentials and associated TTL pulses for 3 motor units (A) and their associated instantaneous firing frequency (B). Two motor units within the right FDI (R1 and R2) were recorded simultaneously with a motor unit within the left FDI (L1). C-E, cross-correlations between the smoothed instantaneous firing frequencies between the motor units indicated. Note the lack of correlation between motor units recorded within the left and right FDI indicating a lack of common drive to the left and right intrinsic hand muscles.
Figure 7. Instantaneous firing frequency and common drive coefficient for motor units situated within PSP.
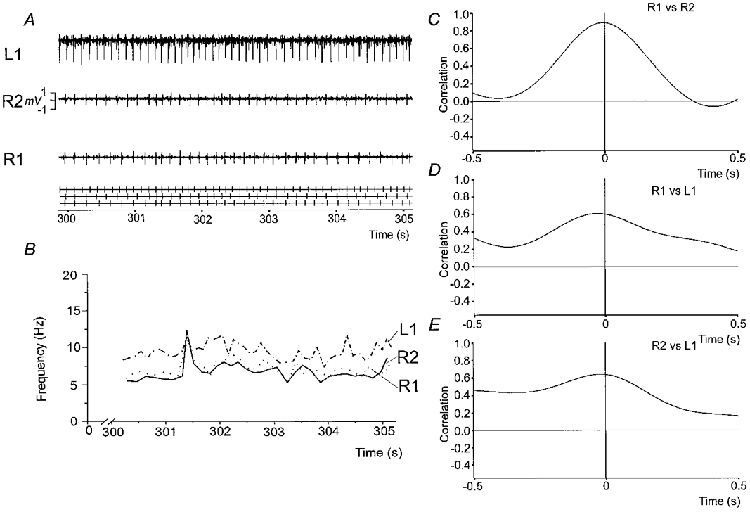
Individual motor unit potentials and associated TTL pulses for 3 motor units (A) and their associated instantaneous firing frequency (B). Two motor units within the right PSP (R1 and R2) were recorded simultaneously with a motor unit within the left PSP (L1). C-E, cross-correlations between the smoothed instantaneous firing frequencies between the motor units indicated. Note that there is a correlation between the two motor units in the right PSP and a smaller correlation between right and left motor units.
Figure 8. Summary of the average cross-correlograms obtained in each condition.
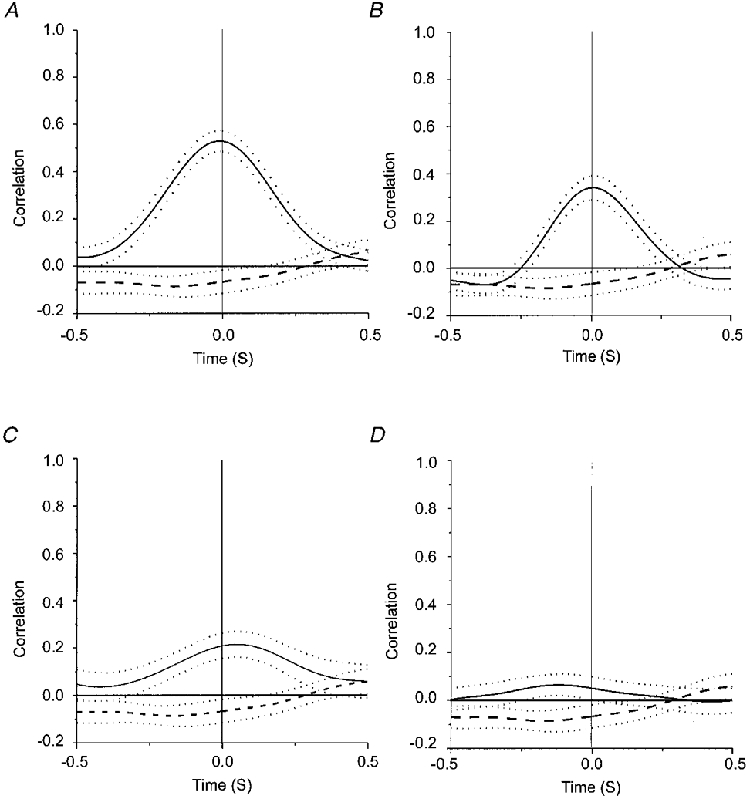
Cross-correlograms averaged from all records for IPSIPSP (A), BIPSP (B), IPSIFDI (C) and BIFDI (D). Dotted lines indicate the standard error of the mean. A dashed line indicates the average cross-correlogram calculated between 50 unrelated data pairs.
DISCUSSION
The present study investigated common presynaptic drives to separate motoneurones, assessing linear interactions only. We have shown that three of the rhythmic presynaptic drives so far identified in man may be present in the same task in a muscle of the distal limb and are associated with synchronisation between motor units. In contrast, in a similar isometric contraction task, only those drives of lower frequency are seen in the discharge of the lumbar paraspinal muscles, and synchronisation between motor units in the back is much weaker than in the hand. The estimated coherence values are around 0.025-0.05 in magnitude and never exceeded 0.3. Due to the normative nature of coherence this indicates that around 2.5-5 % of the variability of spike timing in one motor unit discharge can be predicted by the spike times in the second motor unit discharge; we attribute this to the effects of common inputs.
Comparison between IPSIFDI and IPSIPSP - high frequency oscillations
A high frequency band of coherence between two motor units from 12-40 Hz was present in the unilateral FDI. High frequency coherence was seen only once between motor units within the paraspinals despite their producing a higher percentage of maximal contraction. Strong contractions may favour the detection of high frequency oscillations (Merton, 1981). The differences in the high frequency drives are, in turn, reflected by differences in the degree of motor unit synchronisation which has previously been shown to be significantly correlated with the presence of coherence in the 15-30 Hz band. The degree of synchronisation seen was not felt to be significantly influenced by the differences in the firing rate seen between the motor units in FDI and PSP. Bremner et al. (1991a, b) found no relationship for motor units within FDI between the degree of synchronisation and the firing rate in the range 6-15 Hz which includes the range seen in the present study.
Previously, Farmer et al. (1993a) found a lower incidence of significant coherence in the 16-32 Hz frequency band between two motor units in biceps brachii compared with FDI, both muscles contracting at 10 % of their maximum. These, and the present results, suggest that the more proximally located the muscle, the lower the chance of finding any significant coherence at high frequencies during isometric contractions.
The present findings are in agreement with the known anatomy and physiology of the neural control of distal and axial muscles. Although evidence of direct corticomotoneuronal projections to proximal and axial muscles exists in man (Plassman & Gandevia, 1989), they are felt to be more numerous to the intrinsic hand muscles (Ferbert et al. 1992; Palmer & Ashby, 1992). These connections may be important in the production of fine fractionated finger movements (Lemon, 1993). Much of the supply to the axial muscles from the cortex is indirect via relays in the brain stem (Kuypers, 1981). Our results suggest that coherence at higher frequencies reflect the relative activation of direct corticomotoneuronal input to the motoneurones in a given muscle.
Nevertheless, it is possible that tasks different to those used in the present study may show high frequency coherence between paraspinal muscles. Task-specific changes in synchronisation have been observed within both distal (Bremner et al. 1991a; Huesler et al. 1998) and axial muscles (Adams et al. 1989; Gibbs et al. 1995). Gibbs et al. (1995), for example, showed that synchronisation between the erector spinae muscles was more common when standing and balancing than when activating the muscles voluntarily in lying. The pattern of coherence between motor units or muscles in tasks such as these remains to be established.
Comparison between FDI and PSP - low frequency oscillations
There may be two drives to motor units of low frequency. The first at around 10 Hz was more evident in the hand than the back. This drive may contribute to physiological tremor (Conway et al. 1995), and both coherence at low frequency (Amjad et al. 1997) and physiological tremor (Wade et al. 1982) show a large intersubject variability. The second drive had a frequency of under 4 Hz and may be due to a central ‘common drive’ which co-modulates the firing rates of motor units (De Luca & Erim, 1994). Only the latter common drive was manifest in the bilateral PSP condition. Voluntary activation and relaxation of muscle activity or variations in activity due to fatigue (Krogh-Lund & Jorgensen, 1992) could have contributed to low frequency coherence, but a similar pattern was also seen after cross-correlation of short epochs during which the firing rate of motor units remained more or less stable.
Comparison of bilateral control of the hand and paraspinal muscles
The lack of any significant coherence between the firing times of motor units or correlation between their instantaneous firing frequency in bilateral FDI is in agreement with previous findings examining the presence or absence of peaks in cross-correlograms constructed from two motor unit trains (Farmer et al. 1990) or multiunit data (Carr et al. 1994). The neural control of the hand is predominantly from the contralateral hemisphere. In the monkey only an estimated 5.9 and 1.6 % of corticospinal fibres project ipsilaterally from area 4 and 6, respectively (Toyoshima & Sakai, 1982). In contrast, axial muscles receive a substantial ipsilateral as well as contralateral supply from several levels of the neuraxis. Indeed, those corticospinal fibres that project ipsilaterally in the monkey tend to arise from the areas of cortex representing trunk and axial muscles (Kuypers & Brinkman, 1970). In humans, an early bilateral response can be seen in the erector spinae following transcranial stimulation of the motor cortex (Ferbert et al. 1992). Similarly, descending brain stem projections to the motoneurones of the axial musculature, such as those from the nucleus reticularis gigantocellularis, course the spinal cord bilaterally or send collaterals to both sides of the cord (Kuypers, 1981; Mori et al. 1995). The correlation between instantaneous firing frequencies in the bilateral PSP condition provides support for a bilateral innervation of axial musculature in humans.
Functional significance of common oscillatory drives
It has been suggested that oscillations within the band 15-30 Hz may participate in binding the activities of separate, segregated sensorimotor areas through facilitation of neuronal synchronisation (Farmer, 1998). Synchronisation may lead to more effective summation at later stages of processing, similar to that suggested for perceptual binding within visual areas (Gray, 1994). This may underlie the task-dependant synchronisation seen within a given hand muscle and between separate hand muscles within the same limb during a functional task (Bremner et al. 1991a, b). However, the present finding of a lack of common drive at any frequency to the hand muscles during a bimanual task suggests that oscillations may not be involved in the binding of activity between the left and right hemispheres. This is supported by the demonstration of a lack of increased interhemispheric synchronisation between neurones with bimanual tasks as opposed to unimanual tasks in the monkey (Murthy & Fetz, 1996a,b).
The lower frequency common drive acts on a large number of motor units within and between muscles (De Luca et al. 1982, 1993; De Luca & Mambrito, 1987). The drive has been proposed to negate any need for the central nervous system to control motor unit recruitment directly (De Luca & Erim, 1994). The lack of common drive between homologous hand muscles may allow independent control of the two hands. In contrast, axial muscles are influenced by a common drive allowing the axial skeleton to act as a functional whole during postural control.
Acknowledgments
We thank Mr P.Asselman for his technical support.
References
- Adams L, Datta AK, Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. The Journal of Physiology. 1989;413:213–231. doi: 10.1113/jphysiol.1989.sp017650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. Journal of Neuroscience Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependant modulation. The Journal of Physiology. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Effect of task on the degree of synchronisation of intrinsic hand muscle motor units in man. Journal of Neurophysiology. 1991a;66:2072–2083. doi: 10.1152/jn.1991.66.6.2072. [DOI] [PubMed] [Google Scholar]
- Bremner FD, Baker JR, Stephens JA. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. The Journal of Physiology. 1991b;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Salenius S, Rothwell JC, Hari R. The cortical correlate of the Piper rhythm in man. Journal of Neurophysiology. 1998;80:2911–2917. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. The Journal of Physiology. 1994;475:217–227. doi: 10.1113/jphysiol.1994.sp020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Farmer SF, Halliday DM, Rosenberg JR. On the relation between motor-unit discharge and physiological tremor. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York: Plenium Press; 1995. pp. 596–598. [Google Scholar]
- Datta AK, Farmer SF, Stephens JA. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. The Journal of Physiology. 1991;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AK, Stephens JA. Short-term synchronization of motor unit activity during voluntary contraction in man. The Journal of Physiology. 1990;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Friedland CL, Short DJ. Motor unit discharge characteristics and short term synchrony in paraplegic humans. Journal of Neurology, Neurosurgery and Psychiatry. 1990;53:764–769. doi: 10.1136/jnnp.53.9.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends in Neurosciences. 1994;17:299–304. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. The Journal of Physiology. 1982;329:129–142. doi: 10.1113/jphysiol.1982.sp014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Mambrito B. Voluntary control of motor units in human antagonist muscles: Coactivation and reciprocal activation. Journal of Neurophysiology. 1987;58:525–542. doi: 10.1152/jn.1987.58.3.525. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Roy AM, Erim Z. Synchronisation of motor-unit firings in several human muscles. Journal of Neurophysiology. 1993;70:2010–2023. doi: 10.1152/jn.1993.70.5.2010. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. Journal of Neurophysiology. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Randall JE. Motor-unit activity responsible for 8- to 12-Hz component of human physiological finger tremor. Journal of Neurophysiology. 1976;39:370–383. doi: 10.1152/jn.1976.39.2.370. [DOI] [PubMed] [Google Scholar]
- Farmer SF. Rhythmicity, synchronization and binding in human and primate motor systems. The Journal of Physiology. 1998;509:3–14. doi: 10.1111/j.1469-7793.1998.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary contraction in man. The Journal of Physiology. 1993a;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. The Journal of Physiology. 1990;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous system lesions in man. The Journal of Physiology. 1993b;463:83–105. doi: 10.1113/jphysiol.1993.sp019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Caramia D, Priori A, Bertolasi L, Rothwell JC. Cortical projection to erector spinae muscles in man as assessed by focal transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 1992;85:382–387. doi: 10.1016/0168-5597(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Organisation of inputs to motoneurone pools in man. The Journal of Physiology. 1995;485:245–256. doi: 10.1113/jphysiol.1995.sp020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM. Synchronous oscillations in neuronal systems: Mechanisms and Functions. Journal of Computational Neuroscience. 1994;1:11–38. doi: 10.1007/BF00962716. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neuroscience Letters. 1998;241:1–4. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Progress in Biophysics and Molecular Biology. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Huesler EJ, Hepp RM, Dietz V. Task dependence of muscle synchronization in human hand muscles. NeuroReport. 1998;9:2167–2170. doi: 10.1097/00001756-199807130-00003. [DOI] [PubMed] [Google Scholar]
- Kamen G, De Luca CJ. Firing rate interactions among human Orbicularis Oris motor units. International Journal of Neuroscience. 1992;64:167–175. doi: 10.3109/00207459209000542. [DOI] [PubMed] [Google Scholar]
- Krogh-Lund C, Jorgensen K. Modification of myo-electric power spectrum in fatigue from 15 % maximal voluntary contraction of human elbow flexor muscles, to limit of endurance: Reflection of conduction velocity variation and/or centrally mediated mechanisms? European Journal of Applied Physiology and Occupational Physiology. 1992;64:359–370. doi: 10.1007/BF00636225. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 597–666. part 1. [Google Scholar]
- Kuypers HGJM, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in the rhesus monkey. Brain Research. 1970;24:29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Cortical control of the primate hand. The 1992 G. L. Brown Prize Lecture. Experimental Physiology. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Merton PA. Neurophysiology on man. Journal of Neurology, Neurosurgery and Psychiatry. 1981;44:861–870. doi: 10.1136/jnnp.44.10.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Iwakiri H, Homma Y, Yokoyama T, Matsuyama K. Neuroanatomical and neurophysiological bases of postural control. In: Fahn S, Hallett M, Luders HO, Marsden CD, editors. Negative Motor Phenomena. Philadelphia, USA: Lippincott-Raven; 1995. pp. 289–303. [PubMed] [Google Scholar]
- Murthy V, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronisation of local field potentials and relation to behaviour. Journal of Neurophysiology. 1996a;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Murthy V, Fetz EE. Synchronisation of neurones during local field potential oscillations in sensorimotor cortex of awake monkeys. Journal of Neurophysiology. 1996b;76:3968–3982. doi: 10.1152/jn.1996.76.6.3968. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. The Journal of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Gandevia SC. Comparison of human motor cortical projections to abdominal muscles and intrinsic hand muscles of the hand. Experimental Brain Research. 1989;78:301–308. doi: 10.1007/BF00228901. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Progress in Biophysics and Molecular Biology. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Experimental Physiology. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. Journal of Neurophysiology. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proceedings of the National Academy of Sciences of the USA. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. The Journal of Physiology. 1976;263:357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JD, Nordstrom MA, Wallace CJ. Relationship between motor unit short-term synchronisation and common drive in human first dorsal interosseous muscle. Brain Research. 1997;767:314–320. doi: 10.1016/s0006-8993(97)00621-5. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neuroscience Letters. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Sakai H. Exact cortical extent of the origin of the corticospinal tract (CST) and the quantitative contribution to the CST in different cytoarchitectonic areas. A study with horseradish peroxidase in the monkey. Journal für hirnforschung. 1982;23:257–269. [PubMed] [Google Scholar]
- Wade P, Gresty MA, Findley LJ. A normative study of postural tremor of the hand. Archives of Neurology. 1982;39:358–362. doi: 10.1001/archneur.1982.00510180036009. [DOI] [PubMed] [Google Scholar]


