Abstract
The effects of the naturally occurring neurosteroid tetrahydrodeoxycorticosterone (THDOC) on GABAA receptor-mediated miniature, spontaneous and evoked IPSCs was tested using patch-clamp techniques in slices of hippocampus and cerebellum from rats at two developmental stages (≈10 and ≈20 days postnatal). The cells studied were hippocampal granule cells and cerebellar Purkinje and granule cells.
Most miniature GABAergic currents (mIPSCs) decayed with two exponentials and neurosteroids caused a ≈4-fold increase in the decay time constant of the second exponential at the highest concentration used (2 μm). Similar effects were seen at high concentrations of THDOC (1-2 μm) in all cell groups tested. No effects were seen on amplitude or rise time of mIPSCs.
The effects of THDOC (1 μm) were shown to be stereoselective and rapidly reversible, indicating that the neurosteroid binds to the GABAA receptor, rather than acting genomically.
At concentrations of THDOC likely to occur physiologically (50–100 nm), the decay time of IPSCs was also enhanced (25–50 %) in all cerebellar cell groups tested. In contrast, at 100 nm THDOC, seven of 11 hippocampal granule cells were sensitive from the 10 day group but the 20 day hippocampal granule cells showed no significant enhancement in the presence of these lower concentrations of THDOC.
The differences in sensitivity of hippocampal and cerebellar cells to THDOC are compared to data reported in the literature on regional development of expression of different receptor subunits in the brain and it is suggested that the progressive relative insensitivity of the 20 day hippocampal cells may depend on increasing expression of the δ subunit of the GABAA receptor and possibly an increase in the α4 subunit.
The GABAA receptor is found all over the brain and mediates most of the fast inhibitory neurotransmission. A notable feature of the receptor is that it can be modulated by a wide range of compounds. Various anxiolytic and anaesthetic agents including benzodiazepines, barbiturates and anaesthetic steroids work by binding to different sites on this receptor (for review see Hevers & Lüddens, 1998; Mehta & Ticku, 1999).
Over the last decade considerable evidence has emerged that various progesterone metabolites, which are active and in some cases can be synthesised in the brain (Akwa et al. 1991), act directly on the GABAA receptor (Majewska et al. 1986; Turner et al. 1989; for review see Baulieu, 1997) in a stereospecific manner (Harrison & Simmonds, 1984). The most potent neurosteroids reported to date are 5α-pregnane-3α-ol-20-one (tetrahydroprogesterone, THP) and 5α-pregnane-3α,21-diol-20-one (tetrahydrodeoxycorticosterone, THDOC). The enhancing effects of such neurosteroids on GABAergic currents have recently led to the development of related compounds with the aim of developing improved anticonvulsants for clinical use as an alternative therapy to benzodiazepines (Carter et al. 1997; Rupprecht & Holsboer, 1999).
In this study we observe the effect of bath applied tetrahydrodeoxycorticosterone (THDOC) on GABA released synaptically onto GABAA receptors. It is thus not relevant to this study whether the source of THDOC, in studies to which we refer, is from the breakdown of peripherally produced steroids or from synthesis in the brain. To avoid complication, we will thus refer to steroids which have stereoselective modulatory actions on GABAA receptors as neurosteroids throughout this study, irrespective of their putative source in different reported studies.
As well as their clinical relevance, the effects of neurosteroids on GABAA receptors are likely to have important physiological significance. For example, levels of steroid hormones rise in relation to acute stress, (e.g. Barbaccia et al. 1996) and, conversely, fluctuation of such hormones, due to other causes such as the menstrual cycle (Bixo et al. 1997; Bicikova et al. 1998), can cause fluctuation in mood and changes in stress-like tension (Dennerstein et al. 1985; Smith et al. 1998). Moreover injection of THDOC has been shown to increase exploratory behaviour in mice between a dark and light chamber and to inhibit the effects of application of mild electric shocks in rats (Majewska, 1990).
Other examples of modulators of the GABAA receptors which occur physiologically are various cations, in particular H+ ions (Pasternack et al. 1996) and Zn2+ (e.g. Westbrook & Mayer, 1987), both of which certainly vary under normal or pathological conditions and are dependent in their effects on the specific subunit combination of the receptor. Neurosteroids are, however, probably the first physiologically occurring substances to be considered as potential therapeutic agents in this context.
While it seems clear that fluctuations in neurosteroids in the brain result in changes in stress-related behaviours, the mechanism is far from clear. Various steroid hormones have been shown to have genomic effects under chronic conditions but others exhibit non-genomic effects, such as the direct effect on GABAA receptors and these are probably particularly important under acute conditions of hormonal imbalance. Under conditions of acute stress, various neurosteroids have been detected in rat brain up to about 20 nm (e.g. Purdy et al. 1991), though the highest levels measured were not in stress but rather during the 3rd trimester of pregnancy (100 nm THP; Paul & Purdy, 1992). The types of stress which can reasonably be imposed under experimental conditions are, however, relatively mild and it is likely that much higher levels of neurosteroids could occur in situations which cause extreme pain or other stress related conditions.
Various studies have investigated the actions of neurosteroids on receptors of the central nervous system and it has been repeatedly shown with both biochemical and electrophysiological assays that, at micromolar concentrations, neurosteroids greatly increase chloride flux through the GABAA receptor-channel (Majewska et al. 1986; Harrison et al. 1987). On the single channel level this effect has been shown to be due to an increase in the receptor mean open time rather than channel conductance (Twyman & Macdonald, 1992) as a result of binding to a steroid-specific site on the GABAA receptor (Gee et al. 1988; Lan et al. 1990). This effect, which is rapid and fully reversible, has been suggested to be due to a change in the kinetics of a desensitised state (Zhu & Vicini, 1997). Twyman & Macdonald (1992) also showed an increase in open frequency of single channels during steady state application of GABA but this is probably not relevant to the brief pulse of GABA seen by postsynaptic receptors which are largely saturated at the peak of a synaptic current (Edwards et al. 1990).
Very few studies have observed the effects of levels of neurosteroids at concentrations likely to occur physiologically. However, Belelli et al. (1996) have demonstrated nanomolar sensitivity to a range of neurosteroids of recombinant human GABAA receptors expressed in Xenopus oocytes. At even lower concentrations Dayanithi & Tapia-Arancibia (1996) have reported increases in [Ca2+]i in response to picomolar concentrations of allopregnanolone in primary cultures of fetal rat hypothalamic neurones. They suggest this is due to enhancement of the effects of background GABA in the culture.
Few studies have reported the effects of neurosteroids on synaptic signals. One report in toad spinal cord (Reith & Sillar, 1997) demonstrated that micromolar concentrations of the steroid 5β-pregnan-3α-ol-20-one increased both frequency and decay time of GABAergic potentials without affecting glycinergic signals. The observed effect on decay time was in agreement with an earlier study by Harrison et al. (1987), who showed in rat that the synthetic steroid alphaxalone increased the decay time of GABAergic synaptic currents in hippocampal cultures with no effect on rise time or amplitude. A more recent study showed an enhancement of a long-lasting depolarising component of the IPSP in adult rat hippocampus with very high concentrations of THDOC (10-20 μm, Burg et al. 1998). Another functional study (Brussaard et al. 1997, 1999) showed that in the hypothalamus the neurosteroid allopregnanolone decreased the firing rate of female rat magnocellular neurones, which would be expected to result in a decrease in oxytocin release. At micromolar concentrations it also increased the decay time constant of spontaneous IPSCs, during pregnancy, but not after parturition. This change was attributed to a subunit switch in the GABAA receptor. In contrast to these findings, Poisbeau et al. 1997 observed an increase in both amplitude and frequency of spontaneous GABAergic synaptic currents in cocultures of hypothalamic and intermediate lobe pituitary neurones. This latter effect was observed at the physiological relevant concentrations of 10 nm but no change in kinetics of the currents was observed (Poisbeau et al. 1997). The authors concluded this to be a presynaptic effect of the neurosteroid.
Thus although the previous studies clearly show a non-genomic effect of neurosteroids and related compounds via the GABAA receptor, which is likely to be relevant to the anaesthetic effects of such compounds as alphaxalone, it is not at all clear whether endogenous neurosteroids have such effects under physiological conditions. The only study of synaptic currents which uses a physiologically relevant concentration of a neurosteroid was performed on cultured cells at a single concentration. Moreover in the light of recent evidence that different GABAA receptors congregate under synaptic specialisations from those which are found extrasynaptically (Nusser et al. 1998), it seems particularly important to study the synaptic signal in situ in brain slices.
In the present study we address this question by observing the effects of the neurosteroid THDOC on GABAergic synaptic transmission, both at concentrations likely to occur physiologically (50 and 100 nm) and at the higher concentrations used in previous studies (1 and 2 μm). Further, in the light of many studies showing the wide range of GABA receptors which can be expressed in the brain at different stages of development and in different cell types, dependent on the different subunits expressed (e.g. Wisden et al. 1992), we have selected cell types which show different subunit distributions between the cell types and, in the case of hippocampal granule cells, different subtypes over development. We thus investigate whether our observations are general or specific to particular cell types by observing the effect of this neurosteroid in three cell types and at two stages of postnatal development.
METHODS
Electrophysiology
Male Wistar rats of 9-13 days postnatal (P9-P13, denoted as the 10 day group) and P17-P21 (denoted as the 20 day group) were used for all experiments. Methods for making whole-cell patch-clamp recordings in brain slices have been described in detail previously (Edwards et al. 1989). Briefly, animals were instantly killed by decapitation and the brain rapidly removed and immersed in ice-cold solution. Slices (300 μm thick) were cut with a vibratome (Camden Instruments) and placed in an incubating chamber at 33-35°C for at least 30 min before recording at room temperature (21-25°C). Individual neurones were visually identified using Nomarski optics on a modified Olympus BHS light microscope with a × 40 water immersion objective lens. Before approaching the cell with the patch electrode, tissue debris was removed by suction through a cleaning electrode. Extracellular solution contained (mm): NaCl 125, KCl 2.5, NaH2PO4 1, NaHCO3 25, MgCl2 1, CaCl2 2, glucose 25, and was bubbled with 5 % CO2-95 % O2 (300-320 mosmol kg−1, pH 7.4). Internal solution contained (mm): CsCl 140, Hepes 10, EGTA 10, CaCl2 2, MgATP 1 (diluted to 270-280 mosmol kg−1, pH 7.3). All chemicals including steroids were purchased from Sigma Chemicals, except for 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) which was purchased from Tocris Cookson. Electrodes were made of borosilicate glass (o.d. 1.5 mm, i.d. 0.8 mm, Kwik-fil) and fire-polished to a resistance of 4-7 MΩ. The currents were low pass filtered at 10 kHz (Axoclamp 1-D, Axon Instruments), recorded onto videotape, sampled at 10 kHz and further filtered at 2 kHz (low pass Bessel filter, Frequency Devices) onto a personal computer for analysis with WCP software, kindly supplied by John Dempster (University of Strathclyde). In all traces downward deflections denote inward currents; all error bars denote standard error of the mean (s.e.m.) and are presented with the mean unless otherwise stated.
THDOC was superfused for around 10 min with a flow rate of 0.5-1.5 ml min−1 through a gravity-feed polyethylene tubing system (bath volume ∼1 ml). At least 20 min were required for washout of THDOC. Silanized glass holding containers were used for steroid solutions and the actual concentration of THDOC in the bath was verified with radioimmunoassay (see below). Steroids were dissolved in 100 % ethanol, and the stock solution was frozen (-4°C) and then thawed and diluted in fresh Krebs solution on the day of use, resulting in a maximum ethanol concentration of 0.04 % (i.e. 6.8 mm ethanol). Effects of ethanol on synaptic GABAergic currents reported in the literature have been shown at minimum concentrations of 10-100 mm (generally greater than 50 mm) (Reynolds et al. 1992). Concentrations of 0.1 and 0.01 % ethanol, corresponding to 17 and 1.7 mm, respectively, were tested on cells from both age groups and both cell types and found to have no significant effects on the amplitude, rise time or decay of currents (P > 0.05, one-way ANOVA).
All IPSCs were recorded in the presence of the glutamate antagonist CNQX (10 μm). IPSCs were reversibly blocked by the GABAA antagonist bicuculline (10 μm), indicating that they were mediated by GABAA receptor channels (n = 12 cells). Miniature IPSCs (mIPSCs) were recorded in the presence of the Na+ channel blocker tetrodotoxin (TTX, 1 μm) and compared between cell types. To assess presynaptic effects of THDOC, monosynaptically evoked IPSCs (eIPSCs) were also recorded in hippocampal granule (HG) cells by extracellular stimulation of nearby cells in the granular layer, presumably inducing antidromic firing of local inhibitory interneurones, such as basket cells. Miniature IPSCs were collected for analysis if they crossed a threshold of ∼4 pA from baseline and subsequently individually checked and discarded if the decay phase was contaminated by a subsequent current. They were then selected for 10-90 % rise times of less than 2 ms. IPSCs (n ≥ 30) were digitally averaged through alignment of the mid-point of the rising phase and the averaged IPSC decay fitted with either one or two exponentials (least squares). Goodness of fit was determined by eye and where appropriate verified with the F value statistic which calculated residual variance over background variance. In the majority of cells, mIPSCs showed a double exponential decay similar to results from previous studies (Edwards et al. 1990; Puia et al. 1994) and consistent with stochastic modelling of the GABAergic IPSC decay (Jones & Westbrook, 1996; McClellan & Twyman, 1999). Single exponential decays may be a result of cable filtering in the cases when the rise times were relatively slow (Johnston & Brown, 1983). However, exceptions to this suggested that there may be other factors influencing the decay such as differences in underlying kinetics of GABAA receptors (De Koninck & Mody, 1994; Puia et al. 1994). Single exponential decay time constants were slightly faster than the decay time constants for the second exponential, where two exponentials could be well distinguished, (e.g. HG20 cells: single exponential τ= 30.3 ± 2.4 ms (mean ±s.e.m.), n = 9 cells) vs. two exponential decays τ1 = 11.9 ± 1.3, τ2 = 43.5 ± 4.3 ms (mean ±s.e.m.), n = 13 cells). The relative changes induced by high concentrations of THDOC were not, however, significantly different between IPSCs of single exponential decays and the second exponential of double exponential decays within a cell group (P > 0.05, two-way ANOVA) and thus these relative changes were combined in the results.
Radioimmunoassay
To quantify concentrations of THDOC used in bath perfusion, a 50 nm solution was perfused from silanized glass holding containers through polyethylene tubing and collected for analysis with radioimmunoassay (RIA). The concentration in the top reservoir was compared to the bath concentration after the steroid solution had run through the perfusion tubing (Bicikova et al. 1995). Briefly, the THP antibody (dilution factor 1/2000) was incubated with 2 nm[3H]THP (NEN-Dupont) in a phosphate buffer with 0.1 % gelatin and 0.01 % azide (pH 7.3). Extraction of free radiolabel was achieved with a 0.05 % concentration of dextran-coated charcoal (Norit, activated charcoal, Sigma). Both silanized glassware and siliconized Eppendorf tubes were used to reduce loss of steroid. This assay was suitable for THDOC, as the antibody showed a high degree of cross-reactivity between THP and THDOC (results not shown).
There was no significant loss of steroid compared to the calculated concentration in the top reservoir or in the recording chamber.
Statistics
Unless otherwise stated all comparisons used one- or two-way ANOVA, using Tukey's method for post hoc tests where appropriate (Graph Pad Prism, Graphpad Software Inc., version 2 October 1995).
RESULTS
Whole-cell patch-clamp recordings were made at room temperature (21-25°C) with a nearly symmetrical chloride concentration (external, 130.5 mm, internal, 142 mm), resulting in inward chloride currents at a holding potential of -74 mV (corrected for a liquid junction potential of 4 mV; Fig. 1A). Hippocampal granule cells and cerebellar Purkinje cells from rats of two age groups, 10 day and 20 day groups, are denoted as HG10, HG20, CP10 and CP20, respectively. For comparison, as outlined below, some experiments were also made from cerebellar granule cells from animals in the 10 day group (CG10). The aim of these experiments was to investigate the sensitivity of synaptic GABAergic currents to THDOC in two different brain regions and compare these at different stages of development.
Figure 1. Comparison of GABAergic IPSCs of different cell types and age groups.
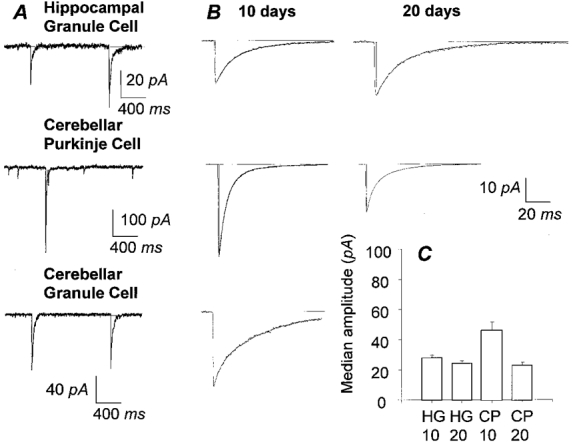
A (from top to bottom), raw data traces showing miniature IPSCs of a hippocampal granule cell and a cerebellar Purkinje cell in the presence of TTX (1 μm) and spontaneous IPSCs of a cerebellar granule cell from 10 day rats at a holding potential of -74 mV with nearly symmetrical chloride concentrations. Records are filtered at 2 kHz (8 pole low pass Bessel) and digitised at 10 kHz. B, comparison of decay kinetics of averaged IPSCs of 10 day group and 20 day group (left and right panels, respectively) amongst cell types (as labelled in A). Decay kinetics of mIPSC of HG10 cell (τ1 = 12.7 ms; τ2 = 45.2 ms; rise time = 0.8 ms) are slower than in a CP10 cell of the same rat (left middle panel, τ1 = 6.3 ms; τ2 = 25.3 ms; rise time = 0.8 ms). Spontaneous IPSCs of CG10 cell (τ1 = 5.4 ms; τ2 = 43.8 ms; rise time = 0.4 ms) have similar decay kinetics to HG10 cells. Decay kinetics of mIPSCs are similar between age groups of the same cell type: HG10 cell (kinetics data above) vs. HG20 cell (τ1 = 13.8 ms; τ2 = 43.1 ms; rise time = 0.9 ms) and CP10 cell (kinetics data above) vs. CP20 cell, (τ1 = 4.9 ms; τ2 = 21.1 ms; rise time = 0.6 ms). Statistical differences in the decay kinetics occur between HG and CP cells and CG and CP cells but not between age groups of the same cell types. Scale bar refers to all panels; n > 50 for all panels. C, comparison of median amplitude of HG10, HG20, CP10 and CP20 cells (n = 11, 18, 17 and 8 cells, respectively) shows a decrease in amplitude of mIPSCs in CP cells with postnatal development and a similar but much smaller trend is seen in HG cells.
Comparison of mIPSC characteristics between cell type and age group (Figs 1 and 2)
Figure 2. Effect of the neurosteroid THDOC on amplitude, rise time and τ1 of mIPSCs.
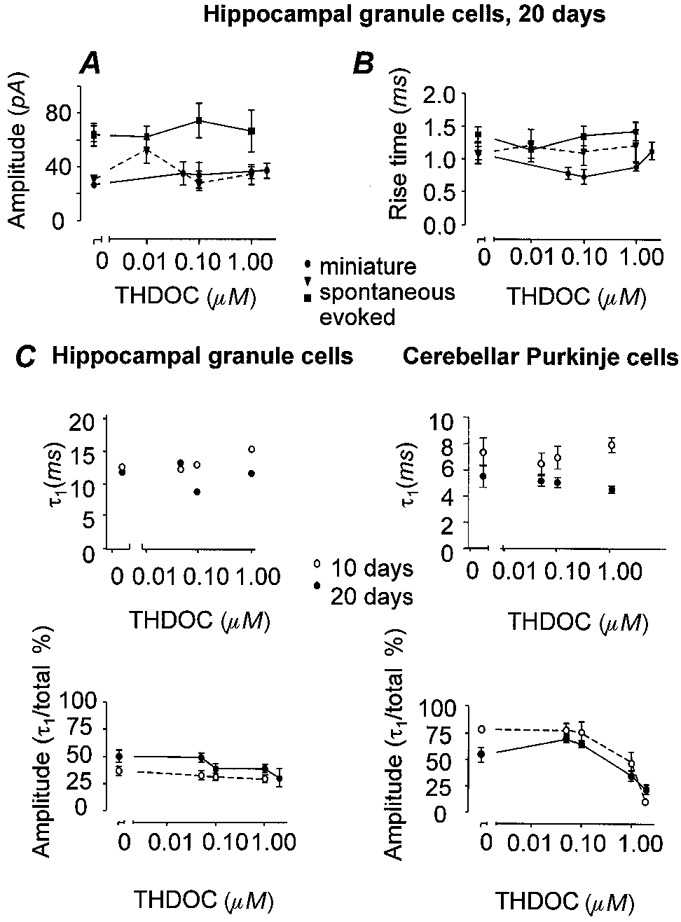
There was no effect of THDOC at concentrations from 0.05 to 2.0 μm on amplitude (A) or rise time (B) of IPSCs as shown in HG20 cells. •, miniature IPSCs (n = 5); ▾, spontaneous IPSCs (n = 3); ▪, evoked IPSCs (n = 4). C, at increasing concentrations of THDOC, the first exponential of the decay of the synaptic currents (τ1) was also unchanged in both its time course and in the proportion of the mIPSC fitted by the first exponential in HG cells (left panels). In CP cells (right panels) the proportion fitted by the first exponential decreased at high concentration but the time course of this component remained unaffected.
There was no significant difference in the frequency of mIPSCs of HG and CP cells from the 20 day group, (3.06 ± 0.95 Hz, n = 4, in HG20 cells compared to 2.3 ± 0.65 Hz, n = 4, in CP20 cells). However, the frequency was lower in the younger age group of both cell types (0.25 ± 0.05 Hz, n = 10, in HG10 cells and 0.7 ± 0.21 Hz, n = 5, in CP10 cells), indicating an increase in frequency of mIPSCs during this period of postnatal development (from P9 to P21) in both hippocampus and cerebellum (P < 0.05; two-way ANOVA).
Amplitude distributions of mIPSCs were heavily skewed in all cells, with the majority in the low amplitude range, as previously described (Edwards et al. 1990). The means and standard errors quoted represent the mean of the medians of all cells recorded in the group referred to. Although in HG cells, the median amplitude of mIPSCs was marginally larger in the 10 day group compared to the 20 day group (27.9 ± 1.94 pA, n = 11, HG10 cells vs. 24.4 ± 1.7 pA, n = 18, HG20 cells), mIPSC amplitudes ranged from around 5 to 100 pA in individual cells in both groups (Fig. 1C). Hence there seems to be no substantial change in mIPSC amplitudes in hippocampal granule cells. In contrast, the median amplitude of mIPSCs in CP cells in the 10 day group was twice that in the 20 day group (46.19 ± 5.33 pA, n = 17, CP10 cells vs. 23.11 ± 1.87 pA, n = 8, CP20 cells) (Fig. 1C). This difference was due to a considerably greater spread of amplitudes in CP10 cells (6-300 pA) compared to CP20 cells (6-100 pA), suggesting a marked change in synaptic strength of GABAergic connections during synaptic development in CP cells.
Under control conditions, the decay kinetics of mIPSCs did not show any differences between age groups in cells from each cell type (P > 0.05, two-way ANOVA) (Fig. 1B). However, there was a marked difference between HG cells and CP cells (P < 0.001, two-way ANOVA). Comparison of mIPSCs of HG and CP cells from the 20 day age group indicated that the decay was significantly slower in HG cells than in CP cells, although the rise time kinetics were similar (Fig. 1B). To test whether this difference could have been due to the presence of a local factor in the slice, we also observed control currents from granules cells in cerebellar slices from the 10-day-old group. In order to collect a sufficient number of currents in cerebellar granule cells, where the frequency tended to be low, spontaneous currents (rather than miniatures) were used for this comparison. The decay rates of spontaneous and miniature currents were not significantly different for currents under control conditions in any cell type tested. The decay of spontaneous IPSCs (sIPSCs) of cerebellar granule cells of 10 day rats (CG10 cells) was not significantly different from that of HG cells, but was significantly slower than that of CP cells (P < 0.05, one-way ANOVA). Note that these cells are in close proximity to CP cells but the currents show similar slow kinetics to HG cells. Hence it is unlikely that the difference in decay of mIPSCs seen between CP and HG cells is due to background levels of GABAA receptor modulators in the slice, such as endogenous neurosteroids.
Decay rates of mIPSCs of both cell types were described with two exponentials in the majority of cells, with the exception of CP10 cells in which the majority were described with single exponentials (see Methods). The relative proportion of the mIPSC amplitudes fitted by the first exponential of the decay was around 50 % in CP20 cells and HG20 cells but slightly less (around 35 %) for HG10 cells and CG10 cells. In contrast, where the amplitudes were fitted with two exponentials, CP10 cells showed a first exponential with an amplitude of approximately 80 % of the total amplitude of the current.
Characteristics of potentiation of mIPSCs by THDOC
In most experiments three or four increasing concentrations of the neurosteroid could be used for recordings of miniature currents. This limitation was due to the time necessary to equilibrate THDOC in the bath and to record sufficient mIPSCs. Moreover, to ensure that the effects seen were really due to application of THDOC, rather than changes in recording conditions, it was important to check for washout of the effects in each case. It was thus not possible to complete a more detailed dose-response curve in individual cells and so the observations were divided into low concentration (50 and 100 nm) effects, to test for the possibility of physiological effects and high concentration (1 and 2 μm) effects for comparison with previously reported data.
Throughout this concentration range no effect of the neurosteroid was seen on the rise time, amplitude or first exponential of the decay of synaptic currents in any cell type tested (Fig. 2), nor in the frequency of miniature currents (e.g. in CP10 cells, control 0.7 ± 0.2 Hz vs. 1 μm THDOC 0.6 ± 0.2 Hz, n = 5 cells; in CP20 cells, control 2.3 ± 0.7 Hz vs. 1 μm THDOC 2.2 ± 0.3 Hz, n = 4 cells). The effects seen were on the second exponential of the decay of the currents (Fig. 3A) as outlined below.
Figure 3. Comparison of effect of THDOC on decay rate constant between cell groups.
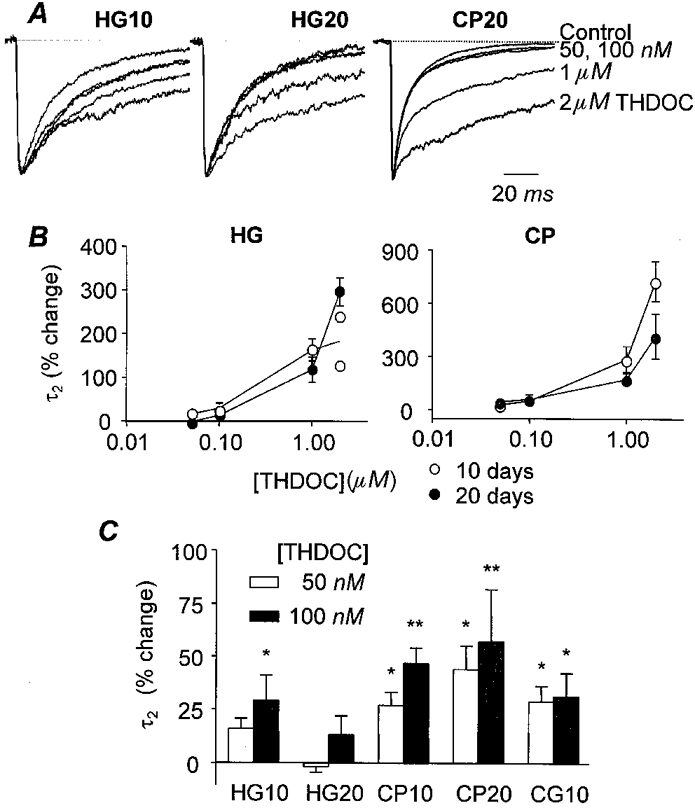
Overlay of averaged mIPSCs of (from left to right) HG10, HG20 and CP20 cells (n > 50) at progressively increasing concentrations (control, 0.05, 0.1, 1.0, 2.0 μm) of THDOC indicates the dose-dependent potentiating effect of THDOC. Note the lack of effect of 50 or 100 nm THDOC in the HG20 cell. Concentrations of THDOC are indicated on the right, amplitudes are scaled for comparison of decays. B, dose-response curve for THDOC effect on the time constant of the second exponential fitted to the decay time of mIPSCs (τ2). Note the difference in scale for the y-axis between CP cells and HG cells. The potentiation is considerably greater in Purkinje cells but this is due to a faster decay of control currents (Table 1). ○, 10 day group (HG10 n = 7, CP10 n = 3); •, 20 day group (HG20 n = 5, CP20 n = 5); in CP10 cells the time constants for single exponential fits (τ) and τ2 for averaged currents fitted with two exponentials are combined. Note that not all cells were exposed to 2 μm THDOC and hence these points represent 2 of 7 HG10 cells, 4 of 5 HG20 cells, 3 of 3 CP10 cells and 3 of 5 CP20 cells. C, effects of lower (possibly physiological) concentrations of THDOC (50 and 100 nm) reveal a developmental change in HG cells. Note there is considerable variation between cells, particularly in the HG10 group as detailed in the text. *P < 0.05; **P < 0.01, one-way ANOVA. HG10, n = 11; HG20, n = 17; CP10, n = 7; CP20, n = 4; CG10, n = 4.
High concentrations of THDOC (Fig. 3A and B)
At micromolar concentrations, THDOC potentiated the decay phase of mIPSCs in both cell types in both age groups. Moreover wherever two exponential were fitted, only the second exponential time constant was affected. Again, at micromolar concentrations, the second exponential decay time constant was increased without measurable changes in the fast time constant, rise times or amplitudes, at any concentration tested. This is consistent with a change in postsynaptic receptor kinetics, without changes in presynaptic release parameters. Interestingly, despite the fact that in the absence of THDOC the mIPSCs in CP cells decayed considerably faster than in HG cells (see above), decay times in the presence of micromolar concentrations of THDOC were very similar (Table 1). This resulted in a substantially greater proportional change in the kinetics of mIPSCs in CP cells than in the kinetics of mIPSCs in HG cells. Evoked GABAergic IPSCs were also recorded in HG cells and the effects of THDOC were evaluated for comparison with mIPSCs (Table 2).
Table 1. Effects of 2 μm THDOC on miniature IPSC decay (τ2, ms).
| Cell group | n | Control | 2 μm THDOC |
|---|---|---|---|
| HG20 | 3 | 39.3 ± 2.6 | 157.6 ± 17.5 |
| CP20 | 4 | 25.5 ± 3.3 | 123.3 ± 20.5 |
Comparison of kinetics of mIPSCs under control conditions vs. 2 μm THDOC. The decay rate constant of second exponential (τ2, ms) of mIPSCs is significantly different (P < 0.05) between controls of HG and CP cell types but no longer different when the decay is potentiated by 2 μm THDOC (see text). This is illustrated for the 20 day group, showing that the relatively larger percentage potentiation in Purkinje cells, compared to hippocampal granule cells, reflects the faster control kinetics rather than slower kinetics of the decay of the current under conditions of the maximum potentiation.
Table 2. Evoked IPSCs in hippocampal cells.
| HG10 cells (n = 3 cells) | HG20 cells (n = 6–9 cells) | |||||||
|---|---|---|---|---|---|---|---|---|
| THDOC(μm) | Rise time(ms) | τ1(ms) | τ2(ms) | τ1/τ2(%) | THDOC(μm) | Rise time(ms) | Amplitude(pA) | t50%(ms) |
| 0 | 1.2 ± 0.1 | 11.5 ± 1.9 | 54.9 ± 6.1 | 46.8 ± 10.0 | 0 | 1.5 ± 0.1 | 66.8 ± 8.6 | 17.8 ± 1.1 |
| — | — | — | — | — | 0.1 | 1.7 ± 0.1 | 77.2 ± 14.1 | 20.1 ± 2.4 |
| 1 | 1.9 ± 0.3 | 10.9 ± 1.3 | 123.2 ± 11.0 | 31.0 ± 7.2 | 1 | 1.6 ± 0.2 | 72.3 ± 18.2 | 46.3 ± 10.4 |
Kinetics of evoked currents (>30 currents per cell per concentration) in hippocampal granule cells. Cells in which two exponentials were detected were analysed in detail in CG10 cells for comparison with miniature currents. Note that similarly to miniature currents only the second exponential component was affected (P < 0.05). All HG20 cells, whether with single or double exponential decay kinetics, were pooled and time to decay to half-amplitude (t50%) was averaged to illustrate that a similar proportional change was detected in the decay time of evoked currrents to the change observed for miniature currents and that amplitude and rise time were unaffected.
The only additional factor which was changed by high doses of THDOC, and which showed another difference between the cell types tested, was the proportion of the amplitude fitted by the first versus the second exponential of the decay (Fig. 2C). In HG cells, potentiation of the decay phase induced by THDOC had no significant effect on the relative amplitude of the two exponentials, but, in CP cells, there was a significant reduction in the proportion fitted by the first exponential at high concentrations of THDOC (Fig. 2C).
Lower, possibly physiological concentrations of THDOC (Fig. 3A)
As with higher concentrations of THDOC, effects on mIPSCs were confined to the second exponential component of the fitted decay (τ2), with no effects on the first component (τ1) or on the amplitude or rise times of the currents. The effects of these lower concentrations (50 and 100 nm) were, however, different between cell types. Bath infusion of 50 or 100 nm THDOC significantly increased the decay time for cerebellar Purkinje cells from both age groups (two-way ANOVA; P < 0.001). In contrast, in HG cells, concentrations of THDOC which might occur physiologically had little, if any, effect on decay time of mIPSCs. No significant changes were seen in the HG20 cells (one-way ANOVA P > 0.05) and, while 100 nm did cause significant potentiation of the decay of mIPSCs in the HG10 group, the effect was small, with only a 25 % potentiation of τ2 compared to around 50 % potentiation of τ in CP10 cells (P < 0.05, one-way ANOVA). The effects of these lower concentrations of THDOC were significantly greater in HG10 cells than in HG20 cells (P < 0.01, two-way ANOVA), which may represent a developmental change in the receptor subtype underlying these synaptic currents. Note that these comparisons are of the means and standard error across cells of the mean potentiation for each cell tested. It is, however, also of interest to note the effects in individual cells as this further underlines the developmental change which seems to be occurring in the HG10 cells.
Amongst the HG10 cells tested with 50 and 100 nm THDOC (n = 11), three showed significant potentiation (∼45 %) by THDOC, even at the lowest concentration and seven showed substantial potentiation at 100 nm (50-80 %). Little or no potentiation was seen in the other cells tested. Currents in HG10 cells were also significantly potentiated without exception by 1 μm THDOC. In contrast, in the 20 day group, none of the HG cells tested showed potentiation of mIPSC decay time constants at nanomolar concentrations of THDOC and even at 1 μm THDOC only 11 of 17 cells showed potentiation. The variability between cells of the 10 day group, compared to the 20 day group, suggests that the 10 day group may represent a period of development during which a change is occurring, so that some neurones have already developed to adult form, while others are still maturing. Unfortunately it is not possible to use younger age groups to see if greater sensitivity is observed uniformly, because the frequency of miniature synaptic currents becomes too low.
It is worth noting that spontaneous IPSCs of cerebellar granule cells from 10-day-old rats were also sensitive to low concentrations of THDOC, showing potentiation of the decay of 28.7 ± 7.4 % (n = 4) and 31.2 ± 10.8 % (n = 6) for 50 and 100 nm, respectively.
Evidence for action via a specific membrane receptor
The potentiation of the decay by THDOC was completely reversible in both cell types (Fig. 4). This is consistent with actions at membrane receptors rather than intracellular steroid receptors.
Figure 4. Stereospecificity of THDOC (1 μm).
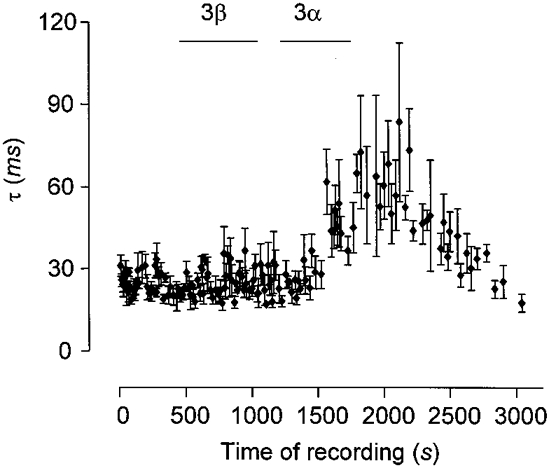
The graph shows effect of the stereoisomer of THDOC 3β-THDOC (3β) compared to potentiation induced by THDOC (3α). Both isomers were used at 1 μm. Each symbol represents the mean ±s.e.m. of the τ2 of 5 consecutive mIPSCs recorded from an HG10 cell. During the period indicated by the bars either the 3β or the 3α isomer of THDOC was perfused into the bath. Note the lack of effect of the 3β isomer despite the fact that in this cell the GABAA receptors mediating the mIPSCs are clearly sensitive to the 3α isomer. Also note the reversal of potentiation with washout.
Further evidence for action via a receptor, rather than non-specific membrane effects, came from the stereospecificity of the changes observed (Fig. 4). In contrast to the potentiating effect of THDOC on the decay, the 3β isomer, 5α-pregnan-3β, 21-diol-20-one (3β-THDOC), at an equivalent concentration of 1 μm, did not potentiate the decay of mIPSCs, nor did it have any effect on other mIPSC parameters or baseline noise in any of the cells tested (n = 8). In fact, there was some tendency for the decay to be slightly decreased by 3β-THDOC, with an overall relative change of -19.8 ± 2.6 % (n = 8). Negative modulation was significant in three of eight cells (P < 0.001, one-way ANOVA). Although we would not like to draw substantial conclusions from this rather inconsistent observation, it may suggest that 3β-THDOC may have competitively antagonised endogenous neurosteroids in the slice or had a direct negative modulatory effect at an alternate steroid site on the GABAA receptor complex. 3β-THDOC has also recently been observed to antagonise L-type voltage-gated calcium channels at picomolar concentrations (ffrench-Mullen, 1999). The lack of effect on mIPSC frequency is, however, in agreement with the observation that the L-type calcium channel blocker nicardipine does not effect vesicular release in the presence of tetrodotoxin unless K+ concentrations were raised above the level used in the present study (Momiyama & Takahashi, 1994).
Voltage dependence
The potentiation of mIPSCs induced by THDOC showed no voltage dependence. The voltage dependence of the effect of THDOC was investigated by collecting IPSCs over a 2 min period at each of a series of holding potentials (Vh) from -74 to +26 mV (n = 6) (Fig. 5A). Both HG10 (n = 6) and HG20 cells (n = 3) were included and the amplitudes were normalised for comparison. There was no change in the current-voltage relationship in the presence of 1 μm THDOC and no shift in the IPSC reversal potential (P > 0.05, two-way ANOVA) (Fig. 5B).
Figure 5. Lack of voltage dependence of the THDOC effect.
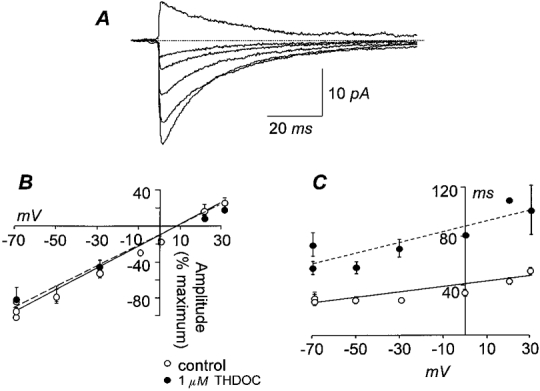
A, overlay of averaged mIPSCs of a HG10 cell at increasing holding potentials, from -74 to +26 mV, demonstrates the voltage dependence of the IPSC conductance. B, THDOC (1 μm) did not change the current-voltage (I-V) relationship of mIPSCs in hippocampal granule cells (HG10 and HG20 grouped; n = 6; ○––○, control, fitted by linear regression, reversal potential (Erev) = 5.8 mV, slope = 1.26 ± 0.07; •––––•, 1 μm THDOC, Erev = 6.9 mV, slope = 1.13 ± 0.09). The amplitude of the averaged mIPSCs was normalised to the maximum amplitude. C, the slow decay rate constant (τ)-voltage relationship of the same currents as in B shows that application of 1 μm THDOC produced no change in the slope (○––○, control, fitted by linear regression, slope = 0.15 ± 0.02; •––––•, 1 μm THDOC, slope = 0.41 ± 0.12). The parallel shift indicates potentiation of the decay by THDOC.
As has been previously reported by others (e.g. Otis & Mody, 1992), the decay rate constant was slightly greater at positive potentials, as shown by the slope of the decay-voltage relationship being significantly different from zero (P < 0.05, two-way ANOVA). In the presence of THDOC, the shift of the decay-voltage relationship was not accompanied by a significant change in the slope (P > 0.05, two-way ANOVA), indicating that there was no voltage dependence of the effect of THDOC on the decay rate constant (Fig. 5C).
DISCUSSION
The results of this study clearly indicate that at concentrations of the neurosteroid THDOC that are likely to occur under physiological conditions, GABAA receptor-mediated synaptic currents can be potentiated in the rat brain via an increase in the slow decay time constant. Moreover as this effect is reversible and stereospecific, it is highly unlikely that the mechanism is genomic but rather demonstrates that it is the effect of binding to a specific membrane receptor. It is interesting to note that the fast decay time constant was unaffected by the application of neurosteroids. Although the reason for the double exponential decays of GABA synaptic currents is still controversial; a possible explanation is that the fast decay is related to the rate of initial closing within a burst, perhaps due to rapid desensitisation of the channel, while the slow decay is determined by inactivation of the channel after dissociation of the transmitter (Jones & Westbrook, 1995; McClelland & Twyman, 1999). The latter component may be controlled in part by recovery from desensitisation but also by the unbinding kinetics in the non-desensitised state. Thus the present results suggest that the neurosteroids affect the long closed-time components of channel kinetics rather than the short closed intervals. Note that the fast decay in the average synaptic current will be particularly susceptible to dendritic filtering and thus will be measured as being slower than the within-burst openings measured in outside-out patches. Where the selection criteria for rise time are more stringent the first decay time constant is faster (see discussion below and Edwards et al. 1990).
The effect of neurosteroids is, however, not uniform, with cerebellar Purkinje cells showing a greater sensitivity at concentrations which might be expected to occur physiologically than hippocampal granule cells which, though somewhat sensitive at 10 days, become virtually insensitive to low concentrations (50 or 100 nm) of neurosteroids by 20 days. It should be noted that the true physiological decay times would be considerably faster than those measured here as, like most of the studies quoted, the measurements in this study were made at room temperature (22-25°C).
A difference in sensitivity to neurosteroids of different brain regions at different stages of development has been suggested from a variety of biochemical studies (e.g. Nguyen et al. 1995). For example, Wilson & Biscardi (1997) recently demonstrated an increase in GABA-induced Cl− uptake into ‘microsacs’ prepared from various brain regions including hippocampus and cerebellum in the presence of THDOC or allopregnanolone (100 nm-3 μm). In contrast to the present study, these authors reported that the microsacs prepared from the hippocampus were more sensitive than those from cerebellum. This apparent difference may reflect the fact that it was not possible, in their study, to differentiate between cell types within the preparations or indeed between synaptic and extrasynaptic receptors on individual cells and thus the result reflects an average of a range of receptor types. This may suggest that a particular subtype of neurones, not sampled in the present study, has a different profile of steroid sensitivity. We cannot completely discount the possibility that temperature plays a role in the difference seen, as the study by Wilson & Biscardi was carried out at 30°C.
Interestingly the concentration of neurosteroids and the enzymes which produce them also vary in different brain areas (Bixo et al. 1997). This regional variation suggests that, whether produced locally in glia or entering the brain from peripheral sources, the neurosteroids could have their effects in very specific areas, i.e. only where the appropriate receptor is present.
Potentiation of GABAergic transmission via an increase in the synaptic decay time constant, as observed here, is consistent with the previously observed electrophysiological effects of this and related compounds. Belelli et al. (1996) demonstrated that GABAA receptors expressed in oocytes were sensitive to the neurosteroid 5α-pregnan-3α-ol-20one (THP) and other related compounds, regardless of whether the α1, 2 or 3 subunit was included. This is one of the few groups to have used physiological concentrations of neurosteroid and they were able to demonstrate effects of THP at concentrations as low as 3 nm with an EC50 of 90 nm. Most other studies have only tested effects of high concentrations (≥ 1 μm). However in these high-concentration studies the neurosteroids and related compounds have consistently been shown to increase the open time of GABA receptor channels without changing the conductance of the channel (Harrison et al. 1987). Moreover after it was demonstrated by Jones & Westbrook (1996) that desensitisation of the GABAA receptor can buffer the protein in a bound state, so that the rate of final closing is slowed, Zhu & Vicini (1997) demonstrated that the presence of neurosteroids may slow the recovery of the receptor from the desensitised state and hence prolong currents by this mechanism.
It seems likely that the differences in kinetics seen in the absence of neurosteroids and the different efficacy of steroids on GABAergic synaptic currents, reported here, are due to the presence of different subunits in the GABAergic synapses recorded in different brain areas and at different stages of development. A wide range of subunit combinations show neurosteroid sensitivity, though some specific subunit combinations have been shown to increase the sensitivity of the GABAA receptor to neurosteroids (Shingai et al. 1991; for review Lambert et al. 1999). Hauser et al. (1995) demonstrated an increase in sensitivity if the α6 subunit was included. Although it has not been directly tested, this suggests a role for the α4 subunit, which shows a high degree of homology with α6. Moreover a recent study has demonstrated that withdrawal from progesterone in rats results in a decrease in decay time constant of GABAergic currents and is associated with an increase in α4 subunit RNA (Smith et al. 1998). A recent study also compared various recombinant receptors containing different α or γ subunits. They concluded that a lack of α subunits greatly decreased the efficacy of allopregnenolone for enhancing GABAergic currents, with α2 being more effective than α1 subunits and γ3 imparting both greater sensitivity and greater efficacy than γ1 or γ2 subunits (Maitra & Reynolds, 1999). Other studies have suggested involvement of various other subunits. Zhu et al. (1996) showed a strong inverse correlation between steroid sensitivity and δ subunit transfection in HEK293 cells, causing a complete loss of neurosteroid sensitivity. Moreover, interestingly, studying cerebellar granule cells in primary cultures, they showed a very similar result to that seen here in hippocampal granule cells in brain slices. They reported a decrease in the potentiation of GABAA-mediated currents by THDOC (100 nm-10 μm) in cells which had been in culture for 14 days compared to those after 4 days in culture (Zhu et al. 1996). They concluded from RT-PCR on these cells and comparison with sensitivities in transfected HEK cells that this change was due to the increased number of cells expressing the δ subunit in the 14 day cultures.
The α4 and δ subunits may be particularly relevant here. During postnatal development the dentate gyrus has been shown to express α1, α3 and α4, as well as β1 and γ2, until around day 12. Around day 12 there is a decline in α4 and β1 subunits and the δ subunit becomes detectable for the first time (Laurie et al. 1992). This correlates well with the relative insensitivity in the granule cells of the 20 day group shown in the present study and the variation in sensitivity seen in different cells of the 10 day group (P9-P13) where some synapses may have already switched and others may be still in the neonatal form. In contrast to the hippocampus, cerebellar Purkinje cells show no change in subunits expressed during development and do not express a δ subunit. This is again in agreement with the present study where the Purkinje cell synapses remained sensitive at both stages of development.
While the parallels which can be drawn from the literature concerning subunit expression and neurosteroid sensitivity are highly suggestive, other mechanisms are also possible. In particular cAMP-dependent phosphorylation of GABAA receptors has been shown to decrease whole-cell responses to GABA application and to change the desensitisation kinetics, which could also be compatible with the present result (e.g. Moss et al. 1992). All intracellular solutions contained MgATP (2 mm) but no other phosphate-regenerating solutions. Although no run-down was observed in the experiments, it is possible that different synapses could have localised supplies of phosphatases and/or kinases which can cause local regulation of GABAA receptors or that these factors can affect different subtypes of the receptor differentially. However, we consider the difference in subunit combination a more likely explanation.
Note that the decay kinetics of the control currents reported here for HG20 cells are slightly different from those reported previously with very close to identical recording conditions (Edwards et al. 1990). This is probably due to two main factors. (1) From experience of using Wistar rats for similar experiments in Germany, UK and Australia, there seem to be genuine differences between GABAergic synaptic currents (F. A. Edwards, unpublished observation). This may be a true factor of breeding, suggesting that differences occur within one strain as generations are bred in isolation from each other. Alternatively the exact breeding and holding conditions, though similar in different animal houses, may be sufficiently different to cause acute or chronic differences in neuromodulators which affect GABA channels or the exact proportions of particular subunits expressed. (2) The apparently slower fast exponential may be an artefact of the selection procedure. In the earlier study (Edwards et al. 1990) the important measured factor was the exact amplitude of individual currents. Recordings were thus highly selected, only being continued on cells where rise times were very fast (< 1 ms) and background noise extremely low. In the present study such selection was not necessary as the main factor affected appears to be the slow decay time of the currents. Thus this study represents a wider sample of the population, with all cells being accepted for recording. Currents were afterwards selected for the somewhat less stringent criterion of a < 2 ms 10-90 % rise, which only excluded currents where artefactual noise or overlap of consecutive currents impeded the measurement. Hence a certain degree of dendritic or series resistance filtering may have slowed the recorded fast component, which would be much more sensitive than the slow component, in which we were primarily interested in this study. Note that it is unlikely that differences in dendritic filtering between ages was a determining feature in the developmental effects seen as the rise times between the two age groups was unchanged in control conditions for hippocampal cells. Moreover changes in dendritic spread would be expected to be considerably greater in Purkinje cells where in fact no change was seen in the effects of THDOC with development.
It is interesting to note that, in the first postnatal week, GABAergic transmission is excitatory in many cells of both the hippocampus and cerebellum (Ben-Ari et al. 1989) due to lack of expression of the chloride transporter KCC2 (Rivera et al. 1999). This results in a relatively high intracellular chloride concentration in neurones of young animals, thus changing the reversal potential of chloride currents such as GABAergic IPSCs. In hippocampal slices from newborn animals, GABA-activated currents result in depolarising potentials which change to hyperpolarising inhibitory potentials during the P9-P12 period. Thus again the P9-P13 period of the 10 day groups in our present study is shown to be a transition period in which the adult pattern of inhibitory transmission is being established in the hippocampus. The effect of the steroid would therefore presumably be to enhance excitability in young animals, and in the dentate gyrus the inhibitory currents mediated by GABAA receptors seem to be largely insensitive to the effects of neurosteroids. In contrast, in the cerebellum, the neurosteroid sensitivity remains so that these intrinsic compounds would have an opposite effect on modulation of excitability in new born versus adult animals in Purkinje cells. It is perhaps relevant that the Purkinje cells, which were the most sensitive cell type tested, are themselves GABAergic and it would be interesting in future studies to investigate whether there is any systematic difference between the sensitivity of excitatory and inhibitory neurones to neurosteroids at physiological concentrations.
In summary, we have shown that the neurosteroid THDOC is likely to have important modulatory effects at concentrations which occur naturally in the brain, in some but not all GABAergic synapses of the central nervous system. The differential sensitivity of different cell types to the neurosteroids is of particular interest in terms of development of potential therapeutic compounds which may be able to be targeted to specific brain areas without causing a general effect across the whole brain.
Acknowledgments
We would like to thank Dr Jonathan Fry, Ms Bonnie Collins and Dr John Honour (University College London) for their time and help in developing the methods for assaying steroid levels and Dr Richard Hampi (Institute of Endocrinology, Prague) for providing the THP antibody. We are also grateful to Dr Delia Belleli (University of Dundee), Dr Kai Kaila (University of Helsinki) and Drs Susan Robertson, Jonathan Fry and Martin Ebner (University College London) for very useful comments on the manuscript. This project was funded by the Australian National Health and Medical Research Council, the Australian Research Council, the British Council and the BBSRC.
References
- Akwa Y, Young J, Kabbadj K, Sancho MJ, Zucman D, Vourc'h C, Jung-Testas I, Hu ZY, Le Goascogne C, Jo D H, Corpechot C, Simon P, Baulieu EE, Robel P. Neurosteroids: biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. Journal of Steroid Biochemistry and Molecular Biology. 1991;40:71–81. doi: 10.1016/0960-0760(91)90169-6. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Baulieu E-E. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Progress in Hormone Research. 1997;52:1–32. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology. 1996;35:1223–1231. doi: 10.1016/s0028-3908(96)00066-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. The Journal of Physiology. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Dibbelt L, Hill M, Hampl R, Stárka L. Allopregnanolone in women with premenstrual syndrome. Hormone and Metabolic Research. 1998;30:227–230. doi: 10.1055/s-2007-978871. [DOI] [PubMed] [Google Scholar]
- Bicikova M, Lapcik O, Hampl R, Starka L, Knuppen R, Haupt O, Dibbelt L. A novel radioimmunoassay of allopregnanolone. Steroids. 1995;60:210–213. doi: 10.1016/0039-128x(94)00039-f. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Research. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. The Journal of Physiology. 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Burg M, Heinemann U, Schmitz D. Neuroactive steroids induce GABAA receptor-mediated depolarizing postsynaptic potentials in hippocampal CA1 pyramidal cells of the rat. European Journal of Neuroscience. 1998;10:2880–2886. doi: 10.1111/j.1460-9568.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- Carter DB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White H S, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterisation of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Dayanithi G, Tapia-Arancibia L. Rise in intracellular calcium via a nongenomic effect of allopregnanolone in fetal rat hypothalamic neurons. Journal of Neuroscience. 1996;16:130–136. doi: 10.1523/JNEUROSCI.16-01-00130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. Journal of Neurophysiology. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Spencer-Gardner C, Gotis G, Brown JB, Smith MA, Burrows GD. Progesterone and the premenstrual syndrome: a double blind crossover trial. British Medical Journal. 1985;290:1617–1621. doi: 10.1136/bmj.290.6482.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. The Journal of Physiology. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- ffrench-Mullen JMH. Neuroactive steriod modulation of neuronal voltage-gated calcium channels. In: Baulieu E-E, Robel P, Schumacher M, editors. Contemporary Endocrinology: Neurosteroids: A New Regulatory Function in the Neurosystem. Totowa, NJ, USA: Humana Press; 1999. chap. 13. [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. Journal of Pharmacology and Experimental Therapeutics. 1988;246:803–812. [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Research. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. Journal of Neuroscience. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser CAE, Chesnoy-Marchais D, Robel P, Baulieu EE. Modulation of recombinant α6β2gamma2 GABAA receptors by neuroactive steroids. European Journal of Pharmacology: Molecular Pharmacology. 1995;289:249–257. doi: 10.1016/0922-4106(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Hevers W, Lüddens H. The diversity of GABAA receptors—Pharmacological and electrophysiological properties of GABAA channel subtypes. Molecular Neurobiology. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Johnston D, Brown TH. Interpretation of voltage-clamp measurements in hippocampal neurons. Journal of Neurophysiology. 1983;50:464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends in Neurosciences. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Shepherd SE, Pistis M, Peters JA. The selective interaction of neurosteroids with the GABAA receptor. In: Baulieu E-E, Robel P, Schumacher M, editors. Contemporary Endocrinology: Neurosteroids: A New Regulatory Function in the Nervous System. Totowa, NJ, USA: Humana Press Inc.; 1999. chap. 7. [Google Scholar]
- Lan NC, Chen J-S, Belelli D, Pritchett DB, Seeburg PH, Gee KW. A steroid recognition site is functionally coupled to an expressed GABAA-benzodiazepine receptor. European Journal of Pharmacology: Molecular Pharmacology. 1990;188:403–406. doi: 10.1016/0922-4106(90)90201-8. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. Journal of Neuroscience. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. The Journal of Physiology. 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra R, Reynolds JN. Subunit dependent modulation of GABAA receptor function by neuroactive steroids. Brain Research. 1999;819:75–82. doi: 10.1016/s0006-8993(98)01316-x. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Steroids and Neuronal Activity. Chichester: Wiley; 1990. Steroid regulation of the GABAA receptor: ligand binding, choride transport and behaviour; pp. 83–106. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Research Reviews. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Momiyama A, Takahashi T. Calcium channels responsible for potassium-induced transmitter release at rat cerebellar synapses. The Journal of Physiology. 1994;476:197–202. doi: 10.1113/jphysiol.1994.sp020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABA-A receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Nguyen Q, Sapp DW, Van Ness P C, Olsen R W. Modulation of GABAA receptor binding in human brain by neuroactive steroids: Species and brain regional differences. Synapse. 1995;19:77–87. doi: 10.1002/syn.890190203. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis T, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Pasternack M, Smirnov S, Kaila K. Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology. 1996;35:1279–1288. doi: 10.1016/s0028-3908(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. Modulation of GABAA receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. The Journal of Physiology. 1997;500:475–485. doi: 10.1113/jphysiol.1997.sp022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul S M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences of the USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith CA, Sillar KT. Pre- and postsynaptic modulation of spinal GABAergic neurotransmission by the neurosteroid, 5β-pregnan-3α-ol-20-one. Brain Research. 1997;770:202–212. doi: 10.1016/s0006-8993(97)00809-3. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, Macdonald JF. Ethanol modulation of GABA receptor-activated Cl− currents in neurons of the chick, rat and mouse central nervous system. European Journal of Pharmacology. 1992;224:173–181. doi: 10.1016/0014-2999(92)90802-b. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl−co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuropsychopharmacological properties of neuroactive steroids. Steroids. 1999;64:83–91. doi: 10.1016/s0039-128x(98)00101-9. [DOI] [PubMed] [Google Scholar]
- Shingai R, Sutherland ML, Barnard EA. Effects of subunit types of the cloned GABAA-receptor on response to a neurosteroid. European Journal of Pharmacology: Molecular Pharmacology. 1991;206:77–80. doi: 10.1016/0922-4106(91)90149-c. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Turner DM, Ransom RW, Yang JSJ, Olsen RW. Steroid anesthetics and naturally occurring analogs modulate the gamma-aminobutyric acid receptor complex at a site distinct from barbiturates. Journal of Pharmacology and Experimental Therapeutics. 1989;248:960–966. [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. The Journal of Physiology. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sciences. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. Journal of Neuroscience. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. Journal of Neuroscience. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Krueger KE, Vicini S. δ subunit inhibits neurosteroid modulation of GABAA receptors. Journal of Neuroscience. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


