Abstract
Intracellular recordings and quantal analysis of synaptic transmission were made at neuromuscular junctions receiving stable convergent innervation in reinnervated rat lumbrical muscles, following recovery from chronic nerve conduction block. The polyneuronally innervated motor endplates (π-junctions) were identified by vital staining of lateral plantar nerve (LPN) and sural nerve (SN) motor terminals, using the activity-dependent staining properties of the aminostyryl dyes RH414 and FM1-43, respectively.
Endplate depolarisation and quantal content per unit area varied by more than a factor of ten (≈0.1-1.4 quanta μm−2) between fibres. However, the stable π-junctions produced nearly equivalent endplate depolarisations and quantal content per unit area, suggesting that synaptic strengths were co-regulated at these motor endplates. Quantal content per unit area was also independent of the size of individual synaptic inputs, or whether one, both or neither input was judged sufficient to produce suprathreshold or subthreshold endplate depolarisations.
Simultaneous excitation of convergent LPN and SN inputs from some π-junctions resulted in profound non-linear summation, and in some cases complete occlusion of the response of the smaller input. The amplitude of the smaller, test responses recovered with a time constant of 2.1 ± 0.5 ms (mean ±s.e.m.) on varying the interval between paired stimuli, of similar order to the time constant of repolarisation of the conditioning endplate potential.
The data show that it is not necessary for a motor nerve terminal to occupy most of an endplate, or to produce a suprathreshold response in order to become stable. The occlusion of linear summation, similar to that described previously at polyneuronal junctions in neonates, suggests that convergent inputs comprising interdigitated synaptic boutons evoke self-contained synaptic responses at endplates, and that these are non-co-operative with respect to overall endplate depolarisation or safety margin for synaptic transmission.
Activity exerts powerful, selective effects on the organisation, stability and strength of synaptic connections in all parts of the nervous system, including neuromuscular junctions (Lohof et al. 1996; Sanes & Lichtman, 1999). For example, neonatal rat muscle fibres are innervated at single motor endplates by motor nerve terminals supplied by several motoneurones (polyneuronal innervation; π-junctions), and a similar pattern is re-established in adult muscle after nerve injury and regeneration (Brown et al. 1976; Betz et al. 1979; Ribchester, 1988). With time, synaptic boutons and axonal inputs are progressively eliminated, eventually leaving most endplates innervated by only one motor axon (Gan & Lichtman, 1998). Progressive and disproportionate weakening of synaptic transmission, induced by differences in pre- and postsynaptic activity, has been presumed to underlie this competitive process (Ribchester & Taxt, 1983; Balice-Gordon & Lichtman, 1994; Coleman et al. 1997). However, activity may not be sufficient to induce elimination from all π-junctions. Previous studies of paralysed neonatal or reinnervated adult muscle suggest that significant polyneuronal innervation persists once activity resumes (Hoffman, 1953; Brown et al. 1982; Barry & Ribchester, 1995). The properties that allow convergent synapses of different size and efficacy to persist at a motor endplate are unknown. Equally, it is unclear whether apparently stable, convergent synaptic inputs in reinnervated muscles may in fact be slowly undergoing heterosynaptic repression and progressive elimination, as in development.
If small synaptic inputs to π-junctions in reinnervated adult muscles were actually undergoing elimination then they should be disproportionately weak: that is, transmitter release per unit area should be less than normal; or the synapses should show a reduced receptor density; or a combination of both (Balice-Gordon & Lichtman, 1994; Coleman et al. 1997). To test this prediction, we have used vital dyes and intracellular recording to correlate fractional endplate occupancy with synaptic efficacy, measured in terms of overall depolarisation and transmitter release per unit area. We also studied the interactions between convergent inputs, by comparing the amplitudes of the endplate potential responses evoked by separate and combined stimulation. Our findings indicate that converging inputs to reinnervated mammalian neuromuscular junctions are indeed stable; that these stable inputs have nearly equivalent synaptic strengths; that the strengths of converging inputs are regulated up or down together; that it is not necessary for small inputs to generate suprathreshold responses in muscle fibres in order to become stable; but that the synaptic responses derived from different inputs can be largely self-contained, and mutually occlusive.
A preliminary account of these data was presented to The Physiological Society (Costanzo et al. 1999).
METHODS
Surgery
Adult female Sprague-Dawley rats were anaesthetised with halothane (2.5 % in 1:1 N2O/O2), and the lateral plantar nerves (LPN) were exposed and crushed bilaterally, leading to sprouting of intact sural nerve (SN) axons innervating the fourth deep lumbrical (4DL) muscles (Betz et al. 1979; Barry & Ribchester, 1995). Nineteen to twenty-one days later, animals were reanaesthetised and osmotic minipumps (Alzet 2002) containing tetrodotoxin (TTX; 500 μg ml−1) were implanted intraperitoneally. Minipumps were connected via silicone tubing to a cuff loosely fitted around the right sciatic nerve. All surgical procedures were carried out under licence and in accordance with UK Home Office regulations. Animals were monitored daily following surgery, and showed no signs of pain or distress. Nerve block was maintained continuously for 14-34 days, as assessed each day by testing for withdrawal of reflex responses to pinching the plantar surface of the foot, and toe spread reflex responses to lifting the animal by the tail. The contralateral foot always gave reflex responses. The TTX supply in the minipump was eventually exhausted and the day when pinch responses and/or toe-spread reflexes returned on the formerly blocked side was recorded as the first day of recovery from nerve block. Animals were killed on the day of the acute experiments by stunning and cervical dislocation. The 4DL muscles were thus dissected with their intact nerve supplies 2-10 weeks (median, 6 weeks) after the resumption of these signs of activity; that is, from 7 to 21 weeks after the original nerve crush. Our previous studies (Barry & Ribchester, 1995) have shown that this procedure leads to enduring polyneuronal innervation in about 30 % of the reinnervated 4DL muscle fibres.
Vital staining of π-junctions
LPN and SN terminals were stained by repetitive stimulation in the presence of RH414 (30 μm; 20 Hz, 12 V, 10 min) and FM1-43 (4 μm; 20 Hz, 12 V, 10 min; both dyes obtained from Molecular Probes), respectively, and washed with oxygenated physiological saline for at least 15 min between each dye application (Betz et al. 1992; Barry & Ribchester, 1995). Preparations were viewed in a fluorescence microscope (Micro Instruments M2B) using a Zeiss ×40 water immersion objective (NA 0.75) and a Nikon filter block fitted with 400-440 nm excitation, 515 nm IF emission filters and a 455 nm dichroic mirror. Images were captured with a Hamamatsu C5810 chilled colour CCD camera. Regions covered by LPN and SN terminals were analysed using OpenLab Software (Improvision, Coventry, UK). Briefly, areas occupied by SN and LPN terminals were estimated from binary masks judged to exactly overlap the respective areas of FM1-43 and RH414 fluorescence.
Electrophysiological recording
Intracellular endplate potential (EPP) recordings were made from identified π-junctions using conventional microelectrode techniques. Muscle action potentials were blocked with μ-conotoxin (2 μm for 20 min; Scientific Marketing Associates, Barnet, UK). In some recordings, microelectrodes were filled with Lucifer Yellow (4 % in 1 M LiCl; Sigma) and the muscle fibre was ionophoretically injected with the dye at the end of the recording period, in order to verify the source of its nerve supply, by briefly overcompensating the electrode capacitance. Here we use the terms ‘synaptic efficacy’ to denote the amount of depolarisation (EPP amplitude) produced by a motor nerve input to the endplate, and ‘synaptic strength’ to indicate the amount of transmitter released (quantal content) per unit area. Evoked EPPs were digitised with a CED 1401+ interface (Cambridge Electronic Design, Cambridge, UK). EPP amplitudes were corrected to a standard resting membrane potential (-80 mV) to allow comparison of synaptic efficacies and strengths between fibres. Quantal contents were measured using the variance and (where appropriate) failures methods, after automatically correcting EPP amplitudes for non-linear summation according to the formula derived by McLachlan & Martin (1981) setting f = 0.8, using WinWCP software kindly provided by Dr J. Dempster (University of Strathclyde, UK). Efficacy:occupancy indices (e:o) were calculated for each input by dividing the normalised synaptic efficacy by the normalised input area, e:o = (EPPi/EPPT)(AreaT/Areai), where i and T denote the input and the summed total of the two inputs, respectively. Reanalysis of the data without first correcting synaptic potentials for non-linear summation did not alter the statistical significance of the correlations between input size and strength.
Immunocytochemistry
Following fixation (4 % paraformaldehyde in phosphate-buffered saline for 15 min) muscles were incubated (20 min) in α-bungarotoxin conjugated to tetramethylrhodamine isothiocyanate (TRITC-α-BTX, 5 μg ml−1; Molecular Probes) to label junctional acetylcholine receptors (AChRs). The muscles were then permeabilised in methanol at -20°C (7 min). Visualisation of axons was achieved by addition of antibodies directed against the 165 kDa neurofilament protein (diluted 1:250) and the synaptic vesicle antigen SV2 (1:500; Feany et al. 1992). Both primary antibodies were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA). Binding of both antibodies was visualised using fluorescein isothiocyanate (FITC)-conjugated sheep anti-mouse secondary antibody (SAPU, Law Hospital, Carluke, UK). Some junctions studied by intracellular recording were relocated in a Leica confocal microscope.
RESULTS
Synaptic efficacies at polyneuronally innervated junctions
As in a previous study (Barry & Ribchester, 1995) about 30 % of junctions were innervated by regenerating axons and/or sprouts from the LPN and SN, more than 2 weeks after recovery from chronic nerve block and more than 8 weeks after regenerating axons returned to the partially denervated 4DL muscles (Fig. 1A). Vital staining with FM1-43 and RH414 revealed that sometimes very few synaptic boutons (1-4) were provided by one of the two converging axons, covering less than 20 % of total endplate area (Fig. 1B, F, H and I). At other π-junctions, the areas occupied by SN and LPN boutons were about equal (Fig. 1D; see also Fig. 4C).
Figure 1. Morphology and electrophysiology of stable π-junctions.
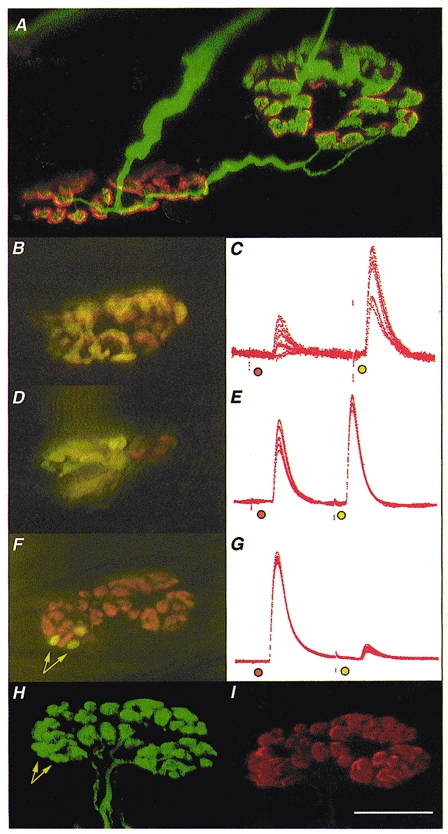
Immunocytochemistry was used to visualise nerve terminals (FITC) and TRITC-α-BTX was used to visualise AChRs in fixed preparations; FM1-43 and RH414 were used to label synaptic boutons supplied by SN and LPN motor axons in freshly isolated preparations, staining their recycled synaptic vesicles fluorescent green/yellow and orange, respectively. A, confocal microscope image of two motor endplates in a reinnervated muscle, 9 weeks after LPN crush (including 4 weeks recovery from a 2 week nerve conduction block, applied 3 weeks after the original crush). The endplates are bridged by an axonal sprout, probably arising from the motor nerve terminal on the left. The endplate on the right is also innervated by a slender regenerating axon. Thus, this endplate is a stable π-junction. B, D and F, composite digital images from three different, vitally stained stable π-junctions innervated by LPN (orange, RH414 labelled) and SN (yellow/green, FM1-43 labelled) terminal boutons, several weeks after regeneration and recovery from nerve conduction block. The image in F is a montage made up from two original digital images taken in slightly different focal planes. C, E and G, corresponding intracellular recordings of EPPs evoked from these π-junctions after bathing the preparations in μ-conotoxin to abolish muscle (but not axonal) action potentials. In each case the orange spot indicates LPN stimulation and the yellow/green spot indicates SN stimulation. The relative EPP amplitudes varied in proportion to the relative areas covered by LPN and SN synaptic boutons. The π-junction shown in F was relocated using a confocal microscope after fixing and staining for neurofilament/SV2 (H; FITC-conjugated secondary antibody, green fluorescence) and ACh receptors (I; TRITC-α-BTX, red fluorescence). Data from this endplate were as follows: in response to stimulating the nerve supplies repeatedly at 1 Hz, the LPN produced a large amplitude EPP (40 ± 0.167 mV, n = 110; uncorrected amplitudes; 93 % of the total synaptic response; mean quantal content, 47.4) and the SN produced a proportionally smaller amplitude EPP (2.9 ± 0.168 mV; 7 % of the total synaptic response; 13.8 times smaller than LPN response; mean quantal content, 3.1). The synaptic area covered by the LPN was 137 μm2 (93.2 % of total area) and the SN covered 10 μm2 (arrows in F; 6.8 % of total area; 13.7 times smaller than LPN). The confocal images confirmed that the differently coloured boutons were supplied by distinct axons directed to the same endplate, and that all boutons visualised immunocytochemically were also stained with the vital dyes before fixation. Arrows in H point to the location of the same boutons as indicated in F. There was no discernible difference in the fluorescence intensity of receptors in the region of the endplate occupied by the three SN boutons compared with receptors occupied by LPN boutons. Calibrations: scale bar in I corresponds to the following measures: A, 10 μm; B, D and F, 20 μm; H and I, 15 μm; C, E and G, 25 ms. The electrophysiological records were scaled to match the largest EPP in each case. These were as follows: C, 6 mV; E, 24 mV; G, 42 mV.
Figure 4. Some convergent inputs are mutually occlusive.
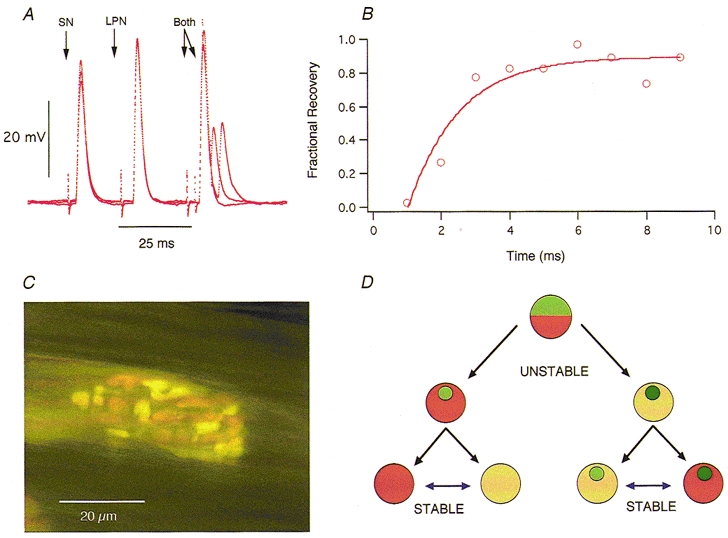
A, three superimposed records showing EPPs evoked by SN, LPN and combined (Both) stimulation, with variable intervals between the combined stimuli in two different π-junctions. Stimulation timed to make the two peaks coincide produced no additional increment in EPP amplitude (occlusion). Assuming conventional non-linear summation of endplate conductance (McLachlan & Martin, 1981) the amplitude of the combined response should have been at least 10 mV greater than the response to LPN stimulation alone. By increasing the interval between the two stimuli, the LPN EPP was used as the conditioning input and the SN EPP as the test input. EPP occlusion was still virtually complete even when many of the AChRs were blocked, following addition of α-bungarotoxin (10 μg ml−1) to the bathing medium (not shown). B, time course of recovery from occlusion (○) and best non-linear least-squares single exponential curve fit of the form Vt = V∞(1 - e-t/τ) for the SN EPP shown in A. The recovery time constant was longer than the decay time constant of the conditioning EPPs. C, example of a stable π-junction where the boutons derived from the SN (green/yellow) and LPN (orange) are interdigitated. D, mononeuronal and polyneuronal innervation represented as alternative stable states, in which synaptic boutons belonging to one or more inputs may be co-regulated. Saturation of orange or green colour is used to represent synaptic strengths of two different inputs in terms of overall efficacy (EPP amplitude) and/or quantal content per unit area. The relative areas covered by orange or green, and the relative synaptic efficacies or strengths (colour saturation), may initially change due to synaptic competition and synapse elimination. Data on the effects of chronic use or disuse at mono-innervated junctions, once established, have shown that synaptic strength continues to be labile (see references in text). The present study extends this to show that, with time, stable synaptic boutons supplied by different motoneurones to the same muscle fibre become equivalent. The data further suggest that the synaptic efficacies or strengths may be scaled up or down together, i.e. they may be interconvertible (reversible blue arrows). Mismatches in the strengths of convergent inputs (light green/dark orange or dark green/light orange; upper icons) may represent a transient state characteristic of a critical period leading to synapse elimination at some junctions (left) and mononeuronal innervation as in development, or to stable polyneuronal innervation with equivalent, co-regulated synaptic strengths (light with light, or dark with dark; right) as shown by the present study.
In two of the preparations, the areas and pixel intensities of endplate AChRs labelled with TRITC-α-BTX were measured before staining with FM1-43 and RH414. In one of these endplates only a single synaptic bouton was supplied by the LPN and the rest of the endplate was innervated by the SN. In the other, three boutons were supplied by the SN and the remainder of the endplate was supplied by the LPN (Fig. 1F). In both instances, there was no discernible difference in the TRITC-α-BTX fluorescence intensity beneath the smaller input compared with the average fluorescence intensity over the rest of the endplate (Fig. 1I). Intracellular recordings were subsequently made from one of these endplates (Fig. 1F and G) and the π-junction was finally relocated in a confocal microscope after staining immunocytochemically for neurofilaments and the synaptic membrane antigen SV2 (Fig. 1H). This, and all other junctions in the same muscle, showed coincidence of synaptic boutons, i.e. there were no terminals with immunostained boutons that had not also been vitally stained, and vice versa.
There were also no discernible differences in the synaptic efficacies per unit area at the reinnervated π-junctions. First, intracellular recordings showed that π-junctions supplied by terminals approximately equal in area produced EPPs about equal in amplitude. Junctions supplied by inputs that differed in area gave larger EPP responses to the larger of the two inputs and proportionally smaller EPPs to the smaller input (Fig. 1C, E and G). Thus, there was a very strong correlation between the fractional occupancy (area) and efficacy (corrected EPP amplitudes) indicating that the small synaptic inputs were weaker than the large inputs (Pearson's r = 0.94; Fig. 2A). The rise times of the EPPs from the smaller inputs (2.19 ± 0.27 ms; mean ±s.e.m., n = 18) were slightly but significantly slower than those of the larger inputs (1.50 ± 0.12 ms; P < 0.03, paired t test). But the differences did not correlate with fractional occupancies of the endplate (r = -0.23; P > 0.3); and the times to half-decay of the EPPs were not statistically different (3.24 ± 0.25 and 2.82 ± 0.23 ms, respectively; P > 0.05, paired t test). The differences in rise times of the smaller and larger EPPs are most probably due to the reduced quantal content of the smaller inputs rather than weak sensitivity to transmitter beneath the small inputs (see below). EPP rise times are also expected to reduce as quantal content increases, assuming the time course of the endplate membrane conductance change is the same for all responses (Martin, 1979).
Figure 2. Convergent inputs to stable π-junctions have equivalent synaptic strengths.
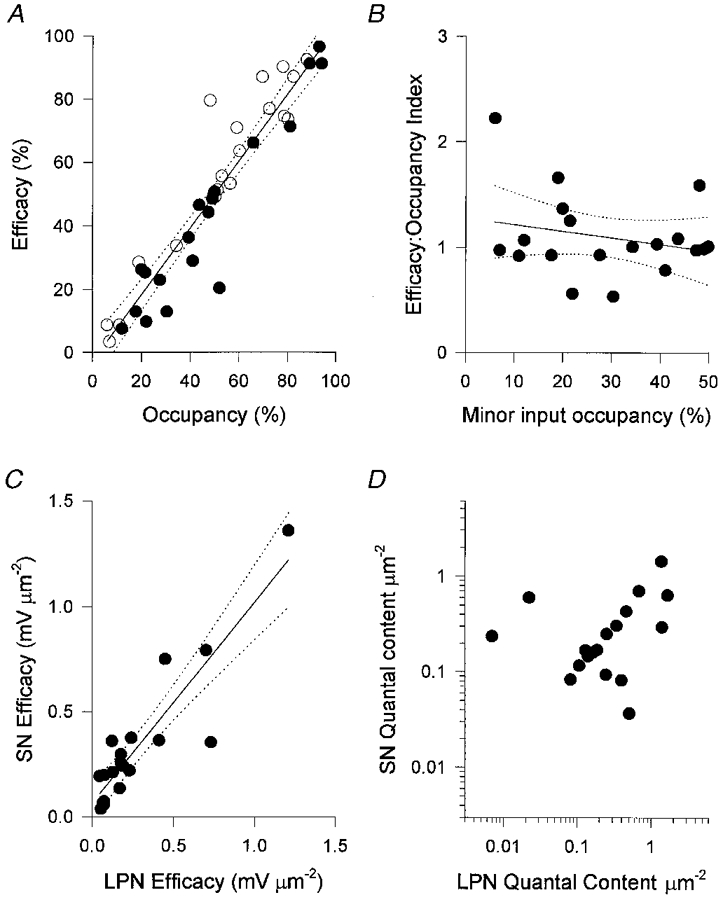
A, EPP amplitudes corrected for non-linear summation plotted against nerve terminal area for LPN (○) and SN (•). The two measures are expressed as a percentage of the arithmetic sum of the EPP amplitudes and terminal areas, respectively. The correlation coefficient was 0.94 and regression analysis (continuous line) and 95 % confidence limits (dotted lines) showed no significant deviation from linearity over the entire range of percentage occupancies. B, efficacy:occupancy (e:o) indices calculated for the minor inputs (less than 50 % occupancy). The lack of correlation, or consistent evidence that small inputs produce EPPs smaller than others for their size suggests that even those inputs with fractional occupancies of less than 20 % of the endplate are not disproportionately weak. C, the specific efficacies of converging inputs, calculated as the EPP amplitude per unit area - corrected for non-linear summation and normalised to a resting membrane potential of -80 mV - were highly correlated (r = 0.90). This correlation could not be attributed to passive electrical properties of the muscle fibres alone, because the quantal content per unit area of the converging inputs was also highly correlated (D; r = 0.62; P < 0.005).
Small synaptic inputs were not disproportionately weak
Other studies have shown that synapses undergoing elimination release less neurotransmitter and have a weaker postsynaptic effect per unit area than synapses that persist (Balice-Gordon & Lichtman, 1994; Coleman et al. 1997). However, further analysis of our data showed that the synaptic efficacies and strengths of converging inputs were equivalent. We calculated a dimensionless efficacy:occupancy (e:o)index for each input (Fig. 2B). We reasoned that if terminals occupying less than 50 % of the total synaptic area were undergoing elimination they would have e:o indices significantly less than unity, and this measure would decline significantly with decreasing fractional occupancy. In fact most small terminals had calculated e:o indices greater than 1.0; but the slope of the regression line was not significantly different from zero (r = -0.24).
Synaptic strengths of convergent inputs were co-regulated
The data in Fig. 2A and B suggest there was a very strong positive correlation between the efficacies of convergent LPN and SN terminals. To examine this further, we recalculated synaptic efficacy in terms of millivolts of membrane depolarisation per unit area for each input to the 19 SN-LPN dually innervated motor endplates (Fig. 2C). The synapses with the greatest efficacies per unit area were those with about equal fractional occupancies, but the two inputs had equivalent efficacies over the entire range (Fig. 2C; r = 0.90; P < 0.001). Furthermore, calculations of quantal content per unit area (synaptic strength) ranged from less than 0.1 quanta μm−2 to about 1.4 quanta μm−2, similar to the range reported in species as diverse as snakes and man (Slater et al. 1992; Wilkinson et al. 1996). The LPN-SN correlation coefficient for synaptic strength was less than that for normalised EPP amplitude but nevertheless highly significant (Fig. 2D; r = 0.62; P < 0.005). Thus, irrespective of their relative sizes, strong synapses appeared to co-exist with other strong synapses, or weak co-existed with weak at the same neuromuscular junctions.
Small, subthreshold inputs were also stable
Neuromuscular junctions normally operate within a large safety margin of neurotransmitter release but when endplate size or occupancy is reduced, small synaptic inputs would be expected to release insufficient transmitter to reach the action potential thresholds of the motor endplates they innervate (Wood & Slater, 1997). The present experiments were carried out using preparations pre-treated with μ-conotoxin, which blocked muscle action potentials whilst leaving axonal action potentials and synaptic transmission intact (Hong & Chang, 1989). Thus, we calculated whether EPPs were subthreshold or suprathreshold (see Fig. 3 legend). We concluded that three muscle fibres in the sample would have given consistent subthreshold responses to both inputs, twelve fibres would have given suprathreshold responses to only one of the two nerves, and four fibres would have given suprathreshold responses to both SN and LPN inputs. These proportions are similar to the numbers of supra- and subthreshold inputs to π-junctions measured directly in reinnervated muscle (Barry & Ribchester, 1995). Comparing these data with fractional occupancy, it appeared that only inputs covering more than about 40 % of the endplate would have given rise to consistent suprathreshold responses (Fig. 3A), although some terminals occupying more than 60 % of an endplate were predicted to give consistent subthreshold responses. This suggests first, that the safety margin for synaptic transmission in reinnervated junctions may be significantly lower than that of intact junctions (Wood & Slater, 1997), but second, it is not necessary for an input to either be large or produce a suprathreshold response in order to become stable.
Figure 3. Suprathreshold and subthreshold synaptic inputs have similar synaptic strengths.
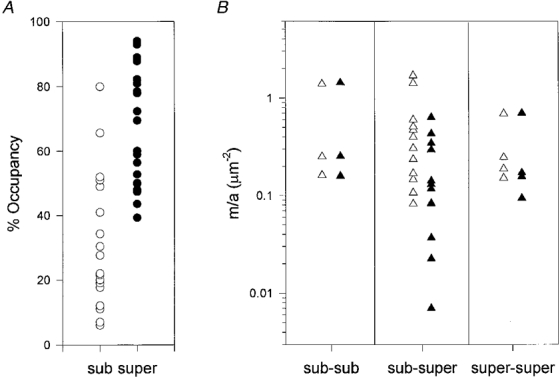
A, the size of terminals (expressed as fractional occupancy of the endplate) required to evoke suprathreshold (super) responses in the muscle fibres was estimated assuming an action potential threshold of -63 mV from a resting potential of -75 mV (Wood & Slater, 1997). None of the inputs with fractional occupancies less than 40 % of total synaptic area were predicted to give suprathreshold responses, although some inputs occupying more than this fraction were predicted from their EPP amplitudes to give subthreshold (sub) responses. B, transmitter release per unit area (m/a) plotted for the three categories of endplate based on the capacity of their inputs to evoke suprathreshold responses: sub-sub, both inputs calculated to produce subthreshold responses; sub-super, one of the two inputs only calculated to give suprathreshold responses; super-super, both inputs calculated to be suprathreshold. In some of the sub-super fibres the subthreshold inputs showed greater and the suprathreshold inputs showed weaker synaptic strengths per unit area, but the differences were not quite statistically significant (P > 0.05, paired t test).
Disproportionate strengthening of small or ineffective neuromuscular synapses has been observed in other studies (Ding, 1982; Tsujimoto et al. 1990; Plomp & Molenaar, 1996). But here we found that junctions where both inputs were suprathreshold, or both inputs were subthreshold, did not have significantly greater quantal content per unit area compared with junctions where only one of the inputs was suprathreshold (Fig. 3B). Subthreshold inputs had numerically higher quantal contents per unit area at eight of the endplates where only one of the two inputs was suprathreshold, but the differences were not statistically significant (P > 0.05, paired and unpaired t tests).
Convergent inputs were reciprocally occlusive
Given the correlation between size and efficacy of the convergent inputs, it was of interest to determine the extent of their summation in response to simultaneous stimulation. Our initial intent was in fact to use the two inputs to an endplate as probes, to measure and check the formula for conventional non-linear summation derived by McLachlan & Martin (1981). Some SN and LPN EPPs produced summated responses on combined nerve stimulation. In five out of eight π-junctions, however, we found that EPPs from the larger convergent input almost completely occluded the response of the smaller input on combined stimulation (Fig. 4A). For instance, if the LPN response was the larger EPP, simultaneous stimulation of the SN produced very little or no additional response. As the interval between paired (conditioning-test) stimulation was increased, the amplitude of the test EPP recovered, and was restored completely to its original mean amplitude with intervals greater than 20 ms. The time constant of recovery from occlusion of the smaller inputs (2.1 ± 0.5 ms, n = 5; see Fig. 4B) was of similar order to the recovery time constants of the conditioning EPPs (see above). Reversing the order of stimulation revealed a similar proportional occlusion of the larger EPP, with a similar recovery time constant (2.5 ± 0.8 ms, n = 3 fibres). If the occlusion had been due to conventional non-linear summation of synaptic potentials then it should have been relieved after reducing the voltage driving force at the endplate (McLachlan & Martin, 1981). However, there was no change in the degree of occlusion or the time constant of its recovery during progressive block of ACh receptors by bath application of α-bungarotoxin (10 μg ml−1; data not shown).
This group of recordings was not obtained from π-junctions identified directly by vital staining. However, subsequent loading of SN and LPN terminals with FM1-43 and RH414 revealed π-junctions in the vicinity of the recording electrode, with interdigitated synaptic boutons from the two nerves (see, for example, Fig. 4C).
DISCUSSION
The data from the present study, taken together with previous reports (Hoffman, 1953; Brown et al. 1982; Barry & Ribchester, 1995) suggest that recovery of muscle activity is not sufficient to re-establish the mononeuronal pattern of muscle fibre innervation after nerve injury and regeneration at all motor endplates. Our present findings also suggest, surprisingly, that it is not necessary for a motor nerve terminal to occupy most of an endplate or produce suprathreshold responses in order to become stable. Polyneuronal innervation, sometimes viewed as an unstable and transient situation in skeletal muscle fibre innervation, should thus be viewed as an alternative, stable endpoint on one trajectory leading from an initially unstable pattern (Fig. 4D). The physiological occlusion of the convergent inputs, similar to that reported in neonatal muscles by Betz et al. (1989), raises interesting questions about the functional consequences of persistent, stable polyneuronal innervation.
Stable π-junctions have also been observed in adult muscles after neonatal treatment with neuromuscular blockers (Brown et al. 1982) or hormones (Lubischer et al. 1992), as well as in normal and reinnervated frog muscle (Werle & Herrera, 1987; Harada & Grinnell, 1996). These findings contrast, however, with those of Balice-Gordon & Lichtman (1994) who showed that focal blockade of small fractions (< 20 %) of adult, mononeuronally innervated endplates with α-bungarotoxin inevitably led to synapse withdrawal; and that during development, mononeuronal innervation is established by inexorable attrition of small, weak inputs (Coleman et al. 1997). Could the converging inputs we observed have become stable because they were synchronously active and therefore indistinguishable from a set of synaptic boutons belonging to a single axon? This seems unlikely, first, because the convergent inputs arose from one regenerating and one intact axon, differing in their conduction velocities and synaptic latencies, and second, because the activity of adult motor units is not synchronised during voluntary movement (Hennig & Lømo, 1985). It is therefore not yet clear how these disparate findings will be resolved. One difference may be that during reinnervation of partially denervated muscle, synapses appear to compete for occupancy of existing synaptic sites, unlike synapses in neonatal muscle where vacated sites are not reoccupied by terminals that remain (Sanes & Lichtman, 1999).
It will be interesting to establish whether small, stable neuromuscular synaptic inputs represent those that withstand a critical period of activity-dependent competition, beyond which relative size or efficacy of synaptic transmission by converging inputs also no longer acts as a decisive, destabilising influence; and whether stable polyneuronally innervated endplates display cytochemical or biophysical characteristics that distinguish them from mononeuronally innervated muscle fibres. One such property is suggested by the mutual occlusion of synaptic potentials with paired stimulation. A similar form of heterosynaptic, inhibitory interaction was reported by Betz et al. (1989) in their studies of SN and LPN motor terminals in neonatal lumbrical muscle, but there the recovery time constant was about 25 ms, approximately 10-fold longer than at the reinnervated junctions we studied here. Betz et al. (1989) considered the most plausible explanation to be changes in sodium and potassium ionic concentrations in the synaptic cleft, reducing ionic current through endplate channels without altering the reversal potential (see also Attwell & Iles, 1979). Alternatively, the activation of each convergent input might concomitantly gate a membrane conductance that would shunt synaptic current from the sites of other synaptic inputs or the recording microelectrode. Betz et al. (1989) concluded on the basis of voltage-clamp experiments that such a conductance would have to be very rapidly activated and inactivated to account for the time course of recovery of synaptic current, and this caveat is underscored by the even more rapid recovery time constants observed in the present study. Equally, Betz et al. (1989) ruled out a presynaptic effect on the basis that the amount of occlusion decayed during the tail of the endplate current. In accordance with their suggestion that the occlusive effects would be expected to act over a very close range, FM1-43 and RH414 co-staining has revealed that synaptic boutons at π-junctions in the reinnervated muscles are often interdigitated (Fig. 4C; see also Barry & Ribchester, 1995). Perhaps some π-junctions comprise self-contained domains whose electrical properties allow (non-linear) summation of synaptic responses within a domain, but not between domains. Further studies, combining voltage-clamp analysis with optical measurement of extracellular ion concentrations within junctional folds, may help to explain how the arrangement of synaptic boutons gives rise to mutual occlusion of heterosynaptic responses.
Finally, perhaps the most important finding of general significance in our data is that the individual synaptic strengths per unit area of convergent inputs were equivalent whatever their relative size, indicating that the strengths of converging inputs are scaled up or down together - that is, they are co-regulated (Fig. 4D). These findings accord with recent reports suggesting that the synaptic strengths of all the inputs to a postsynaptic neurone are scaled concomitantly (Turrigiano et al. 1998). Further studies of stable polyneuronally innervated muscle fibres in adult muscle may therefore provide opportunities for insight into general mechanisms of heterosynaptic integration and co-operation in the nervous system (Zhang et al. 1998), as well as the competitive processes that allow regenerating axons to restore functionally appropriate connections after injury.
Acknowledgments
This work was supported by grants from the Medical Research Council, Action Research, the Wellcome Trust and the Royal Society. We are grateful to Mr Derek Thomson and Ms Linda Sharp for expert technical assistance; and Professor Richard G. M. Morris, Dr Clarke R. Slater and Dr Seth G. N. Grant for discussions and valuable comments on the manuscript.
References
- Attwell D, Iles JF. Synaptic transmission: ion concentration changes in the synaptic cleft. Proceedings of the Royal Society B. 1979;206:115–131. doi: 10.1098/rspb.1979.0095. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon R, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Barry JA, Ribchester RR. Persistent polyneuronal innervation in partially denervated rat muscle after reinnervation and recovery from prolonged nerve-conduction block. Journal of Neuroscience. 1995;15:6327–6339. doi: 10.1523/JNEUROSCI.15-10-06327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Caldwell JH, Ribchester RR. The size of motor units during postnatal development of rat lumbrical muscle. The Journal of Physiology. 1979;297:463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Chua M, Ridge RMAP. Inhibitory interactions between motoneurone terminals in neonatal rat lumbrical muscle. The Journal of Physiology. 1989;418:25–51. doi: 10.1113/jphysiol.1989.sp017827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. Journal of Neuroscience. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Hopkins WG, Keynes RJ. Short-term and long-term effects of paralysis on the motor innervation of two different neonatal mouse muscles. The Journal of Physiology. 1982;329:439–450. doi: 10.1113/jphysiol.1982.sp014312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Jansen JKS, Van Essen DC. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. The Journal of Physiology. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman H, Nabekura J, Lichtman JW. Alterations in synaptic strength preceding axon withdrawal. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- Costanzo EM, Barry JA, Ribchester RR. Synaptic transmission at persistent polyneuronally innervated neuromuscular junctions in reinnervated rat muscle. The Journal of Physiology. 1999;515.P:52–53P. doi: 10.1111/j.1469-7793.1999.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R. Comparison of morphology and physiology of synapses formed at ectopic and original endplate sites in frog muscle. Brain Research. 1982;253:57–63. doi: 10.1016/0006-8993(82)90673-4. [DOI] [PubMed] [Google Scholar]
- Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Gan W-B, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- Harada Y, Grinnell AD. Regeneration of specific innervation in Xenopus pectoralis muscle. Journal of Neurobiology. 1996;13:433–448. doi: 10.1002/(SICI)1097-4695(199612)31:4<433::AID-NEU4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hoffman H. The persistence of hyperneurotized end-plates in mammalian muscles. Journal of Comparative Neurology. 1953;99:331–345. doi: 10.1002/cne.900990207. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Chang CC. Use of geographutoxin-II(μ-conotoxin) for the study of neuromuscular-transmission in mouse. British Journal of Pharmacology. 1989;97:934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Delhaye-Bouchaud N, Mariani J. Synapse elimination in the central nervous system: functional significance and cellular mechanisms. Reviews in Neuroscience. 1996;7:85–101. doi: 10.1515/revneuro.1996.7.2.85. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Jordan CL, Arnold AP. Transient and permanent effects of androgen during synapse elimination in the levator ani muscle of the rat. Journal of Neurobiology. 1992;23:1–9. doi: 10.1002/neu.480230102. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Martin AR. Non-linear summation of end-plate potentials in the frog and mouse. The Journal of Physiology. 1981;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. The effect of membrane capacitance on non-linear summation of synaptic potentials. Journal of Theoretical Biology. 1979;59:179–187. doi: 10.1016/s0022-5193(76)80031-8. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Molenaar PC. Involvement of protein kinases in the upregulation of acetylcholine release at endplates of α-bungarotoxin-treated rats. The Journal of Physiology. 1996;493:175–186. doi: 10.1113/jphysiol.1996.sp021373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR. Activity-dependent and -independent synaptic interactions during reinnervation of partially denervated rat muscle. The Journal of Physiology. 1988;401:53–75. doi: 10.1113/jphysiol.1988.sp017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR, Taxt T. Competition between active and inactive motor axons after reinnervation of partially denervated rat muscle. The Journal of Physiology. 1983;344:89–111. doi: 10.1113/jphysiol.1983.sp014926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Slater CR, Lyons PR, Walls TJ, Fawcett PRW, Young C. Structure and function of neuromuscular junctions in the vastus lateralis of man—a motor point biopsy study of two groups of patients. Brain. 1992;115:451–478. [PubMed] [Google Scholar]
- Tsujimoto T, Umemiya M, Kuno M. Terminal sprouting is not responsible for enhanced transmitter release at disused neuromuscular-junctions of the rat. Journal of Neuroscience. 1990;10:2059–2065. doi: 10.1523/JNEUROSCI.10-07-02059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Werle MJ, Herrera AA. Synaptic competition and the persistence of polyneuronal innervation at frog neuromuscular-junctions. Journal of Neurobiology. 1987;18:375–389. doi: 10.1002/neu.480180405. [DOI] [PubMed] [Google Scholar]
- Wilkinson RS, Son Y-J, Lunin SD. Release properties of isolated neuromuscular boutons of the garter snake. The Journal of Physiology. 1996;495:503–514. doi: 10.1113/jphysiol.1996.sp021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fast- and slow-twitch muscles. The Journal of Physiology. 1997;500:165–176. doi: 10.1113/jphysiol.1997.sp022007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo MM. A critical window for co-operation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]


