Abstract
The effect of intraneural electrical stimulation of the lateral femoral cutaneous nerve on lipolysis in the innervation territory of the stimulated nerve fascicle was studied in seven healthy women. Lipolysis was evaluated by microdialytic measurement of the interstitial glycerol concentration in subcutaneous adipose tissue.
Ten minutes of unilateral intraneural stimulation elicited a 22 ± 8 % (mean ±s.e.m.s) increase in glycerol levels in the stimulated region (P < 0.05), whereas no change was registered in the corresponding area of the contralateral unstimulated leg.
Significantly higher glycerol levels in the stimulated vs. contralateral unstimulated region (47 ± 13 %, P < 0.05) were already observed at baseline (30 min resting period preceding the 10 min stimulation), in all probability as a consequence of the nerve searching procedure and trial stimulations. After the 10 min stimulation, the overall glycerol increase was 72 ± 17 % compared with the contralateral leg, illustrating the degree of lipolysis induced by the whole experimental procedure.
The sympathetic discharge in the lateral femoral nerve (6 recordings) showed typical characteristics of skin sympathetic activity, and the firing pattern was strikingly similar to simultaneously recorded sympathetic discharge in cutaneous nerve fascicles innervating regions without prominent subcutaneous fat stores (2 double nerve recordings). Thus, no component of cutaneous sympathetic outflow specific for the nerve innervating prominent subcutaneous fat stores could be identified.
Our findings suggest that sympathetic nerve fibres travelling in cutaneous nerve fascicles exert a regulatory influence on subcutaneous fat tissue in humans. The combination of intraneural recording/stimulation and subcutaneous microdialysis provides a model for evaluating neural control of human fat metabolism.
Catecholaminergic stimulation of adipose cells is considered to be a key mechanism in the regulation of fat tissue metabolism, involved in the adaptation during starvation (Klein et al. 1989), with ketogenesis (Bahnsen et al. 1984), exercise (Arner et al. 1990) and stress (Hagstrom-Toft et al. 1993). Consequently, a dysfunction of the sympathetic nervous system (SNS) could theoretically contribute to the development of obesity. However, recent studies comparing healthy subjects to patients with spinal cord injury as a model for abolished or altered sympathetic nervous control suggest that neural regulation of human lipolysis is normally active under conditions where sympathetic nerves are stimulated but not during postabsorptive rest (Karlsson et al. 1995, 1997).
In brown adipose tissue, catecholamines are released by a dense network of sympathetic nerves and their activation increases lipolysis (see Bartness & Bamshad, 1998). More relevant for human fat tissue metabolism, stimulation of sympathetic nerves to white adipose tissue (WAT) also increases lipolysis both in vitro (Correll, 1963) and in vivo (Rosell, 1966), despite the sparse innervation of adipocytes in WAT (Slavin & Ballard, 1978).
In humans, the route of sympathetic innervation of WAT has not been described. A putative sympathetic regulation of fat tissue metabolism has mainly been investigated with global measurements, such as plasma and urine catecholamine concentrations (Peterson et al. 1988). The relevance of such global measurements for sympathetic neurotransmission within WAT is, however, clearly limited since noradrenaline (norepinephrine) release in different organ systems is highly differentiated (Esler et al. 1990). WAT is probably a minor contributor to total body catecholamine spillover, and a specific determination of WAT catecholamine spillover is not possible since the arterial supply and venous drainage cannot be defined in humans.
Microneurography allows the recording of sympathetic nerve activity in peripheral nerves of humans (Vallbo et al. 1979; Wallin & Elam, 1997a), and intraneural electrical stimulation of defined cutaneous nerve fascicles has been used to study sympathetic vaso- and sudomotor effects (Wallin & Elam, 1997b). It is not known, however, whether cutaneous sympathetic fibres can affect subcutaneous fat metabolism in their innervation area. Furthermore, recordings from nerve fascicles supplying areas with prominent subcutaneous fat stores (e.g. the lower abdominal region in men or the gluteal/femoral region in women) have not been reported.
To evaluate whether sympathetic fibres in cutaneous nerve fascicles can affect human subcutaneous lipolysis, we stimulated the lateral cutaneous femoral nerve electrically in healthy women. Lipolysis was monitored via microdialysis in the defined innervation area, and compared with the corresponding unstimulated area on the contralateral leg. In a subgroup of the subjects, the pattern of sympathetic activity in the cutaneous femoral nerve was compared with simultaneously recorded sympathetic traffic in nerves innervating areas without prominent subcutaneous fat stores, to evaluate whether a specific lipomotor firing pattern could be discerned.
METHODS
Subjects
Seven lean healthy females (age range, 20-39 years; body mass index, 21.9 ± 0.5 kg m−2), who were not under any medication, participated in the study. They had been weight-stable for at least 3 months, had fasted overnight and abstained from smoking and alcohol for 1 and 3 days prior to the experiment, respectively. The study was approved by the university ethical committee and participants gave written informed consent.
Main study protocol
Experiments started at 08.00 h and were conducted in the laboratory of the Department of Clinical Neurophysiology. Room temperature was kept at 24 ± 2°C. Subjects were supine for the investigation. An intravenous cannula was inserted into a dorsal hand vein for blood sampling, and the hand was warmed with heating pads wrapped in cotton blankets to increase blood flow and induce arterialization of the venous blood. By this approach a 93 ± 1 % oxygen saturation was achieved in sampled blood.
Intraneural stimulation and recording
This was performed with insulated tungsten microelectrodes (shaft diameter, 0.2 mm; uninsulated tip of a few micrometres). A reference electrode was placed a few centimetres from the stimulating/recording electrode. For electrical stimulation, the intraneural electrode was connected to a Grass S 48 stimulator. For recording, signals were amplified (gain, 50 000), filtered (band width, 0.7-2 kHz), passed through a window discriminator for noise reduction and passed through a resistance-capacitance circuit with adjustable time constant to obtain a mean voltage display of the nerve signal. Both filtered and mean voltage neurograms were stored on VHS tape (Racal V-Store; Racal Recorders Ltd, Southhampton, UK), together with recordings of electrocardiogram (via standard chest leads) and respiratory movements (strain gauge). Cutaneous nerve fascicles were identified by three criteria: (1) electrical stimuli through the electrode elicited skin paresthesias in the receptive field of the impaled fascicle but no muscle contractions, (2) neural activity (in sensory afferent fibres) was evoked by touch stimuli within the receptive field and (3) spontaneous nerve activity showed the firing pattern characteristic for skin sympathetic nerve fibres, with irregular bursts of activity without obvious pulse rhythmicity but readily activated by arousal or respiratory stimuli (Vallbo et al. 1979; Wallin & Elam, 1997b). To control for effects on regional blood flow during intraneural stimulation, skin perfusion was monitored with laser Doppler flowmetry (Periflux, Perimed AB, Stockholm, Sweden) within the innervation territory of the stimulated nerve fascicle and the corresponding area on the contralateral leg.
Microdialysis
This was performed with subcutaneous catheters (30 mm × 0.3 mm, cuprophane, 3000 MV cutoff), perfused with isotonic saline with 2.5 mmol l−1 glucose at a rate of 2.5 μl min−1 using a microinjection pump (Carnegie Medicine, Stockholm, Sweden). Relative recovery of glycerol was assessed with the internal reference technique previously described (Lönnroth & Strindberg, 1995). [U-14C]Glycerol (∼3000 c.p.m. μl−1; Amersham, USA) was added to the perfusate. Relative recovery was calculated as dialysate concentration/interstitial concentration of the analysed substance.
Experimental procedure
The lateral cutaneous femoral nerve was located at the outer third of the inguinal fossa, using transcutaneous electrical stimulation (40-70 V, 1 Hz, Grass S 48 stimulator) guided by the subject's reported sensations. Subsequently, a microneurography electrode was inserted towards the nerve, guided by electrical stimuli (3-7 V, 1 Hz) through the electrode. As soon as subjects reported parasthesias in the innervation territory of the nerve, stimulation was switched off and nerve traffic was recorded. While the skin was mechanically stimulated by stroking, to evoke mechanoreceptive afferent impulses, the electrode was slightly adjusted to impale a nerve fascicle which supplied a receptive field with a size of at least 10 cm × 10 cm. In four out of seven subjects, skin sympathetic nerve activity was recorded from the intraneural site used for the stimulation protocol, whereas only afferent nerve traffic could be recorded from the remaining three subjects.
When an electrode position representing a suitable innervation territory had been achieved, repeated short ramps (< 30 s) of intraneural stimulation with increasing intensity were used to determine an optimal level of stimulus intensity, judged by the subjects to be bearable during prolonged stimulation. Subsequently, microdialysis catheters were placed in the subcutaneous tissue within the receptive field of the impaled nerve fascicle and in the corresponding area of the contralateral leg (see Fig. 1 for schematic representation of the experimental setup). Our aim was to place two catheters, at least 2 cm apart, in the stimulated leg and this was successful in six of our seven subjects, whereas only one functioning catheter in the stimulated area was achieved in one subject (due to a malfunctioning second catheter).
Figure 1. Schematic drawing of the experimental setup.
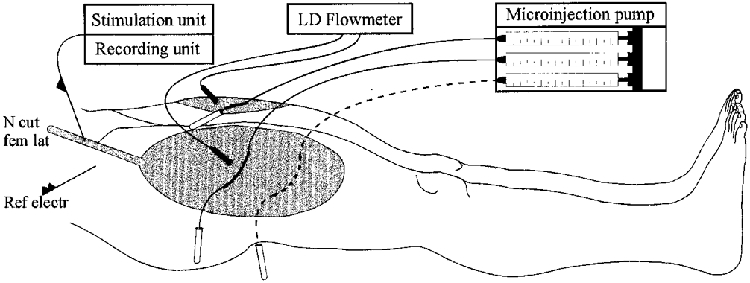
After a suitable stimulation/recording site within the lateral cutaneous femoral nerve (N cut fem lat) was established, 2 microdialysis probes were inserted in the receptive field of the impaled nerve fascicle and one additional control probe in a corresponding area of the contralateral leg. Microdialysis probes were perfused at a constant rate of 2.5 μl min−1. Ref electr, reference electrode; LD, laser Doppler.
After a microdialysis equilibration period of 45 min, a baseline period of 30 min was started and microdialysate samples were collected every 10 min, followed by an intraneural stimulation period of 10 min duration (2 Hz; mean stimulus intensity, 4.7 ± 1.8 V). During stimulation and a subsequent 30 min period, glycerol dialysates were sampled every 5 min.
Blood for the determination of glycerol, noradrenaline and adrenaline (epinephrine) concentration was drawn before the baseline period as well as prior to and after the stimulation period, and at the end of the experiment. Blood samples were immediately centrifuged and stored at -20°C (-70°C for catecholamines).
Laboratory analysis
Glycerol in dialysates and blood samples was analysed in a CMA 600 Microdialysis Analyser (Carnegie Medicine; enzymatical method; coefficient of variation, 3 %). To determine the radioactivity of the marked substances, samples were put in a liquid scintillation analyser (1900CA TRI-CARB, Packard, USA). Plasma noradrenaline and adrenaline were determined by high-performance liquid chromatography with electrochemical detection (Eriksson & Persson, 1982). The sensitivity was 6.03 pg ml−1 for noradrenaline and 6.5 pg ml−1 for adrenaline. The interassay coefficients of variation were 6.1 and 5.6 % for noradrenaline and adrenaline, respectively.
Data analysis
For statistical evaluation of interstitial glycerol release, analysis of variance with time (3 control values, 2 peri-stimulus values and 6 post-stimulus values) and leg (stimulated vs. unstimulated leg) as repeated measures factors was performed, followed by a Scheffépost hoc test. P < 0.05 was considered significant. Results are presented as means ±s.e.m. In subjects with two catheters in the stimulated leg, data from these were averaged before comparison with data from the unstimulated leg.
Double nerve recordings
Two of the subjects participated in an additional experiment in which sympathetic nerve activity of the lateral cutaneous femoral nerve was compared with skin sympathetic activity simultaneously recorded from the peroneal nerve at the level of the fibular head and the median nerve at the wrist. In these experiments, both intraneural recording positions were located as described above and sympathetic activity was recorded during a 15 min resting period, followed by a 2 min period of mental stress elicited by verbally administered arithmetic testing. Subjects were asked to rapidly subtract a one- or two-digit number from a three-digit number, and were repeatedly urged to perform better and harrassed for incorrect, and sometimes for correct, answers.
Apart from visual comparisons of the nerve records, frequency domain analysis was used to evaluate respiratory and cardiac modulation of sympathetic discharge at rest. Power spectral density was estimated using Welch's method (Welch, 1967) with a Hanning window and no overlap. Nine minute resting periods were divided into 18 sections and detrended.
Relative increases in sympathetic activity during mental stress were quantified by calculating the area under the curve in the mean voltage neurogram for the 2 min stress period and a 2 min resting period immediately prior to stress.
RESULTS
Effect of nerve stimulation on interstitial glycerol levels
The ANOVA on all glycerol data showed that both time and leg factors (P < 0.01 for both), as well as the time-leg interaction (P < 0.05) were statistically significant. A 10 min intraneural stimulation elicited a 22 ± 8 % increase in mean interstitial glycerol levels in the innervation territory of the stimulated nerve fascicle (mean of the baseline period, 334 ± 31 vs. 404 ± 83 μmol l−1 after stimulation, P < 0.05), whereas no significant effect was registered in the contralateral unstimulated leg (232 ± 29 vs. 240 ± 52 μmol l−1, n.s.). The interstitial glycerol levels were already significantly higher (47 ± 13 %, P < 0.05) in the stimulated innervation territory vs. the unstimulated control area of the contralateral leg at baseline (before the 10 min stimulation but after the search procedure and trial stimulations), and was 72 ± 17 % higher after the 10 min stimulation, illustrating the degree of lipolysis induced by the whole experimental procedure (Fig. 2). The change in interstitial glycerol release in the stimulated innervation area was not related to whether skin sympathetic nerve activity (SSA) could (n = 4) or could not (n = 3) be recorded at the intraneural site.
Figure 2. Interstitial glycerol concentrations in the stimulated subcutaneous area and the unstimulated control area in the contralateral leg in 7 healthy female subjects.
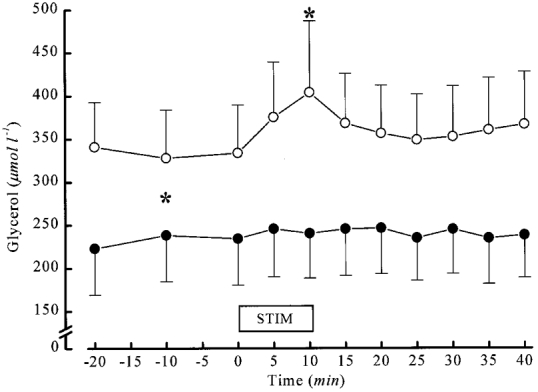
Glycerol concentrations were significantly higher in the stimulated subcutaneous area (○) from the start of measurement, in all probability due to the preceding neural search procedure and trial stimulations, and was further increased during 10 min of intraneural stimulation while no effect was seen in the control area of the contralateral leg (•). * P < 0.05.
Regional skin perfusion
On average, regional skin perfusion did not change significantly in either leg during nerve stimulation. Perfusion did increase 2700 % during stimulation in one stimulated leg, skewing the mean for stimulated legs from all subjects to 476 ± 372 % of the prestimulation perfusion level (n.s.). With this outlier excluded, the perfusion during stimulation was 103 ± 7 % of the prestimulus control level in the remaining six stimulated legs, and 98 ± 12 % in unstimulated legs (n.s. for both legs). The subject showing a stimulation-induced increase in perfusion in the stimulated leg did not differ from the other subjects with regard to the local lipolytic response, two subjects showing a stronger and four subjects a weaker increase in glycerol release during stimulation.
Plasma glycerol and catecholamines
Compared with the plasma glycerol level directly prior to stimulation (47.1 ± 6.0 μmol l−1), glycerol levels after 10 min of intraneural stimulation were insignificantly elevated (to 51.9 ± 8.8 μmol l−1). Plasma noradrenaline concentrations were 221 ± 18 pg ml−1 before and 262 ± 29 pg ml−1 at the end of the stimulation period (n.s.). Plasma adrenaline concentrations also remained unaffected by stimulation (42.1 ± 20.8 vs. 44.7 ± 21.9 pg ml−1).
Pattern of cutaneous sympathetic nerve activity
Sympathetic discharge in the lateral femoral nerve (6 recordings) showed the typical characteristics of cutaneous sympathetic activity (Wallin & Elam, 1997b), consisting of an irregular bursting activity, a clear respiratory modulation, but little or no cardiac rhythmicity, and was strikingly similar to simultaneously recorded sympathetic outflow in nerves innervating regions without prominent subcutaneous fat stores (simultaneous recordings in 2 subjects; Fig 3 and Fig 4). In the two double nerve investigations illustrated at rest in Fig 3 and Fig 4, mental stress elicited a parallel increase of sympathetic discharge in both nerves. Compared with the preceding resting period, sympathetic activity increased by 130 % in the femoral nerve and by 150 % in the peroneal nerve in subject A. The corresponding increases in subject B were 460 % in the femoral nerve and 540 % in the median nerve.
Figure 3. Double nerve recordings.
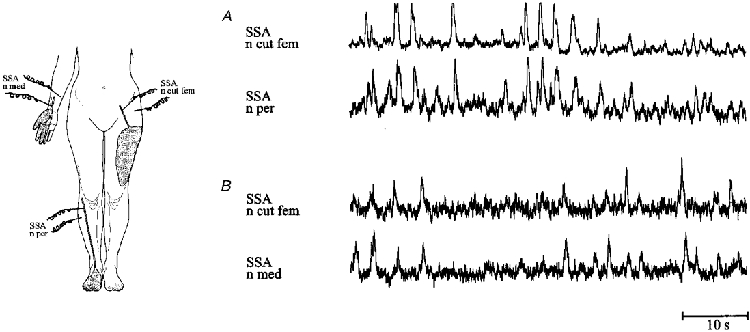
Excerpts from simultaneous recordings of sympathetic nerve activity in the lateral cutaneous femoral nerve (n cut fem) and a cutaneous fascicle of the peroneal nerve (n per) (subject A, A), and in the lateral cutaneous femoral nerve and a cutaneous fascicle of the median nerve (n med) (subject B, B). No specific lipomotor activity could be discerned within the mean voltage neurogram of the lateral cutaneous femoral nerve, when compared with the firing pattern in fascicles innervating territories without prominent subcutaneous fat stores.
Figure 4. Power spectra of resting sympathetic nerve activity in the two double nerve recordings in Fig. 3, as well as respiration and ECG.
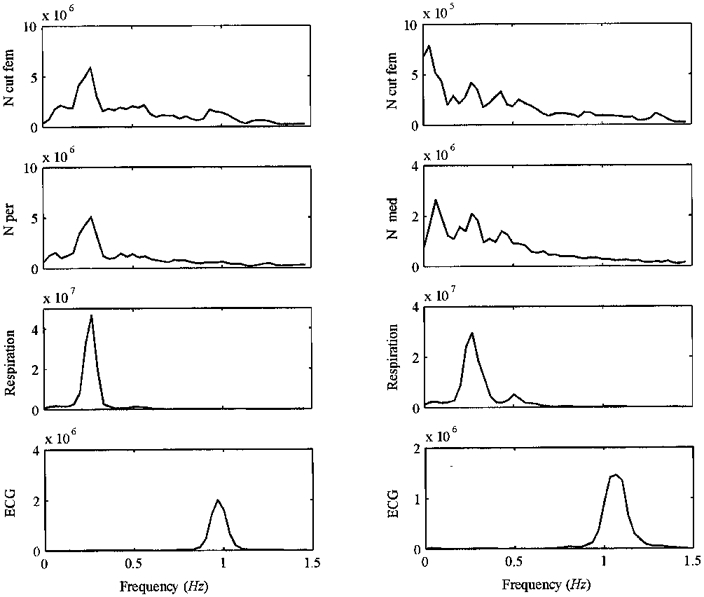
Note that the two nerve pairs show similar spectral peaks corresponding to the respiratory frequency peak (at 0.2 Hz), whereas neither of the nerves show a significant peak related to heart rate (at 1 Hz). Arbitrary units on y-axis.
DISCUSSION
The present study shows that electrical stimulation of the lateral cutaneous femoral nerve induced a regional increase in interstitial glycerol levels in the innervation territory of the stimulated nerve fascicle. This finding suggests that sympathetic nerve fibres travelling in cutaneous nerve fascicles exert a regulatory influence on subcutaneous fat tissue in humans.
In experiments using isolated perfused canine adipose tissue (Rosell & Belfrage, 1975, 1979), sympathetic stimulation has been shown to elicit changes in both regional blood flow and lipolysis, the latter indicated by an increase in glycerol levels. Our in vivo stimulation did not affect local skin perfusion in six out of seven subjects, and the subject in whom local perfusion did increase did not differ from the others in lipolytic response. However, the laser Doppler signal is dominated by perfusion in skin rather than underlying WAT, and a moderate perfusion change specific for WAT cannot be unequivocally excluded. Stimulation-induced blood flow changes could per se modify the interstitial concentration of tissue-derived glycerol, but such an indirect flow-related effect is unlikely to explain our present finding of regionally increased glycerol levels, for several reasons. (1) In subcutaneous tissue, catecholamines cause vasodilatation (Samra et al. 1996) and this dilatation leads to a decrease in interstitial glycerol concentration, not an increase (Enoksson et al. 1995). (2) Experimental nerve stimulation of isolated canine WAT has shown that vascular resistance changes depend on stimulation frequency, with no vasoconstriction induced at 2 Hz stimulation (the frequency used in our protocol), whereas vasoconstriction starts to dominate at stimulation rates above 3 Hz (Linde, 1976). (3) Blood flow changes would be expected to subside after termination of the stimulation and can therefore hardly explain the sustained increase in glycerol levels found in our study. We therefore interpret the increase in interstitial glycerol levels in our experiments as reflecting a regionally stimulated lipolysis. Although the intraneural stimulation was moderately painful, the unchanged interstitial glycerol level in the unstimulated leg argues against a generalized lipolytic effect due to stress, and the lack of change in plasma catecholamine levels further supports this conclusion.
Human sympathetic nerve discharge in skin-innervating nerve fascicles is generally considered to consist of a mixture of impulses from fibres innervating blood vessels and sweat glands (Hagbarth et al. 1972; Wallin & Elam, 1997a). Nerves supplying hairy skin should also contain pilomotor fibres, but only indirect evidence for a contribution of impulses from such fibres has been found in microneurographic recordings (Bini et al. 1980). In the present study, the sympathetic discharge in the lateral femoral nerve showed the typical characteristics of skin sympathetic activity, and the firing pattern was strikingly similar to the simultaneously recorded sympathetic discharge in cutaneous nerve fascicles innervating regions without prominent subcutaneous fat stores. Thus, no component of cutaneous sympathetic outflow specific for the nerve innervating prominent subcutaneous fat stores could be identified. Whether the lipolytic function described in the present paper is carried out by the vasomotor fibres reaching subcutaneous fat tissue or by a separate set of specific lipomotor fibres remains to be established (cf. Bartness & Bamshad, 1998).
Lipolysis induced by in vivo electrical nerve stimulation is blocked by β-adrenoceptor antagonists (Rosell, 1966). Adrenergic receptors responsible for the transduction of catecholaminergic influence on WAT have been further characterized in studies on isolated fat cells. β1-, β2- and β3-receptors have been shown to increase lipolytic rate, while the α2-receptor mediates an inhibitory effect (Arner, 1992; Lafontan & Berlan, 1993). Our finding of a regionally increased lipolysis suggests that our present intraneural stimulation procedure predominantly activates β-adrenoceptors in lean female subjects, while a putative inhibitory α2-adrenoceptor effect was overridden. It should be stressed, however, that a regular 2 Hz nerve stimulation bears little resemblance to a physiological firing pattern of cutaneous sympathetic nerve fibres. Future studies will have to determine to what extent the degree, and possibly the direction, of this effector response is sensitive to the frequency and pattern of nerve stimulation. Such studies will also have to address the possibility that non-adrenergic sympathetic co-transmitters may participate in this effector response since neuropeptide Y has been shown to inhibit lipolysis in human fat cells (Valet et al. 1990) whereas vasoactive intestinal polypeptide, at least in pharmacological concentrations, may increase lipolysis (Richter et al. 1989).
The sustained lipolytic effect, in all probability already initiated during the search procedure and trial stimulations, suggests that short periods of neuronal activation may modify the subcutaneous lipolytic rate for prolonged periods after stimulation has been terminated. The mechanism for such an effect remains to be elucidated. It could be argued that injury of, or mechanical pressure on, nerve fibres in the impaled nerve fascicle may have induced ectopic firing which persisted as long as the electrode was intraneurally inserted. Such an artefactual mechanism is highly unlikely, however, since no ectopic firing was recorded by our intraneural electrodes. Furthermore, no sustained effects of electrode insertion have been identified during the extensive use of intraneural recording/stimulation techniques in studies of other effector organs, such as cutaneous vaso- and sudomotor function (Wallin & Elam, 1997b).
A 72 % increase in lipolytic rate might be considered to be small in comparison with the severalfold increase in glycerol release registered from isolated adipocytes exposed to β-adrenoceptor-stimulatory agents (Fain & Garcia-Sainz, 1983; Arner, 1992; Lafontan et al. 1997). However, in contrast to these in vitro data, the present in vivo results are influenced by additional endocrine, paracrine and autocrine factors, all potentially modifying the lipolytic response to neural stimulation (Fredholm, 1978; Fain & Shepherd, 1979). Furthermore, the putative local vasodilatation elicited by the stimulation could lead to an underestimation of the stimulation-induced lipolysis (see above). Finally, to accurately interpret the present data it is important to note that these were obtained in the postabsorptive state, per se characterized by an activated lipolysis. Low insulin levels may be regarded as the main driving factor for the activated lipolysis prevailing at rest in the postabsorptive state, because β-adrenoceptor-blocking agents (Wijnen et al. 1993; Felländer et al. 1996) or reduced sympathetic outflow to adipose tissue (Karlsson et al. 1995, 1997) do not alter postabsorptive interstitial glycerol levels, whereas insulin exposure (Hagstrom-Toft et al. 1991, 1992) or oral glucose (Jansson et al. 1992) effectively reduces interstitial glycerol by ∼75 %. The additional activation of lipolysis induced by our nerve stimulation agrees well with the magnitude of increase in interstitial glycerol obtained in postabsorptive subjects during sympathoexcitatory manoeuvres such as static muscle contraction or mental stress (Hagstrom-Toft et al. 1993; Karlsson et al. 1997).
A role for sympathetic outflow to skeletal muscle in human obesity has been proposed (Spraul et al. 1993; Snitker et al. 1998), but this sympathetic subdivision modulates glycerol concentrations in muscle and not in subcutaneous fat tissue (Henry et al. 1998). Our present demonstration of a neural modulation of subcutaneous fat tissue metabolism mediated via cutaneous nerve fascicles, in conjunction with the well established fact that sympathetic activity at rest and in response to various stimuli differs substantially between fascicles innervating muscle and skin (Vallbo et al. 1979; Wallin & Elam, 1997a), indicates that a sympathetically mediated mobilization of fat stores may be highly differentiated and may involve different tissues depending on the type of stimulus.
Limitations of the study
The number of nerve fibres activated by intraneural electrical stimulation increases with increasing stimulus intensity and strong (i.e. painful) stimuli probably activate most fibres (myelinated and unmyelinated, efferent and afferent) within an impaled nerve fascicle (Wallin & Elam, 1997b), many fibre types having a lower threshold for activation than that of sympathetic efferent fibres. The lateral cutaneous nerve contains no myelinated efferent fibres but the possibility of effects induced by antedromic activation of sensory nerve fibres cannot be excluded, although neither the existence nor possible functions of a sensory innervation of WAT are known (Bartness & Bamshad, 1998).
Conclusions
In conclusion, our study indicates that skin nerve fascicles convey lipomotor activity to subcutaneous fat tissue. Combining the stimulation/recording of cutaneous sympathetic nerves with microdialytic evaluation of subcutaneous fat mobilization may provide new insights into the physiology and pathophysiology of human fat metabolism in vivo.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Foundation (project no. 10864 and 12170) and the medical faculty, University of Göteborg. The excellent technical assistance of Tomas Karlsson, Lena Strindberg, Göran Pegenius and Christiane Zinke is gratefully acknowledged.
References
- Arner P. Adrenergic receptor function in fat cells. American Journal of Clinical Nutrition. 1992;55:228–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- Arner P, Kriegholm E, Engfeldt P, Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. Journal of Clinical Investigation. 1990;85:893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnsen M, Burrin JM, Johnston DG, Pernet A, Walker M, Alberti KG. Mechanisms of catecholamine effects on ketogenesis. American Journal of Physiology. 1984;247:E173–180. doi: 10.1152/ajpendo.1984.247.2.E173. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. American Journal of Physiology. 1998;44:R1399–1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Bini G, Hagbarth K-E, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. The Journal of Physiology. 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll JW. Adipose tissue: ability to respond to nerve stimulation in vitro. Science. 1963;140:387–388. doi: 10.1126/science.140.3565.387. [DOI] [PubMed] [Google Scholar]
- Enoksson S, Nordenstrom J, Bolinder J, Arner P. Influence of local blood flow on glycerol levels in human adipose tissue. International Journal of Obesity and Related Metabolic Disorders. 1995;19:350–354. [PubMed] [Google Scholar]
- Eriksson BM, Persson BA. Determination of catecholamines in rat heart tissue and plasma samples by liquid chromatography with electrochemical detection. Journal of Chromatography. 1982;228:143–154. doi: 10.1016/s0378-4347(00)80427-2. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Fain JN, Garcia-Sainz JA. Adrenergic regulation of adipocyte metabolism. Journal of Lipid Research. 1983;24:945–966. [PubMed] [Google Scholar]
- Fain JN, Shepherd RE. Hormonal regulation of lipolysis: role of cyclic nucleotides, adenosine, and free fatty acids. Advances in Experimental Medicine and Biology. 1979;111:43–77. doi: 10.1007/978-1-4757-0734-2_3. [DOI] [PubMed] [Google Scholar]
- Felländer G, Eleborg L, Bolinder J, Nordenstrom J, Arner P. Microdialysis of adipose tissue during surgery: effect of local alpha- and beta-adrenoceptor blockade on blood flow and lipolysis. Journal of Clinical Endocrinology and Metabolism. 1996;81:2919–2924. doi: 10.1210/jcem.81.8.8768852. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Local regulation of lipolysis in adipose tissue by fatty acids, prostaglandins and adenosine. Medical Biology. 1978;56:249–261. [PubMed] [Google Scholar]
- Hagbarth K-E, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiologica Scandinavica. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E, Arner P, Johansson U, Eriksson LS, Ungerstedt U, Bolinder J. Effect of insulin on human adipose tissue metabolism in situ. Interactions with beta-adrenoceptors. Diabetologia. 1992;35:664–670. doi: 10.1007/BF00400260. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E, Arner P, Naslund B, Ungerstedt U, Bolinder J. Effects of insulin deprivation and replacement on in vivo subcutaneous adipose tissue substrate metabolism in humans. Diabetes. 1991;40:666–672. doi: 10.2337/diab.40.6.666. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E, Arner P, Wahrenberg H, Wennlund A, Ungerstedt U, Bolinder J. Adrenergic regulation of human adipose tissue metabolism in situ during mental stress. Journal of Clinical Endocrinology and Metabolism. 1993;76:392–398. doi: 10.1210/jcem.76.2.8381801. [DOI] [PubMed] [Google Scholar]
- Henry S, Trueb L, Sartori C, Scherrer U, Jequier E, Tappy L. Effects of a sympathetic activation by a lower body negative pressure on glucose and lipid metabolism. Clinical Physiology. 1998;18:562–569. doi: 10.1046/j.1365-2281.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Jansson P-A, Larsson A, Smith U, Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. Journal of Clinical Investigation. 1992;89:1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A-K, Attvall S, Jansson P-A, Sullivan L, Lönnroth P. Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: a study in spinal cord-injured subjects. Metabolism. 1995;44:52–58. doi: 10.1016/0026-0495(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Karlsson A-K, Elam M, Friberg P, Biering-Sörensen F, Sullivan L, Lönnroth P. Regulation of lipolysis by the sympathetic nervous system: a microdialysis study in normal and spinal cord-injured subjects. Metabolism. 1997;46:388–394. doi: 10.1016/s0026-0495(97)90053-6. [DOI] [PubMed] [Google Scholar]
- Klein S, Peters EJ, Holland B, Wolfe RR. Effect of short- and long-term beta-adrenergic blockade on lipolysis during fasting in humans. American Journal of Physiology. 1989;257:E65–73. doi: 10.1152/ajpendo.1989.257.1.E65. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpene C, Bousquet-Melou A, Berlan M. Adrenergic regulation of adipocyte metabolism. Human Reproduction. 1997;12(suppl. 1):6–20. doi: 10.1093/humrep/12.suppl_1.6. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. Journal of Lipid Research. 1993;34:1057–1091. [PubMed] [Google Scholar]
- Linde B. Studies on the vascular exchange function in canine subcutaneous adipose tissue. Acta Physiologica Scandinavica. 1976;(suppl. 433):1–43. [PubMed] [Google Scholar]
- Lönnroth P, Strindberg L. Validation of the ‘internal reference technique’ for calibrating microdialysis catheters in situ. Acta Physiologica Scandinavica. 1995;153:375–380. doi: 10.1111/j.1748-1716.1995.tb09875.x. [DOI] [PubMed] [Google Scholar]
- Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. New England Journal of Medicine. 1988;318:1077–1083. doi: 10.1056/NEJM198804283181701. [DOI] [PubMed] [Google Scholar]
- Richter WO, Robl H, Schwandt P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides. 1989;10:333–335. doi: 10.1016/0196-9781(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Rosell S. Release of free fatty acids from subcutaneous adipose tissue in dogs following sympathetic nerve stimulation. Acta Physiologica Scandinavica. 1966;67:343–351. doi: 10.1111/j.1748-1716.1966.tb03320.x. [DOI] [PubMed] [Google Scholar]
- Rosell S, Belfrage E. Adrenergic receptors in adipose tissue and their relation to adrenergic innervation. Nature. 1975;253:738–739. doi: 10.1038/253738a0. [DOI] [PubMed] [Google Scholar]
- Rosell S, Belfrage E. Blood circulation in adipose tissue. Physiological Reviews. 1979;59:1078–1104. doi: 10.1152/physrev.1979.59.4.1078. [DOI] [PubMed] [Google Scholar]
- Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, Frayn KN. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. American Journal of Physiology. 1996;271:E834–839. doi: 10.1152/ajpendo.1996.271.5.E834. [DOI] [PubMed] [Google Scholar]
- Slavin BG, Ballard KW. Morphological studies on the adrenergic innervation of white adipose tissue. Anatomical Record. 1978;191:377–390. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- Snitker S, Tataranni PA, Ravussin E. Respiratory quotient is inversely associated with muscle sympathetic nerve activity. Journal of Clinical Endocrinology and Metabolism. 1998;83:3977–3979. doi: 10.1210/jcem.83.11.5265. [DOI] [PubMed] [Google Scholar]
- Spraul M, Ravussin M, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity: a potential mechanism to body weight gain. Journal of Clinical Investigation. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valet P, Berlan M, Beauville M, Crampes F, Montastruc JL, Lafontan M. Neuropeptide Y and peptide YY inhibit lipolysis in human and dog fat cells through a pertussis toxin-sensitive G protein. Journal of Clinical Investigation. 1990;85:291–295. doi: 10.1172/JCI114425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Elam M. Microneurography and autonomic dysfunction. In: Low P, editor. Clinical Autonomic Disorders: Evaluation and Managment. Boston: Little, Brown & Co.; 1997a. pp. 233–243. [Google Scholar]
- Wallin BG, Elam M. Cutaneous sympathetic nerve activity in humans. In: Gibbins IL, Morris JL, editors. Autonomic Innervation of the Skin. Amsterdam: Harwood Academic Publishers; 1997b. pp. 111–132. [Google Scholar]
- Welch PD. The use of Fast Fourier Transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Transactions on Audio and Electroacoustics. 1967;AU-15:70–73. [Google Scholar]
- Wijnen JA, Van Baak MA, De Haan C, Boudier HA, Tan FS, Van Bortel LM. Beta-blockade and lipolysis during endurance exercise. European Journal of Clinical Pharmacology. 1993;45:101–105. doi: 10.1007/BF00315488. [DOI] [PubMed] [Google Scholar]


