Abstract
Synaptic responses of 46 substantia gelatinosa (SG) neurones in the spinal dorsal horn to cutaneous mechanical and/or thermal stimuli were investigated in an in vivo rat preparation with whole-cell patch-clamp recordings. The clamped neurones were identified as being in the SG based on either their morphological features by intrasomatic injection of biocytin or the depth of the neurones from the surface of the spinal cord.
In all SG neurones examined where spontaneous EPSCs occurred, pinch (noxious) and air (innocuous) stimuli applied to the ipsilateral hindlimb elicited a barrage of EPSCs (some of which initiated an action potential under current-clamp conditions), which subsided just after cessation of the stimuli without any residual slow current (or after-discharge). The spontaneous and evoked EPSCs were reversibly abolished by a non-N-methyl-D-aspartate (non-NMDA) receptor antagonist, CNQX (20 μm).
Noxious (≥ 45 °C) or innocuous (≤ 40 °C) thermal stimuli did not elicit any synaptic responses in all 18 SG neurones tested which were sensitive to mechanical stimuli. Noxious cold stimulation (≤ 10 °C) also failed to produce any responses (n = 6).
It is concluded that both noxious and innocuous mechanical information to SG neurones are transmitted primarily by activation of non-NMDA receptors, probably without any involvement of slow synaptic transmission, and that thermal information is conveyed to areas of the dorsal horn other than SG.
Substantia gelatinosa (SG) neurones in the dorsal horn (lamina II of Rexed; Rexed, 1952) of the spinal cord are of great interest because of their possible role in the modulation of nociceptive transmission from the periphery to the CNS (Cervero & Iggo, 1980; Brown, 1982). Primary afferent inputs to the SG are thought to derive both from high-threshold mechanoreceptive afferents conducting in the Aδ-fibre range and from polymodal nociceptors conducting in the C-fibre range (Light & Perl, 1979; Sugiura et al. 1986); these central terminals contain various neurotransmitters, e.g. L-glutamate (Greenamyre et al. 1984) and substance P (Hökfelt et al. 1977; see Willis & Coggeshall, 1991, for review). Microelectrode and patch-clamp techniques have been adapted to SG neurones in a spinal cord slice with an attached dorsal root to investigate synaptic responses to peripheral nerve stimulation (Schneider & Perl, 1988; Yoshimura & Jessell, 1989; Randic et al. 1993; Yoshimura & Nishi, 1993; Baba et al. 1994; Jeftinija & Urban, 1994; Yajiri et al. 1997; Yang et al. 1999; Nakatsuka et al. 1999). These studies revealed that SG neurones exhibit a variety of excitatory and inhibitory synaptic responses which range in duration from milliseconds to minutes (see Yoshimura, 1996, for review). It remains, however, to be settled what kinds of stimulation applied to the skin elicit these responses.
In vivo intracellular recordings from SG neurones have been used to measure synaptic responses evoked by cutaneous stimuli (Bennett et al. 1980; Woolf & Fitzgerald, 1983; Light & Kavookjian, 1988). However, attempts to record synaptic activities from them have rarely been sufficiently stable for prolonged investigation because of their small size (∼10 μm in diameter; Woolf & Fitzgerald, 1983) and also because of spinal cord pulsations caused by respiration and heart beat. Furthermore, a limitation of microelectrode methods is the trade-off between the small electrode-tip size needed to impale neurones and the low resistance needed to pass currents through the microelectrode. This compromise is largely avoided by the whole-cell patch-clamp technique (Sakmann & Neher, 1983). In the present study, we have developed a method for making whole-cell recordings from SG neurones in vivo in order to analyse functional synaptic responses by cutaneous mechanical and thermal stimuli.
METHODS
Preparation
Experiments were carried out in 26 male Sprague-Dawley rats (7-10 weeks old). All the experiments involving rats were conducted in accordance with the Saga Medical School Guidelines for Animal Experimentation and the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science of the Physiological Society of Japan. Anaesthesia was induced with either urethane (1.2-1.5 g kg−1, i.p.) or pentobarbital (40 mg kg−1, i.p.); in the latter case, pentobarbital (4 mg kg−1 h−1) was continuously supplemented. There were no differences in synaptic responses recorded from SG neurones between rats anaesthetized by either method. Under an artificial ventilation, a lumbar laminectomy was performed at the level of L4 or L5 and the animal was then placed in a stereotaxic apparatus (Fig. 1A). Under a binocular microscope with ×8 to ×40 magnification, the dura was cut and reflected, and then, since the dorsal roots enter the spinal cord above the level at which recordings of SG neurones were made, a dorsal root was lifted using a glass hook so that a recording electrode could be advanced into the SG from the surface of the spinal cord. The superficial dorsal grey matter lateral to the dorsal root entry zone was discernible as a relatively translucent band under Lissauer's tract. The pia-arachnoid membrane was cut to make a window large enough to allow the patch electrode into the spinal cord. The surface of the exposed spinal cord was irrigated with Krebs solution (mm: NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, glucose 11, NaHCO3 25) equilibrated with 95 % O2-5 % CO2 at 38 ± 0.5°C. At the end of the experiments the animals were killed by exsanguination.
Figure 1. Schematic diagram of anaesthetized rat preparation and identification of SG neurones from which in vivo patch-clamp recordings were taken.
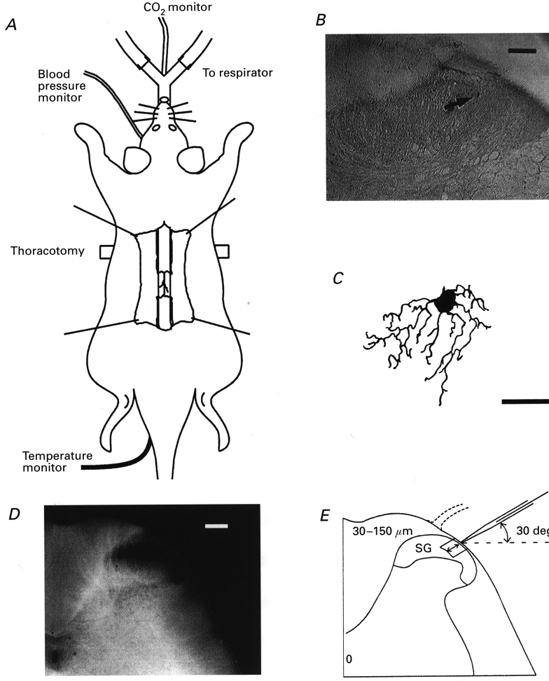
A, a urethane anaesthetized rat which was artificially ventilated by cannulation of the trachea, where end-tidal CO2 partial pressure was maintained between 3.5 and 4.5 %; under this ventilation, a bilateral pneumothorax was made to reduce a respiration-induced movement of the spinal cord. Blood pressure was monitored from a cannulated carotid artery; the mean values ranged from 80 to 120 mmHg. Rectal temperature was kept at 37-38 °C by means of a heating pad placed beneath the animal. The lumbar spinal cord at the level of L4 or L5 was exposed by laminectomy. B, location of a recorded cell which was identified with an intracellular injection with biocytin. After recording synaptic responses, the spinal cord was fixed and then cut into 50 μm slices. The tissue was incubated with a streptavin-biotin-peroxidase complex solution and then reacted with diaminobenzidine (0.3 mg ml−1). C, camera lucida drawing of the neurone shown in B, which was reconstructed from seven serial transverse sections. D, transverse slice of the spinal cord at the level of L4. The SG was clearly discernible as a relatively translucent band across the dorsal horn. E, schematic drawing of the transverse slice shown in D and of the arrangement of a recording electrode. The electrode was advanced into the SG at an angle of 30 deg. Recordings were made from cells at a depth of 30-150 μm (shown by arrows) from the surface of spinal cord. Scale bars in B, C and D are 100, 20 and 200 μm, respectively.
Penetration of the patch electrode into the spinal cord and cell identification
The electrodes used were pulled from thin-walled borosilicate glass capillaries (o.d. 1.5 mm) using a puller (p-97, Sutter Instrument, USA), and were filled with a patch-pipette solution of the following composition (mm): potassium gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, ATP-Mg 5, Hepes-KOH 5; pH 7.2. This had a resistance of 10-15 MΩ. As shown in Fig. 1E, the electrode was advanced at an angle of 30 deg into the SG through the window using a micromanipulator (Model WR-88, Narishige, Japan), and a gigaohm seal was then formed with a cell at a regular depth of 30-150 μm measured from the point of contact with the cell to the the dorsal surface of the spinal cord. This distance was within the SG which was identified using transverse slices obtained from the spinal cords of 7- to 10-week-old rats in the same lumbar level (Fig. 1D and E). The location and morphological features of the recorded cells were further confirmed in some instances by an intrasomatic injection of biocytin (0.5 % in electrode solution; Fig. 1B) after obtaining synaptic responses. The neurone shown in Fig. 1B was located in the SG and possessed morphological features similar to cells described previously as SG neurones using either Golgi staining (Beal & Bicknell, 1985) or horseradish peroxidase labelling (Woolf & Fitzgerald, 1983) (Fig. 1C). The present recordings were made using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA). Current and voltage data were digitized with an A/D converter (Digidata 1200; Axon Instruments) and stored on a personal computer using the pCLAMP 6 data acquisition program (Axon Instruments). Input resistance was determined in the range -70 to -80 mV by applying a voltage step (duration 100 ms).
Stimulation protocols
Mechanical and thermal stimuli were applied to the skin of the hindlimb. The noxious and innocuous mechanical stimuli used were pinching of skin folds with toothed forceps and puffing air onto the skin, respectively. There were no differences in synaptic responses recorded from SG neurones between cells stimulated with air puffs with and without a preceding pinch stimulus. Using a feedback-controlled thermal stimulator with a radiant heat lamp (DPS-705, Dia Medical System, Japan), noxious (45-60°C; skin temperature measured with a thermometer set on the skin) and innocuous (38-40°C) thermal stimuli were applied for 15-30 s from a baseline temperature of ∼32°C. Noxious cold (2-10°C) stimuli were applied for 15-30 s by placing a cold beaker on the skin. It took 2-5 s to reach the target temperatures from the baseline temperature. The drug used to abolish spontaneous or evoked EPSCs in this work was 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Tocris Neuramin, Bristol, UK). CNQX was added to Krebs solution which perfused the surface of the spinal cord.
Statistics
Data values are presented as means ±s.e.m. Statistical significance was determined as P < 0.05 using Student's paired t test. In all cases n refers to the number of neurones studied.
RESULTS
Obtaining a high seal resistance from an SG neurone and its success rate
While advancing a patch-pipette electrode through the surface of the spinal cord, we applied a continuous positive pressure (with compression of an air volume of 20 ml to 15 ml using a syringe) to the electrode; this prevented clotting of the electrode tip with surface tissue debris. At a depth of more than 30 μm from the dorsal surface of the spinal cord, the pressure in the electrode was reduced by 2-3 ml while the electrode was passed into the SG at a steady speed (2 μm s−1). When a small decrease in the current amplitude (80-90 % of initial amplitude) occurred, the pressure was released, and then a slight negative pressure (1-2 ml) was applied until a high-resistance seal was established. The success rate for forming high seal resistances of more than 3 GΩ (7.3 ± 1.1 GΩ, n = 13) was more than 50 % of all trials. The membrane patch was ruptured by applying an additional negative pressure to obtain the whole-cell recording configuration. The success rate for achieving this configuration was as low as 20 %, presumably because a seal could be made from either the edge of a neurone or a glial cell. However, stable recordings from single SG neurones could be maintained for up to 3 h, once the whole-cell mode was established. Recordings could be obtained from in vivo preparations for more than 8 h. During 1 day of recordings between two and six cells could be thoroughly investigated.
Membrane properties and spontaneous synaptic responses
The data presented in this paper were obtained from 46 SG neurones with resting membrane potentials of -62.9 ± 2.2 mV (n = 15) and input membrane resistances of 569 ± 49 MΩ (n = 14); these values were higher than those recorded with microelectrodes from SG neurones in spinal cord slices (Yoshimura & Jessell, 1989, 1990), presumably due to the improved seal between the electrode and the membrane that is possible with patch electrodes. Under voltage-clamp conditions at a holding potential (VH) of -70 mV, SG neurones exhibited EPSCs with an average amplitude of 21.7 ± 8.7 pA and a frequency of 5.1 ± 2.2 Hz (n = 8; Fig. 2A); these values were not distinct from those obtained from SG neurones in in vitro slice preparations (15.1 ± 0.7 pA and 9.0 ± 0.8 Hz; Kohno et al. 1999; P > 0.05).
Figure 2. Excitatory synaptic responses in SG neurones occurring spontaneously and in response to mechanical but not thermal stimuli.
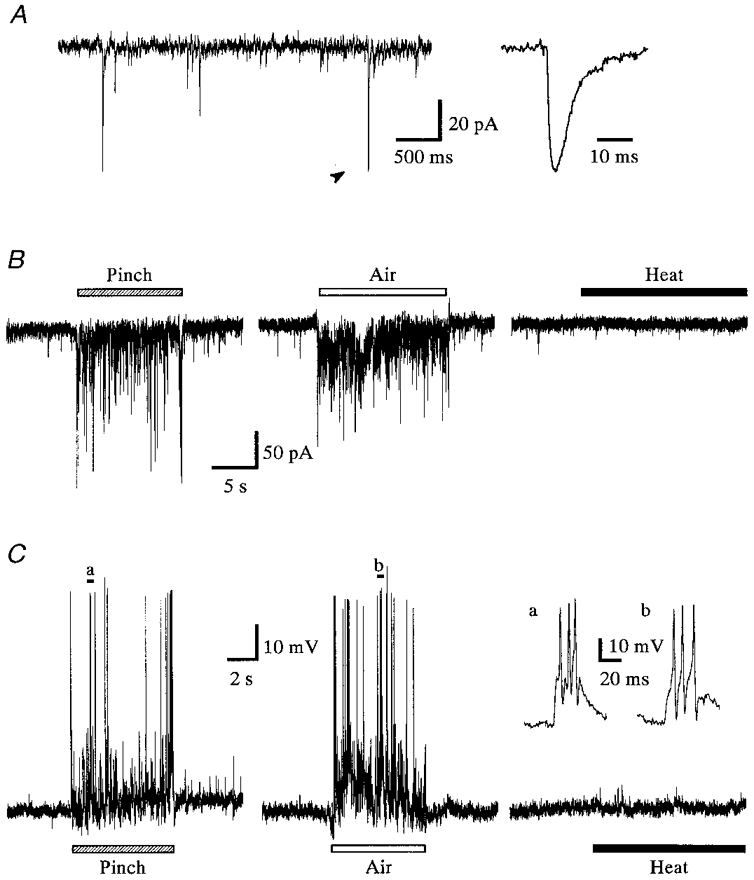
A, spontaneous EPSCs recorded under voltage-clamp conditions at a VH of -70 mV. The EPSC indicated by the arrowhead in the left panel is shown on an expanded time scale in the right panel; this has a duration of ≈20 ms. B, EPSCs elicited by pinch or air stimuli applied to the ipsilateral thigh (VH -70 mV); the responses disappeared immediately after the cutaneous stimuli were terminated. C, under current-clamp conditions (resting potential -65 mV), the pinch or air stimuli also elicited EPSPs, some of which initiated action potentials. SG neurones were more sensitive to pinch than air stimulation, producing EPSCs with larger peak amplitudes. Noxious heat stimulation given to the skin of the hindlimb elicited no detectable response (right panels in B and C). Bars above or below the traces show the duration of the stimulation. Insets in C show the action potentials marked a and b in the left and middle panels on an expanded time scale. Data in B and C were obtained from the same neurone.
Synaptic responses evoked by cutaneous mechanical stimuli, but not thermal stimuli, and the receptive fields
All 46 SG neurones examined responded to both noxious (pinch) and innocuous (air) mechanical stimuli. Either pinch or air stimuli applied to the ipsilateral hindlimb elicited a barrage of EPSCs; these disappeared within 1 s after the stimuli were terminated (Figs 2B and 4). The average frequency of EPSCs during the stimuli was 29.5 ± 8.2 Hz for pinch and 19.0 ± 5.7 Hz for an air puff (n = 8); they were not significantly different (P > 0.05). The peak amplitudes were not determined, because multiple summation resulting from the high-frequency bursting of EPSCs made it difficult to obtain an accurate estimation. Under current-clamp conditions, pinch or air stimuli elicited EPSPs, some of which initiated an action potential, as shown in Fig. 2C. Each SG neurone had an excitatory receptive field throughout the thigh and sole of the foot, although the evoked EPSCs were different in amplitude and frequency at each point stimulated (Fig. 3). The point most sensitive to stimulation was different for each cell tested. Stimulating the contralateral hindlimb did not elicit any synaptic responses (data not shown). These evoked and spontaneous EPSCs were suppressed by CNQX (20 μm), where no slow response was noted as seen in Fig. 4. The effect of CNQX in abolishing the EPSCs was rapid, as was recovery after washout, indicating that the effect was due to a direct action in the spinal cord and was not achieved via blood circulation.
Figure 4. Effect of CNQX on the responses evoked in SG neurones by cutaneous mechanical stimuli.
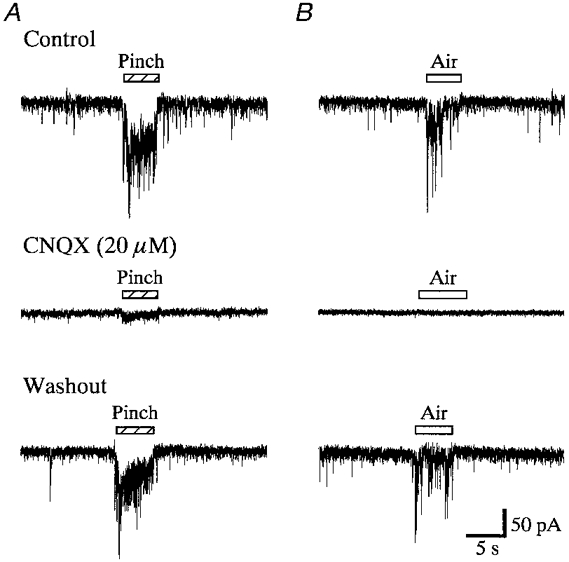
EPSCs evoked by pinch (A) or air (B) stimuli were substantially suppressed by CNQX (20 μm) in a reversible manner. Middle and lower records were obtained after 1 min in the presence of CNQX and 2 min after its washout, respectively. Note that spontaneous EPSCs were also blocked by CNQX. Bars above the traces show the duration of the stimulation. Records in A and B were obtained from the same neurone. VH = -70 mV.
Figure 3. Representative receptive field of an SG neurone in response to pinch stimuli.
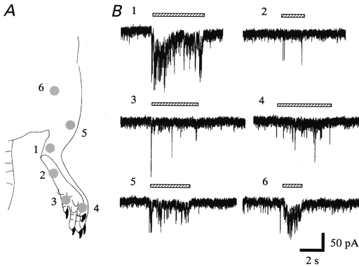
A, locations of pinch stimuli applied to the hindlimb; they were applied to the lower leg (1), foot (2), toes (3, 4), upper leg (5) and thigh (6). B, response to the stimulus applied to each of the locations marked in A. Although stimuli applied between the thigh and the toe elicited EPSCs in SG neurones, the lower leg (1) was most sensitive. VH = -70 mV. Another three SG neurones also had receptive fields extending from the thigh to the toes.
Next we tested the effect of thermal stimuli since the SG has been reported to receive abundant inputs from C-afferent fibres which are thought to convey thermal as well as mechanical information (Sugiura et al. 1986). Unexpectedly, noxious heat stimuli did not elicit any synaptic responses (see Fig. 2B and C). All SG neurones examined (n = 18), which were located throughout the SG, did not respond to a change in temperature from ∼32°C to 38-60°C in the skin of the hindlimb. When neurones in other laminae were examined, deep dorsal horn neurones (existing at a depth of more than 300 μm from the dorsal surface of the spinal cord; see Methods) responded to thermal stimuli with a barrage of EPSCs (n = 5; not shown). Noxious cold stimulation (temperature change from ∼32°C to 2-10°C) also failed to elicit any responses in SG neurones (n = 6).
DISCUSSION
We report here for the first time that the whole-cell patch-clamp technique can be applied to spinal cord neurones of the in vivo adult rat. Though in vivo patch recordings from cat visual cortex neurones have been reported (Pei et al. 1991), the tight-sealing appears to be incomplete. The higher gigaohm seal achieved on establishing the whole-cell patch-clamp configuration in the present study may have resulted from either a striking absence of myelinated axons in the SG (Willis & Coggeshall, 1991) or smaller pulsations of the spinal cord placed in our stereotaxic apparatus. Light & Willcockson (1996) have produced a preliminary reportof in vivo patch-clamp recordings from SG neurones in the rat spinal cord.
In the present study, all SG neurones examined responded to both noxious and innocuous mechanical stimuli and their receptive fields extended through the whole hindlimb. This indicates that SG neurones receive excitatory inputs from larger receptive fields throughout the hindlimb than was previously thought. In previous extra- and intracellular recordings from rats, SG neurones were classified into innocuous, noxious and wide dynamic range neurones based on their response profiles; the receptive field investigated was restricted to either the foot or the toes in many neurones (Woolf & Fitzgerald, 1983). The differences in the response profile and field size between these studies are due to the fact that subthreshold EPSCs could be recorded in the present study. The possibility cannot be ruled out that neurones from a specific region (medial or lateral) of the SG were examined, resulting in a responsiveness to both noxious and innocuous stimuli. It is unlikely that the responsiveness to both noxious and innocuous stimuli is due to selective recordings from SG neurones located in the inner layer of the SG, since SG neurones stained with biocytin were found in both inner and outer layers and furthermore exhibited morphological features similar to those of islet or stalk cells.
Our present results also revealed that the EPSCs elicited by mechanical stimuli in SG neurones are substantially suppressed by CNQX, indicating that non-NMDA receptors have been activated. A similar action of CNQX on responses evoked by cutaneous mechanical stimuli has been observed in intracellular recordings from dorsal horn neurones in an in vitro preparation of the hamster spinal cord with partially intact innervation from an isolated patch of hairy skin (Schneider & Perl, 1994). Many Aδ- and C-primary afferent terminals establish monosynaptic connections with dendrites of SG neurones (Light & Perl, 1979; Sugiura et al. 1986). In our previous studies using slice preparations of the spinal cord, Aδ- and C-afferent fibres were primarily responsible for mono- and polysynaptic EPSCs in SG neurones and the contribution of Aβ-afferents appeared to be quite small (see Yoshimura & Nishi (1993) for Aδ-fibre data, and Nakatsuka et al. (1999) for Aδ- and C-fibre data). The mechanical information is, therefore, conveyed to the SG neurones by the activation of non-NMDA receptors through the glutamatergic Aδ- and C-primary afferents. It is possible that the activation of NMDA receptors is also involved in the transmission, since SG neurones in in vitro spinal cord slices exhibit an NMDA component of synaptic responses (Yoshimura & Jessell, 1990). This remains to be examined.
Although Sugiura et al. (1986) have reported that thermoreceptive C-afferent fibres terminate in the SG, SG neurones did not respond to thermal stimuli applied to the skin. Consistent with the present observation, a previous study using extracellular unit recordings from the rat dorsal horn has demonstrated that neurones sensitive to cutaneous thermal stimuli exist only in laminae I, IV and V (Menétrey et al. 1979). The thermoreceptive C-afferent fibres terminating in the SG may transmit the information to neurones located in other, possibly deeper laminae; this possibility is suggested by results in the present study. This notion is supported by the following observations.
Central terminals of the C-afferent fibres contain one or more neuropeptides, such as substance P (SP), and excitatory amino acids (see Willis & Coggeshall, 1991, for review). SP terminals are found in abundance in the SG in the rat and cat (Hökfelt et al. 1975a,b) and SP is considered to be mainly involved in innocuous sensation. However, in our previous studies using in vitro slice preparations of the rat spinal cord, SG neurones were insensitive to SP (Yoshimura et al. 1993). In the present study, SG neurones in vivo also did not exhibit any responses to SP (2 μm, n = 3; not shown). On the other hand, in vivo intracellular recordings from cat deep laminae neurones combined with immunocytochemical techniques have shown that noxious mechanical stimuli evoke a slow synaptic response that can last for 20 s to 1 min, through the activation of SP-containing fibres (De Koninck & Henry, 1991; De Koninck et al. 1992). Immunohistochemical studies have revealed that neurokinin-1 receptors are almost completely absent within lamina II and that noxious thermal stimuli produce neurokinin-1 receptor internalization in lamina I cells (Allen et al. 1997). It is suggested that C-afferents containing SP and probably also excitatory amino acids make a synaptic connection with dendrites of neurones which are located in laminae other than SG. This hypothesis remains to be examined in detail.
Acknowledgments
We are grateful to Professor Masuko and Dr Murata for histological support. This work was supported in part by the Human Frontier Science Program and Grants-in-Aids for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan to M.Y.
References
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. Journal of Neuroscience. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. The Journal of Physiology. 1994;478:87–99. doi: 10.1113/jphysiol.1994.sp020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal JA, Bicknell HR., Jr . Development and maturation of neurons in the substantia gelatinosa (SG) of the rat spinal cord. In: Rowe M, Willis WD Jr, editors. Development, Organization, and Processing in Somatosensory Pathways. New York: Wiley-Liss; 1985. pp. 23–30. [Google Scholar]
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. Journal of Comparative Neurology. 1980;194:809–827. doi: 10.1002/cne.901940407. [DOI] [PubMed] [Google Scholar]
- Brown AG. The dorsal horn of the spinal cord. Quarterly Journal of Experimental Physiology. 1982;67:193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A. The substantia gelatinosa of the spinal cord. A critical review. Brain. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Henry JL. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proceedings of the National Academy of Sciences of the USA. 1991;88:11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Ribeiro-da-Silva A, Henry JL, Cuello AC. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proceedings of the National Academy of Sciences of the USA. 1992;89:5073–5077. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, Young AB, Penney JB. Quantitative autoradiographic distribution of L-[3H]glutamate-binding sites in rat central nervous system. Journal of Neuroscience. 1984;4:2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Kellerth J-O, Nilsson G, Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Research. 1975a;100:235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Kellerth J-O, Nilsson G, Pernow B. Substance P: localization in the central nervous system and in some primary sensory neurons. Science. 1975b;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proceedings of the National Academy of Sciences of the USA. 1977;74:3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S, Urban L. Repetitive stimulation induced potentiation of excitatory transmission in the rat dorsal horn: an in vitro study. Journal of Neurophysiology. 1994;71:216–228. doi: 10.1152/jn.1994.71.1.216. [DOI] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. The Journal of Physiology. 1999;518:803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III-V) Journal of Comparative Neurology. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. Journal of Comparative Neurology. 1979;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Light AR, Willcockson HH. In vivo, whole-cell recordings from spinal substantia gelatinosa (Lamina II) neurons of adult rats. Society for Neuroscience Abstracts. 1996;22:861. [Google Scholar]
- Menétrey D, Chaouch A, Besson J-M. Responses of spinal cord dorsal horn neurones to non-noxious and noxious cutaneous temperature changes in the spinal rat. Pain. 1979;6:265–282. doi: 10.1016/0304-3959(79)90048-4. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Park J-S, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999;82:39–47. doi: 10.1016/S0304-3959(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Pei X, Volgushev M, Vidyasagar TR, Creutzfeldt OD. Whole cell recording and conductance measurements in cat visual cortex in vivo. NeuroReport. 1991;2:485–488. doi: 10.1097/00001756-199108000-00019. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. Journal of Neuroscience. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. Journal of Comparative Neurology. 1952;96:415–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Neher E. Single Channel Recording. New York: Plenum Press; 1983. pp. 3–51. [Google Scholar]
- Schneider SP, Perl ER. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. Journal of Neuroscience. 1988;8:2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Synaptic mediation from cutaneous mechanical nociceptors. Journal of Neurophysiology. 1994;72:612–621. doi: 10.1152/jn.1994.72.2.612. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Coggeshall EE. Sensory Mechanisms of the Spinal Cord. 2. New York: Plenum Press; 1991. pp. 79–107. [Google Scholar]
- Woolf CJ, Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. Journal of Comparative Neurology. 1983;221:313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Yajiri Y, Yoshimura M, Okamoto M, Takahashi H, Higashi H. A novel slow excitatory postsynaptic current in substantia gelatinosa neurons of the rat spinal cord in vitro. Neuroscience. 1997;76:673–688. doi: 10.1016/s0306-4522(96)00291-6. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Li Y-Q, Yoshimura M. Action of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Research. 1999;830:268–273. doi: 10.1016/s0006-8993(99)01408-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M. Slow synaptic transmission in the spinal dorsal horn. Progress in Brain Research. 1996;113:443–462. [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. Journal of Neurophysiology. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. The Journal of Physiology. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Shimizu T, Yajiri Y, Inokuchi H, Nishi S. Primary afferent-evoked slow EPSPs and responses to substance P of dorsal horn neurons in the adult rat spinal cord slices. Regulatory Peptides. 1993;46:407–409. doi: 10.1016/0167-0115(93)90102-e. [DOI] [PubMed] [Google Scholar]


