Abstract
Ionic current responses elicited by acetylcholine (ACh) in follicle-enclosed Xenopus oocytes (follicles) were studied using the two-electrode voltage-clamp technique. ACh generated a fast chloride current (Fin) and inhibited K+ currents gated by cAMP (IK,cAMP) following receptor activation by adenosine, follicle-stimulating hormone or noradrenaline. These previously described cholinergic responses were confirmed to be of the muscarinic type, and were independently generated among follicles from different frogs.
Inhibition of IK,cAMP was about 100 times more sensitive to ACh than Fin activation; the half-maximal effective concentrations (EC50) were 6.6 ± 0.4 and 784 ± 4 nm, respectively.
Both responses were blocked by several muscarinic receptor antagonists. Using the respective EC50 concentrations of ACh as standard, the antagonist 4-diphenylacetoxy-N-methylpiperidine methiodide blocked the two effects with very different potencies. Fin was blocked with a half-maximal inhibitory concentration (IC50) of 2.4 ± 0.07 nm, whilst the IC50 for IK,cAMP inhibition was 5.9 ± 0.2 μm.
Oxotremorine, a muscarinic agonist, preferentially stimulated IK,cAMP inhibition (EC50= 15.8 ± 1.4 μm), whilst Fin was only weakly activated. In contrast, oxotremorine inhibited Fin generated by ACh with an IC50 of 2.3 ± 0.7 μm.
Fin elicited via purinergic receptor stimulation was not affected by oxotremorine, indicating that the inhibition produced was specific to the muscarinic receptor, and suggesting that muscarinic actions do not exert a strong effect on follicular cell-oocyte coupling.
Using reverse transcription-PCR, transcripts of a previously cloned muscarinic receptor from Xenopus (XlmR) were amplified from the RNA of both the isolated follicular cells and the oocyte. The pharmacological and molecular characteristics suggest that XlmR is involved in IK,cAMP inhibition.
In conclusion, follicular cells possess two different muscarinic receptors, one resembling the M2 (or M4) subtype and the other the M3 subtype. These receptors are coupled to distinct membrane mechanisms leading to independent regulation of two membrane conductances.
Follicle-enclosed Xenopus oocytes (follicles) are endowed with cholinergic receptors which, when stimulated by acetylcholine (ACh), generate various ionic current responses (Kusano et al. 1977, 1982; Dascal et al. 1985; Arellano & Miledi, 1993; for a review see Arellano et al. 1996). At least five different responses to ACh have been characterized. One response arises in the membrane of the oocyte itself, and results from the opening of Ca2+-dependent Cl− channels (e.g. Miledi, 1982; Miledi et al. 1989). The other four types of cholinergic response have their origin in the membrane of the follicular cells and can be monitored via electrodes inserted into the oocyte, because of its strong electrical coupling with the enveloping follicular cells (Browne & Werner, 1984; van den Hoef et al. 1984; Woodward & Miledi, 1987; Arellano & Miledi, 1993, 1995). For the present study, we focused on two cholinergic current components that are the most ubiquitous responses in follicles. One is the fast inward Cl− current (Fin; Arellano & Miledi, 1993), which can also be activated by purinergic agents (e.g. ATP, UTP). Fin activation by ACh cross-inhibits that generated by ATP, and vice versa, strongly suggesting that the transmitters act on a common channel-activation pathway (Arellano et al. 1998). The second cholinergic component studied here is the ACh-induced inhibition of K+ currents (IK,cAMP) that are activated by an increase in cAMP synthesis in the follicular cells stimulated by various compounds, including follicle-stimulating hormone (FSH), noradrenaline, adenosine (ADE) or dopamine (Kusano et al. 1977, 1982; Van Renterghem et al. 1984, 1985; Dascal et al. 1985; Stinnakre & Van Renterghem, 1986; Woodward & Miledi, 1987; Greenfield et al. 1990). An important feature of the cholinergic inhibition of IK,cAMP is that when that K+ current is generated by superfusion of a membrane-permeant cAMP analogue, or by intra-oocyte injection of cAMP, ACh is still able to inhibit the current completely, thus suggesting that the cholinergic action takes place downstream of cAMP synthesis (Dascal et al. 1985; Miledi & Woodward, 1989).
The follicle responses elicited by ACh are of the muscarinic type (Kusano et al. 1977, 1982; Arellano & Miledi, 1993). Studies aimed at determining the pharmacological and molecular profile of the receptors involved have all focused on the receptors responsible for activation of the typical oscillatory current due to opening of Ca2+-dependent Cl− channels in the oocyte membrane (Kusano et al. 1982; Van Wezenbeek et al. 1988; Davidson et al. 1991), with little attention paid to the other response types.
Currently, little is known about the molecular nature of the muscarinic receptors mediating the follicular cell-based cholinergic responses, or about the membrane mechanisms being activated. It is not known how many receptor subtypes are involved in generating the multiple components of the cholinergic response of the follicle, knowledge that would help to elucidate their role in follicular cell-oocyte physiology. However, one muscarinic receptor (XlmR) from Xenopus laevis oocytes has been cloned and characterized. Based on its amino acid sequence, XlmR is homologous to the human m4 subtype (Herrera et al. 1994, 1997).
Although a specific physiological role has not yet been clearly shown for any of the muscarinic responses, it appears that cholinergic actions on Xenopus follicles modulate important events, such as the maturation induced by progesterone, where ACh accelerates the process (Dascal et al. 1984). Also, it has been suggested that muscarinic activation of osmolarity-dependent Cl− currents plays a role in follicle volume regulation (Arellano & Miledi, 1993). Moreover, knowledge of the pharmacological and physiological membrane mechanisms activated in Xenopus follicles may serve as a model towards a comprehensive understanding of muscarinic actions in other cellular systems, especially in ovarian systems of other species, including human, where muscarinic effects have also been shown (Eusebi et al. 1984).
METHODS
Cell preparation
Xenopus laevis frogs were obtained from Xenopus I (Ann Arbor, MI, USA) and Xenopus Express (Homosassa, FL, USA). Three to four ovarian lobules were surgically removed under sterile conditions from frogs anaesthetized with 1 g l−1 tricaine, and rendered hypothermic. After surgery, the frogs were allowed to recover consciousness. No further oocytes were taken for at least 2 months. After the final taking of oocytes, the anaesthetized frogs were killed by decerebration and pithing. The procedure was approved by the institutional animal use committees. The lobules were placed in sterile modified Barth's medium (containing (mm): 88 NaCl, 0.2 KCl, 2.4 NaHCO3, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 0.88 KH2PO4, 2.7 Na2HPO4, pH 7.4; with 70 μg ml−1 gentamicin).
Follicle-enclosed oocytes (stage VI; Dumont, 1972) were removed from the ovary by peeling away the inner ovarian epithelium, together with the thecal blood vessels, with sharp watchmaker's forceps. This procedure leaves the follicular cell basement membrane, thus providing protection and a natural environment for the follicular cells. Moreover, removal of the epithelium facilitates electrode insertion, improves the stability of electrophysiological recording and simplifies the interpretation of results by eliminating the possible participation of the epithelium or other surrounding thecal tissues in the responses (Arellano et al. 1998). These ‘epithelium-removed’ follicles were incubated (18-20°C) in sterile Barth's medium supplemented with glucose (5 mm) and fetal bovine serum (0.1-0.2 %). Under these conditions, follicular cell-oocyte electrical coupling and follicular responses can be maintained for more than 10 days.
For follicular cell isolation, the follicle-enclosed oocytes (stage VI) were dissected from the ovary as above except that the external layers were removed together with the basal membrane of the follicular cells. In this way, the follicular cell layer was exposed and remained attached to the vitelline envelope (cf. plate I, Miledi & Woodward, 1989), thus allowing isolation of the follicular cells by enzymatic treatment. For this purpose, 200-1000 of these ‘unzipped’ follicles were incubated (5 min) in Hanks’ balanced salt solution containing 0.05 % trypsin and 0.5 mm EDTA, then gently washed in Barth's medium containing 10 % fetal bovine serum. The follicular cells were dislodged from the oocyte by repeatedly drawing the treated follicles into a polished Pasteur pipette. The oocytes were discarded and the dislodged follicular cells were recovered from the supernatant and maintained at -80°C prior to RNA purification. Using this method we obtained a homogeneous population of follicular cells, corresponding to those that are in immediate contact with the oocyte (R. Reyes, S. Alshihabi, R. O. Arellano & R. Miledi, unpublished observations).
Defolliculated oocytes were prepared by treatment with collagenase (0.5-1 mg ml−1) at room temperature for 40-50 min in normal frog Ringer (NR) solution containing (mm): 115 NaCl, 2 KCl, 1.8 CaCl2, 5 Hepes, pH 7.0. After washing of the oocytes, all their remaining external envelopes, except for the vitelline layer, were removed using fine forceps. The defolliculated oocytes maintained in Barth's medium were then used for purification of RNA (see below), or for electrophysiological recording.
Electrophysiological techniques
Follicular electrical responses were monitored using a two-microelectrode voltage clamp (Miledi, 1982). Unless otherwise stated, the follicles were voltage clamped at -60 mV for studies of Fin, and at -40 mV for IK,cAMP, for better differentiation of the responses. Follicles were bathed in NR solution, and drugs were applied by superfusion (10 ml min−1, chamber volume of 0.4 ml). Current-voltage relationships were obtained by changing the membrane potential from +20 to -120 mV, in 20 mV steps of 80 ms, before (control membrane current) and during the peak of the current response activated by the different drugs. For each voltage step, the control membrane currents were subtracted from those obtained during drug-elicited currents, and these values were plotted, as in Fig. 1A.
Figure 1. Current-voltage and dose-response relationships of follicular responses generated by ACh.
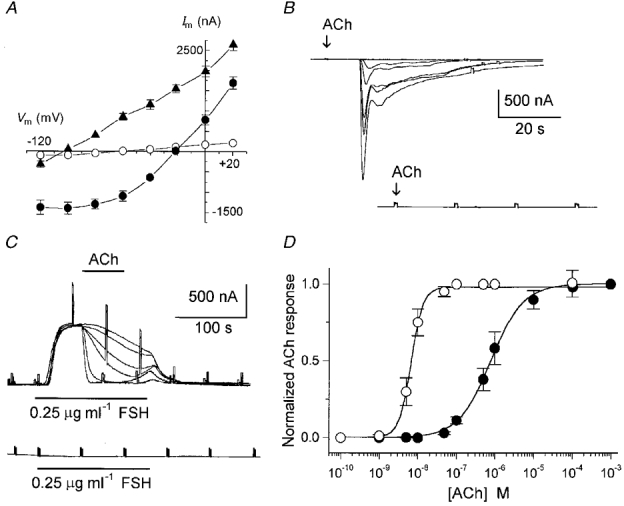
Intra-oocyte injections of EGTA were made by pneumatic pressure ejection from micropipettes; the injection solution (100 mm EGTA) was made up in 5 mm Hepes, pH adjusted to 7.0 with KOH. A similar injection apparatus was used for extracellular applications of a brief pulse jet of ACh (100 μm in NR solution) from a pipette positioned close (ca 50 μm) to the follicle (Arellano et al. 1998).
Reverse transcription-PCR (RT-PCR) and hybridization analysis
Amplification of XlmR (Herrera et al. 1994) transcripts from total RNA purified from isolated follicular cells or from defolliculated oocytes was performed using the RT-PCR technique. Total RNA was purified using the guanidinium isothiocyanate method (Chomczynski & Sacchi, 1987). First strand cDNA was synthesized using SuperScript II RNase H− reverse transcriptase with oligo-dT (100 pM) and random hexamers (10 pM) as primers in a total volume of 25 μl (1 h, 42°C). Aliquots of 2 μl were then amplified by a step cycle (94°C for 1 min; 55°C for 1 min; and 72°C for 1 min), for thirty cycles. In control reactions the reverse transcriptase was omitted. The primers designed to amplify 430 bp of the XlmR cDNA (bases 18 to 449) were based on the sequence reported by Herrera et al. (1994) and were as follows: forward, 5′-CTT CTT GCT GGG TAA GTC AGT G-3′; and reverse, 5′-GGA- AAA CGA CAC TTG GGA AAA T-3′. The RT-PCR products were then resolved in 1 % agarose gels, and were further analysed by Southern blotting, using standard procedures (Sambrook et al. 1989). DNA was transferred to Hybond N membrane (Amersham, NJ, USA), and the XlmR cDNA, generously supplied by Dr Juan Olate (Laboratorio de Genética Molecular, Facultad de Ciencias Biológicas, Universidad de Concepción, Chile), was used as a specific hybridization probe.
Substances
follicle-stimulating hormone was purchased from Calbiochem (La Jolla, CA, USA). Pilocarpine hydrochloride, oxotremorine sesquifumarate, 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), pirenzepine dihydrochloride (PZP), metoctramine tetrahydrochloride (MTT), tropicamide (TPC) and pertussis toxin were obtained from RBI (Natick, MA, USA). Hanks’ balanced salt solution, SuperScript II RNase H− reverse transcriptase, primers, and oligo-dT were from Gibco BRL (Gaithersburg, MD, USA). Random hexamers were from Boehringer (Mannheim, Germany). All other compounds, such as collagenase Type I, ATP, ACh, carbachol, nicotine, noradrenaline, ADE, dopamine, atropine, tubocurarine, tricaine, trypsin and salts, were from Sigma Chemical Co. (St Louis, MO, USA).
RESULTS
Dose-response relationships of follicular cholinergic responses
The follicles used in this study had a mean resting potential in NR solution of -43 ± 6 mV (234 follicles, 27 frogs) (all data given as means ±s.e.m.), and a mean input resistance of 0.62 ± 0.14 MΩ and, as reported previously, ACh-activated current responses made up of several components whose amplitudes varied greatly among follicles from different frogs (Kusano et al. 1977, 1982; Arellano & Miledi, 1993). The two cholinergic responses studied here, Fin activation and IK,cAMP inhibition, were generated in ca 90 % of the donors. For all frogs, both cholinergic responses were routinely confirmed to be of follicular cell origin, since oocyte defolliculation (n = 2-5 per donor) completely eliminated Fin and IK,cAMP (Fig. 1B and C). Moreover, the ACh responses were confirmed to be potently blocked by atropine (1 μm; 5 follicles, 2 frogs), and were not affected by intra-oocyte (n = 9, 4 frogs) injections of EGTA, indicating that both currents were fundamentally Ca2+ independent, as has been shown previously (Dascal et al. 1985; Miledi & Woodward, 1989; Arellano & Miledi, 1993). Some other important characteristics of Fin and IK,cAMP were confirmed in this study, for comparison with previous work. Briefly, Fin was follicular cell-based current with a reversal potential of -21.6 ± 3 mV (Fig. 1A), and with an onset delay of ca 400 ms, measured by application of a brief jet of ACh (100 μm) from a micropipette positioned 50 μm from the follicle (5 follicles, 2 frogs). Fin inactivated (50 % from the peak) in approximately 10-25 s (e.g. Fig. 1B), and was not dependent on the external osmolarity (15 follicles, 6 frogs). Fin elicited by ACh was inhibited (50 %) by overnight incubation with pertussis toxin (5 μg ml−1), suggesting a G protein-mediated receptor channel pathway (7 follicles, 2 frogs). All of these characteristics were consistent with those of Fin responses elicited by ATP (Arellano et al. 1998). Fin activation by ATP cross-inhibited Fin activated by ACh, suggesting that both agonists acted on a common channel-activation pathway.
On the other hand, IK,cAMP had a mean reversal potential of -98 ± 2 mV (Fig. 1A), and the inhibitory effect of ACh on IK,cAMP had the same characteristics as those described before (Dascal et al. 1985; Van Renterghem et al. 1985; Stinnakre & Van Renterghem, 1986; Woodward & Miledi, 1987) (Fig. 1C). For example, inhibition of IK,cAMP was associated with a decrease in membrane conductance (Fig. 1A), was Ca2+ independent as mentioned above, and was mimicked by 4β-phorbol 12,13-dibutyrate (1 μm; 3 follicles, 2 frogs).
Thus, in full accord with previous studies, the two ACh responses studied here shared certain characteristics: (1) both were abolished by defolliculation, (2) both were blocked by atropine, a non-specific muscarinic antagonist, and (3) both cholinergic actions were Ca2+ independent. Despite these common general characteristics, some other properties suggested that they were elicited independently. For example, the amplitudes of the two cholinergic actions in follicles from different frogs were not correlated. In follicles (n = 12) from one particular frog, ACh (10 μm) elicited a strong inhibition (95 ± 3 %) of IK,cAMP activated by FSH (0.25 μg ml−1) or ADE (10 μm), while in the same follicles ACh (10-100 μm) elicited weak Fin responses of 48 ± 15 nA. Similar results were obtained in follicles from two other frogs. In contrast, in follicles (n = 11) from another frog, ACh inhibited IK,cAMP by only 63 ± 5 % while it generated robust Fin of 947 ± 152 nA; a similar relationship was also found in follicles from two other donors. Also, in both follicles and oocytes where endogenous acetylcholinesterase (Moya et al. 1991) was specifically blocked, the amplitudes of the muscarinic currents were still highly variable (R. Miledi & R. O. Arellano, unpublished observations), suggesting that this characteristic was not due to the activity of the enzyme. Thus, as has been amply shown for several different types of receptor in Xenopus follicles (Arellano et al. 1996), the amplitudes of the two responses to ACh studied here were independently expressed among follicles from different frogs.
The latter indirect evidence for independence of the two cholinergic responses was then investigated in more detail. Dose-response curves were constructed for both ACh effects (Fig. 1B-D) in 12-16 follicles from 5 frogs; the Fin amplitude and percentage of IK,cAMP inhibition were normalized and plotted as in Fig. 1D. In these experiments, follicles from two frogs were tested for ACh-generated inhibition of IK,cAMP (elicited by 0.25 μg ml−1 FSH) when the K+ current was at its maximum (Fig. 1C). In follicles from another frog, curves were generated by superfusing ACh for approximately 1 min before and during stimulation by FSH (same concentration) or ADE (e.g. Fig. 2B). Both stimulation protocols gave the same results. The follicular ACh responses were dose dependent, with IK,cAMP inhibition being much more sensitive to ACh than Fin activation. The half-maximal effective concentration (EC50) for IK,cAMP inhibition was 6.6 ± 0.4 nm, while for Fin activation the EC50 was 784 ± 4 nm. A clear difference was also observed in the Hill slope coefficients (nH) of the dose-response curves, which were approximately 3 for IK,cAMP inhibition and 1 for Fin activation. This suggests a highly cooperative mechanism in the case of the K+ current inhibition, and a more direct mechanism for Fin activation. The sensitivity to ACh inhibition was independent of the agonist used for activating IK,cAMP; thus ADE (0.2 μm), dopamine (0.5 μm) and noradrenaline (10 μm) gave similar results, and dose-response curves obtained at a holding potential of -60 mV showed no differences (not shown).
Figure 2. Dose-response relationships of muscarinic antagonists on follicular responses to ACh.
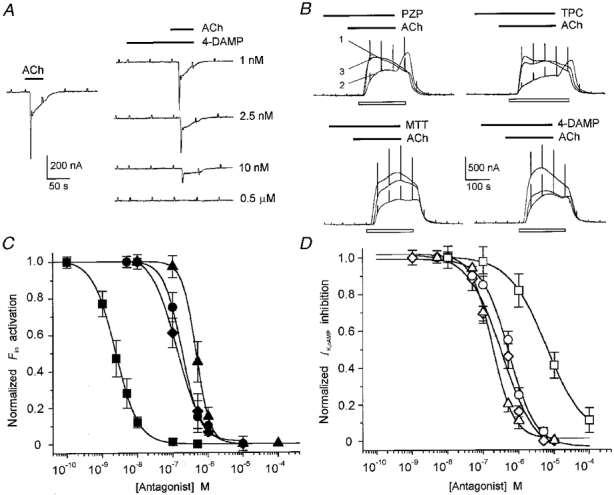
A, effect of 4-DAMP on Fin generated by 800 nm ACh. The traces on the right show 4-DAMP blockade of Fin elicited by ACh; the trace on the left is the control current. Wash periods were of 10 min. Data from a single follicle. B, blocking effect of 1 μm PZP, TPC, MTT or 4-DAMP on 8 nm ACh inhibition of IK,cAMP generated by 2 μm ADE (open bar below each trace). Superimposed traces in each group correspond to control current (ADE alone; 1), IK,cAMP inhibition in the presence of ACh and ADE (2), and the same as 2 plus the antagonist which was superfused for 100 s before ACh application (3). Wash intervals were of 15 min. Data from different follicles (2 frogs). C and D, dose-antagonist relationships for the effect of 4-DAMP (squares), MTT (circles), PZP (triangles) and TPC (diamonds) on Fin activation (C) and IK,cAMP inhibition (D) elicited by ACh at the EC50 concentration. Curves were fitted using the same equation as for Fig. 1D, and each point represents the mean of 4-5 follicles from 2 frogs. IK,cAMP was generated by 2 μm ADE or 2 μg ml−1 FSH.
Blocking effects of cholinergic antagonists
As a first approach to discriminate the different cholinergic responses at a membrane receptor level, the effects of various antagonists were analysed. Neither of the follicle currents was affected by tubocurarine (1-10 μm) and neither was mimicked by nicotine (0.1-1 mm) (6 follicles, 3 frogs), and as noted before both were blocked by atropine; thus the responses were confirmed to be of the muscarinic type. The results of previous pharmacological studies in Xenopus oocytes suggested the presence of muscarinic receptors of the M1 and M3 subtypes (Van Wezenbeek et al. 1988; Davidson et al. 1991), whereas the amino acid sequence of XlmR shows that it is more comparable to the human m4 subtype (Herrera et al. 1994), although competitive inhibition binding curves show that XlmR exhibits similarities to the m2 subtype (Herrera et al. 1997). In view of this, we studied the antagonistic effects of PZP, MTT, 4-DAMP and TPC (Fig. 2), which have, respectively, a high affinity for M1, M2, M3 and M4 receptors in other preparations (e.g. Caulfield & Birdsall, 1998). For these experiments, the follicular responses were elicited by ACh doses around their respective EC50, i.e. 7-10 nm ACh for IK,cAMP inhibition and 0.8-1 μm ACh for Fin activation. To study the effects on Fin, a given concentration of an antagonist was applied alone for 100-120 s, before ACh and the antagonist (same concentration) were coapplied (Fig. 2A). To examine the effects of antagonists on the ACh-induced inhibition of IK,cAMP, a mixture of ACh plus antagonist and ADE or FSH was applied (Fig. 2B). Fin was more potently blocked by 4-DAMP than the ACh-induced inhibition of IK,cAMP; all other compounds blocked the responses over a similar submicromolar range. The half-maximal inhibitory concentrations (IC50) for Fin of PZP, MTT, 4-DAMP and TPC (Fig. 2C) (slope factor (nH) in parentheses) were, respectively, 457 ± 3 nm (2), 183 ± 15 nm (1.7), 2.4 ± 0.07 nm (1.3) and 134 ± 9 nm (1.1). The IC50 values for block of the inhibition of IK,cAMP were 183 ± 20 nm (1.4), 530 ± 11 nm (1), 5900 ± 200 nm (0.8) and 341 ± 15 nm (1) (see Fig. 2C and D). The relative potencies for block of Fin activation or IK,cAMP inhibition by PZP, MTT and TPC were fairly similar; however, 4-DAMP was about 2000 times more potent in blocking Fin, thus supporting the notion that cholinergic actions upon Fin activation and IK,cAMP inhibition involve distinct muscarinic receptors. The potency sequences were 4-DAMP >> TPC ≈ MTT > PZP for the effect on Fin, and PZP > TPC ≈ MTT >> 4-DAMP for the effect on IK,cAMP inhibition. The higher sensitivity to 4-DAMP compared with that to PZP and MTT suggests that a population of M3 receptors is involved in the activation of Fin.
In follicles from the same frogs used to study the antagonism of ACh effects, Fin activation or IK,cAMP inhibition produced by ATP (10 μm) through activation of purinergic receptors was not affected by any of the four cholinergic antagonists (100 μm) tested (13 follicles, 4 frogs; data not shown). Thus, these antagonists appear to act specifically on the follicular muscarinic receptors, and not on ion channels or some other molecular component of the membrane pathways involved.
Effects of cholinergic agonists on follicular ACh responses
The generation of follicular responses by activation of muscarinic receptors was then studied using different agonists. Carbachol, a non-specific agonist, elicited both ACh responses (Fig. 3D); dose-response curves gave EC50 values of 51.5 ± 3 and 329 ± 17 nm for IK,cAMP inhibition and Fin activation, respectively. Both responses elicited by carbachol (100 μm; 9 follicles, 3 frogs) were blocked by atropine (1 μm), 4-DAMP or PZP (both at 100 μm). In contrast, when the follicular responses were elicited by oxotremorine, an agonist with higher affinity for the M2 receptor subtype, there was a clear specificity in the response elicited. Oxotremorine did not activate Fin responses as robustly as did ACh or carbachol in the same follicles (Fig. 3A). Indeed, the Fin responses elicited by oxotremorine (0.1-100 μm) were in all cases < 5 % of those activated by the other agonists (same concentration range). For example, in eight follicles from one frog the Fin response elicited by ACh (10 μm) was 1387 ± 340 nA, while the Fin response generated by oxotremorine (100 μm) was 36 ± 8 nA. All follicles (n = 28) from different frogs (n = 6) behaved similarly. However, oxotremorine completely inhibited the IK,cAMP elicited by FSH or ADE (Fig. 3B-D). The EC50 for oxotremorine-elicited inhibition was 15.8 ± 1.4 μm (13 follicles, 5 frogs).
Figure 3. Dose-response relationships of muscarinic agonists on follicular responses.
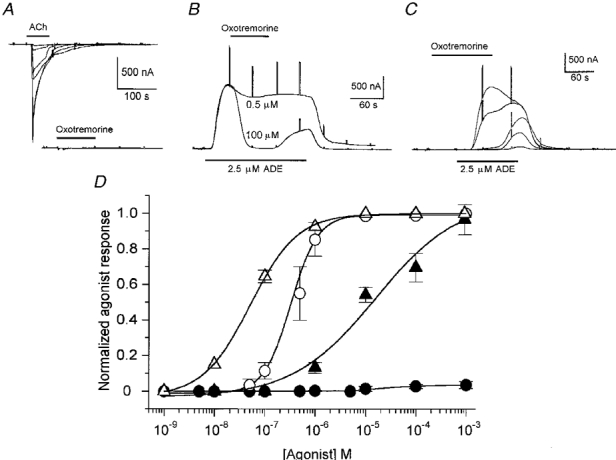
A, Fin generated by ACh and oxotremorine in a single follicle. The ACh concentrations were 0.05, 0.1, 0.5, 1, 10 and 100 μm, and the oxotremorine concentrations were 1, 10 and 100 μm. B, inhibition by oxotremorine of IK,cAMP (elicited by ADE). Oxotremorine (0.5 or 100 μm) was applied at the peak of the K+ current. C, preincubation with oxotremorine also blocked IK,cAMP. The oxotremorine concentrations were 0.1, 1, 10, 100 and 1000 μm. Note that at intermediate concentrations (1-100 μm) IK,cAMP inhibition was reversed rapidly on washout. D, carbachol (open symbols) and oxotremorine (filled symbols) dose-response relationships for Fin activation (circles) and IK,cAMP inhibition (triangles). Mean of 9-13 follicles from 5 frogs.
The inhibition of IK,cAMP induced by 10-100 μm oxotremorine (14 follicles, 6 frogs) was blocked by cholinergic antagonists such as atropine (1 μm), 4-DAMP, PZP and TPC (all at 100 μm), thus indicating that oxotremorine was acting on muscarinic receptors (Fig. 4A). Oscillatory Ca2+-dependent Cl− currents generated by ACh in follicles (n = 8) from some donors (3 frogs) were not mimicked by applications of oxotremorine (0.01-100 μm; Fig. 4B). In addition, oxotremorine itself had a potent inhibitory effect on Fin generated by ACh (10 μm). This effect had an IC50 of 2.3 ± 0.7 μm, and was reversible on washout with NR solution for 7-10 min (Fig. 5A and B).
Figure 4. Oxotremorine effect on follicular responses and activation rates of muscarinic responses.
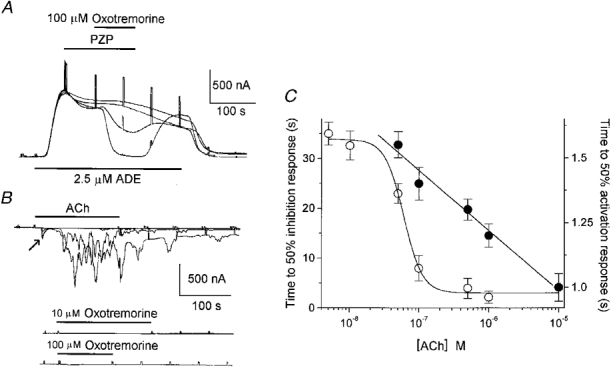
A, PZP blockade of oxotemorine-elicited (100 μm) inhibition of IK,cAMP. Decreasing concentrations of PZP were applied (100, 10, 1 and 0.1 μm), with 15 min wash intervals. Similar results were obtained in 3 other follicles from 2 frogs. B, examples of follicles in which ACh generated both Fin (arrow) and oscillatory Ca2+-dependent Cl− currents (top traces), but in which oxotremorine failed to generate any response (middle and bottom traces). The 4 superimposed traces (top) show currents elicited by ACh (0.1, 1, 10 and 100 μm) applied to the same follicle. Similar results were obtained in follicles from 2 other frogs. C, ACh concentration vs. rate of Fin activation (•) or inhibition of IK,cAMP (○). Rates are expressed as the time necessary to reach 50 % of the maximal response for each concentration. For Fin activation the rate decreased linearly while for IK,cAMP inhibition it increased following a sigmoidal relationship (3 follicles, 2 frogs).
Figure 5. Oxotremorine effect on Fin elicited by ACh or ATP.
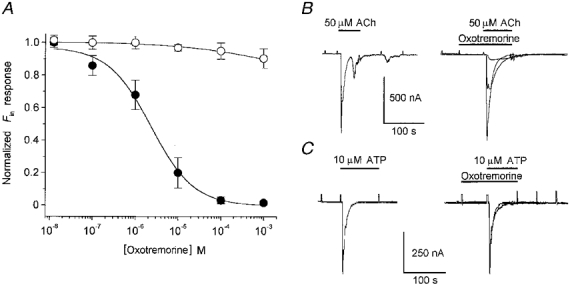
A, dose-response relationships for oxotremorine inhibition of Fin elicited by 50 μm ACh (•) or 10 μm ATP (○). Inhibition curves were adjusted as in Fig. 1D. The IC50 for Fin elicited by ACh was 2.3 ± 0.7 μm. Each point represents the mean of 8 follicles from 3 frogs. B and C, sample traces of inhibition produced by oxotremorine on Fin. The trace on the left in each panel is the control current elicited by the agonist alone, and the superimposed traces on the right show the effects of oxotremorine: for Fin activated by ACh the oxotremorine concentrations were 10 nm, 1 and 10 μm, and for Fin activated by ATP the concentrations were 10 and 100 μm. All traces were obtained from a single follicle.
Cholinergic membrane mechanisms and follicular cell-oocyte communication
Further evidence for the idea that different types of muscarinic receptor are involved in the generation of Fin and in the inhibition of IK,cAMP by ACh was derived from a comparison of the rates of development of both responses. These rates were estimated as the time necessary for the currents to reach 50 % of their maximum. In the case of Fin evoked by ACh, the rate of activation decreased linearly with the logarithm of the increase in agonist concentration, while the rate of IK,cAMP inhibition decreased sigmoidally (Fig. 4C).
To study these responses further, and to obtain information about the possible mechanisms of IK,cAMP inhibition, we designed the following experiment in order to see whether muscarinic stimulation of follicular receptors decreased the coupling between the follicular cells and the oocyte, as has been suggested previously (e.g. Greenfield et al. 1990), and proved for some other cellular systems coupled through gap-junction channels (see Neyton & Trautmann, 1986).
If the ACh inhibition of IK,cAMP is due, at least in part, to a muscarinic uncoupling of follicular cells from the oocyte, one would assume that any other current, such as Fin elicited by ATP, which also originates in the follicular cells, should suffer a similar inhibition. To examine this question, follicles were stimulated with ATP (10 μm) or ACh (50 μm) alone, or with either agonist co-applied with oxotremorine, after a brief (approximately 60 s) preincubation with oxotremorine alone. The concentrations of oxotremorine used ranged from 0.01 to 1000 μm but special attention was given to concentrations (10-100 μm) where oxotremorine completely inhibited IK,cAMP but weakly activated Fin. Under such conditions it would be expected that oxotremorine would not directly cross-affect Fin responses very strongly, in the way that ACh affects ATP-activated Fin (see Arellano et al. 1998). The results of these experiments are illustrated in Fig. 5. Oxotremorine, at doses which produced complete inhibition of IK,cAMP, reduced by only 8 ± 5 % the Fin elicited by ATP. This suggests that muscarinic stimulation did not induce an important closing effect of the follicular cell-oocyte gap-junction channels, and that ACh inhibited IK,cAMP more directly by acting on the K+ channels. Moreover, as we have noted above, concentrations (100 μm) of oxotremorine that almost completely blocked the ACh-elicited Fin inhibited only a small fraction of the Fin elicited by ATP (Fig. 5A and C), indicating that the inhibitory effect of oxotremorine on Fin is more probably a direct effect on the muscarinic receptor.
Molecular detection of XlmR in isolated follicular cells and oocytes
The differences found for the follicular muscarinic receptors stimulated during Fin activation and IK,cAMP inhibition already gave some clues as to the possible receptor subtypes involved. For example, in the case of IK,cAMP inhibition the receptor (1) had a high sensitivity to PZP and MTT, and (2) was fully activated by oxotremorine, a preferential agonist of M2 receptors. The muscarinic receptor (XlmR) encoded by the gene cloned from a Xenopus oocyte cDNA library (Herrera et al. 1994) resembles the human m4 receptor, although a clear functional or pharmacological distinction cannot be made between M2 or M4 subtypes (Herrera et al. 1997). Therefore, as a first step to see whether XlmR plays a role during IK,cAMP inhibition, we examined whether XlmR was present in follicular cells.
Using primers specific for XlmR, RT-PCR amplification of total RNA was performed for two different types of cellular preparations: defolliculated oocytes and isolated follicular cells. RT-PCR amplification resulted in a product of 430 bp in both preparations, as expected for the transcript of XlmR gene (Herrera et al. 1994), while no amplification was obtained in controls in which the reverse transcriptase was omitted (Fig. 6A). This result indicates that the receptor may be expressed not only in the membrane of the oocyte (see Herrera et al. 1994), but also in the membrane of the follicular cells. In order to confirm that the RT-PCR product represented the transcript of XlmR gene and was not due to non-specific amplification, the positive product was then identified by hybridization with an XlmR probe in Southern blots (Fig. 6B). Again, this resulted in a signal of the expected size and thus suggested the presence of XlmR in the follicular cells. All PCR amplifications from 4 frogs gave the same result. Electrophysiological experiments revealed that follicles (n = 6) from the same frogs exhibited ACh-elicited inhibition of IK,cAMP, while defolliculated oocytes (n = 7) did not generate any response to ACh.
Figure 6. RT-PCR amplification of XlmR transcripts in follicular cells.
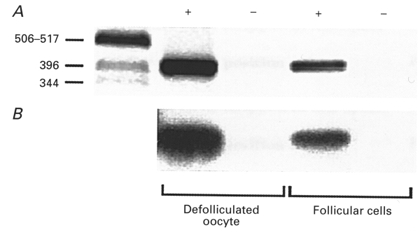
A, ethidium bromide-stained gel of RT-PCR-amplified products. The left-hand lane contains DNA markers (in bp). +, cDNA from defolliculated oocytes or isolated follicular cells. -, negative controls, RNA without reverse transcriptase. B, Southern blot of the same gel as in A, hybridized with XlmR probe.
These results suggest that XlmR may be involved in the cholinergic responses from follicular cells, particularly in the mechanisms that produce IK,cAMP inhibition.
DISCUSSION
Our study shows that in Xenopus follicles there is a molecular heterogeneity in the muscarinic receptors involved in generating the current responses elicited by ACh. Pharmacological and kinetic evaluations of these responses revealed that two different muscarinic receptor subtypes appear to be coupled to different sets of membrane proteins producing completely distinct responses. Both muscarinic receptors seem to be in the membrane of the follicular cells. Their principal differences are: (1) the receptor that produces inhibition of the channel underlying IK,cAMP displayed a 100-fold higher sensitivity to ACh than the receptor involved in Fin activation. (2) The antagonist 4-DAMP was about 2000-fold more potent on the receptor mediating Fin activation. (3) Oxotremorine partially mimicked the actions of ACh and inhibited IK,cAMP. In sharp contrast, this cholinergic agonist potently inhibited Fin generated by ACh. (4) The rate of development of IK,cAMP inhibition was non-linearly dependent on the dose of ACh, while that for Fin generation was linear.
Our results suggest that Fin activation is mediated by an M3-like receptor subtype. This conclusion is based on the pharmacological results which show that 4-DAMP was by far the most effective antagonist of Fin. A similar conclusion was reached for the muscarinic receptor involved in generating the oscillatory Ca2+-dependent Cl− currents arising in the oocyte (Van Wezenbeek et al. 1988). Despite this similarity, these two responses to ACh differ. For example, they are located in different cellular compartments (i.e. oocyte vs. follicular cells), and Fin (and the slow inward current named Sin; Arellano & Miledi, 1993) are not eliminated when the oocytes are loaded with Ca2+ chelators (e.g. EGTA, BAPTA). This strongly suggests that an intracellular Ca2+ increase is not a central element in the activation cascade of Fin.
The effect of oxotremorine on Fin is particularly interesting. As far as we know, this is the first report of an inhibitory action of this drug on the function of muscarinic receptors, but further experiments will be necessary to determine the mechanism of action of oxotremorine on this receptor (e.g. antagonism vs. partial agonism), and to determine whether this effect is specific for follicular muscarinic receptors.
On the other hand, the muscarinic receptor involved in the inhibition of IK,cAMP seems to be an M2-like receptor, because it was fully stimulated by oxotremorine and it was more sensitive to PZP, TPC and MTT, than to 4-DAMP. The molecular characteristics of XlmR, and the functional membrane mechanisms it controls when expressed in transfected cell lines, closely resemble those of both the M4 and M2 subtypes. By using RT-PCR we showed that follicular cells, as well as the oocyte, possess XlmR transcripts. Based on the functional and pharmacological characteristics of the receptor involved in IK,cAMP inhibition, and the possible expression of an M2 or M4-like receptor encoded by the XlmR gene in follicular cells, we suggest that this receptor subtype is responsible for IK,cAMP inhibition, although, in competitive inhibition binding experiments (Herrera et al. 1997), XlmR transiently expressed in COS-7 (African green monkey kidney) cells also exhibited a higher affinity for 4-DAMP than for PZP and MTT.
The XlmR couples efficiently to the system that produces inhibition of adenylyl cyclase and activation of mitogen-activated protein (MAP) kinase in COS-7 and human embryonic kidney 293 cells (Herrera et al. 1997), but its mode of function in the oocyte or in the follicular cells is still unknown. Nevertheless, from our results and those of other studies it is clear that the inhibition of IK,cAMP does not involve a reduction of cAMP synthesis, because IK,cAMP activated by direct intracellular injection of cAMP or cGMP is also blocked by ACh (Dascal et al. 1985; Miledi & Woodward, 1989; Greenfield et al. 1990). Another important fact is that the electrical communication between the oocyte and the follicular cells is not greatly affected by muscarinic stimulation, because oxotremorine-sensitive receptors did not produce a strong effect on Fin generated by ATP, and these responses are dependent on electrical coupling between those cells (Arellano et al. 1998). This lack of effect on electrical coupling supports the idea that the eventual XlmR expression in the oocyte membrane does not play a fundamental role in IK,cAMP inhibition. All this strongly suggests that the ACh inhibitory effect on IK,cAMP is a more direct effect on the K+ channels, a conclusion similar to that reached previously using different experimental procedures (e.g. Miledi & Woodward, 1989; Honoré & Lazdunski, 1993).
The fact that protein kinase C (PKC) activators such as phorbol esters, like ACh, inhibit IK,cAMP suggests that PKC might participate in the cascade stimulated by the M2-like muscarinic receptor of the follicular cells. However, this needs to be reconsidered, since it has been shown that phorbol esters effectively reduce the communication between Xenopus follicular cells and oocytes (Greenfield et al. 1990), as they do in follicles from other species (e.g. Cerdáet al. 1993). Thus, the phorbol ester-induced inhibition of IK,cAMP monitored from the follicle clearly involves a different mechanism (i.e. closing of gap-junction channels) from that activated by ACh.
It still remains to be determined whether different muscarinic receptor subtypes coexist in the membrane of the same follicular cells, or if the heterogeneity of receptors and their membrane responses is a consequence of differences among the 10 000 or so follicular cells that surround the oocyte. Under the latter scheme, there could be at least two types of follicular cells, i.e. a group with M3-like receptors capable of eliciting the Fin and Sin responses, and a second group of follicular cells endowed with channels underlying IK,cAMP and M2-like receptors. It should be noted that whether the receptors are M3 or M2 (or M4) requires further support from pharmacological experiments (i.e. detailed competitive inhibition binding studies), and more thorough information concerning the molecular nature of the receptors involved. In any case, the finding of two different receptors and membrane mechanisms in the whole follicle will help to determine their role in important physiological events such as maturation, as well as their participation in the regulation of other currents such as the osmolarity-dependent Sin. For example, it has been reported that oocyte maturation is modulated by stimulation of muscarinic receptors (Dascal et al. 1984) and by ionic currents carried by K+ and Cl− (Wibrand et al. 1992; Skoblina & Huhtaniemi, 1997). However, the cellular mechanisms involved are poorly understood. It has been suggested that there is an important effect of Cl− currents on the progesterone production pathway, and that the opening of K+ channels favours maturation by increasing the oocyte sensitivity to progesterone. In addition, increasing evidence has accumulated regarding the participation of different neurotransmitters (e.g. noradrenaline, dopamine, ACh) in mammalian ovarian physiology, not only during oocyte growth and maturation, where it has been shown that neurotransmitters modulate ovarian steroidogenesis (e.g. Adashi & Hsueh, 1981), but also in fertilization, where ACh has been proposed to be involved in the activation process stimulated by sperm-egg interaction (Eusebi et al. 1984). Moreover, studies in different species have shown that such neurotransmitters may have various sources in the ovarian follicle. For example: (1) autonomic catecholaminergic and cholinergic innervation (e.g. Sporrong et al. 1985), (2) ovarian intrinsic neuronal-like cells that in non-human primates have been shown to contain tyrosine hydroxylase (Dees et al. 1995), and (3) the synthesis of noradrenaline by the oocyte itself (e.g. Mayerhofer et al. 1998). Thus, information regarding the nature of the receptors present in the membranes of follicular cells and oocytes and their mechanisms of action are important for a comprehensive understanding of their role in reproductive function.
In conclusion, we have shown the presence of two different types of muscarinic receptor in the membrane of Xenopus follicular cells. These receptors are like the M3 and M2 (or M4) subtypes, and they trigger distinct membrane pathways that lead to two types of ionic channel regulation. The characteristics of XlmR suggest that it resembles the receptor involved in IK,cAMP inhibition, and we show here that XlmR transcripts are present in follicular cells, strongly suggesting that XlmR mediates the inhibition. Further information regarding the molecular nature of the receptors, ion channels and membrane mechanisms stimulated is needed to understand their functional role. The results presented here are a step towards that goal, and in the meantime, will also facilitate the rational use of the follicle-enclosed Xenopus oocyte as a cellular model in biology.
Acknowledgments
We are grateful to Drs Ian Parker and Michael Jeziorski for critical comments and help with the manuscript. We also thank Horacio Ramirez Leyva for technical assistance. This work was supported by grants from UNAM-DGAPA (IN209596) and from CONACYT-México (3713-PN) to R. O. Arellano, and from CONACYT (G25775 N) and the National Science Foundation USA (IBN-9604499) to R. Miledi.
References
- Adashi EY, Hsueh AJ. Stimulation of β2-adrenergic responsiveness by follicle-stimulating hormone in rat granulosa cells in vitro and in vivo. Endocrinology. 1981;108:2170–2178. doi: 10.1210/endo-108-6-2170. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Garay E, Miledi R. Cl− currents activated via purinergic receptors in Xenopus follicles. American Journal of Physiology. 1998;274:C333–340. doi: 10.1152/ajpcell.1998.274.2.C333. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Novel Cl− currents elicited by follicle stimulating hormone and acetylcholine in follicle-enclosed Xenopus oocytes. Journal of General Physiology. 1993;102:833–857. doi: 10.1085/jgp.102.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Functional role of follicular cells in the generation of osmolarity-dependent Cl− currents in Xenopus follicles. The Journal of Physiology. 1995;488:351–357. doi: 10.1113/jphysiol.1995.sp020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Woodward RM, Miledi R. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes. In: Narahashi T, editor. Ion Channels. Vol. 4. New York: Plenum Press; 1996. pp. 203–259. [DOI] [PubMed] [Google Scholar]
- Browne CL, Werner W. Intercellular junctions between the follicle cells and the oocytes of Xenopus laevis. Journal of Experimental Zoology. 1984;230:105–113. doi: 10.1002/jez.1402300114. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacological Reviews. 1998;50:279–290. [PubMed] [Google Scholar]
- Cerdá JL, Petrino TR, Wallace RA. Functional heterologous gap junctions in Fundulus ovarian follicles maintain meiotic arrest and permit hydration during oocyte maturation. Developmental Biology. 1993;160:228–235. doi: 10.1006/dbio.1993.1300. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dascal N, Lotan I, Gillo B, Lester HA, Lass Y. Acetylcholine and phorbol esters inhibit potassium currents evoked by adenosine and cAMP in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1985;82:6001–6005. doi: 10.1073/pnas.82.17.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N, Yekuel R, Oron Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. Journal of Experimental Zoology. 1984;230:131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- Davidson A, Mengod G, Matus-Leibovitch N, Oron Y. Native Xenopus oocytes express two types of muscarinic receptors. FEBS Letters. 1991;284:252–256. doi: 10.1016/0014-5793(91)80697-2. [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, Schultea TD, Mayerhofer A, Danilchik M, Dissen GA, Ojeda SR. The primate ovary contains a population of catecholaminergic neuron-like cells expressing nerve growth factor receptors. Endocrinology. 1995;136:5760–5768. doi: 10.1210/endo.136.12.7588334. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin): I. Stages of oocyte development in laboratory maintained animals. Journal of Morphology. 1972;136:153–180. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Pasetto N, Siracusa G. Acetylcholine receptors in human oocytes. The Journal of Physiology. 1984;346:321–330. doi: 10.1113/jphysiol.1984.sp015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield LJ, Jr, Hackett JT, Linden J. Xenopus oocyte K+ current. III. Phorbol esters and pH regulate current at gap junctions. American Journal of Physiology. 1990;259:C792–800. doi: 10.1152/ajpcell.1990.259.5.C792. [DOI] [PubMed] [Google Scholar]
- Herrera L, Carvallo P, Antonelli M, Olate J. Cloning of a Xenopus laevis muscarinic receptor encoded by an intronless gene. FEBS Letters. 1994;352:175–179. doi: 10.1016/0014-5793(94)00957-0. [DOI] [PubMed] [Google Scholar]
- Herrera L, Hinrichs MV, Frías J, Gutkind S, Olate J. Dual transduction signaling by a Xenopus muscarinic receptor: adenylyl cyclase inhibition and MAP kinase activation. Journal of Cellular Biochemistry. 1997;65:75–82. [PubMed] [Google Scholar]
- Honoré E, Lazdunski M. Single-channel properties and regulation of pinacidil-glibenclamide-sensitive K+ channels in follicular cells from Xenopus oocyte. Pflügers Archiv. 1993;424:113–121. doi: 10.1007/BF00374601. [DOI] [PubMed] [Google Scholar]
- Kusano K, Miledi R, Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977;270:739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K, Miledi R, Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. The Journal of Physiology. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer A, Smith GD, Danilchik M, Levine JE, Wolf DP, Dissen GA, Ojeda SR. Oocytes are a source of catecholamines in the primate ovary: evidence for a cell-cell regulatory loop. Proceedings of the National Academy of Sciences of the USA. 1998;95:10990–10995. doi: 10.1073/pnas.95.18.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proceedings of the Royal Society B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I, Sumikawa K. Fidia Research Foundation Neuroscience Award Lectures. New York: Raven Press; 1989. Transplanting receptors from brains into oocytes; pp. 57–90. [Google Scholar]
- Miledi R, Woodward RM. The effect of defolliculation on membrane current responses of Xenopus oocytes. The Journal of Physiology. 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya MA, Fuentes ME, Inestrosa NC. A comparison of the Xenopus laevis oocyte acetylcholinesterase with the muscle and brain enzyme suggests variations at the post-translational level. Comparative Biochemistry and Physiology C. 1991;98:299–305. doi: 10.1016/0742-8413(91)90209-c. [DOI] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. The Journal of Physiology. 1986;377:283–295. doi: 10.1113/jphysiol.1986.sp016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. New York, USA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Skoblina MN, Huhtaniemi I. Involvement of chloride channels in progesterone production during meiotic maturation of follicle-enclosed oocytes of Rana temporaria and Xenopus laevis. Journal of Experimental Zoology. 1997;278:422–428. [PubMed] [Google Scholar]
- Sporrong B, Kannisto P, Owman C, Sjöberg NO, Walles B. Histochemistry and ultrastructure of adrenergic and acetylcholinesterase-containing nerves supplying follicles and endocrine cells in the guinea-pig ovary. Cell and Tissue Research. 1985;240:505–511. doi: 10.1007/BF00216338. [DOI] [PubMed] [Google Scholar]
- Stinnakre J, Van Renterghem C. Cyclic adenosine monophosphate, calcium, acetylcholine and the current induced by adenosine in the Xenopus oocyte. The Journal of Physiology. 1986;374:551–569. doi: 10.1113/jphysiol.1986.sp016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoef MHF, Dictus WJAG, Hage WJ, Bluemink JG. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. European Journal of Cell Biology. 1984;33:242–247. [PubMed] [Google Scholar]
- Van Renterghem C, Pénit-Soria J, Stinnakre J. β-Adrenergic induced potassium current in Xenopus oocyte: Involvement of cyclic AMP. Biochimie. 1984;66:135–138. doi: 10.1016/0300-9084(84)90202-5. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C, Pénit-Soria J, Stinnakre J. β-Adrenergic induced potassium current in Xenopus oocytes: role of cyclic-AMP, inhibition by muscarinic agents. Proceedings of the Royal Society B. 1985;223:389–402. doi: 10.1098/rspb.1985.0008. [DOI] [PubMed] [Google Scholar]
- Van Wezenbeek LACM, Tonnaer JADM, Ruigt GSF. The endogenous muscarinic acetylcholine receptor in Xenopus oocytes is of the M3 subtype. European Journal of Pharmacology. 1988;151:497–500. doi: 10.1016/0014-2999(88)90551-1. [DOI] [PubMed] [Google Scholar]
- Wibrand F, Honoré E, Lazdunski M. Opening of glibenclamide-sensitive K+ channels in follicular cells promotes Xenopus oocyte maturation. Proceedings of the National Academy of Sciences of the USA. 1992;89:5133–5137. doi: 10.1073/pnas.89.11.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward RM, Miledi R. Hormonal activation of membrane currents in follicle enclosed Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1987;84:4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]


