Abstract
Pericytes, which are contractile cells located on the outer wall of microvessels, are thought to be particularly important in the retina where the ratio of these cells to vascular endothelial cells is the highest of any tissue. Retinal pericytes are of interest since they may regulate capillary blood flow and because their selective loss is an early event in diabetic retinopathy, which is a common sight-threatening disorder associated with dysfunction of the blood-retinal barrier.
Although a breakdown in the vascular endothelial barrier is a frequent pathophysiological event, knowledge of the effects of blood-derived molecules on pericyte function is limited. Based on the premise that ion channels play a vital role in cellular function, we examined the effect of serum on the ionic currents of retinal pericytes. To do this, we used the perforated-patch configuration of the patch-clamp technique to monitor the whole-cell currents of pericytes located on freshly isolated rat retinal microvessels.
Exposure to serum reversibly activated inward and outward currents in virtually all of the sampled retinal pericytes. Two types of sustained conductances were induced by serum. These were a calcium-permeable non-specific cation (NSC) current and a voltage-dependent potassium current. In addition, exposure to serum increased the activity of chloride channels which caused transient depolarizing currents.
Associated with the activation of these conductances, the membrane potential showed a sustained decrease of 10 ± 2 mV from −56 mV to −46 mV and, also, transient depolarizations to near −30 mV. The serum-induced depolarizations can activate the voltage-gated calcium channels expressed by the retinal pericytes.
Calcium-permeable NSC channels appear to play a critical role in the response of pericytes to serum-derived molecules. Consistent with this, activation of the chloride and potassium channels was sensitive to SK&F 96365, which is a blocker of NSC channels. In addition, chloride and potassium channel activation was dependent on extracellular calcium.
The effects of serum on the activity of channels in retinal pericytes were qualitatively mimicked by insulin-like growth factor-1 (IGF-1), which is a normal constituent of the blood.
There are significant differences in the effects of serum on retinal pericytes compared with vascular smooth muscle cells. Serum activated sustained conductances in retinal pericytes but not in the vascular smooth muscle cells. This suggests a fundamental difference in the mechanisms by which serum-derived molecules affect these two types of cells.
We conclude that serum-derived molecules, such as IGF-1, can activate several types of ion channels in retinal pericytes. These changes in channel activity are likely to influence pericyte function at sites of a breakdown in the blood-retinal barrier.
Pericytes, the cells which ensheathe the microvessels, are likely to play an important role in vascular biology and pathophysiology. The expression by pericytes of contractile proteins, such as actin, myosin and tropomyosin (Shepro & Morel, 1993) and the demonstration of pericyte contraction (Tildon et al. 1979; Hirschi & D'Amore, 1996; Matsugi et al. 1997; Schonfelder et al. 1998) suggest that these cells regulate the diameter of capillaries and, thereby, control the flow of blood within the microcirculation. In addition to regulating blood flow, other putative functions of pericytes include the maintenance of capillary structure, inhibition of endothelial proliferation and participation in angiogenesis (Hirschi & D'Amore, 1996).
The function of pericytes is thought to be particularly important in the microvasculature of the retina where the ratio of pericytes to vascular endothelial cells is greater than in any other vascular bed (Balabanov & Dore-Duffy, 1998). Interest in the role of retinal pericytes is heightened by the observation that the selective loss of these cells is one of the earliest histopathological events in the course of diabetic retinopathy (Cogan et al. 1961), which is a vision-threatening condition characterized, in part, by a failure of the blood-retinal barrier. At sites where this barrier is defective, serum-derived molecules leak from the blood vessels into the retina. Since pericytes are located on the ablumenal surface of the vascular endothelium, they are among the first cells to be exposed to the molecules leaking from the circulatory system. As a result, the responses of pericytes to blood-derived molecules may help determine how well the retina functions when the vascular endothelial barrier is compromised.
Based on the premise that ion channels play a vital role in cellular function, we examined the effect of blood-derived molecules on the ionic currents of retinal pericytes. We now report that perforated-patch recordings from pericytes located on freshly isolated rat retinal microvessels reveal that serum reversibly activates inward and outward currents in these cells. The serum-induced ionic conductances are due to the activation of a number of types of ion channels, including non-specific cation, outward potassium and chloride channels. These effects on ion channel activity are mimicked qualitatively by insulin-like growth factor-1, which is a normal component of the blood (LeRoith, 1997). Our experimental findings support the idea that the activity of ion channels in retinal pericytes is altered when serum-derived molecules leak from the vascular system at sites where the blood-retinal barrier is dysfunctional.
METHODS
Isolated retinal microvessels
Freshly isolated microvessels were prepared from rat retinae. Five- to eight-week-old rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) were killed by prolonged exposure to carbon dioxide gas and their eyes removed. Animal use conformed to the guidelines of the Society for Neuroscience and Association for Research in Vision and Ophthalmology. All experiments were carried out according to guidelines laid down by the University of Michigan University Committee on the Use and Care of Animals. After the retina was dissected from an eye, it was incubated in 2.5 ml Earle's balanced salt solution supplemented with 0.5 mm EDTA, 1.5 mm CaCl2, 1 mm MgSO4, 20 mm glucose, 26 mm NaHCO3, 15 U papain (Worthington Biochemicals, Freehold, NJ, USA), 0.04 % DNase and 2 mm cysteine for 30 min at 30°C while 95 % oxygen-5 % CO2 was bubbled through to maintain pH and oxygenation. After transfer to solution A (Table 1) the retina was placed with the vitreal side up on a coverslip (diameter, 15 mm; Warner Instrument Corp., Hamden, CT, USA) which was modified by gluing onto its surface a mosaic of approximately two dozen, non-contiguous, irregularly shaped, ∼4 mm2 fragments of a no. 1 glass coverslip. A second coverslip was then positioned over the retina, and the tissue was gently sandwiched between the glass surfaces. Complexes of microvessels adhered to the upper coverslip (Fig. 1A).
Table 1.
Compositions (mm) of the solutions used in this study
| Solution | [NaCl] | [CsCl] | [KCl] | [K2SO4] | [CaCl2] | [BaCl2] | [MgCl2] | [Na-Hepes] | [K-Hepes] | [Cs-Hepes] | [Glucose] | [Mannitol] | [EGTA] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 140 | — | 3 | — | 1.8 | — | 0.8 | 10 | — | — | 5 | 30 | — |
| B | — | — | 50 | 65 | — | — | 6 | — | 10 | — | — | — | — |
| C | — | 30 | — | — | 1.8 | 1.8 | 0.8 | — | — | 10 | 5 | 207 | — |
| D | — | 135 | — | — | — | — | 6 | 10 | — | — | — | — | — |
| E | 133 | — | 10 | — | 1.8 | — | 0.8 | 10 | — | — | 20 | — | — |
| F | 140 | — | 3 | — | — | — | 0.8 | 10 | — | — | 5 | — | 3 |
Figure 1. Scanning electron photomicrograph of freshly isolated microvessels from an adult rat retina.
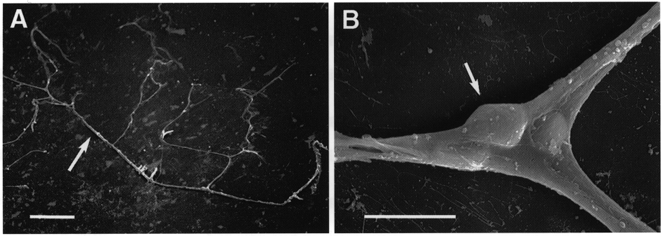
A, a complex of microvessels adhering to a glass coverslip. Scale bar, 100 μm. B, a pericyte (arrow) located on the ablumenal wall of a bifurcating microvessel. Scale bar, 10 μm.
A coverslip with adhering vessels was placed in a recording chamber which was perfused (2 ml min−1) with solutions from a gravity-fed system using multiple reservoirs. Unless noted otherwise, solution A was the bathing solution. Vessels were examined at ×400 magnification with an inverted microscope equipped with phase-contrast optics. Pericytes were identified by their characteristic location on the ablumenal wall of microvessels (Fig. 1B; Kuwarbara & Cogan, 1960). In addition to small vessels (diameter, 5–7 μm) with pericytes, these preparations often included larger vessels (15–45 μm) that were encircled by doughnut-shaped smooth muscle cells, which we also studied electrophysiologically. Experiments were performed within 3 h after the isolation of the retinal vessels.
Solutions
Table 1 lists the composition of the various solutions used in this study. The pH of all solutions was 7.4. The osmolarity was adjusted to 310 mosmol l−1 for the bathing solutions (i.e. A, C, E and F) and to 280 mosmol l−1 for the pipette solutions.
Patch-clamp recordings
The perforated-patch configuration of the patch-clamp technique was used to monitor the ionic currents and the membrane potentials of pericytes located on freshly isolated rat retinal microvessels. As detailed previously (Kusaka & Puro, 1997), micropipettes were pulled from Corning No. 7052 glass tubing (Gardner Glass Co., Claremont, CA, USA) using a multistage programmable puller (Sutter Instruments, San Rafael, CA, USA) and were heat polished to tip diameters of 2–3 μm. A pipette tip was filled to approximately 400 μm from the tip by applying negative pressure to the back end of the pipette while briefly dipping the tip into the pipette solution. Unless stated otherwise, the pipette contained solution B. The remainder of the pipette was then backfilled with this solution supplemented with freshly mixed amphotericin B (240 μg ml−1) and nystatin (240 μg ml−1) or gramicidin (100 μg ml−1). The resistances of the pipettes used were 2–5 MΩ when tested in the bathing solution.
The pipettes were mounted in the holder of a Dagan 3900 (Dagan Corp., Minneapolis, MN, USA) or Axopatch 200B (Axon Instruments, Foster City, CA, USA) patch-clamp amplifier and sealed to the cell bodies of pericytes. Similar methods were used to record from vascular smooth muscle cells located on freshly isolated retinal vessels which had diameters of 15–45 μm. Seals generally formed over a period of 1–30 s and reached resistances of greater than 1 GΩ. As amphotericin and nystatin perforated the patch, the access resistance to the cell usually decreased to less than 20 MΩ within 20 min for the pericytes analysed. Cells with membrane potentials more negative than −30 mV were used in this study since the relatively rapid deterioration of cells with lower membrane potentials suggested cell injury. Capacitative compensation was applied via circuits within the amplifier; we did not correct for series resistance. Cell capacitance was estimated using circuits of the Dagan 3910 expander module (Dagan Corp.). Currents and voltages were filtered at 1 kHz and digitally sampled (at 400 μs) using a Lab Master DMA acquisition system (Axon Instruments), an IBM-compatible microcomputer and pCLAMP software (version 6, Axon Instruments), which also controlled the voltage protocols. Currents were evoked by a voltage step protocol. Due to the presence of transiently occurring currents, generation of current-voltage (I–V) plots of the sustained conductances required the use of long duration (> 3 s) voltage steps; periods without transient events were used to establish the steady-state level of the sustained current. For voltage-clamp recordings, the zero-current potential for the sustained conductances was defined as the resting membrane potential of the cell.
After data collection, the recorded membrane potentials were corrected for liquid junction potentials, which were calculated using a computer program (Barry, 1993). Data analysis was facilitated with the use of pCLAMP software (Axon Instruments) and a scientific plotting program (Origin, MicroCal, Northhampton, MA, USA). The net charge transfer associated with transiently occurring events was quantified in coulombs during fourteen 3.5 s sampling periods; this was calculated by multiplying the duration of each sampling period by the difference between the mean current amplitude and the amplitude of the steady-state current, which was defined as the lowest absolute value of the current amplitude detected during the sampling period. Unless otherwise stated, this calculation was made for current recorded at a holding potential of −57 mV.
Scanning electron microscopy
Standard techniques (Agardh et al. 1985) were used to prepare gluteraldehyde-fixed retinal microvessels for scanning electron microscopy, which was performed by the University of Michigan Anatomy and Cell Biology Core Facility.
Dialysis
Cellulose membrane tubing (Spectrum Medical Industries, Laguna Hills, CA, USA) with molecular weight (MW) cut-off of 6000 was used for dialysis. The serum to be dialysed was placed in the dialysis tubing, which was tightly clipped at both ends and carefully checked for leaks. The tube was placed in a beaker containing ∼800 ml of Dulbecco's phosphate buffer solution, which was continuously stirred and totally replaced three times during the first 8 h. Dialysis was performed for ∼24 h at 4°C.
Chemicals
Charybdotoxin and apamin were purchased from RBI (Natick, MA, USA). Other chemicals and fetal bovine serum were from Sigma (St Louis, MO, USA) unless noted otherwise.
Statistics
Unless otherwise stated, data are given as means ±s.e.m. Probability was evaluated using Student's t test. The level of significance was set at P < 0.05.
RESULTS
Serum-induced currents
We hypothesized that serum, which enters the retina upon breakdown of the blood-retinal barrier, may alter the physiology of the pericytes. To test this idea, we developed an experimental preparation in which the patch-clamp technique was used to analyse the activity of ion channels in pericytes located on microvessels freshly isolated from the rat retina (Fig. 1). In this study, the whole-cell currents of pericytes were monitored via perforated-patch pipettes. This recording configuration minimizes the disruption of intracellular contents and the subsequent loss of second messengers and other molecules vital for cellular function.
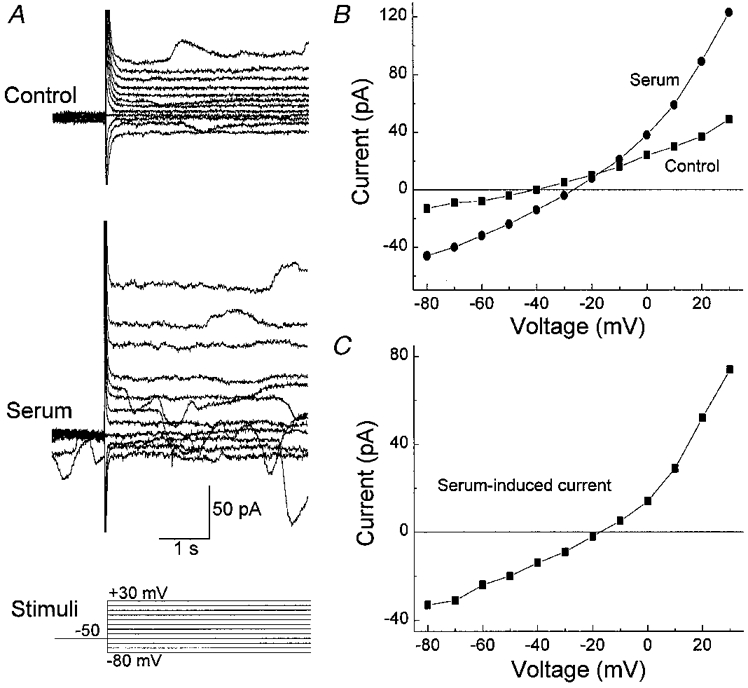
As illustrated in Fig. 2, exposure of fresh pericytes to serum (10 % v/v) reversibly increased both sustained and transiently occurring currents. All 22 pericytes sampled under the recording conditions used in Fig. 2 showed conductance changes with exposure to serum. The induced currents were inward at hyperpolarized potentials and outward at depolarized potentials. During exposure to serum, the resting membrane potential depolarized significantly (P < 0.001) from a mean of −56 ± 2 to −46 ± 2 mV.
Figure 2. Effect of serum on retinal pericyte currents.
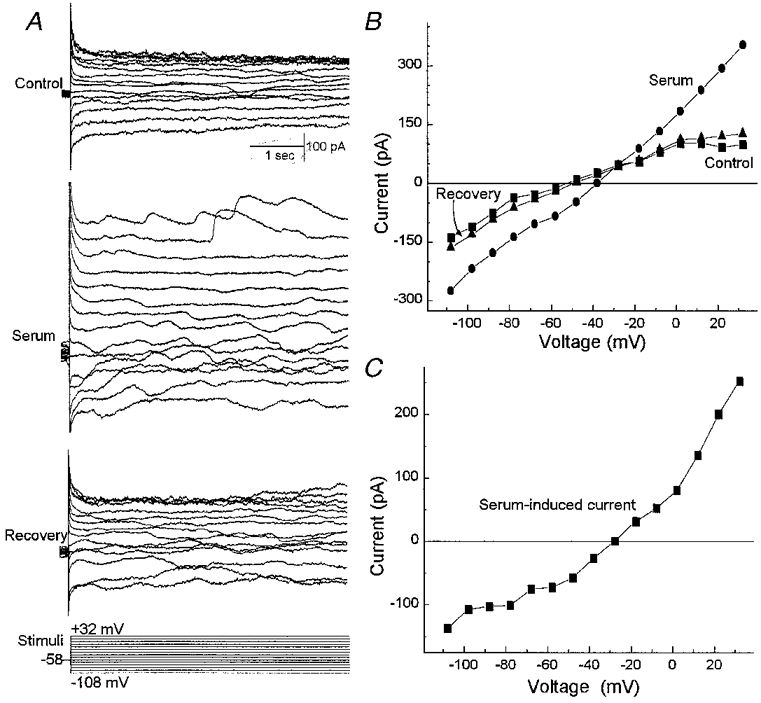
A, currents evoked before (Control), during (Serum) and after (Recovery) exposure to 10 % serum. The clamp protocol is shown below. Currents were monitored using the perforated-patch recording technique. Bathing solution A and pipette solution B were used. B, I–V plots of the sustained currents measured from the traces in A. C, the difference between the I–V curves obtained in the absence (Control) and presence of serum. Exposure of retinal pericytes to serum is associated with an increase in both sustained and transiently occurring currents.
Transient currents
A characteristic electrophysiological feature of fresh retinal pericytes is the presence of transiently occurring currents (Fig. 3 and also Figs 2A, 4, 7A, 9A, and 11A and B). We observed spontaneous transient currents in 68 % (15 of 22) of the pericytes sampled under the recording conditions used in Fig. 2. Since these currents appear similar to the spontaneous transient inward currents caused by calcium-activated chloride currents in smooth muscle cells (Wang et al. 1992), we tested the possibility that the transiently occurring events in retinal pericytes are also due to a chloride conductance. In a series of experiments, the equilibrium potential for chloride was changed by varying the ionic composition of the pipette and bathing solutions. We found that the reversal potential of the spontaneously occurring transient currents in retinal pericytes changed as predicted for a chloride-selective conductance (Fig. 3B and C).
Figure 3. Spontaneous transient currents in fresh retinal pericytes.
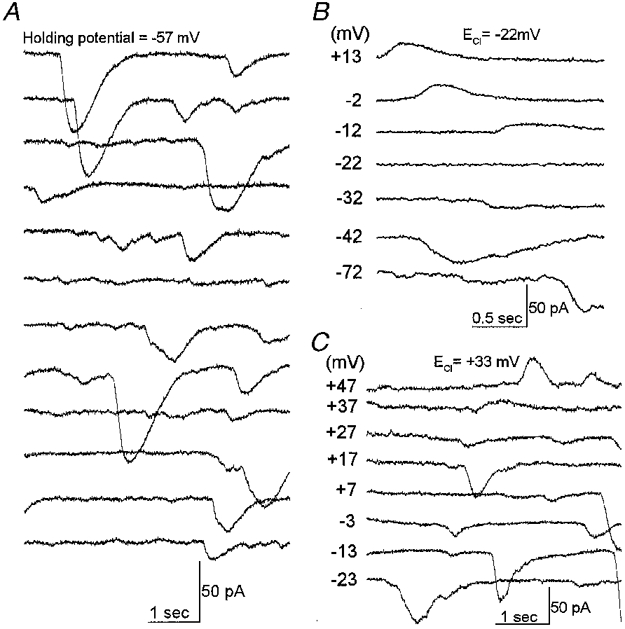
A, a continuous 1 min record of the currents in a retinal pericyte. The holding potential was −57 mV. Currents were monitored via a perforated patch pipette. Numerous transient inward currents are evident. B, currents in a fresh rat retinal pericyte held at the various membrane potentials listed adjacent to each trace. The Nernstian value for the equilibrium potential of chloride (ECl) was −22 mV. The bathing solution was solution E; the pipette solution was solution B (Table 1). C, currents of a rat retinal pericyte at various holding potentials; the calculated ECl was +33 mV. In the bath was solution C; solution D was used in the pipette. The reversal potential for the spontaneous transient currents in retinal pericytes is close to ECl.
Figure 4. Effect of a chloride channel blocker on the transient inward currents in a rat retinal pericyte.
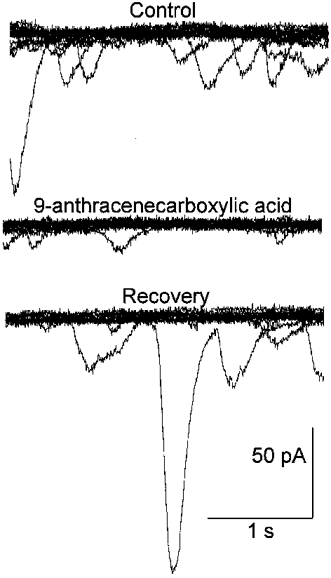
In each panel, ten current traces are superimposed. Currents were recorded via a perforated-patch pipette before (Control), during and after (Recovery) exposure to 1 mm 9-anthracenecarboxylic acid (9-ACA), which is a chloride channel blocker. The holding potential was −57 mV. Pipette solution B and bathing solution A supplemented with 10 % serum were used. The transient currents in retinal pericytes are sensitive to a chloride channel blocker.
Figure 7. Effect of serum on pericyte currents recorded under conditions in which potassium channels are blocked.
A, currents evoked before (Control) and during exposure to 10 % serum. The clamp protocol is shown below. B, I–V relations of the steady-state currents before (▪) and during (•) exposure to 10 % serum. C, the difference of the I–V curves in B. Bathing solution C and pipette solution D were used to block potassium channels. Blockage of potassium channels reveals that serum activates an outwardly rectifying conductance in retinal pericytes.
Figure 9. Effect of IGF-1 on the currents of a rat retinal pericyte.
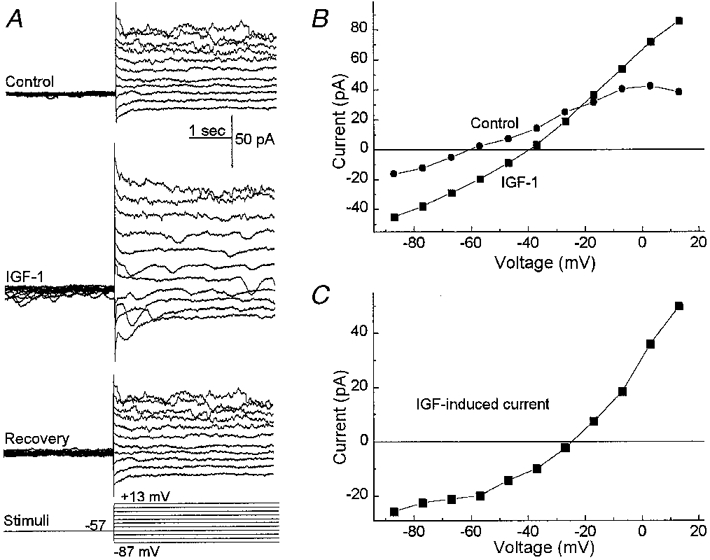
A, currents evoked before (Control), during (IGF-1) and after (Recovery) exposure to IGF-1 (50 ng ml−1). The clamp protocol is shown below. B, I–V plots of the sustained currents measured from the traces in A. C, the difference of the I–V curves in B. IGF-1 induces transient and sustained currents in retinal pericytes that are qualitatively similar to those induced by serum.
Figure 11. Effect of lowering the extracellular calcium concentration on the serum-activated currents in retinal pericytes.
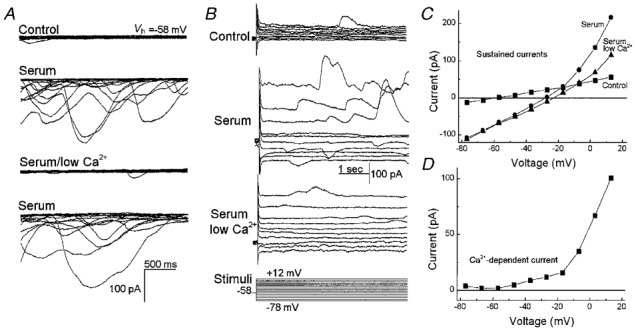
A, each panel shows ten successive current traces recorded from a rat retinal pericyte held at −58 mV (Vh, holding potential). In the top panel, currents were recorded with bathing solution A and pipette solution B. The second panel shows currents recorded 4 min after the onset of exposure of the pericyte to bathing solution A supplemented with 10 % serum. The traces in the next panel were obtained after switching to serum-containing solution F (Table 1), which lacked added CaCl2 and contained 3 mm EGTA. The currents in the bottom panel show traces after returning to bathing solution A, which contained serum and calcium. B, panels show currents evoked in bathing solution A (Control), in solution A supplemented with 10 % serum (Serum) or in solution F with serum (Serum low Ca2+). The clamp protocol is shown below. C, I–V plots of the sustained currents shown in B. D, difference between the I–V plots shown in C which were generated during exposure to serum in the presence or absence of added calcium. In fresh retinal pericytes, an influx of calcium is required for the serum-induced activation of the transient chloride channels and a sustained outward potassium conductance.
Exposure to serum was associated with an increase in the transient currents (Figs 2A, 7A, and 11A and B) in 95 % (21 of 22) of the pericytes recorded under the conditions used in Fig. 2. With exposure to serum, the net charge transfer associated with the transient inward currents increased significantly (P= 0.01) from −9 ± 1 to −31 ± 9 pC. As we observed for the spontaneous transient currents, the reversal potential for the transient currents recorded in serum shifted as the equilibrium potential for chloride was systematically changed. Specifically, these currents reversed from inward to outward at +26 ± 2 mV (n= 4) when recorded in bathing solution C and pipette solution D (Table 1). After the addition of 30 mm NaCl to the external solution (the mannitol concentration was adjusted to maintain osmolarity), the reversal potential for the transient currents was +12 ± 2 mV (n= 4). This −14 mV shift was significant (P= 0.007) and was close to the −15 mV Nernstian value for a channel that is selectively permeable to chloride. Further evidence for a chloride conductance was the observation (Fig. 4) that the serum-induced transient events were reduced by a chloride channel blocker, 9-anthracenecarboxylic acid (9-ACA). Specifically, the net charge transfer associated with transient inward currents decreased significantly (P= 0.04) from −19 ± 3 to −7 ± 0.4 pC (n= 3) with the addition of 1 mm 9-ACA to the perfusate. Based on these observations, it seems likely that serum activates chloride channels which account for the transiently occurring currents induced in fresh retinal pericytes.
We wished to assess the effects of this chloride channel activity under conditions in which the endogenous intracellular chloride concentration was unperturbed. In the experiments described above, advantage was taken of the fact that patches perforated using amphotericin and nystatin are permeable to chloride ions. Due to this anion permeability, the concentration of chloride in the pipette established the intracellular chloride concentration and, thereby, the reversal potential of the chloride currents. However, it was also important to determine whether the activation of chloride channels causes a depolarization or hyperpolarization of the membrane potential of retinal pericytes under more normal conditions since the endogenous chloride concentration varies widely in various cell types (Rhee et al. 1994; Owens et al. 1996).
To minimally alter the intracellular chloride concentration, we performed a series of perforated-patch recordings using gramicidin, rather than amphotericin and nystatin. An advantage of gramicidin is that it forms pores that are permeable to monovalent cations but not to chloride (Kyrozis & Reichling, 1995). We found that with gramicidin in the pipette, the reversal potential for the transiently occurring currents was −30 ± 2 mV (n= 3) when measured in bathing solution A. Based on this reversal potential, the calculated intracellular chloride concentration in fresh retinal pericytes is 45 mm. Thus, activation of chloride channels under physiological conditions should induce an inward, depolarizing current in retinal pericytes.
Consistent with transiently occurring chloride currents causing intermittent depolarizations, we observed that the membrane potential of fresh pericytes fluctuated considerably during exposure to serum. This is illustrated in Fig. 5 in which depolarizing fluctuations are evident during the initial 30 s of the voltage record. With the addition of 9-ACA to the perfusate, the pericyte membrane potential stabilized. Taken together, our experiments indicate that serum activates a transient chloride conductance which causes intermittent depolarizations of retinal pericytes.
Figure 5. Effect of a chloride channel blocker on the membrane potential of a fresh retinal pericyte.
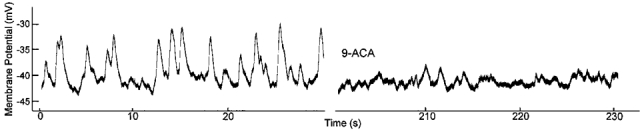
Current-clamp record of a pericyte exposed to 10 % serum. Forty seconds after the onset of this record, the chloride channel blocker, 9-ACA (1 mm), was added to the serum-containing perfusate. The membrane was monitored via a gramicidin-perforated patch. The fluctuating depolarizations that are observed in retinal pericytes exposed to serum are due to chloride channel activity.
Sustained currents
To help characterize the sustained currents induced by serum, we tested the effect of tetraethylammonium (TEA), which is a potassium channel blocker (Hille, 1992). In pericytes exposed to serum TEA significantly reduced the outward current but not the inward current (Fig. 6). The TEA-sensitive current had a threshold of activation of approximately −50 mV (Fig. 6B). Similar findings were observed in three other fresh pericytes. Although the specific type of potassium channel activated by serum remains to be identified, these experiments indicate that serum induces a voltage-dependent outward potassium conductance.
Figure 6. Effect of TEA on the pericyte currents induced by serum.
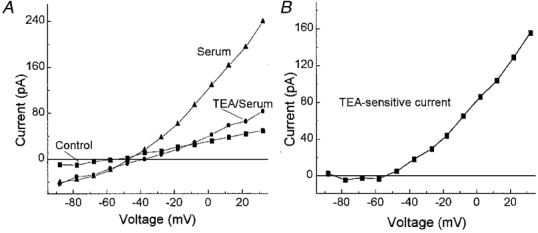
A, I–V relations of the steady-state currents under control conditions (▪) and in the presence of serum without (▴) or with 10 mm TEA (•). B, the difference of the I–V curves generated during exposure to serum without and with TEA. Serum induces an outward potassium current.
Although activation of potassium channels plays a significant role in the serum-induced increase in sustained outward currents, there were sustained inward and outward currents which persisted in 10 mm TEA (Fig. 6A). To better characterize these TEA-insensitive currents, we examined the effect of serum on fresh pericytes which had their potassium channels blocked by caesium and barium (solutions C and D). Under these recording conditions, serum induced a current that showed outward rectification (Fig. 7). To determine the ionic selectivity of this conductance, we varied the ionic composition of the bathing solution (Table 2); osmolarity was maintained by adjusting the mannitol concentration. Under control conditions, the mean reversal potential was −17 mV. Increasing the external calcium concentration from 1.8 to 11.8 mm caused a 6 mV depolarization of the reversal potential. This is consistent with a permeablity to calcium. With the addition of 30 mm KCl to solution C, the reversal potential was −9 mV. This depolarization from −17 mV suggests a permeability to potassium. A sodium permeability was demonstrated when substitution of 30 mm NaCl for KCl resulted in a reversal potential of −5 mV. These experiments indicate that serum activates a non-specific cation (NSC) channel that is sensitive to both monovalent and divalent cations. Based on the extended constant-field voltage equation with assumptions similar to those detailed by Mayer & Westbrook (1987), the serum-induced NSC channels are more permeable to calcium than to monovalent cations (permeability ratio, PCa/PNa,K= 3).
Table 2.
Effect of changes in the ionic composition of the bathing solution on the reversal potential (Vrev) of the serum-induced current
| [Ca2+]o(mm) | [Na+]o(mm) | [K+]o(mm) | [Cl−]o(mm) | Vrev(mV) | n | P | |
|---|---|---|---|---|---|---|---|
| Control | 1.8 | 0 | 0 | 38.8 | −17 ± 2 | 9 | — |
| CaCl2 | 11.8 | 0 | 0 | 58.8 | −11 ± 2 | 7 | 0.03 |
| KCl | 1.8 | 0 | 30 | 68.8 | −9 ± 2 | 3 | 0.03 |
| NaCl | 1.8 | 30 | 0 | 68.8 | −5 ± 1 | 3 | < 0.01 |
As a further demonstration that serum activates NSC channels in retinal pericytes, we tested the effect of SK&F 96365, a blocker of receptor-activated non-specific cation channels (Merritt et al. 1990). Bathing solution C and pipette solution D were used to block potassium channels. As illustrated in Fig. 8, exposure to this blocker reduced the serum-induced NSC conductance. In a series of five cells, the current measured at −50 mV was decreased by 68 ± 4 % in 50 μm SK&F 96365. Taken together, our observations support the idea that exposure of retinal pericytes to serum activates calcium-permeable NSC channels, as well as voltage-dependent potassium channels and chloride channels.
Figure 8. Effect of SK&F 96365 on the serum-induced current in a retinal pericyte recorded under conditions in which potassium channels were blocked.
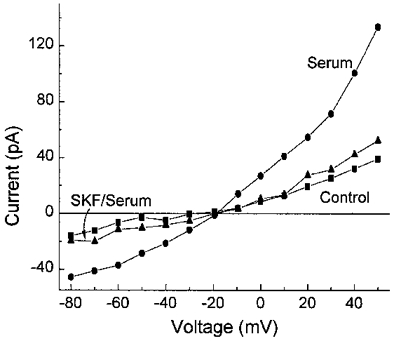
I–V relations of the steady-state currents under control conditions (▪) and in the presence of serum without (•) or with 50 μm SK&F 96365 (▴). Bathing solution C and pipette solution D were used to block potassium channels. The serum-induced NSC conductance is sensitive to SK&F 96365.
Effect of IGF-1 on pericyte currents
Having found that exposure to serum changes the ionic currents of retinal pericytes, we wished to identify at least one of the molecules in serum that can mediate these effects. To help identify a range of molecular weights for candidate molecules, serum was dialysed to remove molecules with molecular weights (MWs) less than 6000. Since we found that the dialysed serum activated the pericyte currents, attention focused on molecules with molecular weights greater than 6000. One candidate molecule is insulin-like growth factor-1 (IGF-1, MW 7500), which is a normal constituent of the blood (LeRoith, 1997) and can bind to the IGF-1 receptors expressed by retinal pericytes (King et al. 1984). As illustrated in Fig. 9, exposure to IGF-1 reversibly altered the sustained and transient ionic currents of fresh retinal pericytes. These effects were qualitatively similar to those induced by serum (Fig. 2). Nine other retinal pericytes were also similarly affected by IGF-1. However, since only 9 of 16 (56 %) sampled pericytes were responsive to this growth factor compared with 22 of 22 pericytes which had currents induced by serum, it seems likely that, in addition to IGF-1, serum contains other molecules which can activate ion channels in retinal pericytes.
Voltage-gated calcium channels in retinal pericytes
Having found that serum-derived molecules cause retinal pericytes to depolarize, we asked whether these cells have voltage-gated calcium channels (VGCCs). These channels would provide additional pathways for a calcium influx that may instigate pericyte contraction or other important cellular responses. To detect calcium currents, we used pipette solution D and bathing solution C, which was supplemented with 10 mm CaCl2; these solutions blocked potassium channels and enhanced the electrochemical gradient for an influx of calcium. Under these experimental conditions, inward currents (Fig. 10A) were activated at depolarized potentials in 12 of 13 sampled pericytes. Nifedipine, which is a dihydropyridine that blocks L-type VGCCs, effectively inhibited the inward currents (Fig. 10B and C). A sensitivity to nifedipine was demonstrated in all of five sampled pericytes.
Figure 10. Voltage-gated calcium currents in a fresh retinal pericyte.
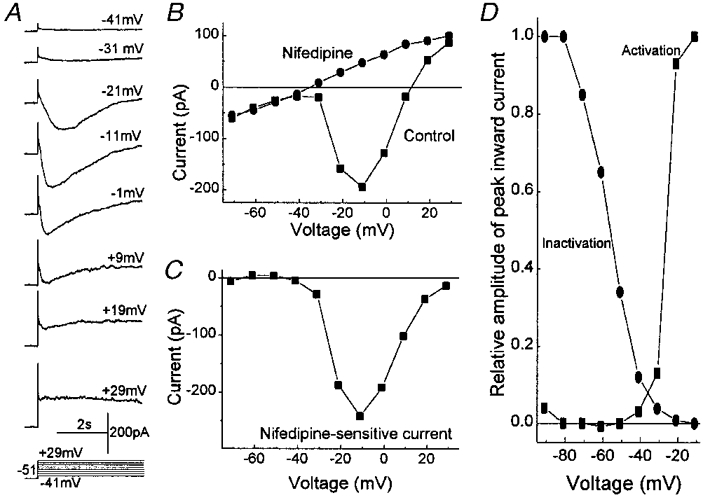
A, currents evoked from a holding potential of −51 mV to test potentials of −41 to +29 mV. Potassium channels were blocked and calcium currents enhanced by using bathing solution C, which was supplemented with 10 mm CaCl2, and pipette solution D. B, plots of the peak inward current versus test potential in the absence (▪) and presence (•) of 10 μm nifidipine. The holding potential was −51 mV. C, the difference of the I–V curves in B. D, squares show the normalized peak amplitudes of the inward currents evoked by stepping from a holding potential of −101 mV to various test potentials. Currents were normalized to the maximal inward current detected with a voltage step to −10 mV and are plotted against the test potential. Circles show the peak amplitudes of the inward currents evoked by voltage steps from various holding potentials to a test potential of −11 mV. Currents were normalized to the maximal current detected with a holding potential of −91 mV and are plotted against the holding potential. Between approximately −50 mV and −20 mV, the activation and inactivation curves overlap and reveal the ‘window of current’ for the L-type calcium channels expressed by the retinal pericyte.
In our perforated-patch recordings, the calcium currents in retinal pericytes showed time-dependent changes suggesting channel inactivation (Fig. 10A). Plots of the activation and inactivation curves for the nifedipine-sensititive current revealed a range of voltages, from approximately −50 mV to −20 mV, over which there was an overlap of these two curves (Fig. 10D). A sustained VGCC conductance could occur within this ‘window of current.’ Similar findings were made in three fresh retinal pericytes. Taken together this series of observations suggest that a serum-induced depolarization can activate L-type VGCCs in retinal pericytes.
Role of NSC channels in retinal pericytes
To examine the role of the NSC channels in the response of retinal pericytes to serum-derived molecules, we tested the effect of the NSC channel blocker, SK&F 96365, under recording conditions in which potassium and chloride channels were not blocked (solutions A and B). As expected, we found that SK&F 96365 diminished the effect of serum on the NSC conductance. To quantify this NSC conductance, we measured the current at −100 mV, which is the equilibrium potential for potassium (EK) and thus a voltage at which NSC currents predominate. The inward current at −100 mV was reduced by 65 ± 6 % (n= 4) in SK&F 96365. In addition, there was also a 75 ± 5 % (n= 4) reduction in the serum-induced current measured at 0 mV, which is near the NSC equilibrium potential (ENSC) and thus a voltage at which the potassium conductance predominates. We also found that the addition of SK&F 96365 to the perfusate diminished by 63 ± 5 % (n= 4) the serum-induced increase in transiently occurring inward currents. These results indicate that exposure of pericytes to SK&F 96365 causes inhibition of the serum-induced increases in potassium and chloride channel activity, as well as the activity of NSC channels. Assuming that SK&F 96365 blocks NSC channels specifically, our findings suggest that the serum-activated NSC channels are essential for the subsequent activation of chloride and potassium channels in retinal pericytes.
Calcium dependence of chloride and potassium channels
Since we found that calcium-permeable NSC channels were needed for the activation of the chloride and potassium channels, we hypothesized that an influx of calcium played a role. Consistent with this idea, the serum-induced transient currents, which are due to the activity of chloride channels, were markedly reduced when the external calcium concentration was low (Fig. 11A and B). In addition, a component of the sustained outward current with a threshold of approximately −50 mV was also dependent on external calcium (Fig. 11B, C and D). However, an NSC conductance could be activated by serum in the low calcium bathing solution. Similar effects on transient and sustained currents were observed in three pericytes. These experiments are consistent with a mechanism in which NSC channel activation and calcium influx are critical for the serum-induced activation of chloride and potassium channels.
Effects of serum on retinal vascular smooth muscle cells
Since pericytes are known to share a number of characteristics with vascular smooth muscle cells, we considered the possibility that serum affects the ion channels of these cells similarly. To assess this possibility, we used the perforated-patch technique to monitor the whole-cell currents of vascular smooth muscle cells located on freshly isolated retinal vessels, which had diameters of 15–45 μm. In contrast to our findings with pericytes, we did not detect a significant (P > 0.229) effect of serum on the sustained ionic conductances or the resting membrane potential of vascular smooth muscle cells (Table 3). However, both cell types showed a significant (P < 0.01) increase in the transient inward currents during exposure to serum (Table 3). Thus, chloride channels, but not NSC and potassium channels, are activated when retinal vascular smooth muscle cells are exposed to serum-derived molecules.
Table 3.
Comparison of the effects of serum on ionic currents and the membrane potential of fresh retinal pericytes and vascular smooth muscle cells
| Sustained currents | Transient current | |||
|---|---|---|---|---|
| NSC (pA) | K (pA) | Cl (pC) | Vm (mV) | |
| Pericytes | ||||
| Control | −35 ± 10 | 68 ± 17 | −9 ± 1 | −56 ± 2 |
| Serum | −72 ± 13* | 111 ± 21* | −31 ± 9* | −46 ± 2* |
| Myocytes | ||||
| Control | −187 ± 28 | 121 ± 35 | −29 ± 8 | −52 ± 3 |
| Serum | −174 ± 35 | 118 ± 35 | −41 ± 8* | −49 ± 4 |
The non-specific cation (NSC) conductance was measured at voltages near EK; the potassium current was measured at 0 mV, which is close to ENSC. The net charge transfer associated with the transient chloride currents was measured at −57 mV. Membrane potentials (Vm) were determined from current-voltage plots of the sustained currents.
Significant (P < 0.01) difference between the mean value in serum and the corresponding control value. Sample sizes were 22 for the pericytes and 9 for the vascular smooth muscle cells. The sampled pericytes had a membrane capacitance of 11.9 ± 1.2 pF, for the vascular smooth muscle cells the value was 10.8 ± 1.5 pF (P= 0.7). In contrast to pericytes, serum neither induced sustained currents nor significantly changed the resting membrane potential of retinal vascular smooth muscle cells.
DISCUSSION
Serum-activated ion channels in retinal pericytes
The results show that exposure of retinal pericytes to serum alters the activity of several types of ion channels. Our perforated-patch recordings from pericytes located on freshly isolated microvessels revealed that one of the channels activated by serum is permeable to monovalent cations and calcium. In addition, serum increases the frequency and size of transient inward currents that are caused by the opening of chloride channels. Serum also induces a voltage-dependent potassium conductance. These effects of serum on ion channel activity are mimicked by IGF-1, suggesting that this may be one of the serum-derived molecules regulating the physiology of pericytes when a breakdown of the vascular endothelial barrier occurs.
Associated with these serum-induced changes in ion channel activity, the membrane potential of a retinal pericyte decreases. Although limited by the hyperpolarizing effect of the serum-induced potassium channels, the activation of the NSC channels caused a sustained depolarization of 10 mV, from −56 to −46 mV. This change in membrane potential is likely to be functionally significant since we observed that, between approximately −50 and −20 mV, there is a ‘window of current’ for the voltage-gated calcium channels (VGCCs) expressed by these cells. At voltages within this window there is enough depolarization to activate the VGCCs, but not so much as to cause complete inactivation. VGCCs can also be activated during the serum-induced transient depolarizations caused by the intermittent activity of chloride channels. Thus, exposure to serum-derived molecules activates voltage-dependent (i.e. VGCCs) and voltage-independent (i.e. NSC channels) pathways for the influx of calcium into retinal pericytes.
Role of NSC channels
Activation of the NSC channel may be a critical step in the response of retinal pericytes to serum (Fig. 12). For example, our experiments suggest that the calcium-permeable NSC channels are also necessary for the activation of chloride and potassium channels. This is supported by the finding that SK&F 96365, which is a blocker of receptor-activated NSC channels (Merritt et al. 1990), diminished the serum-induced chloride and potassium currents, as well as the NSC conductance. Also consistent with a primary role for these calcium-permeable channels, we found that an influx of calcium was required for the activation of chloride and potassium channels. In addition, these NSC channels appear to be necessary for the activation of VGCCs since the serum-induced depolarization of the pericytes was prevented by SK&F 96365. Taken together, these observations suggest that the activation of NSC channels and the subsequent influx of calcium play important roles in the response of retinal pericytes to serum-derived molecules.
Figure 12. Schematic outline of the proposed effects of serum on pericyte physiology.
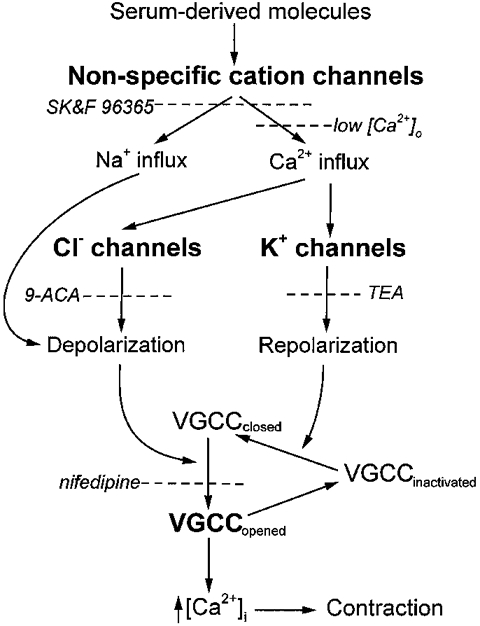
As detailed in the text, serum-derived molecules, such as IGF-1, activate several types of ion channels in fresh retinal pericytes. Activation of NSC channels appears to be required for the subsequent opening of chloride and potassium channels. The depolarizations induced by NSC and chloride channel activity can open VGCCs. The transient nature of the depolarizations induced by the serum-activated chloride channels may enhance the net influx of calcium by allowing periods of repolarization during which inactivated VGCCs can transition to the closed state. The dashed lines show steps at which the response of pericytes to serum can be blocked.
Transient depolarizations in retinal pericytes
A dramatic feature of the electrophysiology of fresh retinal pericytes is the presence of transient depolarizing currents. Upon exposure of fresh retinal pericytes to serum, these transient currents increased markedly. Our experimental findings indicate that calcium-activated chloride channels are responsible for these currents. Specifically, we observed that the reversal potential for the transient currents is close to the equilibrium potential for chloride, that the chloride channel blocker 9-ACA inhibits these currents and that these events are dependent on extracellular calcium. Future studies are needed to determine whether calcium release from intracellular stores, uptake of calcium into mitochondria and/or inactivation of channels by calcium-calmodulin-dependent protein kinase II have roles in establishing the time course of the transient inward currents in pericytes as they do in smooth muscle cells (Wang et al. 1992; Greenwood et al. 1997; Wang & Kotlikoff, 1997).
At present, the functional role of the transiently active chloride channels in vascular cells is uncertain. One possibility is that the repetitive depolarizations caused by the opening of these channels serve to enhance the net influx of calcium during a prolonged exposure of pericytes to serum-derived molecules. This possibility is supported by our observation that the VGCCs of retinal pericytes inactivate during a sustained depolarization. Thus, with a relatively long-lasting decrease in the membrane potential, channel inactivation may limit the influx of calcium via VGCCs. However, in contrast to a sustained depolarization, frequent transient depolarizations allow for the repetitive opening of a pericyte's VGCCs. In this scenario (Fig. 12), activation of chloride channels induces a depolarization which causes VGCCs to open. After opening, these calcium channels begin to inactivate, and the influx of calcium decreases. However, since the chloride channels are only transiently active, the pericyte subsequently repolarizes. With repolarization of the membrane potential, inactivated VGCCs can return to the closed state and be ready to reopen when the pericyte is again transiently depolarized. Thus, multiple transient depolarizations may be an effective mechanism for maximizing the net influx of calcium during a prolonged exposure to serum.
Channel activity and pericyte function
Activation of ion channels by serum-derived molecules is likely to alter the function of pericytes. One mechanism by which ion channels may affect cellular function is by providing pathways for an influx of calcium. We found that exposure to serum activates two types of calcium-permeable ion channels in retinal pericytes. One is an NSC channel; the other is an L-type calcium channel, which can be activated by the depolarization caused by the opening of the NSC and chloride channels.
A probable response of pericytes to a serum-induced influx of calcium is contraction. This seems to be a reasonable possibility since pericytes express contractile proteins, such as tropomysin (Joyce et al. 1985), myosin (DeNofrio et al. 1989) and cGMP-dependent protein kinase (Joyce et al. 1984), which are activated by calcium. In addition, Kelley et al. (1987) documented that retinal pericytes in culture contract when exposed to serum. Furthermore, we have observed (unpublished observations) that the diameter of freshly isolated microvessels decreases in the presence of serum.
Serum-induced contraction of pericytes may be a successful adaptive response to a breakdown of the blood-retinal barrier. Contraction of leaky microvessels would shunt blood away from areas with a defective vascular endothelial barrier. This shunting could reduce leakage from the vascular system and, thereby, lessen sight-threatening retinal oedema. On the other hand, an extensive shunting of blood may cause ischaemic damage. In addition, a persistent influx of calcium caused by chronic exposure to serum may require a substantial metabolic response in order to prevent cellular injury from an excessive accumulation of calcium. Eventually, pericytes may die if they fail to meet this metabolic demand. A possible clinical correlate is the selective loss of pericytes early in the course of diabetic retinopathy, a condition associated with a chronic failure of the blood-retinal barrier (Cunha-Vaz et al. 1975; Mathews et al. 1997).
Comparison of pericyte and vascular smooth muscle physiology
Traditionally, pericytes have been thought to be similar in many ways to vascular smooth muscle cells. For example, both cell types are derived from the mesenchyme, are closely associated with the vascular endothelium and are known to express similar ion channels (Weiderholt et al. 1995) and cytoplasmic proteins (Hirschi & D'Amore, 1996). In addition, both cell types can contract (Kelley et al. 1987; Das et al. 1988), although evidence of pericyte contraction in vivo is limited. Further, with regard to function, pericytes are postulated to regulate blood flow in the microvasculature much as vascular smooth muscle cells do in larger vessels (Tilton, 1991; Anderson, 1996; Hirschi & D'Amore, 1996). However, despite these developmental, biochemical and functional similarities, we found that pericytes and vascular smooth muscle cells respond differently to serum-derived molecules. Namely, serum activates sustained ionic conductances in retinal pericytes, but not in vascular smooth muscle cells.
We suspect that the activation of NSC channels in pericytes and not in vascular smooth muscle cells reflects a fundamental difference in the mechanisms by which serum-derived molecules affect these two types of cells. In retinal pericytes, the calcium-permeable NSC channels appear to play a vital role in the subsequent opening of chloride, potassium and voltage-gated calcium channels. However, vascular smooth muscle cells lack a serum-induced NSC conductance, although serum does activate transient depolarizing currents that are caused by the opening of calcium-activated chloride channels. To account for these observations, we postulate that a serum-induced release of calcium from intracellular stores is an early step in the response of vascular myocytes. In contrast, our experiments indicate that an event early in the response of pericytes is an influx of calcium through serum-activated NSC channels.
These physiological differences in the responses of pericytes and vascular smooth muscle cells suggest that these two types of vascular cells have different functional roles in the regulation of the retinal circulation. In addition, there may be pathobiological consequences. Perhaps, under conditions in which there is a chronic breakdown in the blood-retinal barrier, a persistent influx of calcium via serum-activated channels renders retinal pericytes selectively vulnerable to a cytotoxic overload of calcium.
Acknowledgments
This work was supported by grants from the NIH, EY12505, EY07003, DK-20572, MH14279, and the Michigan Eye Bank and Transplantation Center. D.G.P. received a Research to Prevent Blindness Senior Scientist Award. We thank Chris Edwards of the University of Michigan Anatomy and Cell Biology Core Facility for performing the scanning electron microscopy, S. Kusaka for preparing dialysed serum and B. Hughes for helpful discussions.
References
- Agardh E, Yeh HH, Herrmann R, Puro DG. γ-Aminobutyric acid-mediated inhibition at cholinergic synapses formed by cultured retinal neurons. Brain Research. 1985;330:323–328. doi: 10.1016/0006-8993(85)90692-4. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Glaucoma, capillaries and pericytes 1. Blood flow regulation. Ophthalmologica. 1996;210:257–262. doi: 10.1159/000310722. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. Journal of Neuroscience Research. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1993;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Archives of Ophthalmology. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, DeAbreu JRF, Campos AJ, Figo GM. Early breakdown of the blood-retinal barrier in diabetes. British Journal of Ophthalmology. 1975;59:649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Frank RN, Weber ML, Kennedy A, Reidy CA, Mancini MA. ATP causes retinal pericytes to contract in vitro. Experimental Eye Research. 1988;46:349–362. doi: 10.1016/s0014-4835(88)80025-3. [DOI] [PubMed] [Google Scholar]
- DeNofrio D, Hook TC, Herman I. Functional sorting of actin isoforms in microvascular pericytes. Journal of Cell Biology. 1989;109:191–202. doi: 10.1083/jcb.109.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Helliwell RM, Large WA. Modulation of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle by an inhibitor of mitochondrial Ca2+ uptake. The Journal of Physiology. 1997;505:53–64. doi: 10.1111/j.1469-7793.1997.053bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 1992. [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovascular Research. 1996;32:687–698. [PubMed] [Google Scholar]
- Joyce NC, DeCamilli P, Boyles J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvascular Research. 1984;28:206–219. doi: 10.1016/0026-2862(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. Journal of Cell Biology. 1985;100:1379–1386. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C, D'Amore P, Hechtman HB, Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. Journal of Cell Biology. 1987;104:483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GL, Goodman DA, Buzney S, Moses A, Kahn RC. Receptors and growth-promoting effects of insulin and insulin-like growth factors on cells from bovine retinal capillaries and aorta. Journal of Clinical Investigation. 1984;75:1028–1039. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka S, Puro DG. Intracellular ATP activates inwardly rectifying K+ channels in human and monkey retinal Müller (glial) cells. The Journal of Physiology. 1997;500:593–604. doi: 10.1113/jphysiol.1997.sp022045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Studies of retinal vascular patterns. Part 1. Normal architecture. Archives of Ophthalmology. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. Journal of Neuroscience Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- LeRoith D. Insulin-like growth factors. New England Journal of Medicine. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- Mathews MK, Merges C, McLeod DS, Lutty GA. Vascular endothelial growth factor and vascular permeability changes in human diabetic retinopathy. Investigative Ophthalmology and Visual Science. 1997;38:2729–2741. [PubMed] [Google Scholar]
- Matsugi T, Chen Q, Anderson DR. Contractile responses of cultured bovine retinal pericytes to angiotensin II. Archives of Ophthalmology. 1997;115:1281–1285. doi: 10.1001/archopht.1997.01100160451011. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. The Journal of Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JE, Armstrong WP, Benhan CD, Hallan TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochemistry Journal. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA-responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. Journal of Neuroscience. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J-S, Ebihara S, Akaike N. Gramicidin perforated patch-clamp technique reveals glycine-gated outward chloride current in dissociated nucleus solitarii neurons of the rat. Journal of Neurophysiology. 1994;72:1103–1108. doi: 10.1152/jn.1994.72.3.1103. [DOI] [PubMed] [Google Scholar]
- Schonfelder U, Hofer A, Paul M, Funk RHW. In situ observation of living pericytes in rat retinal capillaries. Microvascular Research. 1998;56:22–29. doi: 10.1006/mvre.1998.2086. [DOI] [PubMed] [Google Scholar]
- Shepro D, Morel NL. Pericyte physiology. FASEB Journal. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Tilton RG. Capillary pericytes: Perspectives and future trends. Journal of Electron Microscopy Technique. 1991;19:327–344. doi: 10.1002/jemt.1060190308. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Kilo C, Williamson JR, Murch DW. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvascular Research. 1979;18:336–352. doi: 10.1016/0026-2862(79)90042-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. The Journal of Physiology. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-Y, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1997;94:14918–14923. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederholt M, Berwick S, Helbig H. Electrophysiological properties of cultured retinal capillary pericytes. Progress in Retinal Research. 1995;14:437–451. [Google Scholar]


