Abstract
Unlike in normal rabbits, pulmonary rapidly adapting receptors (RARs) in rabbits with chronic mitral regurgitation (MR) do not respond to small changes in extravascular fluid (EVF) volume in major airways. The present study examined the effect of shrinking the EVF volume in rabbits with chronic MR by infusing hypertonic albumin, to see whether this response of RARs is restored. The effect of raising the left atrial pressure (LAP) acutely above 25 mmHg (to cause pulmonary oedema) on RARs was also investigated.
Mean RAR activities in rabbits with MR (n= 6) at initial control, LAP +5 mmHg, LAP +10 mmHg and final control periods were 20·9 ± 9·5, 18·8 ± 11·3, 27·0 ± 11·2 and 17·2 ± 9·8 action potentials min−1, respectively (P> 0·05, ANOVA). After infusion of 35 % bovine serum albumin i.v. these values were 9·4 ± 3·2, 30·6 ± 14·6, 48·9 ± 10·1 and 18·4 ± 7·3 action potentials min−1, respectively (P < 0·01, ANOVA). In rabbits with chronic MR (n= 7) raising the LAP above 25 mmHg stimulated RARs.
EVF content of the airways and lungs was measured in rabbits with MR and in control rabbits, at baseline and after elevation of the LAP by 10 or 25 mmHg for 20 min. In control rabbits the EVF contents in the lower trachea, carina and bronchi at baseline and at LAP +10 mmHg were 52·1 ± 1·2 and 57·8 ± 1·7 %, respectively (P < 0·05, Student's t test). In rabbits with MR these values were 58·3 ± 1·5 and 56·9 ± 1·9 %, respectively. When the LAP was elevated by 25 mmHg the EVF content increased to 62·4 ± 1·1 % (P < 0·05, t test compared with baseline and LAP +10 mmHg).
We concluded that in rabbits with chronic MR, RARs are unable to respond to acute, small elevations of LAP because there is no concomitant increase in EVF content in the vicinity of these receptors. Furthermore, these receptors can be activated in these animals by elevating the LAP above 25 mmHg or can be made sensitive to acute small elevations of LAP by shrinking the chronically expanded EVF compartment.
Pulmonary rapidly adapting receptors (RARs) in anaesthetized intact rabbits are activated by small changes in the extravascular fluid spaces of the major airways where they are located (Hargreaves et al. 1991). It has also been shown that stimulation of RARs in this manner causes reflex increases in respiratory rate (Kappagoda et al. 1989), tracheal tone (Kappagoda et al. 1988) and mucus production by the respiratory epithelium (Yu et al. 1989). All these reflex effects collectively form the syndrome of cardiac asthma which is characterized by wheezing, an increased respiratory rate and increased mucus production. Therefore, it was suggested that in chronic left ventricular dysfunction, intermittent, small acute elevations of left atrial pressure (LAP) cause these exacerbations in symptoms through activation of RARs (Hargreaves et al. 1991).
However, in a recent study from our laboratory performed on rabbits maintained in a state of chronic pulmonary venous congestion (PVC) secondary to surgically induced mitral regurgitation (MR), acute small elevations (+10 mmHg) of LAP failed to activate RARs (Gunawardena et al. 1998). It was speculated that in these animals this lack of response of RARs could be due to a change either in the receptor per se, or in the extravascular space surrounding the receptor. These issues were addressed in the present study by testing the following hypotheses: (i) shrinking the extravascular space by an intravenous infusion of hypertonic albumin restores the sensitivity of previously insensitive RARs (to small, acute elevations of LAP) in rabbits with chronic PVC, (ii) the increase in extravascular fluid volume in major airways resulting from small elevations of LAP is less in animals with chronic PVC than in age-matched control animals, and (iii) enhancing the stimulus to RARs by increasing the LAP above 25 mmHg stimulates previously insensitive RARs in rabbits with chronic PVC. As in the previous study (Gunawardena et al. 1998), chronic PVC was induced by surgically damaging the mitral valve.
METHODS
Experiments were performed in 38 New Zealand White rabbits weighing 3.8 ± 0.04 kg (range, 3.2-4.3 kg). Experiments were divided into two categories: (a) electrophysiological studies on the activity of RARs (n= 13) and (b) assessment of extravascular water content in the major airways, the lungs and the left ventricle (n= 27). (Two animals with chronic MR were used both for electrophysiological studies and the assessment of water content, hence the total of 38 rabbits.) Mean age (at the time of the final experiment) of the rabbits who underwent surgical damage to the mitral valve was 30.4 ± 0.5 weeks. Mean age of the age-matched control rabbits was 31.1 ± 0.8 weeks (P > 0.05, t test). All the protocols were approved by the Animal Use and Care Committee of the University of California, Davis.
Electrophysiological studies on RARs
Thirteen rabbits were studied. Their mitral valves were damaged surgically under anaesthesia (described below). The animals were allowed to develop chronic PVC over the next 12 weeks after which they were re-anaesthetized and prepared for recording of action potentials in the cervical vagus nerve.
Surgical induction of mitral regurgitation
Mitral valves of these rabbits were damaged through a left-sided thoracotomy, as described in an earlier study done in the same laboratory (Gunawardena et al. 1998). Briefly, rabbits were pre-medicated with ketamine HCl (50 mg kg−1, Mallinkrodt Veterinary Inc., Mundelin, IL, USA) and xylazine (5 mg kg−1, Bayer, Shawnee Mission, KA, USA) i.m. and were then anaesthetized with pentobarbital sodium (5 mg kg−1, Veterinary Laboratories Inc., Lenexa, KA, USA) given through the dorsal marginal ear vein. The chest was opened at the left fourth intercostal space using aseptic techniques. The mitral valve was damaged using a pair of micro-scissors (Straight 14.5 cm, item 15030-14, Fine Science Tools, Inc., CA, USA) introduced through the left atrial appendage. The chest was then closed in layers after reducing the pneumothorax by suction. Surgery was completed within 20–30 min. An antibiotic (enrofloxacine, Baytril; 6 mg kg−1, i.m. for 5 days) was given to prevent postoperative infections.
Electrophysiological studies
Twelve weeks after the mitral valve surgery, the rabbits were pre-medicated with ketamine HCl (50 mg kg−1i.m.) and xylazine (5 mg kg−1i.m.) and anaesthetized with pentobarbital sodium (5 mg kg−1i.v.); anaesthesia was maintained with the same dose of pentobarbital sodium given i.v. every half an hour. They were ventilated (Model 55–0798; Harward Apparatus, South Natic, MA, USA) at a tidal volume of ∼8 ml kg−1 and a rate of ∼20 min−1, through a tracheostomy performed just below the larynx. The right femoral artery was cannulated (i.d. 1.14 mm, Intramedic polyethylene tubing, Becton Dickinson Co., Sparks MD, USA) to measure the blood pressure and to obtain blood samples for blood gas analysis. The right femoral vein was cannulated (i.d. 0.86 mm, Intramedic polyethylene tubing) to introduce anaesthetic agents and other intravenous infusions. Animals were paralysed with gallamine triethiodide (1.5 mg kg−1, Sigma) given i.v. every half an hour. This was necessary as artifacts from muscle movements could interfere with the recording of action potentials. Adequacy of anaesthesia in paralysed animals was tested by allowing them to recover from the effects of gallamine at approximately hourly intervals and testing them for absence of withdrawal reflex and an increase in arterial blood pressure and heart rate. The arterial PCO2, pH and bicarbonate concentration were maintained within the physiological range by adjusting the respiratory rate and tidal volume and by infusing sodium bicarbonate (8.4 % w/v, Abbott Laboratories, North Chicago, IL, USA) as needed. The body temperature of the animals was maintained at 37 ± 1°C using heating pads.
The chest was opened by a midline sternal incision and a balloon-tipped cannula (i.d. 0.86 mm, Intramedic polyethylene tubing) was introduced to the left atrium through its appendage. Another cannula (i.d. 1.14 mm) was also introduced to measure the LAP. Inflation of this balloon was used to increase the LAP acutely by obstructing the mitral valve partially. After opening the chest, the expiratory line of the ventilator was kept 1–2 cm under water to prevent the collapse of the lungs. LAPs reported in this paper are referenced at the mid-heart level by exposing the tip of the cannula to air at the end of each experiment.
Recording action potentials from the vagus
The right cervical vagus nerve was carefully dissected away from the carotid artery and a small pool was created around it using the surrounding tissues, which was then filled with mineral oil. The vagus nerve was placed on a dissecting platform and desheathed under a dissecting microscope (D. F. Vasconcellos, Model M900, Thousand Oaks, CA, USA) and thin filaments of vagus nerve were dissected away from the nerve. A pair of bipolar platinum electrodes were used to record single unit activity of pulmonary RARs from these nerve filaments. The raw signals were amplified and filtered (Grass Instruments Co., Model CP511B, W. Warwick, RI, USA) and then fed to a recorder with light-sensitive paper (Gould Instrument Systems, Model TA 11, Valley View, OH, USA), audio device and an electronic counter with an amplitude discriminator. Action potentials arising from RARs were identified by their burst of activity and rapid adaptation (over 70 % adaptation in 1 s) in response to hyperinflation of the lung by clamping the expiratory line of the ventilator for three consecutive breaths. Conduction velocity of the nerve fibres was measured at the end of each experiment by stimulating the nerve electrically (strength, 1.5-4 V; duration, 0.07-2 ms) 1.5-2.5 cm caudal to the recording electrode (Grass Instruments Co., Model SMD 9J). Location of the RARs was identified at the end of each experiment by gently probing the bronchial tree externally with a wet cotton-tipped applicator. At the end of the experiment, the animal was killed with an overdose of pentobarbital sodium (60 mg kg−1i.v.).
The mitral valve apparatus was visually examined post mortem in all the rabbits in order to confirm the mitral valve damage. The left ventricular weight was measured after trimming the right ventricle, atrial tissues and the ascending aorta away from it.
Experimental protocols
Effect of shrinking the extravascular compartment (by using a hypertonic albumin infusion) on the activity of RARs
After identifying an RAR, it was allowed to stabilize for 10 min. The activity of the receptor was then recorded each minute for 10 consecutive minutes. Next, the left atrial balloon was inflated and the LAP was elevated by 5 mmHg followed by a further 5 mmHg (each for a period of 5 min). The activity of RARs was recorded each minute during these two 5 min periods. Then the balloon was deflated and the final control activity was recorded each minute for 10 min. Then a hypertonic albumin infusion (10 ml of 35 % bovine serum albumin solution) was given i.v. over a 2–3 min time period and the above protocol was repeated (n= 6). A 2 ml arterial blood sample was withdrawn immediately before and 1 min after the completion of hypertonic albumin infusion to analyse plasma protein, albumin and sodium concentrations (Boehringer Mannheim/Hitachi, Biochemical analyser, Model 912, Indianapolis, IN, USA). Plasma osmolality (Advanced Digimatic Osmometer, Model 3DII, Needham Heights, MA, USA) and haematocrit (Fisherbrand heparinized micro-haematocrit capillary tubes) were also measured.
Effect of acute elevation of LAP above 25 mmHg on the activity of RARs
After identifying an RAR, it was allowed to stabilize for 10 min (n= 7). Then, initial control activity was recorded each minute for 10 consecutive minutes. Next, the LAP was elevated above 25 mmHg by inflating the balloon in the left atrium and the activity was recorded each minute for 15 min. The left atrial balloon was then deflated and the final control counts were recorded for the next 10 min. In two of these rabbits, after recording the final control activity, the LAP was again elevated above 25 mmHg for 20 min and then the tissues were obtained for analysis of the water content.
Measurement of the water content in the trachea, bronchi, lungs and the left ventricle
Seventeen mitral valve-damaged rabbits and 10 age-matched control rabbits were used to measure the water content of the the airways, lungs and the left ventricular muscle. Water content of the tissues was measured under the following conditions: (i) at baseline conditions (7 mitral valve-damaged rabbits and 5 age-matched control rabbits), (ii) after elevating the LAP by 10 mmHg for 20 min (5 mitral valve-damaged rabbits and 5 age-matched control rabbits), and (iii) after elevating the LAP by 25 mmHg for 20 min (5 mitral valve-damaged rabbits).
Twelve rabbits were investigated under baseline conditions (5 control rabbits and 7 mitral valve-damaged rabbits). The animals were pre-medicated with a mixture of ketamine HCl (50 mg kg−1) and xylazine (5 mg kg−1) i.m. and killed with pentobarbital sodium (60 mg kg−1i.v.). The airways, lungs and the heart were removed en bloc. Fifteen other rabbits whose tissues were removed after elevating the LAP acutely by either 10 mmHg (5 control rabbits and 5 rabbits with mitral valve damage) or 25 mmHg (5 rabbits with mitral valve damage) for 20 min were prepared as follows. Animals were pre-medicated with ketamine HCl (50 mg kg−1i.m.) and xylazine (5 mg kg−1i.m.) and anaesthetized with pentobarbital sodium (5 mg kg−1i.v.). Anaesthesia was maintained with pentobarbital sodium (5 mg kg−1i.v. every 30 min). As in the electrophysiological studies, adequacy of anaesthesia was assessed by carefully monitoring the blood pressure and the heart rate for absence of spontaneous fluctuations and fluctuations in response to paw pinch. Artificial ventilation and haemodynamic and blood gas monitoring were undertaken as described above. Immediately after induction of anaesthesia some of the mitral valve-damaged rabbits (n= 8) underwent ultrasound examination of the heart (Hewlett Packard Model 5500 Echo machine, 8 MHz probe), in order to confirm the mitral regurgitation.
The chest was opened by a midline incision anteriorly and a balloon-tipped cannula and another cannula were introduced to the left atrium as described earlier. In these animals, after 20 min of acute elevation of LAP, animals were killed with pentobarbital sodium (60 mg kg−1) and the tissues were removed.
Once removed, tissues were immediately placed in a humidified chamber and further dissected to obtain tissue samples from: (1) upper trachea (up to 1 cm from the carina), (2) lower trachea, carina and major bronchi, (3) middle and lower lobes of right lung, and (4) left ventricular muscle. Each of these samples was further divided into two (trachea and airways were divided longitudinally) and were placed in pre-weighed glass vials which were closed immediately with a screw cap. The vials were then weighed and the wet weight of the tissues was obtained. The weights of the two samples were similar (e.g. trachea, carina and major bronchi samples: 0.36 ± 0.02 vs. 0.37 ± 0.02 g, P > 0.05, t test). The balance had a sensitivity of 0.1 mg (Sartorius, model BP 61, Goettingen, Germany).
One sample from each area was kept in an oven at ∼95°C for 5–7 days (until the weight became constant) to obtain the dry weight. The difference between the wet and dry weights provided the total water content which was expressed as a percentage of the wet weight.
The second sample of tissue was used to estimate the amount of blood in the wet tissue. These tissues were homogenized after adding a known amount of distilled water, using a tissue homogenizer (Tissue Tearer, model 985-370, Biospec Products, Inc., Racine, WI, USA). The homogenates were centrifuged at 4000 r.p.m. (2000 g) for 30 min and the supernatants were removed for analysis of haemoglobin concentration using spectrophotometric method (plasma haemoglobin measurement kit, Sigma, catalogue item 527A and Hewlett Packard model HP 8453, Biochemical UV-Vis system spectrophotometer). This method of measurement of the blood volume is a modification of the method used by Pearce et al. (1965) and Hemingway (1950) in calculating the blood volume in the lung. The residue was also dried and weighed to establish a second estimate of the dry weight (see below). A sample of blood (1.5-3 ml) obtained from the left ventricle was also weighed and dried in the same manner and the water content in the blood was obtained. The haemoglobin (Hb) concentration in the blood was also measured (Acid Base Laboratory, model ABL3, Radiometer, Copenhagan, Denmark).
Calculation of the extravascular water content in the tissues
The extravascular water content was expressed as a percentage of the wet weight of the tissue. The total water content (TW, in g) was determined from the difference in the wet and dried weights of the tissue.
| (1) |
where EVWS is the weight of the extravascular water in the tissue sample, TWS is the total weight of water in the tissue sample and X is the factor that will be derived to account for the weight of water in the blood in the wet tissue.
The volume of blood in the homogenized tissue (BVH) was established as:
| (2) |
where [Hb]H is the haemoglobin concentration of the tissue homogenate and [Hb]LVB is the haemoglobin concentration of the left ventricular blood sample.
The weight of water present in this volume of blood (IVWH) is given by:
| (3) |
where WWLVB and DWLVB are the wet and dry weights, respectively, of the left ventricular blood sample, and the specific gravity of the water is assumed to be 1.
The total weight of water in the sample which was homogenized (TWH) was obtained from the difference in its wet weight before homogenization and the weight of the dried residue of the homogenate. The weight of extravascular water in the homogenized sample (EVWH) is thus given by:
| (4) |
and the factor X can be derived for the homogenized tissue as:
| (5) |
and can be substituted in eqn (1) to obtain EVWS.
Statistical analysis
All the group data are expressed as the mean ±s.e.m. unless mentioned otherwise. Activity of RARs during acute elevations of LAP was compared with initial and final control periods (as a group) using analysis of variance (ANOVA). Response of RARs to acute elevation of LAP, before and after the infusion of hypertonic albumin solution, was compared using ANOVA for repeated measures and Dunnett's post hoc test. Multiple t tests and correction of resultant P values with a Bonferroni test were also used to compare the action potentials at different levels of LAP. Student's unpaired t test was used to determine the significance in all the other instances. P≤ 0.05 was considered as statistically significant.
RESULTS
The arterial blood PO2, PCO2 and pH from all the animals were 332.4 ± 24.9 mmHg, 34.3 ± 0.9 mmHg and 7.43 ± 0.01, respectively. The mean arterial pressure, mean LAP, heart rate and peak intra-tracheal pressure at the beginning of the experiments were 60.4 ± 3.0 mmHg, 4.6 ± 0.4 mmHg, 187.7 ± 5.5 beats min−1 and 5.0 ± 0.3 mmHg, respectively.
Evidence for mitral valve damage
The mitral valve apparatus was examined in all the animals, post mortem. The anteroseptal cusp of the mitral valves was perforated in all the rabbits who had undergone surgical damage to their mitral valves. The posterior leaflet of the mitral valve was also perforated in five rabbits. The mitral valve apparatus appeared normal to visual inspection in intact control rabbits. Doppler echocardiography examination 12 weeks after undergoing surgery on the mitral valve showed a mild to moderate degree of mitral regurgitation based upon visual inspection of the recordings (Fig. 1). No additional quantification was attempted on the regurgitant jet. The procedure was performed on eight rabbits and mitral regurgitation was demonstrated on seven. The LAP in rabbits with mitral valve damage was significantly higher than that in age-matched control rabbits (5.1 ± 0.5 and 2.4 ± 0.3 mmHg, respectively). This difference is statistically significant (P < 0.01, t test). The left ventricular weights in rabbits with mitral regurgitation and in age-matched control rabbits were 5.4 ± 0.1 and 4.4 ± 0.1 g, respectively (P < 0.01, t test). The left ventricular weight to body weight ratio in rabbits with mitral regurgitation was 1.45 ± 0.03 g kg−1. This ratio in age-matched control rabbits was 1.14 ± 0.03 g kg−1. This difference is also statistically significant (P < 0.01, t test).
Figure 1. An example of a colour Doppler echocardiograph in a rabbit with mitral regurgitation.
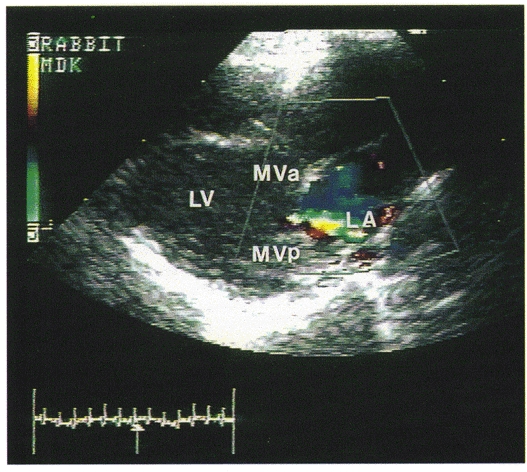
The picture is a frozen frame during ventricular systole (as indicated by the arrow pointing to the ECG tracing at the bottom left) when the blood is regurgitating back to the left atrium, which is seen as a blue jet inside the left atrium. LV, left ventricle; LA, left atrium; MVa, mitral valve (anterior cusp); MVp, mitral valve (posterior cusp).
Electrophysiological studies on RARs
Effect of intravenous infusion of hypertonic albumin solution on the activity of RARs in rabbits with MR
Six RARs were studied in six rabbits. Two of the receptors were located in the right main bronchus, and one receptor was located in each of the right upper, middle and lower lobe bronchi. The remaining receptor was located in the left lower lobe bronchus. The calculated conduction velocity of their axons was 25.9 ± 2.7 m s−1. Mean LAP values during the initial control, LAP +5 mmHg, LAP +10 mmHg and final control periods were 5.9 ± 1.5, 10.8 ± 1.4, 16.3 ± 1.4 and 5.8 ± 1.3 mmHg, respectively. Mean values for RAR activity during the corresponding periods were 20.9 ± 9.5, 18.8 ± 11.3, 27.0 ± 11.2 and 17.2 ± 9.8 action potentials min−1, respectively (P > 0.05, ANOVA and Dunnett's post hoc test). After the infusion of hypertonic albumin, the corresponding values for RAR activity were 9.4 ± 3.2, 30.6 ± 14.6, 48.9 ± 10.1 and 18.4 ± 7.3 action potentials min−1, respectively (P < 0.01, ANOVA and Dunnett's post hoc test; Figs 2 and 3). RAR activity increased significantly during acute elevation of LAP by 10 mmHg after albumin infusion compared with initial (P < 0.01) and final (P < 0.05) controls (t test with Bonferroni correction). The initial control activities before and after albumin infusion were not statistically significantly different (P > 0.05, t test with Bonferroni correction). After albumin infusion, the corresponding LAP values were 7.3 ± 2.0, 11.8 ± 2.1, 16.8 ± 1.8 and 4.6 ± 0.6 mmHg, respectively. Hypertonic albumin infusion increased the plasma albumin concentration by 1.42 ± 0.12 g dl−1 (P < 0.01, t test; Table 1). RAR activity, LAP, heart rate, mean arterial blood pressure and peak intra-tracheal pressure during each phase of the experiment are shown in Table 2.
Figure 2. Effect of hypertonic albumin infusion.
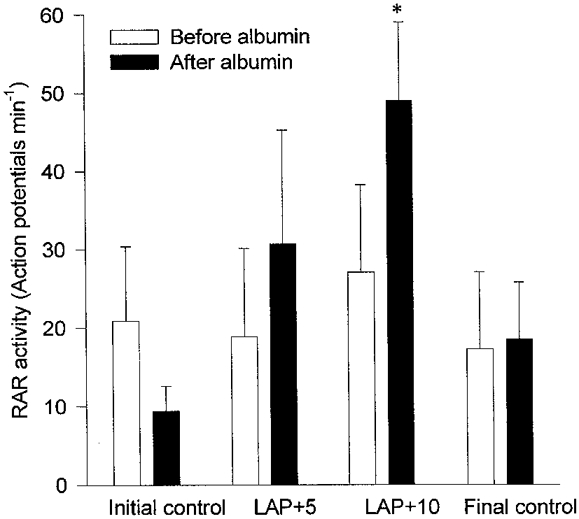
Effect of hypertonic albumin infusion on the response of rapidly adapting receptors to acute elevation of left atrial pressure by 5 mmHg (LAP +5) and 10 mmHg (LAP +10) in rabbits with chronic pulmonary venous congestion for 12 weeks (n= 6). Note that the activity significantly increased after hypertonic albumin infusion when the LAP was acutely elevated by 10 mmHg. * Significantly higher than initial and final control periods after albumin infusion (P < 0.01 and 0.05, respectively; t test with Bonferroni correction).
Figure 3. An example of a rapidly adapting receptor from a rabbit with chronic pulmonary venous congestion.
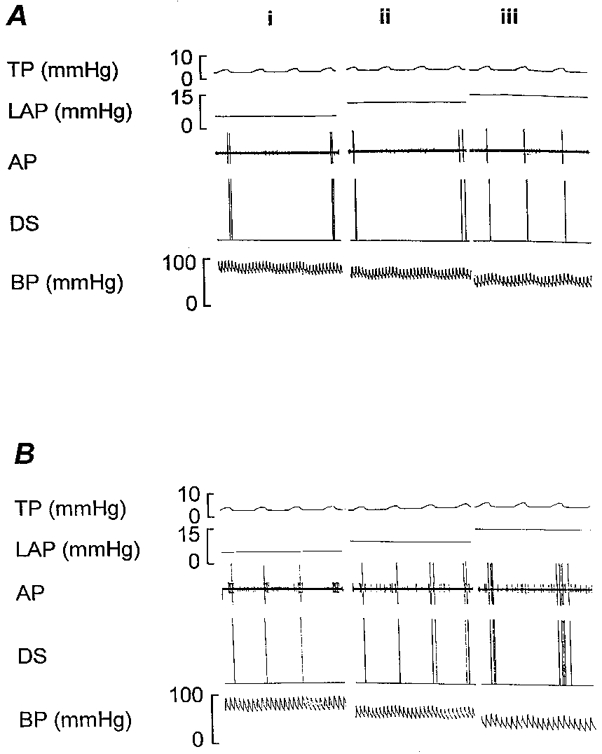
Response of the rapidly adapting receptor to acute elevation of left atrial pressure by 5 and 10 mmHg, before (A) and after (B) the hypertonic albumin infusion. (i) Initial control period, (ii) LAP +5 mmHg and (iii) LAP +10 mmHg. Note that after the hypertonic albumin infusion, there was a significant increase in receptor activity when the LAP was elevated. TP, tracheal pressure; LAP, left atrial pressure; AP, action potentials; DS, discriminator signal; BP, blood pressure.
Table 1.
Plasma albumin, total protein, plasma osmolality, plasma sodium and haematocrit values before and after the infusion of hypertonic albumin solution
| Before albumin infusion | After albumin infusion | |
|---|---|---|
| Plasma albumin (g dl−1) | 3.6 ± 0.1 | 5.0 ± 0.1† |
| Plasma total protein (g dl−1) | 4.6 ± 0.2 | 6.1 ± 0.2† |
| Plasma osmolality (mosmol kg−1) | 307.7 ± 6.7 | 320.3 ± 9.7 |
| Plasma sodium (mmol l−1) | 143.8 ± 3.0 | 144.5 ± 1.7 |
| Haematocrit (%) | 32.2 ± 1.5 | 28.8 ± 0.9 |
Significantly higher than the corresponding value before albumin infusion (P < 0.01, t test).
Table 2.
Mean rapidly adapting receptor activity (RAR activity; action potentials min−1), mean left atrial pressure (MLAP), mean arterial blood pressure (MABP), heart rate (HR) and peak intratracheal pressure (PITP) during graded increase in left atrial pressure (LAP) when rapidly adapting receptors were investigated before and after an infusion of hypertonic albumin
| Initial control | LAP +5 mmHg | LAP +10 mmHg | Final control | |
|---|---|---|---|---|
| Before hypertonic albumin infusion | ||||
| RAR activity | 20.9 ± 9.5 | 18.8 ± 11.3 | 27.0 ± 11.2 | 17.2 ± 9.8 |
| MLAP (mmHg) | 5.9 ± 1.5 | 10.8 ± 1.4 | 16.3 ± 1.4 | 5.8 ± 1.3 |
| MABP (mmHg) | 72.8 ± 6.1 | 60.4 ± 5.3 | 53.4 ± 5.2† | 64.1 ± 3.4 |
| HR (beats min−1) | 180.4 ± 13.9 | 182.2 ± 8.9 | 175.0 ± 5.1 | 164.0 ± 7.0 |
| PITP (mmHg) | 4.8 ± 0.5 | 5.2 ± 0.6 | 5.8 ± 0.6 | 5.2 ± 0.5 |
| After hypertonic albumin infusion | ||||
| RAR activity‡ | 9.4 ± 3.2 | 30.6 ± 14.6 | 48.9 ± 10.1 | 18.4 ± 7.3 |
| MLAP (mmHg) | 7.3 ± 2.0 | 11.8 ± 2.1 | 16.8 ± 1.8 | 4.6 ± 0.6 |
| MABP (mmHg) | 79.4 ± 4.1 | 65.0 ± 5.0 | 63.2 ± 5.3† | 72.2 ± 4.1 |
| HR (beats min−1) | 153.0 ± 11.1 | 155.4 ± 9.8 | 151.0 ± 12.5 | 154.8 ± 8.1 |
| PITP (mmHg) | 4.5 ± 0.3 | 4.7 ± 0.3 | 5.2 ± 0.3 | 5.0 ± 0.5 |
Significantly lower than initial control period (P < 0.05, t test).
Significant change in activity when LAP is increased (P < 0.01, ANOVA and Dunnett's post hoc test).
Effect of acute elevation of LAP above 25 mmHg on the activity of RARs
Seven RARs were studied in seven rabbits with MR. Three of these receptors were located in the right lower lobe bronchus and two other receptors were located in the right upper lobe bronchus. One receptor each was located in the right middle lobe bronchus and the left lower lobe bronchus. Their nerve conduction velocity was 17.9 ± 3.1 m s−1. LAPs during the initial control period, LAP +25 and the final control periods were 5.2 ± 0.4, 28.1 ± 1.2 and 6.8 ± 1.2 mmHg, respectively. The mean activity of the receptors during 10 min of initial control period was 22.5 ± 2.8 action potentials min−1. The mean RAR activity increased to 58.7 ± 1.8 action potentials min−1 when the LAP was elevated above 25 mmHg (causing pulmonary oedema). All seven units showed an increase in activity during elevation of LAP. RAR activity increased from the first minute of this period and was sustained throughout the 15 min period of increased LAP. During the final control period, the mean activity was 29.6 ± 2.7 action potentials min−1. This increase in activity with elevation of LAP above 25 mmHg was statistically significant (P < 0.01, ANOVA; see Figs 4 and 5). Mean LAP, mean arterial blood pressure, heart rate and the mean peak intra-tracheal pressure values during experiments are shown in Table 3.
Figure 4.
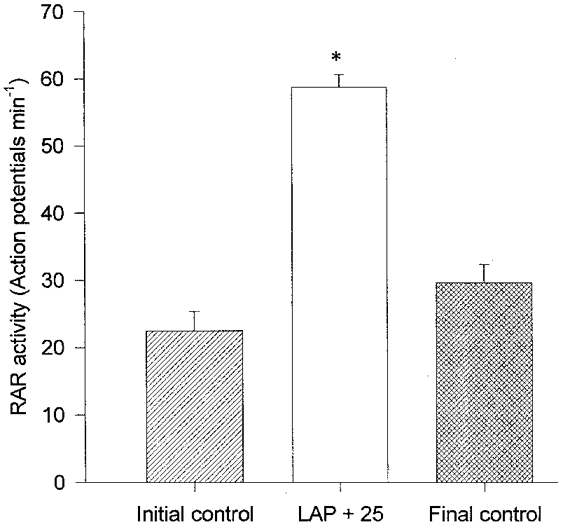
Response of rapidly adapting receptors to elevation of left atrial pressure (LAP) by 25 mmHg for a period of 15 min in rabbits with chronic pulmonary venous congestion for 12 weeks (n= 7). * Significantly higher than initial and final control periods (P < 0.01, ANOVA).
Figure 5.
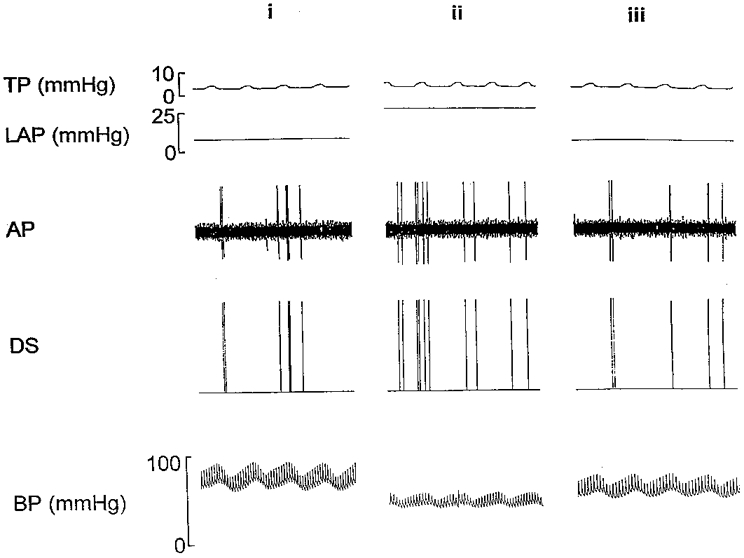
An example of a rapidly adapting receptor from a rabbit with chronic pulmonary venous congestion for 12 weeks, showing the stimulation of the receptor when the left atrial pressure (LAP) was acutely elevated above 25 mmHg. (i) Initial control period, (ii) LAP +25 mmHg and (iii) final control period. See the legend to Fig. 3. for a description of abbreviations used in this figure.
Table 3.
Mean left atrial pressure (MLAP), mean arterial blood pressure (MABP), heart rate (HR) and the peak intra-tracheal pressure (PITP) values when investigating the effect of acutely elevating the left atrial pressure (LAP) above 25 mmHg on the activity of rapidly adapting receptors in rabbits with chronic pulmonary venous congestion
| Initial control period | LAP +25 mmHg | Final control period | |
|---|---|---|---|
| MLAP (mmHg) | 5.2 ± 0.4 | 28.1 ± 1.2† | 6.8 ± 1.2 |
| MABP (mmHg) | 58.0 ± 4.5 | 21.0 ± 4.3† | 57.2 ± 8.2 |
| HR (beats min−1) | 211.0 ± 9.9 | 152.3 ± 18‡ | 200.6 ± 16.4 |
| PITP (mmHg) | 4.1 ± 0.1 | 5.1 ± 0.1† | 4.2 ± 0.2 |
Significantly different from initial control and final control periods (P < 0.01, t test).
Significantly different from the initial control period (P < 0.05, t test).
Water content in the airways and lungs
In rabbits with mitral regurgitation, extravascular water content in the region of the lower trachea, carina and major bronchi (where the RARs are located) was 58.3 ± 1.5 % of the wet weight at baseline conditions (n= 7). The LAP was not measured in this group of rabbits. In five other rabbits with mitral valve damage the left atrial pressure was found to be 4.37 ± 0.2 mmHg. Then it was elevated to 13.86 ± 0.5 mmHg (elevated by ∼10 mmHg) for a period of 20 min and the tissues were removed for analysis of water content. In the area where the RARs are mainly located, the extravascular water content was 56.9 ± 1.9 %. There was no significant change in extravascular water content by elevating the LAP acutely by 10 mmHg in rabbits with chronic PVC for 12 weeks (P > 0.05, t test). Measurement of extravascular water content in the same region in age-matched control rabbits showed a significant increase when LAP was elevated by 10 mmHg for 20 min (52.1 ± 1.2 % at baseline conditions, increased to 57.8 ± 1.7 %. P < 0.05, t test, n= 5 in each group). The corresponding LAPs were 2.4 ± 0.3 and 13.6 ± 0.5 mmHg, respectively. The extravascular water content in the region of the carina at baseline conditions in rabbits with chronic PVC was significantly higher than that of age-matched control rabbits (58.3 ± 1.5 and 52.1 ± 1.2 %, respectively; P < 0.01, t test).
In five other rabbits with chronic mitral valve damage the initial LAP was found to be 5.8 ± 0.7 mmHg. It was then acutely elevated to 27.1 ± 3.9 mmHg for 20 min and tissues were removed to study the water content. In this group of animals, extravascular water content in the carina and major bronchi was increased to 62.4 ± 1.1 % which is significantly higher than the corresponding values in rabbits with chronic mitral regurgitation at both baseline conditions and after increasing LAP by 10 mmHg (P < 0.05, t test). The extravascular water content in the lungs showed a significant increase (from 74.6 ± 0.4 to 77.9 ± 1.1 %, P < 0.05, t test) in these rabbits indicating that there was development of pulmonary oedema when the LAP was elevated above 25 mmHg. Complete information on the total water content and extravascular water content in other regions of the airways, lungs and the left ventricular muscle is shown in Table 4.
Table 4.
Total water content and the extravascular water content in the major airways, lungs and the left ventricular muscle, expressed as a percentage of the wet weight
| Upper and Mid-trachea | Lower trachea, carina, bronchi | Right lung (middle and lower lobes) | Left ventricle | |
|---|---|---|---|---|
| Age-matched control rabbits | ||||
| Baseline | 59.4 ± 1.4 | 56.6 ± 1.0 | 80.0 ± 0.6 | 80.0 ± 0.6 |
| n= 5 | (56.1 ± 1.3) | (52.1 ± 1.2) | (75.1 ± 0.9) | (77.5 ± 0.2) |
| LAP +10 mmHg | 59.1 ± 1.0 | 62.5 ± 1.3‡ | 81.3 ± 0.3 | 77.5 ± 0.3 |
| n= 5 | (55.0 ± 1.3) | (57.8 ± 1.7)† | (76.2 ± 0.9) | (76.1 ± 0.3)¶ |
| Rabbits with mitral regurgitation | ||||
| Baseline | 62.4 ± 0.7 | 61.1 ± 1.5† | 80.2 ± 0.2 | 77.6 ± 1.3 |
| n= 7 | (58.4 ± 0.9) | (58.3 ± 1.5)‡ | (74.6 ± 0.4) | (76.7 ± 1.4) |
| LAP +10 mmHg | 63.8 ± 1.5 | 61.0 ± 2.1 | 80.1 ± 1.1 | 78.8 ± 0.4 |
| n= 5 | (59.8 ± 1.5) | (56.9 ± 1.9) | (74.1 ± 1.0) | (77.2 ± 0.6) |
| LAP +25 mmHg | 60.7 ± 1.3 | 65.7 ± 1.1 ‖ | 85.0 ± 1.1§ | 75.8 ± 1.7 |
| n= 5 | (57.3 ± 1.7) | (62.4 ± 1.1)§ | (77.9 ± 1.1)§ | (74.5 ± 1.6) |
Values for the extravascular water content are given in parentheses. LAP, left atrial pressure.
Significantly higher than the corresponding value in age-matched control rabbits at baseline conditions (P < 0.05, t test).
Significantly higher than the corresponding value in age-matched control rabbits at baseline conditions (P < 0.01, t test).
Significantly higher than the corresponding value in rabbits with mitral regurgitation at baseline conditions and at LAP +10 mmHg (P < 0.05, t test).
Significantly higher than the corresponding value in rabbits with mitral regurgitation at baseline conditions (P < 0.05, t test).
Significantly lower than the corresponding value in agematched control rabbits at baseline conditions (P < 0.05, t test).
The mean arterial blood pressure, mean LAP, heart rate and peak intra-tracheal pressure during the experiments when tissues were obtained for analysis of water content are shown in Table 5.
Table 5.
Mean left atrial pressure (MLAP), mean arterial blood pressure (MABP), heart rate (HR) and the peak intra-tracheal pressure (PITP) values in rabbits whose tissues were harvested for analysis of water content
| MLAP (mmHg) | MABP (mmHg) | HR (beats min−1) | PITP (mmHg) | |
|---|---|---|---|---|
| Rabbits with chronic mitral regurgitation | ||||
| Baseline | 4.1 ± 0.2† | 54.6 ± 7.5 | 174.2 ± 8.8 | 6.8 ± 0.4‖ |
| LAP +10 mmHg | 13.9 ± 0.5‡ | 28.6 ± 2.5§ | 172.6 ± 8.3 | 7.4 ± 0.4 |
| Rabbits with chronic mitral regurgitation | ||||
| Baseline | 5.8 ± 0.7† | 56.8 ± 9.3 | 196.8 ± 9.0 | .6 ± 0.6 |
| LAP +25 mmHg | 27.1 ± 3.9‡ | 27.7 ± 5.5§ | 144.0 ± 6.0‡ | 6.0 ± 0.8 |
| Age-matched intact control rabbits | ||||
| Baseline | 2.4 ± 0.3 | 58.2 ± 4.5 | 169.0 ± 8.0 | 5.0 ± 0.5 |
| LAP +10 mmHg | 13.6 ± 0.5‡ | 32.6 ± 4.9‡ | 170.6 ± 5.8 | 5.8 ± 0.4 |
Significantly higher than that of age-matched control rabbits at baseline (P < 0.01, t test).
Significantly different from the baseline value of the same group of rabbits (P < 0.01, t test).
Significantly lower than the baseline value of the same group of rabbits (P < 0.05, t test).
Significantly higher than that of the baseline value of the age-matched control group and the other group of rabbits with chronic mitral regurgitation (P < 0.05, t test).
DISCUSSION
Pulmonary RARs are extremely sensitive to manipulations of Starling forces in the pulmonary microvasculature in intact control animals of several species (Kappagoda et al. 1987; Hargreaves et al. 1991; Ravi et al. 1995). Location of these receptors in close proximity to the bronchial venules (Elftman, 1943; Kappagoda et al. 1990) enable them to sense fluid fluxes across the bronchial venules. Manipulation of Starling forces, for example, by elevating the LAP by 10 mmHg, causes about a 100 % increase in activity of these receptors in control animals (Hargreaves et al. 1991).
It was also shown that an increase in activity of these receptors reflexly increases the respiratory rate and the tracheal muscle tone. Stimulation of RARs also increases the mucus production in the airways (Yu et al. 1989). All these reflex effects together constitute a picture of ‘cardiac asthma’ which is seen in patients with heart failure. In patients with heart failure the picture is of chronic pulmonary venous congestion interspersed with intermittent acute episodes of pulmonary oedema during which symptoms of cardiac asthma (e.g. wheezing, increased respiratory rate, increased mucus production etc.) become more severe. In an earlier study (Gunawardena et al. 1998) we showed that RARs in rabbits with chronic PVC for 12 weeks did not respond to small acute increments in LAP (up to +10 mmHg) unlike in intact control animals. This observation questioned whether RARs played a significant role in providing information on the status of the extravascular fluid compartment to the central nervous system in patients with heart failure.
In the current study, RARs in rabbits maintained in a state of chronic PVC for 12 weeks responded to acute elevation of LAP above 25 mmHg. There was an approximately 2.5-fold increase in RAR activity with this stimulus in these animals (increased from 22.5 ± 2.8 to 58.7 ± 1.8 action potentials min−1). Thus, although RARs in rabbits with chronic PVC were stimulated when LAP was elevated above 25 mmHg, the magnitude of the response is similar to that observed in intact control animals when the LAP was elevated by 10 mmHg. Activity during the initial control period in these rabbits is significantly lower compared with that in intact control animals (22.5 ± 2.8 and 48.8 ± 0.9 action potentials min−1, respectively; see Gunawardena et al. 1998 for the latter value). This reduction in activity during the initial control period and attenuation of response to elevation of LAP above 25 mmHg may be due to adaptation of RARs over a period of time, when exposed to chronic PVC.
In the present study, previously unresponsive RARs responded to small elevations of LAP when an intravenous infusion of hypertonic albumin solution was given to shrink the extravascular space. Therefore it is clear that this adaptation of RARs, at least in part, is a function of a chronic change in the volume of the extravascular fluid compartment. Hypertonic albumin infusion probably shrinks the extravascular fluid compartment by moving water from it into the intravascular compartment. The observation that the initial control activity of RARs after this infusion (9.4 ± 3.3 action potentials min−1) was approximately 50 % of that before the infusion (20.9 ± 9.5 action potentials min−1) supports this suggestion. After the extravascular compartment was shrunk by this procedure, the tissue in the vicinity of RARs (in the carina and bronchi) acquired the capacity to accommodate fluid entering it during small acute elevations of LAP. It is suggested that in this situation there was a greater fluid flux from the bronchial capillaries compared with that before the hypertonic albumin infusion and that this change was sufficient to activate the RARs.
In animals with chronic PVC, the extravascular water content in the carinal region was increased under baseline conditions, compared to age-matched controls. An increase of LAP by 10 mmHg caused no change in the water content of the animals with chronic PVC but further increase to 25 mmHg above the control was accompanied by an increase in extravascular fluid content in this region. This increase was comparable to that observed in intact control animals. The RARs in animals with chronic PVC remain unresponsive when the LAP is increased by 10 mmHg from the baseline but do respond when the pressure was raised by 25 mmHg.
Guyton & Lindsey (1959) have shown in dogs that there was no significant increase in lung water content until the LAP was elevated to 23–25 mmHg. The lung water content when the LAP was maintained at 27 mmHg of LAP for 30 min in these dogs was approximately 83.3 %. In the present study in rabbits with chronic PVC, the total lung water content was 85.0 ± 1.1 % when the LAP was elevated to 27.1 ± 3.9 mmHg for 20 min. This percentage was higher than that at the baseline conditions, indicating that there is an accumulation of a significant amount of fluid in the lungs at these pressures. At lower pressures there was no increase in lung water in both normal animals and those with chronic PVC.
Taken collectively, these results suggest that an increase in activity of RARs is observed only in circumstances where the extravascular fluid content increases. In chronic conditions which increase the basal extravascular fluid, RARs will be activated only when there are further large increases in LAP to levels which produce pulmonary oedema.
Acknowledgments
This project is supported by a program project grant from the National Institutes of Health (HL52165). The authors wish to thank Milind Dhond, MD, for performing the echocardiography recordings. This work was undertaken as partial fulfilment of the requirements for a PhD thesis at the University of Ruhuna, Sri Lanka by Dr S. Gunawardena.
References
- Elftman AG. The afferent and parasympathetic innervation of the lungs and trachea of the dog. The American Journal of Anatomy. 1943;72:1–27. [Google Scholar]
- Gunawardena S, Bravo E, Kappagoda CT. Effect of chronic mitral valve damage on activity of pulmonary rapidly adapting receptors in the rabbit. The Journal of Physiology. 1998;511:79–88. doi: 10.1111/j.1469-7793.1998.079bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circulation Research. 1959;VII:649–657. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Ravi K, Kappagoda CT. Response of slowly and rapidly adapting receptors in the airways of rabbits to changes in the Starling forces. The Journal of Physiology. 1991;432:81–97. doi: 10.1113/jphysiol.1991.sp018377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway A. A method of chemical analysis of guinea pig lung for the factors involved in pulmonary edema. Journal of Laboratory Clinical Medicine. 1950;35:817–822. [PubMed] [Google Scholar]
- Kappagoda CT, Man GCW, Ravi K, Teo KK. Reflex tracheal contraction during pulmonary venous congestion in the dog. The Journal of Physiology. 1988;402:335–346. doi: 10.1113/jphysiol.1988.sp017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda CT, Man GCW, Teo KK. Behaviour of canine pulmonary vagal afferent receptors during sustained acute pulmonary venous pressure elevation. The Journal of Physiology. 1987;394:249–265. doi: 10.1113/jphysiol.1987.sp016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda CT, Ravi K, Teo KK. Effect of pulmonary venous congestion on respiratory rate in dogs. The Journal of Physiology. 1989;408:115–128. doi: 10.1113/jphysiol.1989.sp017450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda CT, Skepper JN, McNaughton L, Siew E E-L, Navaratnam V. Morphology of presumptive rapidly adapting receptors in the rat bronchus. Journal of Anatomy. 1990;168:265–276. [PMC free article] [PubMed] [Google Scholar]
- Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circulation Research. 1965;XVI:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- Ravi K, Singh M, Julka DB. Properties of rapidly adapting receptors of the airways in monkeys (Macaca mulatta) Respiration Physiology. 1995;99:51–62. doi: 10.1016/0034-5687(94)00072-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Schultz HD, Goodman J, Coleridge JCG, Coleridge HM, Davis B. Pulmonary rapidly adapting receptors reflexly increase airway secretion in dogs. Journal of Applied Physiology. 1989;67:682–687. doi: 10.1152/jappl.1989.67.2.682. [DOI] [PubMed] [Google Scholar]


