Abstract
Monosynaptic extracellular field potentials evoked by electrical stimulation of ipsilateral hindlimb nerves carrying muscle group I, II and cutaneous afferents were examined during fictive locomotion. Fifty-eight field potentials were recorded in the dorsal and intermediate laminae throughout the mid-lumbar to first sacral segments and fictive locomotion was evoked by mesencephalic locomotor region (MLR) stimulation in paralysed decerebrate cats.
The majority (96 %) of group I, II and cutaneous-evoked field potentials were decreased during fictive locomotion. Group I, cutaneous and dorsal group II potentials were reduced on average to about 80 % of control values. Group II field potentials recorded in the intermediate laminae were reduced to a mean of 49 % of control values. Cyclic variations in field potential amplitude between the flexion and extension phases were observed in 24 of 45 cases analysed. Of those 24 field potentials, the two group I and four cutaneous field potentials were smaller during the flexion phase. All eleven group II and the remaining seven cutaneous fields were smaller during extension. In all but two cases, these cyclic variations were smaller than the tonic depression upon which they were superimposed.
In 7/9 group II field potentials examined, reductions (on average to 85 % of control) began with the onset of MLR stimulation that produced tonic activity in the motor nerves before the onset of rhythmic alternating, locomotor discharges. In six of the seven cases the field potential depression increased with the establishment of fictive locomotion. This observation and the cyclic modulation of field potentials during fictive locomotion suggests that the depression was strongly linked to the operation of the spinal locomotor circuitry.
Depression of the monosynaptic components of the field potentials suggests a reduction in synaptic transmission from primary afferents to first-order spinal interneurones during fictive locomotion. Accordingly, the larger depression of intermediate group II field potentials may indicate a preferential reduction in transmission from group II afferents to interneurones located in intermediate spinal laminae.
Flexion reflexes evoked by group II and cutaneous afferents were also depressed during MLR-evoked fictive locomotion. The possibility that this depression results from a reduction in transmission from primary afferents, and in particular from group II afferents, ending on interneurones in the intermediate laminae is discussed.
It has been estimated that during locomotion in the cat, muscle afferents in a single limb would produce as many as 800 000 discharges per second (Prochazka & Gorassini, 1998). Such intense sensory feedback could trigger inappropriate reflex responses and detract from processing the most relevant sensory signals during movement. The need to regulate the action of afferent-evoked reflexes was discussed by Eccles & Lundberg (1959b) with regard to the flexion reflex. Under certain experimental conditions, the flexion reflex is evoked by the activation of group II and higher-threshold muscle, as well as joint and cutaneous afferents (Eccles & Lundberg, 1959a). Activation of some of these afferents during the movement could induce disruptive flexion reflexes (Eccles & Lundberg, 1959b). As detectors of static muscle length, group II afferents are active during many movements (see Prochazka et al. 1989). It would thus seem particularly important to modify group II reflex actions and avoid disruptive flexion reflexes during movement (Eccles & Lundberg, 1959b; Grillner & Shik, 1973). Accordingly during brainstem-evoked fictive locomotion, stimulation of certain group II afferents initiates extensor activity instead of evoking flexion reflexes (Perreault et al. 1995).
There is evidence that one form of reflex regulation during movement involves a reduction in the central effects of sensory feedback. For example, during voluntary contractions in humans the group Ia monosynaptic excitation of heteronymous motoneurones is reduced at the onset of contraction (Hultborn et al. 1987). Furthermore, our ability to consciously detect afferent signals such as those produced by muscle twitches (Collins et al. 1998) and tactile stimuli (Williams et al. 1998) is decreased during voluntary movement. The inhibitory mechanisms responsible for these reduced sensory effects during voluntary movements are unknown but it has been suggested that a reduction in transmitter release via presynaptic inhibition of primary afferent terminals could play an important role (Hultborn et al. 1987; see Rossignol, 1996). Results from animal experimentation provide further evidence for a presynaptic regulation of primary afferent transmission during the execution of some motor programmes. During fictive locomotion, for example, primary afferents are subject to rhythmic depolarization (Gossard et al. 1989, 1991; Gossard, 1996) and the excitability of the terminals of afferents is increased (Dueñas & Rudomin, 1988). Other motor activities in which presynaptic inhibition may modulate excitatory segmental afferent transmission include voiding (Angel et al. 1994; Buss & Shefchyk, 1999), scratching (Bayev & Kostyuk, 1981) and mastication (Kurasawa et al. 1988).
The present study used an analysis of monosynaptic field potentials to determine if primary afferent transmission from hindlimb afferents to first-order spinal interneurones is reduced during fictive locomotion. Analysis of monosynaptic field potentials offers several advantages over intracellular recordings in this regard. Because field potentials result from ionic movements, their changes reflect alterations in the currents produced by synaptic transmission between the afferents and their target neurones. On the other hand, intracellular current clamp recordings do not reveal synaptic currents directly, but rather show the postsynaptic voltage changes which are also influenced by postsynaptic membrane conductance and impalement injury. Although all terminals of individual afferents may not be affected uniformly during locomotion, trends in the changes in synaptic transmission may be better appreciated by an analysis of extracellular recorded population responses than by intracellular recordings from single neurones. The strong association between the depression of monosynaptic field potentials and the presence of primary afferent depolarization (see Sypert et al. 1980, for group I afferents, and Riddell et al. 1995, for group II afferents) should aid in comparing results obtained during fictive locomotion and in other preparations.
Therefore, to assess the regulation of synaptic transmission from hindlimb afferents during fictive locomotion, afferent-evoked extracellular field potentials were examined during locomotion evoked by stimulation of the mesencephalic locomotor region in decerebrate cats. This preparation has an advantage over drug-induced locomotion because the motor state of the animal can be readily changed from one of quiescence into locomotion. Results will show that there is a general reduction in dorsal and intermediate monosynaptic field potentials evoked by muscle and cutaneous afferents throughout the mid-lumbar to rostral sacral segments examined. This depression is most pronounced for group II field potentials recorded in intermediate spinal laminae. Preliminary results have been reported (Perreault et al. 1994; McCrea & Perreault, 1998).
METHODS
Preparation
Data were obtained from 13 cats weighing between 1.9 and 3.2 kg. All surgical and experimental protocols were in compliance with the guidelines set out by the Canadian Council for Animal Care and the University of Manitoba. Under halothane-nitrous oxide anaesthesia, a tracheotomy was performed and cannulas inserted into a carotid artery and jugular vein for blood pressure monitoring and administration of fluids and drugs. Atropine (0.05 mg kg−1 subcutaneous) and dexamethasone (2 mg kg−1 intravenous) were given at the beginning of the surgery and a buffer solution (5 % glucose and 0.85 % NaHCO3) was infused intravenously (5 ml h−1) throughout the experiment. The following left hindlimb nerves were cut and dissected for recording or stimulation: sartorius (Sart, both medial and lateral branches), semimembranosus and anterior biceps (SmAB), posterior biceps and semitendinosus (PBSt), quadriceps (Q, usually with the rectus femoris portion included), lateral gastrocnemius and soleus (LGS) or when combined with medial gastrocnemius (GS), tibialis anterior (TA), extensor digitorum longus (EDL), superficial peroneal (SP), posterior tibialis (Tib; mixed muscular and cutaneous), lateral and caudal cutaneous sural, caudal cutaneous femoralis (CCF), sensory pudendal (SPud), and superficial perineal (SPeri). The nerve abbreviated as FDHL included the innervation of flexor digitorum and hallucis longus muscles as well as branches to interosseous, tibialis posterior and popliteal muscles. The Q and Sart nerves were placed in cuff electrodes and the other nerves were mounted on bipolar silver-silver chloride electrodes. Contralateral SmAB and PBSt nerves were mounted to monitor fictive locomotion. All other nerves as well as the tendons around the hips were cut bilaterally.
After a laminectomy exposing the third lumbar (L3) to second sacral (S2) spinal cord segments, the animal was transferred to a rigid frame, the head positioned in a stereotaxic apparatus, and hindlimb and back mineral oil pools constructed from skin flaps. A precollicular-postmammillary decerebration was performed and all brain tissue rostral to the transection was removed. Anaesthesia was then discontinued and the animal paralysed with gallamine triethiodide (Flaxedil, 2–3 mg kg−1 h−1). The expired CO2 was maintained between 3.0 and 5.0 % by artificial ventilation. Blood pressure decreases below 80 mmHg were counteracted by dextran injection. Core body temperature was kept near 38°C by infrared lamps. In two cats, 4-aminopyridine (4-AP; initial dose 100 μg kg−1; total dose of 200 and 650 μg kg−1, respectively) was administered intravenously to facilitate fictive locomotion (see Dubuc et al. 1986). At the termination of the experiments, the decerebrate, paralysed animals were killed by potassium chloride injection.
Stimulation and recording
In all but three experiments where the dura was cut longitudinally from S2 to L3, small holes were made in the dura and the pia for microelectrode insertion. Extracellular field potentials were recorded using glass micropipettes (tip diameter 1.6-2 μm, resistance 2–5 MΩ) filled with a 1.5 M sodium citrate solution. The depths of the field potential recordings are given as the distance between the dorsal or dorsolateral surface of the spinal cord and the tip of the microelectrode and do not take the angle of insertion (0–30 deg) into account. Peripheral nerves were stimulated (1 or 2 pulses of 0.1 ms duration at 3–5 Hz) before, throughout, and after periods of fictive locomotion induced by MLR stimulation (described in Guertin et al. 1995). The strength of nerve stimulation was expressed in multiples of threshold (T) for the most excitable afferent fibres as measured from the cord dorsum potential recorded in the L4 or L7 segment.
Data analysis
Extracellular field potentials were evoked by peripheral nerve stimulation at 3–5 Hz throughout a period (typically 2 min) in which the first 10–30 s were without MLR stimulation. These records were used to calculate the control field potential area and peak values. A period of about 60 s followed in which continuous MLR stimulation (10–20 Hz, not synchronized to peripheral nerve stimulation) was delivered. The last 30–60 s were without MLR stimulation and locomotion. In some cases the recovery of field potentials after fictive locomotion was followed for a period of 5 min during which data collection continued.
Subsequent analysis consisted of averaging the extracellular microelectrode and cord dorsum records evoked by peripheral nerve stimulation before (control), during, and after MLR-evoked fictive locomotion. The number of sweeps comprising averaged field potentials ranged from about 35 to 300. The central latency of extracellular field potentials was measured from the arrival of the earliest component of the afferent volley (for muscles nerves, the group I volley) at the cord dorsum to the onset of the downward (negative) deflection of the earliest component of the field potential. The baseline for the field potential measurements was chosen as a point before the arrival of the volley and before any stimulus artefact or field component. One to two milliseconds of the record was averaged to determine the baseline. Points on the time axis used for measurement of baseline, field potential amplitude and area were selected using the control record and used for all subsequent measurements of that field. Field potential changes are expressed as percentages of the control field potential measured just before MLR stimulation. Field potential amplitude and the area from the onset to the peak negative deflection of the averaged waveform were calculated. One potential problem was the presence, particularly during locomotion, of interneurone spikes on the field potential. Because area measurements would be less likely to be affected by occasional spikes than would peak amplitude, all field potential data are presented as area measurements. The results with either measure were similar; for the 58 fields sampled, the mean depression of the peak amplitude of fields during locomotion was to 64 % of control and this reduction was similar (P > 0.1) to the mean reduction to 69 % of control of the field potential area. For those fields with group I and II components, the area of each component was calculated separately (e.g. Fig. 3). This procedure emphasized detecting changes in the shorter-latency, predominantly monosynaptic components of the fields. Separate averages of potentials recorded during the flexion and extension phases of the fictive locomotor step cycle were also calculated. This was accomplished by dividing the locomotor cycle into periods of flexion and extension based upon flexor or extensor electroneurogram (ENG) activity and then separating the field potential records into those occurring during flexion or extension. In some cases separate averages were also obtained during the MLR-evoked motor activity that preceded locomotion. Field potentials that were ≤ 100 μV were not analysed for phasic modulation within the locomotor cycle. Integrated and rectified ENGs, stimulus markers (from MLR and peripheral nerve stimulation) as well as the microelectrode and cord dorsum records were digitized at 500 Hz, 2 kHz, 5 kHz and 2.5 kHz, respectively. Except where noted, means and standard deviations are reported. The data capture and analysis software was developed within the Winnipeg Spinal Cord Research Centre to run under QNX or Linux operating systems.
Figure 3. Depression of group I and group II fields recorded in dorsal and intermediate regions during fictive locomotion.
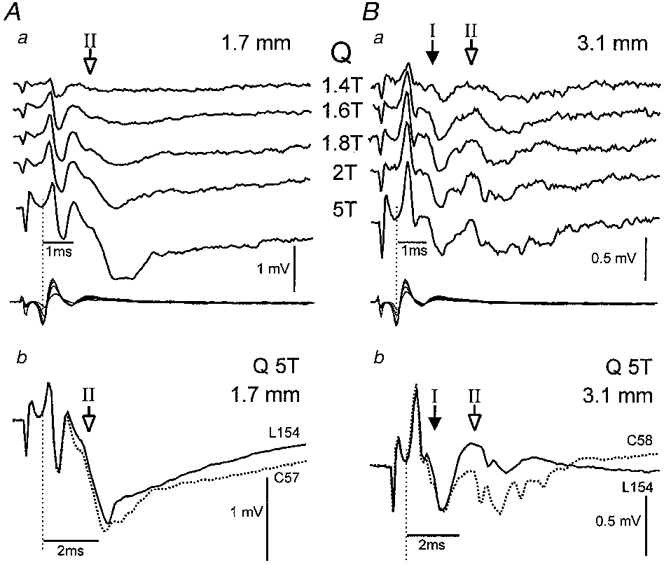
Stimulation of the Q nerve-evoked fields in both dorsal (Aa and b, depth 1.7 mm) and intermediate (Ba and b, depth 3.1 mm) regions of the L4 segment. The lower traces in Aa and Ba are records of the cord dorsum potential; all other traces are averaged fields obtained during non-locomotor control periods (dotted traces; Ab and Bb) and during MLR-evoked fictive locomotion (continuous traces; Ab and Bb). Five sweeps were averaged in Aa and Ba. Filled arrows indicate group I and open arrows group II fields. Note in Bb, the disappearance of the group II intermediate field potential during fictive locomotion while no change in the group I field is evident.
RESULTS
During fictive locomotion in 13 cats, 29 field potentials (13/17 of the Q field potentials, all the Sart and TA and 2/5 of the SP field potentials) were recorded between the L3 and L5 spinal segments and 29 (all of the PBSt, GS, FDHL and 12/14 of the cutaneous field potentials) between the L6 and S1 segments. Of these 58 fields, 42 were evoked by stimulation of muscle nerves at group I (≤ 2T) or group II strengths (5T) and 16 by stimulation of cutaneous nerves (usually 2T). Because there were no obvious differences in the evoked fields recorded from the two preparations receiving 4-AP, data from all experiments were pooled. The shape, latency and location of the field potentials recorded in the present decerebrate preparation correspond closely to the field potentials recorded in the dorsal horn and intermediate zone of the spinal cord in the intact anaesthetized cat (Fu et al. 1974; Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993). More ventrally located group II fields (Edgley & Jankowska, 1987a) were not found in the present series of experiments.
Group I field potentials
Of the 42 field potentials evoked by muscle nerve stimulation, 9 were evoked monosynaptically from group I afferents. These group I fields were recorded in the intermediate regions of the spinal cord (depth 1.8-3.1 mm) and had peak amplitudes of 152–547 μV (mean 391 μV) and central latencies of 0.7-1.0 ms. All five Q fields were recorded in L4-L5 segments, while all of the three GS fields and the FDHL field were in the caudal lumbar or rostral sacral segments. Figure 1A shows an FDHL-evoked group I field potential that grew in amplitude with increasing stimulus intensity from 1.2 to 2T. The appearance of the field at 1.4T suggests that it resulted from activation of Ib afferents and not the lowest-threshold group Ia afferents in the nerve. Raising the stimulation from 2 to 5T evoked a small, longer-latency group II field potential (open arrow, Fig. 1A). In Fig. 1B the pre-locomotor (control) field potential (dotted trace) evoked by 2T stimulation is superimposed on the same field recorded during fictive locomotion (continuous trace). This group I field was depressed during fictive locomotion with its area reduced to 80 % of control and peak amplitude reduced to 83 % of control (standard errors of the mean for both measurements were ± 0.01 %). All but one of the nine group I fields (evoked by 1.8 or 2T stimulation strengths) became smaller during fictive locomotion with the depressions ranging from 60 to 93 % of control values. These values are plotted in the top portion of Fig. 2 with the mean group I field depression to 82 % of control indicated by the vertical dotted line.
Figure 1. Depression of group I field potentials during fictive locomotion.
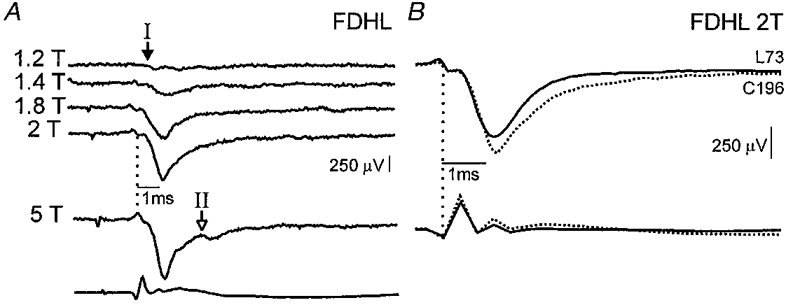
A, the 5 top traces are averages (each of 5 traces) of FDHL-evoked control field potentials (negative deflections downward) recorded in L6 at a depth of 2.0 mm. Stimulus strength varied from 1.2 to 5T. Arrows show the onset of the group I (filled arrow) and II (open arrow) components of the fields. The bottom trace is the cord dorsum (negative deflection upward) evoked by 5T stimulation. B, the averaged field potentials and cord dorsum records produced by stimulation of the FDHL nerve at 2T are shown during control (dotted traces) and locomotor (continuous traces) periods. The number of sweeps used to construct the control (C) and locomotor (L) averages are indicated to the right of the averages.
Figure 2. Depression of group I, group II and cutaneous field potentials during fictive locomotion.
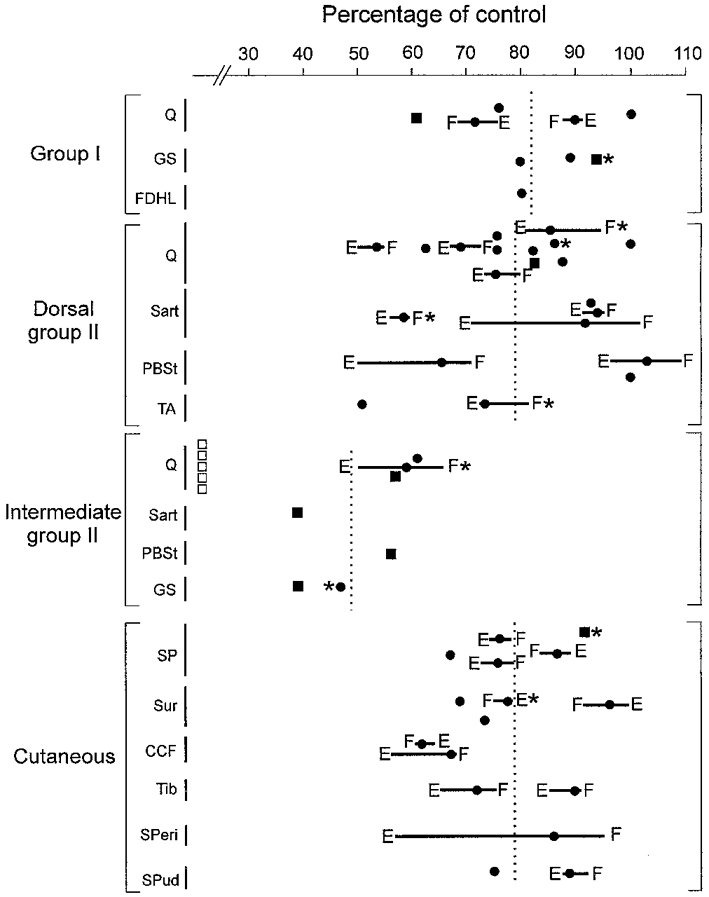
The area of each field potential (measured from onset to peak) during fictive locomotion is expressed as a percentage of the pre-locomotor control value prior to MLR stimulation and locomotion. The nerves used to evoke each field are indicated on the left. Horizontal lines indicate the phasic modulation of the field between the extension (E) and flexion (F) phases of the fictive step cycle. The mean values of the field potential reductions for each of the 4 major groupings of fields are indicated by the vertical dotted lines. □, intermediate Q group II fields that became too small to be measured during fictive locomotion; ▪, fields < 100 μV during locomotion not analysed for phasic modulation; * measurements taken following administration of 4-AP.
In two cases while recording group I fields MLR stimulation produced activity in motor nerves before the onset of rhythmic alternating flexor and extensor nerve activity. In one case, the group I field potential was unaffected before locomotion began and in the other the field was reduced to 93 % of control. During fictive locomotion, these two group I field potentials were reduced to 90 and 71 % of their control values, respectively, suggesting that MLR stimulation per se is not entirely responsible for field potential depression.
The seven group I fields with amplitudes remaining >100 μV during locomotion were analysed for locomotor cycle-dependent modulation (▪ in Fig. 2 represent fields considered too small to be analysed for phasic modulation). Only two of the seven group I fields showed a cyclic modulation and both were smaller during flexion. These two Q-evoked fields are shown by the horizontal bars in Fig. 2 with the letters E and F indicating the extension and flexion phases and the filled circle indicating the mean reduction in the field. Unequal durations of the flexion and extension phases result in a mean reduction not lying halfway between the values calculated during flexion and extension.
Group II field potentials
A substantial growth in the field potential as the stimulus intensity was increased from 1.8 to 5T was taken as evidence that the field was evoked by group II afferents (Edgley & Jankowska, 1987a,b). Because of the lower conduction velocity of group II afferents, the group II components of field potentials occur at longer latencies than the group I fields (Edgley & Jankowska, 1987a). Examples of the appearance of group II fields at longer latencies evoked with increasing stimulation intensities are shown in Figs 1A and 3Aa and Ba. To allow comparison with previous data (Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993; Noga et al. 1995), group II fields were divided into those recorded at dorsal (1.0-1.7 mm) and intermediate depths (1.8-3.1 mm).
Figure 3 shows averaged Q-evoked fields recorded at dorsal (1.7 mm, Fig. 3Aa and b) and intermediate depths (3.1 mm, Fig. 3Ba and b) along the same electrode track in the L5 segment. At the dorsal location there were no group I-evoked fields; 1.8T stimulation evoked a small group II field with an onset at 1.6 ms (open arrow, Fig. 3Aa) that grew as the stimulus was increased to 5T. As the microelectrode was advanced deeper into the intermediate regions of the spinal cord (Fig. 3Ba and b), Q stimulation evoked a substantial monosynaptic group I field potential (0.9 ms, filled arrow Fig. 3Ba and b) that increased only slightly with stimulation > 1.8T. The longer-latency group II components were readily recognized with increasing stimulus intensity from 2 to 5T (open arrow in Fig. 3Ba, latency 2.6 ms). Intermediate fields produced by 5T stimulation are shown expanded in Fig. 3Bb (dotted trace) to illustrate more clearly the distinction between the group I and II fields.
In the absence of fictive locomotion, the amplitudes of dorsal group II field potentials (n= 21) ranged from 110 to 674 μV (mean 362 ± 149 μV; pooled data from all segments). Central latencies were 1.3-3.4 ms (mean 1.8 ± 0.4 ms) and are compatible with a monosynaptic activation of interneurones by group II afferents (Edgley & Jankowska, 1987a,b; Jankowska & Riddell, 1994). The mean amplitude of the intermediate group II fields (n= 12) was 272 ± 110 μV with latencies ranging from 2.2 to 3.0 ms (mean 2.6 ± 0.4 ms). In the rostral L3-L5 segments, dorsal and intermediate group II field potentials were primarily evoked by stimulation of Q and Sart nerves while in the caudal L6-S1 segments the largest fields were evoked by stimulation of PBSt.
All but 2 of the 33 group II field potentials recorded in midlumbar and caudal lumbar-sacral segments were depressed during fictive locomotion. Figure 3 shows depression of both dorsal and intermediate group II fields during MLR-evoked locomotion: compare the superimposed control (dotted) and locomotor records (continuous lines) in Fig. 3Ab and Bb. The area (as measured from the onset to peak amplitude) of this dorsal group II field potential (Fig. 3A) was reduced by 18 % (to 82 % of its control value), whereas the earliest components of the intermediate group II field recorded deeper along the same electrode track became so small that they could not be easily measured (Fig. 3Bb). Figure 2 summarizes the reduction in the area of dorsal and intermediate group II field potentials during fictive locomotion (means indicated by vertical dotted lines). While the group II field potentials recorded in dorsal regions were reduced on average to 79 % of control values, the intermediate group II field potentials were decreased more. The mean reduction of intermediate group II fields to 49 % of control (indicated by the vertical dotted line in Fig. 2) underestimates the locomotor depression since its calculation did not include the five Q-evoked fields that became too small to measure during locomotion (indicated by □ in Fig. 2; see also Fig. 3Bb). Three Q- and one GS-evoked group II field potentials were preceded by group I fields recorded simultaneously at the same intermediate location (e.g. Fig. 3B). All four group II fields were reduced more than the group I fields during fictive locomotion. Figure 3B shows an example of a group I field (filled arrow) that was almost unaffected during fictive locomotion while the group II field potential was markedly attenuated.
Figure 4 illustrates several features of group II field potential depression during fictive locomotion. Figure 4A shows rectified integrated ENG recordings during a 6 s control period followed by continuous MLR stimulation which initially produced a period with considerable activity in flexor nerves (Sart and EDL) and sporadic activity in extensor nerves (SmAB and LGS) prior to the development of stable rhythmic alternating flexor and extensor ENG discharges during fictive locomotion (only the first 10 s are illustrated). Averaged PBSt-evoked group II field potentials recorded during these periods are shown in Fig. 4B. The left panel of Fig. 4B shows field potentials prior to MLR stimulation (control), during tonic activity produced by MLR stimulation and following locomotion (recovery). The panel to the right shows the averaged fields obtained during the flexion and extension phases of locomotion. The field during flexion is similar to that recorded with MLR stimulation producing tonic flexor activity (70 % of control). During the extension phase the area of the field was further decreased to 50 % of control.
Figure 4. Modulation of a PBSt-evoked dorsal group II field potential during fictive locomotion.
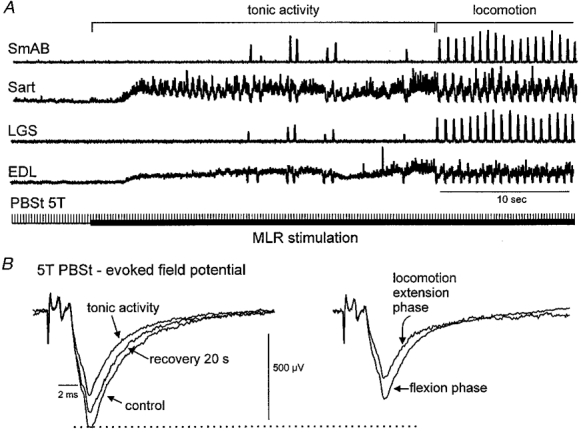
A, integrated, rectified ipsilateral extensor and flexor ENGs before and during MLR stimulation (filled horizontal bar) evoking tonic activity and during fictive locomotion. Stimulation of the PBSt nerve at 5T is indicated by the vertical bars. B, averages of the PBSt-evoked fields (averages from 25–125 traces) recorded at a depth of 1.4 mm in S1 during these periods as well as 20 s after MLR stimulation (recovery). The horizontal dotted line indicates the peak amplitude of the control field.
In nine cases, there was sufficient delay between the onset of MLR stimulation and the development of fictive locomotion to examine the effects of MLR stimulation on group II fields in the absence of fictive rhythmic locomotor activity (e.g. Fig. 4). Seven of these nine fields were depressed by the MLR stimulation before the onset of rhythmic nerve activity. MLR stimulation reduced the amplitude of six of these seven group II fields on average to 85 % of control values before rhythmic alternating activity appeared in the nerves. While one of these six fields returned to its control value once rhythmic alternating nerve activity was evident, five were further depressed (on average to 65 % of control) with the greatest reductions measured during the extension phase as illustrated in the example shown in Fig. 4B. The seventh field became too small to be measured during MLR stimulation before the onset of alternating nerve activity and its phasic modulation was not examined.
Group II field potentials remaining > 100 μV during locomotion were analysed for modulation within the two phases of the locomotor cycle. Four intermediate fields and one dorsal group II field were excluded from this analysis. Ten of the twenty dorsal group II fields and one of the four intermediate group II fields analysed showed a cyclic modulation (mean 12 %) with the maximal depression occurring during extension in all cases (Figs 2 and 4B). This contrasts with the maximal depression during flexion of the two group I fields displaying cyclic modulation. Like the modulation of group I fields, group II field depression usually occurred in both locomotor phases.
Group II field potentials remained depressed for some time after the termination of MLR stimulation. The field potential recorded 20 s after cessation of MLR stimulation shown in Fig. 4B had recovered to only 83 % of control area. In three experiments where the time course was examined, complete recovery of Q and Sart group II field potentials following locomotion took 1–4 min.
Cutaneous field potentials
Sixteen cutaneous-evoked field potentials were recorded mainly throughout the L6-S1 segments at depths between 0.9 and 2.0 mm. Control cutaneous fields had peak amplitudes of 182–1443 μV (mean 565 μV) and latencies ranging from 0.8 to 2.3 ms. The majority (11/16) were evoked at latencies ≤ 1 ms and were thus compatible with a monosynaptic activation from cutaneous afferents. Figure 5A, B, D and E shows monosynaptic field potentials evoked by stimulation of the SP, sural, Tib and SPud cutaneous nerves recorded in the same animal. Control fields (dotted traces) recorded in the absence of MLR stimulation and fictive locomotion are superimposed on cutaneous fields recorded during fictive locomotion (continuous traces).
Figure 5. Depression of cutaneous field potentials during fictive locomotion.
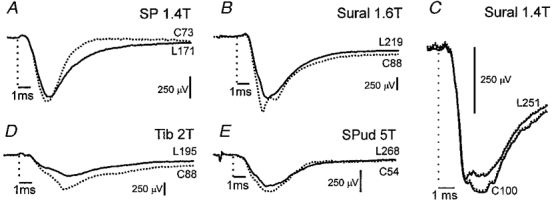
A, B, D and E, depression of fields evoked by stimulation of SP (depth 1.2 mm), sural (depth 2 mm), Tib (depth 2 mm) and SPud (depth 1.7 mm) nerves during fictive locomotion (continuous traces, locomotion; dotted traces, control). These fields were recorded in the L6-S1 segments in the same experiment. C, sural field from another experiment. In this panel only, dotted lines indicate standard errors of the means (for clarity plotted on only one side of the mean). Although the overall depression of this field potential was small, the small standard errors show that it was nevertheless genuine. Same format as in Figs 1 and 3.
As illustrated in Fig. 5 and summarized in Fig. 2, all 16 cutaneous field potentials were depressed during fictive locomotion. Because the depression of the short-latency fields to 75 % of control was similar to the 80 % depression of longer-latency (> 1 ms, n= 5) cutaneous field potentials, the results were pooled. Cutaneous fields during locomotion ranged from 62 to 96 % of control areas with a mean reduction to 78 % of control. The reduction of the sural field illustrated in Fig. 5C to 96 % of control was the smallest change during locomotion considered significant (three group II fields were considered unaffected during locomotion, see Fig. 2). The dotted traces in Fig. 5C are the standard errors of the means for the control and locomotor averages. Note the large sample sizes and lack of overlap between the error and mean traces obtained during control and locomotion. The depression of the cutaneous-evoked field potentials was similar to that of the dorsal group II field potentials and the intermediate group I fields (see Fig. 2). Three cutaneous field potentials were recorded during trials where there was a delay between the onset of MLR stimulation and the beginning of rhythmic nerve activity. One field potential evoked by sural (2T) stimulation was unaffected during MLR-evoked tonic activity and was depressed to 96 % of its control value during fictive locomotion (Fig. 5C). Two SP-evoked fields were depressed with MLR stimulation (98 and 85 %) and further reduced (to 87 and 76 %, respectively) after rhythmic activity began. Seven of the eleven cutaneous fields that were modulated throughout the locomotor cycle were depressed most during the extension phase; four were depressed most during flexion. In all but two cases the difference between the fields recorded in flexion and extension was less than the depression of the least affected phase.
Depression of flexion reflex pathways during fictive locomotion
In the decerebrate, spinal cord-intact preparations used in this study, single shocks to peripheral nerves never evoked flexion reflex discharges detectable in the ENG recordings. However, in the two experiments where fictive locomotion was facilitated by intravenous administration of 4-AP, single shocks to muscle nerves at group II intensity or cutaneous nerves evoked reflex discharges in flexor nerves. Figure 6 shows the effects of continuous 5 Hz stimulation of Q at 5T in an experiment in which 4-AP had been administered (200 μg kg−1). The records in A show the entire 120 s period of data collection. In B portions of the records are replotted with the same vertical scaling and on an expanded time base. In the absence of MLR stimulation, Q stimulation evoked short-latency (5 ms) reflex responses in flexor nerves. These reflexes are the vertical deflections in the TA and PBSt traces occurring before MLR stimulation (the 0–13 s period in A and the response to the first five Q stimuli at the left of B). The top trace in C shows the averaged activity evoked in the TA ENG by Q stimulation before MLR stimulation. Excitation of TA and PBSt is suppressed with the onset of MLR stimulation. In this example, MLR stimulation produced a period (from 13 to 31 s in Fig. 6A) of extensor activity before rhythmic alternating flexor and extensor discharges (48–65 s). The averaged responses in Fig. 6C show that during the flexion phase of the fictive step cycle, the excitation of flexors evoked by Q stimulation at group II strength reversed to a mixed, largely inhibitory effect. Upon termination of MLR stimulation the excitatory responses in TA began to reappear. Full recovery of the reflex was delayed, however, and had not returned to pre-locomotor levels within 53 s of the termination of MLR stimulation and the end of the data collection (compare the amplitude of responses in TA at 0 and 120 s in A). The simultaneously recorded dorsal Q group II field potential (not shown) was first reduced to 97 % of control by MLR stimulation alone and then to 82 % during fictive locomotion. This field potential recovered to control values within seconds after locomotion. Unfortunately, group II fields in intermediate locations were not recorded in the preparations in which flexion reflexes were examined (see Discussion). During the flexion reflex, one might also expect short-latency inhibition of extensors (Eccles & Lundberg, 1959a). Although extensor inhibition would be undetectable from ENGs recordings at rest (FDHL records in Fig. 6A and B), inhibition was evident both during the tonic extensor activity produced by MLR stimulation before locomotion began and during the extensor phase of the fictive locomotor cycle (Fig. 6B and C).
Figure 6. Depression of Q-evoked flexion reflexes during fictive locomotion.
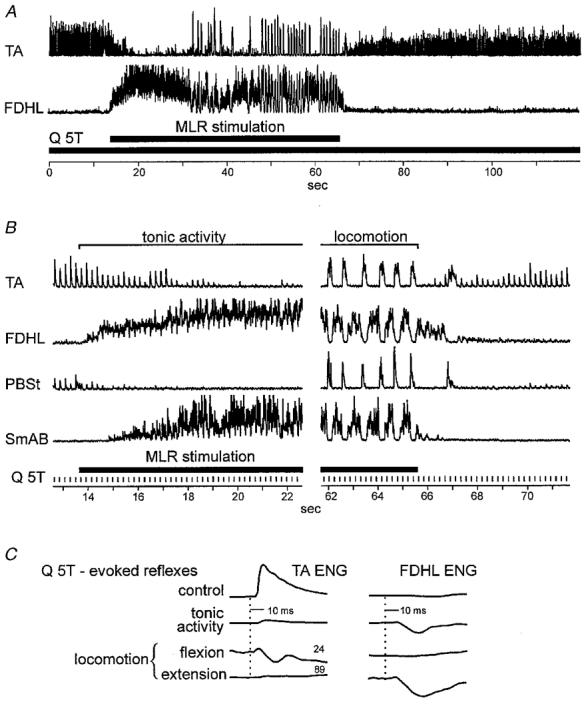
A and B, integrated, rectified ipsilateral extensor and flexor ENGs. The Q nerve was stimulated at 5T (5 Hz) throughout the trial (shown by the longer filled bar in A and vertical ticks in B) and the MLR was stimulated during the period indicated. C, averaged Q-evoked responses recorded in TA and FDHL nerves during these periods.
Flexion reflexes evoked by cutaneous nerve (SP) stimulation in Fig. 7 are from the same experiment illustrated in Fig. 6. Like the effects of Q group II stimulation, SP-evoked excitation of TA (latency about 8 ms; Fig. 7A and B) was suppressed with the onset of MLR stimulation. In contrast to the suppression of group II excitation, cutaneous excitation persisted during the flexion phase of the locomotor cycle (Fig. 7B). The SP-evoked inhibition of the extensor FDHL (Fig. 7B) was similar, albeit smaller, to that evoked by Q stimulation (Fig. 6B) during MLR stimulation both during tonic and rhythmic (fictive locomotor) activity in the nerves. A depression of short-latency flexion reflexes during MLR-evoked fictive locomotion without 4-AP administration has also been reported (Grillner & Shik, 1973).
Figure 7. Depression of cutaneous-evoked flexion reflexes during fictive locomotion.
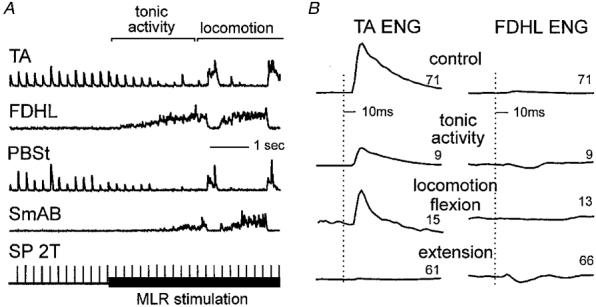
The SP nerve was stimulated at 2T (5 Hz) throughout the trial. A and B, same format as in Fig. 6.
DISCUSSION
The present study did not (1) differentiate between field potentials evoked by group Ia muscle spindle and Ib tendon organ afferents, (2) examine fields evoked by high-strength stimulation of cutaneous nerves, or (3) conduct an exhaustive survey of all fields in all segmental locations. Despite these limitations, however, a striking finding is that almost all of the fields examined were depressed during fictive locomotion (Fig. 2). This depression sometimes varied with the phase of locomotion but often it was unmodulated. During fictive locomotion, fields evoked by group II muscle afferents and recorded in the deeper parts of the dorsal horn (intermediate fields) were depressed the most. This may indicate a specialized control of transmission from group II afferents to some neurones during fictive locomotion. Because stimulation of areas in and around the MLR in anaesthetized cats reduces group II field potentials located in intermediate laminae (Noga et al. 1995) without evoking fictive locomotion, the possibility that field depression results from MLR stimulation per se and not activation of the spinal locomotor pattern generator must be considered. An examination of those cases in which there was a delay between MLR stimulation and the appearance of rhythmic alternating locomotor activity in the muscle ENGs revealed that maximal depression usually occurs with the onset and throughout the rhythmic activity. Furthermore, the depression of fields could vary rhythmically within the fictive locomotor step cycle. The changes in field potential depression occurring in the presence of unchanging MLR stimulation argue strongly for a locomotor-related component to field potential depression.
Origin of field potential depression
The negative polarity and monosynaptic latencies of the extracellular fields studied indicate their origin as afferent-evoked depolarization of first-order spinal neurones. Field potential depression thus reflects a reduction of depolarizing synaptic current into spinal neurones during fictive locomotion. There are three broad mechanisms by which this reduction could occur. The first would be the result of a general depolarization of neurones in the vicinity of the recording electrode during fictive locomotion. The approach of neurone membrane potentials to the excitatory synaptic equilibrium potential would reduce the driving potential for synaptic excitation and result in smaller extracellular fields. This seems an unlikely explanation particularly for the substantial reduction of group II fields in intermediate spinal regions as earlier investigations utilizing the same preparation did not find a tonic depolarization of interneurones in the intermediate regions (Shefchyk et al. 1990). Instead approximately one-half of the interneurones with monosynaptic group II input are rhythmically depolarized during the flexion phase and the other half are tonically depressed (i.e. hyperpolarized) during fictive locomotion. Therefore, depolarization of interneurones with group II input is unlikely to account for the substantial field reductions seen during both locomotor phases. Furthermore, the greater reduction of group II than group I fields recorded simultaneously in the same (intermediate) location is strong evidence that postsynaptic depolarization is unlikely to be the dominant mechanism for field potential depression during fictive locomotion. It is more likely that the depression of the fields involves a reduction in synaptic transmission from segmental afferents to spinal interneurones. This could result from either decreased transmitter release from the afferents (i.e. a presynaptic inhibition) or modification of postsynaptic receptor actions during fictive locomotion. A locomotor-related reduction in receptor responses to excitatory amino acids has not been investigated but depression of excitatory amino acid receptor function has been described in other systems (see Smart, 1997).
Previous studies of primary afferent depolarization (PAD) and dorsal root potentials provide strong support for the existence of rhythmic presynaptic inhibitory mechanisms during fictive locomotion (reviewed in Rossignol, 1996) that could contribute to monosynaptic field potential depression. Furthermore, Dueñas & Rudomin (1988) found a tonic increase in the excitability of Ia afferent terminals upon which a phasic modulation is superimposed and argued that this reflected both a tonic and phasic PAD during fictive locomotion. Field potentials showing a tonic depression upon which cycle-dependent fluctuations were also seen (e.g. Figs 2 and 4). Recent evidence shows that composite Ia EPSPs recorded in motoneurones are tonically depressed during MLR-evoked fictive locomotion (Gosgnach et al. 1998). Both the threshold current for intra-spinal current injection activation of single group I fibres (Dueñas & Rudomin, 1988) and the size of group I fields are decreased to about 80 % of control values during locomotion.
Studies using intra-axonal recordings, while showing a rhythmic PAD, have not commented on the possibility of a tonic depolarization of afferents (Gossard et al. 1989, 1991; Gossard 1996; Ménard et al. 1999). Because of the reported prevalence of rhythmic PAD, we expected a greater incidence of cyclic modulation of field potentials than was found. However, the depression of many field potentials was not modulated during fictive locomotion. Because the peak depolarization of all afferents is usually during flexion (see Rossignol, 1996), we expected that the maximum field depression would also occur during flexion. As indicated in Fig. 2, however, maximum depression occurred during extension in all of the group II and the majority of cutaneous afferents. Moreover, almost all of the modulated field potentials were depressed in both phases. These results could arise from either the summation of processes producing a stepwise (unmodulated) depression upon which phasic modulations may be superimposed or a single process that varies in strength within the step cycle. Other lines of evidence also suggest that PAD recorded in the dorsal horn may not reliably reflect the changes in synaptic transmission occurring during fictive locomotion. Paired recordings from group I afferents and their target motoneurones show that fluctuations in monosynaptic EPSP amplitude and PAD are not well correlated (Gossard, 1996). Finally the amplitudes of composite monosynaptic group Ia EPSPs in motoneurones are not consistently more depressed during flexion (Shefchyk et al. 1984; Angel et al. 1996; Gosgnach et al. 1998). Because some of the observations on PAD were obtained during drug-induced fictive locomotion in acute spinal cats, there is the possibility that some of the discrepancies between the modulation of PAD and synaptic transmission during locomotion are preparation dependent.
Final clarification of the association between PAD and field potential depression during fictive locomotion must await further study. It appears likely, however, that monosynaptic EPSP and field potential reductions may result in part from presynaptic processes not associated with PAD. Recent evidence shows that the presynaptic inhibition produced by conditioning stimulation of flexor nerves has at least two pharmacologically distinct components (Curtis & Lacey, 1998). One appears to be a G-protein-mediated reduction in presynaptic transmitter release (Curtis, 1998; see also Miller, 1998). Such a second messenger-mediated presynaptic process would be in keeping with the prolonged recovery of field potentials following periods of fictive locomotion and contribute to the discrepancy between patterns of PAD and field potential or EPSP depression. While accumulation of extracellular potassium could contribute to PAD (Jiménez et al. 1984) and field potential reduction, its time course and the preferential depression of group II recorded in the same location as group I fields both argue against it being the principal mechanism (see Dueñas & Rudomin, 1988).
Regardless of the mechanism, it is likely that during fictive locomotion in decerebrate cats, there is a reduced efficacy of depolarization of first-order spinal interneurones from muscle and cutaneous afferents. While some of the interneurones responsible for the PAD of group I (Rudomin et al. 1987) and group II fibres (Jankowska & Riddell, 1995) have now been identified, an understanding of their contribution to field potential depression must await assessment of not only their activity, but also the mechanisms by which synaptic transmission is altered during MLR-evoked fictive locomotion. Neither the present results, nor PAD (Gossard et al. 1991; Gossard, 1996; Ménard et al. 1999), nor excitability measurements (Dueñas & Rudomin, 1988) support a previous conclusion that group I afferent fibres are hyperpolarized during fictive locomotion (Bayev & Kostyuk, 1982).
Implications of reduced transmission from segmental afferents during locomotion
As mentioned in the Introduction, the potential for proprioceptive and cutaneous afferent input to evoke inappropriate reflexes during locomotion must be avoided. Field potential depression may reflect one of the ways this is achieved. A general reduction in transmission from sensory afferents to spinal neurones would tend to reduce reflex gain during locomotion but may also contribute to a reorganization of reflex pathways.
Controversy has long existed about whether group II muscle spindle afferents should be considered as part of the flexion reflex system. Their inclusion originated from the observation of common (flexion) reflex actions upon stimulation of group II, joint or high-threshold cutaneous afferents in low spinal preparations (Eccles & Lundberg, 1959a). At the same time, the need to prevent proprioceptive activation of group II afferents from evoking flexion reflexes was recognized (Eccles & Lundberg, 1959b). There is a depression of flexion reflexes during MLR stimulation in decerebrate cats that increases with the onset of locomotion (Figs 6 and 7; Grillner & Shik, 1973). While Grillner & Shik (1973) favoured a postsynaptic control of interneurones in flexion reflex depression during locomotion, we suggest that a presynaptic mechanism also reduces transmission from segmental afferents.
In accord with the more powerful depression of group II fields in the intermediate spinal regions (Fig. 2) is the possibility that a reduction in synaptic transmission from group II afferents contributes to the greater change in group II- than cutaneous-evoked flexion reflexes (Figs 6 and 7). A hypothesis arising from the present study is that a presynaptic suppression of group II input to interneurones located in intermediate regions results in suppression of group II-evoked flexion reflexes during locomotion. In midlumbar segments, intermediate regions contain group II afferent-activated interneurones that produce either monosynaptic excitation or inhibition of motoneurones (Cavallari et al. 1987). About half of the group II-activated interneurones are active during the flexion phase (Shefchyk et al. 1990). The remaining interneurones become more difficult to activate by peripheral nerve stimulation throughout fictive locomotion (Shefchyk et al. 1990). The original interpretation that there was a tonic postsynaptic inhibition of some of the interneurones (Shefchyk et al. 1990) needs to be reconsidered in the light of the present observations on a tonic presynaptic inhibition. It remains unknown if the group II interneurones active during flexion are excitatory or inhibitory to motoneurones nor is there any information concerning the activity during locomotion of group II interneurones located more dorsally or in other spinal segments.
It also remains to be determined if regulation of afferent transmission contributes to the reorganization of group II reflexes into a system in which some group II afferents can reset the locomotor cycle to the extension phase during MLR-evoked fictive locomotion (Perreault et al. 1995). Another interesting possibility is that a reduction in group II excitation of γ-motoneurones during locomotion would reduce γ-drive to the spindles and thus prevent excessive positive feedback from muscle stretch (Jankowska et al. 1998; Gladden et al. 1998). Exploration of these hypotheses must await further information about whether group II interneurones in intermediate spinal laminae contribute to γ-motoneurone excitation or flexor motoneurone excitation during the flexion reflex. It is important to note that during locomotion in intact cats (Hiebert et al. 1996) and fictive locomotion in spinal cats (Schomburg et al. 1998), the actions of some group II afferents remain excitatory to flexor motoneurones and can promote the flexor phase of locomotion. Presumably as yet unidentified descending pathways can control the flexor- and extensor-related actions of the group II reflex system during locomotion.
Differential PAD has been discussed as a mechanism that can select between parallel reflex pathways (see Rudomin, 1990; McCrea, 1992) and there is accumulating evidence that interneurones responsible for sensory-evoked PAD have restricted, local actions on relatively few afferent fibre terminals (Riddell et al. 1992; Eguibar et al. 1994; Quevedo et al. 1995; Lomelíet al. 1998). With such an organization, presynaptic inhibition operating via PAD or other mechanisms could control the access of a particular afferent input to specific interneurones during locomotion. The present observation of depression of intermediate group I fields appears similar to the depression of monosynaptic group Ia EPSPs and field potentials recorded in the ventral horn (Gosgnach et al. 1998). In the case of the group II fields, however, there is good evidence for a more powerful reduction in transmission to interneurones located in intermediate than to those in dorsal laminae (Fig. 2). The extent to which this preferential depression contributes to selection of reflex actions during locomotion must await further study.
Acknowledgments
This work was supported by a program grant from the MRC of Canada to D.A.M. and S.J.S.; M.-C. P. was supported by The Rick Hansen Man in Motion Legacy Fund. Technical assistance was by Sharon McCartney. We thank Michael Angel and Pierre Guertin for help in collecting some of the present data and discussion of the manuscript.
References
- Angel MJ, Fyda D, McCrea DA, Shefchyk SJ. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. The Journal of Physiology. 1994;479:451–461. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jiménez I, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. The Journal of Physiology. 1996;494:851–861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayev KV, Kostyuk PG. Primary afferent depolarization evoked by the activity of spinal scratching generator. Neuroscience. 1981;6:205–215. doi: 10.1016/0306-4522(81)90056-7. [DOI] [PubMed] [Google Scholar]
- Bayev KV, Kostyuk PG. Polarization of primary afferent terminals of lumbosacral cord elicited by the activity of spinal locomotor generator. Neuroscience. 1982;7:1401–1409. doi: 10.1016/0306-4522(82)90253-6. [DOI] [PubMed] [Google Scholar]
- Buss RR, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. The Journal of Physiology. 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Postsynaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. The Journal of Physiology. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Cameron T, Gillard DM, Prochazka A. Muscular sense is attenuated when humans move. The Journal of Physiology. 1998;508:635–643. doi: 10.1111/j.1469-7793.1998.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR. Two types of inhibition in the spinal cord. In: Rudomin P, Romo R, Mendell L, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 150–177. [Google Scholar]
- Curtis DR, Lacey G. Prolonged GABA B receptor-mediated synaptic inhibition of the cat spinal cord: an in vivo study. Experimental Brain Research. 1998;121:319–333. doi: 10.1007/s002210050465. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Rossignol S, Lamarre Y. The effects of 4-aminopyridine on the spinal cord: rhythmic discharges recorded from the peripheral nerves. Brain Research. 1986;369:243–259. doi: 10.1016/0006-8993(86)90533-0. [DOI] [PubMed] [Google Scholar]
- Dueñas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Experimental Brain Research. 1988;70:15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Synaptic action in motoneurones by afferents which may evoke the flexion reflex. Archives Italiennes de Biologie. 1959a;97:199–221. [Google Scholar]
- Eccles RM, Lundberg A. Supraspinal control of interneurons mediating spinal reflexes. The Journal of Physiology. 1959b;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. The Journal of Physiology. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S, Jankowska E. An interneuronal relay for group I and II muscle afferents in the middle lumbar segments of the cat spinal cord. The Journal of Physiology. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguibar JR, Quevedo J, Jiménez I, Rudomin P. Selective cortical control of information flow through different intraspinal collaterals of the same muscle afferent fiber. Brain Research. 1994;643:328–333. doi: 10.1016/0006-8993(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Fu TC, Santini M, Schomburg ED. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiologica Scandinavica. 1974;91:298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Gladden MH, Jankowska E, Czarkowska-Bauch J. New observations on coupling between group II muscle afferents and feline α-motoneurones. The Journal of Physiology. 1998;512:507–520. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea D. Tonic presynaptic reduction of monosynaptic Ia EPSPs during fictive locomotion. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. New York: New York Academy of Sciences; 1998. pp. 505–507. [DOI] [PubMed] [Google Scholar]
- Gossard J-P. Control of transmission in muscle group Ia afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1996;76:4104–4112. doi: 10.1152/jn.1996.76.6.4104. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Cabelguen J-M, Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1989;62:1177–1188. doi: 10.1152/jn.1989.62.5.1177. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Cabelguen J-M, Rossignol S. An intracellular study of muscle primary afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1991;65:914–926. doi: 10.1152/jn.1991.65.4.914. [DOI] [PubMed] [Google Scholar]
- Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the ‘mesencephalic locomotor region’. Acta Physiologica Scandinavica. 1973;87:320–333. doi: 10.1111/j.1748-1716.1973.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR-evoked fictive locomotion in the cat. The Journal of Physiology. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Whelan P, Prochazka A, Pearson KG. Contribution of hindlimb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology. 1996;75:1–12. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. The Journal of Physiology. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Gladden MH, Czarkowska-Bauch J. Modulation of responses of feline α-motoneurones by noradrenaline, tizanidine and clonidine. The Journal of Physiology. 1998;512:521–532. doi: 10.1111/j.1469-7793.1998.521be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. A relay for input from group II muscle afferents in sacral segments of the cat spinal cord. The Journal of Physiology. 1993;465:561–580. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones in pathways from group II muscle afferents in sacral segments of the feline spinal cord. The Journal of Physiology. 1994;475:455–468. doi: 10.1113/jphysiol.1994.sp020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones mediating presynaptic inhibition of group II muscle afferents in the cat spinal cord. The Journal of Physiology. 1995;483:461–471. doi: 10.1113/jphysiol.1995.sp020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I, Rudomin P, Solodkin M, Vyklicky L. Specific and nonspecific mechanisms involved in generation of PAD of group Ia afferents in cat spinal cord. Journal of Neurophysiology. 1984;52:921–940. doi: 10.1152/jn.1984.52.5.921. [DOI] [PubMed] [Google Scholar]
- Kurasawa I, Hirose Y, Sundada T, Nakamura Y. Phase-lined modulation of excitability of presynaptic terminals of low-threshold afferent fibers in the inferior alveolar nerve during cortically induced fictive mastication in the guinea pig. Brain Research. 1988;446:113–120. doi: 10.1016/0006-8993(88)91301-7. [DOI] [PubMed] [Google Scholar]
- Lomelí J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature. 1998;395:600–604. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Can sense be made of spinal interneuron circuits? Behavioral and Brain Sciences. 1992;15:633–643. [Google Scholar]
- McCrea D, Perreault M-C. PAD and modulation of group II evoked flexion reflexes during MLR evoked fictive locomotion. In: Rudomin P, Romo R, Mendell L, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 366–384. [Google Scholar]
- Ménard A, LeBlond H, Gossard J-P. The modulation of presynaptic inhibition in single muscle primary afferent during fictive locomotion in the cat. Journal of Neuroscience. 1999;19:391–400. doi: 10.1523/JNEUROSCI.19-01-00391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annual Review of Pharmacology and Toxicology. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Noga BR, Jankowska E, Skoog B. Depression of transmission from group II muscle afferents by electrical stimulation of the cuneiform nucleus in the cat. Experimental Brain Research. 1995;105:25–38. doi: 10.1007/BF00242179. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II muscle afferents during fictive locomotion. The Journal of Physiology. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Jiménez I, Shefchyk SJ, McCrea D. Depression of monosynaptic group II and other field potentials during MLR-evoked fictive locomotion suggests a reduction of transmission in sensory afferent pathways. Society for Neuroscience Abstracts. 1994;20:716.3. [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. The Journal of Physiology. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Trend P, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Progress in Brain Research. 1989;80:61–74. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Jiménez I, Rudomin P. Raphe magnus and reticulospinal actions on primary afferent depolarization of group I muscle afferents in the cat. The Journal of Physiology. 1995;482:623–640. doi: 10.1113/jphysiol.1995.sp020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. The Journal of Physiology. 1992;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. The Journal of Physiology. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell L, Shepard J, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: The American Physiological Society; 1996. pp. 173–216. chap. 5. [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends in Neurosciences. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Quevedo J, Eguibar J. Presynaptic modulation of spinal reflexes. Current Opinions in Neurobiology. 1993;3:997–1004. doi: 10.1016/0959-4388(93)90173-v. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Solodkin M, Jiménez I. Synaptic potentials of primary afferent fibers and motoneurons evoked by single intermediate nucleus interneurons in the cat spinal cord. Journal of Neurophysiology. 1987;57:1288–1313. doi: 10.1152/jn.1987.57.5.1288. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kreillaars D, Fortier P, Jordan L. Activity of L4 interneurons during brainstem evoked fictive locomotion in the mesencephalic cat. Experimental Brain Research. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Stein RB, Jordan LM. Synaptic transmission from muscle afferents during fictive locomotion in the mesencephalic cat. Journal of Neurophysiology. 1984;51:986–997. doi: 10.1152/jn.1984.51.5.986. [DOI] [PubMed] [Google Scholar]
- Smart T. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Current Opinion in Neurobiology. 1997;7:358–367. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- Sypert GW, Munson JB, Fleshman JW. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. Journal of Neurophysiology. 1980;44:792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]
- Williams SR, Shenasa J, Chapman CE. Time course and magnitude of movement-related gating of tactile detection in humans. I. Importance of stimulus location. Journal of Neurophysiology. 1998;79:947–963. doi: 10.1152/jn.1998.79.2.947. [DOI] [PubMed] [Google Scholar]


