Abstract
Two kinetically and pharmacologically distinct transient outward K+ currents, referred to as Ito,f and Ito,s, have been distinguished in mouse left ventricular myocytes. Ito,f is present in all left ventricular apex cells and in most left ventricular septum cells, whereas Ito,s is identified exclusively in left ventricular septum cells.
Electrophysiological recordings from ventricular myocytes isolated from animals with a targeted deletion of the Kv1.4gene (Kv1.4−/− mice) reveal that Ito,s is undetectable in cells isolated from the left ventricular septum (n= 26). Ito,f density in both apex and septum cells, in contrast, is not affected by deletion of Kv1.4.
Neither the 4-AP-sensitive, slowly inactivating K+ current, IK,slow, nor the steady-state non-inactivating K+ current, ISS, is affected in Kv1.4−/− mouse left ventricular cells.
In myocytes isolated from transgenic mice expressing a dominant negative Kv4.2 α subunit, Kv4.2W362F, Ito,f is eliminated in both left ventricular apex and septum cells. In addition, a slowly inactivating transient outward K+ current similar to Ito,s in wild-type septum cells is evident in myocytes isolated from left ventricular apex of Kv4.2W362F-expressing transgenics. The density of Ito,s in septum cells, however, is unaffected by Kv4.2W362F expression.
Western blots of fractionated mouse ventricular membrane proteins reveal a significant increase in Kv1.4 protein level in Kv4.2W362F-expressing transgenic mice. The protein levels of other Kv α subunits, Kv1.2 and Kv2.1, in contrast, are not affected by the expression of the Kv4.2W362F transgene.
The results presented here demonstrate that the molecular correlates of Ito,f and Ito,s in adult mouse ventricle are distinct. Kv1.4 underlies mouse ventricular septum Ito,s, whereas Kv α subunits of the Kv4 subfamily underlie mouse ventricular apex and septum Ito,f. The appearance of the slow transient outward K+ current in Kv4.2W362F-expressing left ventricular apex cells with properties indistinguishable from Ito,s in wild-type cells is accompanied by an increase in Kv1.4 protein expression, suggesting that the upregulation of Kv1.4 underlies the observed electrical remodeling in Kv4.2W362F-expressing transgenics.
The amplitudes and the durations of action potentials in cardiac cells are largely determined by voltage-gated K+ channels and, in most cells, two types of voltage-gated K+ channel currents have been distinguished based on differences in time- and voltage-dependent properties and pharmacological sensitivities: (1) rapidly activating and inactivating, 4-aminopyridine-(4-AP)-sensitive K+ currents, referred to as Ito (transient outward); and (2) delayed, slowly inactivating and typically 4-AP-insensitive, K+ currents, referred to as IK (delayed rectifiers). These are broad classifications, however, and it is now clear that there are multiple components of IK, and that the densities, properties and functional roles of Ito and IK vary in cardiac cells isolated from different species, as well as in cells from different regions of the heart in the same species (Anumonwo et al. 1991; Antzelevitch et al. 1995; Barry & Nerbonne, 1996). There are also marked changes in the densities and the properties of voltage-gated K+ currents in the heart during normal development (Wetzel & Klitzner, 1996; Nerbonne, 1998) and in various myocardial disease states (Ten Eick et al. 1989; Boyden & Jeck, 1995; Näbauer & Kaab, 1998). For all of these reasons, there is considerable interest in defining the properties and the molecular correlates of cardiac Ito and IK channels, and in delineating the molecular mechanisms regulating the functional expression of these channels.
Rapidly activating and inactivating Ca2+-independent, depolarization-activated transient outward, K+-selective currents, referred to as Ito1, Ito or It, have been identified in a number of cardiac cell types (Campbell et al. 1995; Barry & Nerbonne, 1996; Giles et al. 1996; Nerbonne, 1998). The time- and voltage-dependent properties of Ito in most cells are similar in that activation, inactivation and recovery from steady-state inactivation are all rapid (Campbell et al. 1995; Barry & Nerbonne, 1996; Giles et al. 1996). In rabbit atrial and ventricular myocytes, however, inactivation of Ito (It) is slow and recovery from steady-state inactivation is very slow, with complete recovery requiring seconds (Giles & Imaizumi, 1988; Fedida & Giles, 1991; Wang et al. 1999). Recently, it was also reported that there are regional differences in the properties of Ito in human (Näbauer et al. 1996), ferret (Brahmajothi et al. 1999), rat (Wickenden et al. 1999) and mouse (Xu et al. 1999) ventricles. The rates of Ito inactivation and recovery (from steady-state inactivation), for example, are significantly slower in human and ferret left ventricular endocardial (than epicardial) cells (Näbauer et al. 1996; Brahmajothi et al. 1999). In adult mouse ventricular myocytes, two transient outward K+ currents, similar to those characterized in the human and ferret, have also been distinguished; they have been referred to as Ito,fast (Ito,f) and Ito,slow (Ito,s) (Xu et al. 1999). In addition, Ito,f and Ito,s are differentially distributed in mouse ventricle: Ito,f is present in both left ventricular apex and septum cells, whereas Ito,s is only identified in left ventricular septum cells (Xu et al. 1999). Ferret epicardial Ito and mouse ventricular Ito,f have similar kinetic properties and both are blocked by nanomolar concentrations of Heteropoda toxins (HpTx) (Sanguinetti et al. 1997), whereas ferret endocardial Ito and mouse ventricular Ito,s are characterized by slow inactivation and recovery (from steady-state inactivation) kinetics and both currents are unaffected by HpTx (Brahmajothi et al. 1999; Xu et al. 1999).
In ferret, there are also regional differences in the expression patterns of the voltage-gated (Kv), pore-forming (α) subunits Kv1.4, Kv4.2 and Kv4.3 (Brahmajothi et al. 1999). These observations have been interpreted as suggesting that distinct Kv α subunits underlie Ito in ferret left ventricular endocardial (Kv1.4) and epicardial (Kv4.2/Kv4.3) cells (Brahmajothi et al. 1999). Several recent studies have provided direct evidence to support a role for Kv α subunits of the Kv4 subfamily in the generation of the rapidly activating and inactivating current Ito,f in rat and mouse ventricular myocytes (Fiset et al. 1997b; Johns et al. 1997; Barry et al. 1998), although the molecular identity of mouse ventricular Ito,s has not been determined. To test directly the hypothesis that Kv1.4 underlies Ito,s in mouse left ventricular septum cells, electrophysiological experiments were completed on myocytes isolated from the left ventricles of mice with a targeted deletion of the Kv1.4 gene (Kv1.4−/−; London et al. 1998b). Analysis of whole-cell voltage-clamp recordings from Kv1.4−/− ventricular myocytes revealed that Ito,s in septum cells is selectively eliminated, whereas the currents in apex cells are unaffected by elimination of Kv1.4. In addition, in experiments conducted on left ventricular myocytes isolated from transgenic mice expressing a mutant Kv4.2 α subunit, Kv4.2W362F (Barry et al. 1998), Ito,f is eliminated in both septum and apex cells, whereas Ito,s in septum cells is unaffected. Analysis of the outward K+ currents in Kv4.2W362F-expressing left ventricular apex cells reveals the presence of a ‘novel’ current indistinguishable from Ito,s in septum cells. Importantly, however, Ito,s density in septum cells is unaffected by Kv4.2W362F expression. Western blot analysis also reveals that Kv1.4 protein expression is increased in the ventricles of Kv4.2W362F-expressing animals. Taken together, these results demonstrate that Kv1.4 underlies mouse ventricular Ito,s, and suggest that upregulation of Kv1.4 in apex cells underlies the electrophysiological remodelling observed in Kv4.2W362F-expressing transgenic mice.
METHODS
Isolation of mouse ventricular myocytes
Ventricular cells were isolated from adult (6–8 weeks of age) wild-type C57BL6 mice (n= 5), mice with a targeted deletion of the Kv1.4 gene (Kv1.4−/−, n= 2, London et al. 1998b) and transgenic mice expressing a dominant negative Kv4.2 α subunit, Kv4.2W362F (n= 3, Barry et al. 1998) using a procedure previously developed and utilized to isolate rat cardiomyocytes (Xu et al. 1996). Briefly, hearts were excised from anaesthetized (5% halothane-95% O2) animals, mounted on a Langendorf apparatus, and perfused retrogradely through the aorta with 40 ml of a Ca2+-free Hepes-buffered Eagle's balanced salt solution (Gibco) supplemented with 6 mm glucose, amino acids and vitamins (Buffer A). Hearts were then perfused with 50 ml of Buffer A containing 0.8 mg ml−1 collagenase (Type II, Worthington) and 10 μm CaCl2 (Buffer B), with the temperature of the perfusate and the tissue maintained at 37°C. The enzyme solution was filtered (at 5 μm) and recirculated through the heart for approximately 15–20 min.
The heart was cut open following perfusion, and the ventricular septum and the top approximately 0.3 mm of tissue at the apex of the left ventricle were removed. These tissue pieces were placed separately in Buffer A supplemented with 1.25 mg ml−1 taurine, 5 mg ml−1 bovine serum albumin (BSA, Sigma) and 150 μm CaCl2 (Buffer C). After mechanical dispersion by gentle trituration, cell suspensions were filtered to remove large undissociated tissue fragments, and the cells were collected by sedimentation. Isolated myocytes were resuspended in Buffer C, plated on laminin-coated 35 mm culture dishes, and kept in a 5% CO2-95% air incubator at 37°C. Approximately 30 min after plating, serum-free medium-199 (M-199, Irvine Scientific) supplemented with antibiotics (penicillin- streptomycin) was added. Ca2+-tolerant ventricular myocytes attached to the laminin-coated dishes; damaged cells were removed by replacing the medium with fresh M-199 at 1 h after plating. Cells were examined electrophysiologically within 36 h of isolation. No differences in the properties or in the densities of the currents were evident in cells examined at times varying from 1 to 36 h after isolation.
Electrophysiological recordings
The conventional whole-cell recording technique was employed to record Ca2+-independent, depolarization-activated K+ currents and action potential waveforms in isolated adult mouse left ventricular myocytes. Voltage-clamp and current-clamp recordings were obtained only from Ca2+-tolerant, rod-shaped cells, and all experiments were conducted at room temperature (22–24°C). For voltage-clamp recordings, the bath solution contained (mm): 136 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 5 CoCl2, 10 Hepes, 0.02 tetrodotoxin and 10 glucose; pH 7.4 and 295–305 mosmol l−1. In current-clamp experiments, the CoCl2 and tetrodotoxin were omitted from the bath solution. Recording pipettes contained (mm): 135 KCl, 1 MgCl2, 10 EGTA, 10 Hepes, 5 glucose; pH 7.2 and 300–310 mosmol l−1. 4-Aminopyridine (4-AP, Sigma) and Heteropoda toxin-3 (HpTx-3, NPS Pharmaceuticals) stock solutions were prepared in the bath solution and diluted to the appropriate concentrations immediately before use. 4-AP and HpTx-3 were applied to isolated myocytes during recordings through narrow-bore capillary tubes (inner diameter 300 μm) placed within 200 μm of the cells.
Experiments were conducted using a Dagan 3900A amplifier (headstage gain (β) = 1, Dagan Corporation). Pipettes had resistances of 2–3 MΩ when filled with the recording solution. Junctional potentials were zeroed before gigaohm seals were formed. After establishing the whole-cell configuration, ±10 mV voltage steps from a holding potential of −70 mV were applied to allow measurements of whole-cell membrane capacitances and input resistances. Series resistances were estimated by dividing the time constant of the decay of the capacitive transient by the membrane capacitance. Whole-cell membrane capacitances and series resistances were routinely compensated electronically (≥ 85%); voltage errors resulting from the uncompensated series resistance were ≤ 6 mV and were not corrected. Only data obtained from the cells with input resistance ≥ 0.7 GΩ were analysed. From a holding potential of −70 mV, voltage-gated outward K+ currents were evoked during 400 ms or 4 s depolarizing voltage steps to potentials between −40 and +60 mV in 10 mV increments. The voltage steps were applied at 15 s intervals. To quantify the rates of recovery from steady-state inactivation, cells were first depolarized to +50 mV for 10 s to inactivate the currents (conditioning pulses), subsequently hyperpolarized to −70 mV for varying times ranging from 10 ms to 8 s, and finally stepped to +50 mV to activate the currents and assess the extent of recovery. In current-clamp recordings, action potentials were evoked by 3 ms suprathreshold current pulses at 1.0 Hz and recorded after reaching a steady state. Experimental data were collected using a Gateway microcomputer equipped with a Digidata 1200 Series analog/digital interface (Axon) and pCLAMP 7 software (Axon). Data were acquired at variable sampling frequencies and the current signals were filtered on-line at 5 kHz before digitization and storage.
Data analysis
Analysis of digitized data was completed using Clampfit 6.0.5 (Axon). Whole-cell membrane capacitances were calculated by integrating the capacitive transients elicited during ±10 mV voltage steps from a holding potential of −70 mV. Peak outward K+ currents at each test potential were measured as the difference between the maximal outward current amplitudes and the zero current level. The waveforms of the 4-AP- and HpTx-3-sensitive currents were obtained in the same cells by offline digital subtraction of the currents recorded in the presence of 4-AP or HpTx-3 from the control currents.
The decay phases of the outward K+ currents evoked during 4 s depolarizing voltage steps to test potentials between +10 and +60 mV (from a holding potential of −70 mV) can be fitted by the sum of two or three exponential functions using one of the following equations:
or
where t is time; τ1, τ2, and τ3 are the time constants of decay of the inactivating K+ currents; A1, A2 and A3 are the amplitudes of the inactivating current components; and B is the amplitude of the steady-state, non-inactivating component of the total outward K+ currents. For all analyses, correlation coefficients (r) were determined to assess the quality of fits; r values for the fits reported here were ≥ 0.98. Although the decay phases of the outward K+ currents in all left ventricular apex cells were well described by the sum of two exponentials, this was not the case for septum cells. For the majority of septum cells, the decay phases of the outward K+ currents were not well fitted by two exponentials as judged by eye and by the correlation coefficients of the fits. For these cells, the quality of the fits was markedly improved when three exponentials were included. In contrast, the quality of the fits to the decay phases of the outward K+ currents in apex cells was not improved by the inclusion of a third exponential. As reported previously (Xu et al. 1999), the time constants of decay of Ito,f, Ito,s and IK,slow do not display any appreciable voltage dependence; all mean ±s.e.m. decay time constants (τdecay) reported here were calculated from the decays of the total outward K+ currents evoked at +40 mV in each cell. To quantify the rates of recovery from steady-state inactivation of Ito,f and the slowly inactivating transient outward K+ current (Ito,s) in wild-type and Kv4.2W362F transgenic animals, the decay phases of the currents following each recovery time were fitted to the sum of two exponentials to provide the amplitudes of Ito,f, Ito,s and IK,slow; the amplitudes of Ito,f or Ito,s were normalized to the amplitudes of these components determined during the corresponding conditioning pulses; mean ±s.e.m. normalized recovery data are plotted and fitted with single exponential functions. All average and normalized data are presented as means ±s.e.m. The statistical significance of observed differences between groups of cells or between different parameters describing the properties of the currents were evaluated using a one-way analysis of variance (ANOVA) followed by an F test or a two-tailed Student's t test; P values are presented in the text, and statistical significance was set at the P < 0.05 level.
Western blots
Ventricular membrane proteins were prepared from adult wild-type, Kv1.4−/− and Kv4.2W362F transgenic mice using methods for the preparation of rat ventricular membrane proteins (Barry et al. 1995; Xu et al. 1996). Tissue samples used in the membrane preparations included the lower half of the heart from the apex and included both the left and right ventricles. The protein content of the solubilized membrane preparations was determined by using a Bio-Rad protein assay kit with BSA as the standard. For Western blots, membrane proteins (50 or 180 μg) were fractionated on 10% SDS-polyacrylamide gels and transferred to Polyscreen PVDF membrane (DuPont). PVDF membrane strips were first incubated in 0.2% I-Block (Tropix) in PBS containing 0.1% Tween 20 (blocking buffer) for 1 h at room temperature, followed by overnight incubation at 4°C with polyclonal antibodies directed against Kv1.4 (Alamone Labs), Kv1.2 (Alamone Labs) and Kv2.1 (Upstate Biotechnology). All antibodies were tested for specificity and cross reactivity, as described previously (Barry et al. 1995). The anti-Kv1.4, anti-Kv1.2 and anti-Kv2.1 antibodies were used at dilutions of 1:200, 1:100 and 1:100, respectively, in blocking buffer. After washing (twice), the strips were incubated for 2 h at room temprature in alkaline phosphatase-conjugated goat anti-rabbit IgG (Tropix) diluted in 1:5000 in blocking buffer. Strips were washed three times in blocking buffer and twice in assay buffer (0.1 M diethanolamine and 1 mm MgCl2, Tropix). Bound antibodies were detected by using the CPSD (Tropix) chemiluminescent alkaline phosphate substrate.
RESULTS
Regional differences in action potential waveforms in adult mouse ventricle
Recently it was reported that the waveforms of the Ca2+-independent, depolarization-activated K+ currents in cells isolated from mouse left ventricular apex and septum are distinct (Xu et al. 1999). In addition, peak outward K+ current densities in cells isolated from the apex are significantly (P < 0.001) higher than in cells isolated from the septum (Table 1). In cells isolated from the apex, the rapidly inactivating and the slowly inactivating K+ currents, Ito,f and IK,slow, are evident (Table 1), and there is a steady-state, non-inactivating component of the outward K+ currents, referred to as ISS (Xu et al. 1999). On average, Ito,f, IK,slow and ISS contribute ∼60, 30 and 10%, respectively, to the peak outward K+ currents in adult mouse left ventricular apex cells (Table 1). In approximately 80% of the septum cells studied, the decay phases of the outward K+ currents were well described by the sum of three exponentials reflecting the presence of Ito,f, IK,slow and a distinct, slowly inactivating transient outward K+ current, referred to as Ito,s (Xu et al. 1999). Importantly, Ito,s is present in septum cells but not in apex cells. In the remaining ∼20% of septum cells, Ito,f is absent and only Ito,s, IK,slow and ISS are evident (Table 1). The densities of Ito,f (when expressed) and IK,slow in septum cells are significantly (P < 0.01) lower than in apex cells, whereas the densities of ISS in apex and septum cells are similar (Table 1).
Table 1.
Comparison of K+ current densities in wild-type, Kv1.4 knockout and Kv4.2W362F-expressing mice
| n | Ipeak (pA pF−1) | Ito,f (pA pF−1) | Ito,s (pA pF−1) | IK,slow (pA pF−1) | ISS (pA pF−1) | |
|---|---|---|---|---|---|---|
| Wild-type | ||||||
| Apex | 43 | 57.4 ± 2.9 | 34.5 ± 2.3 | — | 17.4 ± 0.9 | 5.5 ± 0.4 |
| Septum with Ito,f | 28 | 31.1 ± 1.3 | 6.8 ± 0.4 | 7.5 ± 0.6 | 12.2 ± 0.5 | 4.6 ± 0.4 |
| Septum without Ito,f | 8 | 20.5 ± 2.2 | — | 5.5 ± 0.7 | 10.4 ± 1.3 | 4.7 ± 0.4 |
| Kv1.4−/− | ||||||
| Apex | 8 | 56.9 ± 1.4 | 34.7 ± 2.5 | — | 17.0 ± 1.4 | 5.2 ± 0.4 |
| Septum with Ito,f | 20 | 25.0 ± 1.1 | 9.1 ± 0.6 | — | 11.0 ± 0.6 | 4.9 ± 0.2 |
| Septum without Ito,f | 6 | 15.5 ± 0.9 | — | — | 10.2 ± 0.8 | 5.2 ± 0.3 |
| Kv4.2W362F | ||||||
| Apex | 11 | 33.4 ± 3.5 | — | 12.5 ± 1.7 | 15.9 ± 1.8 | 5.0 ± 0.6 |
| Septum with Ito,f | — | — | — | — | — | — |
| Septum without Ito,f | 13 | 21.8 ± 1.9 | — | 6.4 ± 0.6 | 11.4 ± 1.4 | 4.0 ± 0.5 |
Current densities were determined from analyses of records obtained on depolarization to +40 mV from a holding potential of −70 mV.
In mouse ventricular myocytes, Ito,f plays a prominant role in action potential repolarization (Barry et al. 1998). The finding that the density of Ito,f (and IK,slow) is significantly lower in septum cells than in apex cells (Table 1), therefore, suggested that action potentials would probably be prolonged in septum cells, relative to action potentials in apex cells. Current-clamp recordings from isolated adult mouse left ventricular apex and septum cells confirmed this hypothesis (Fig. 1A and B), and quantitive analysis of action potential durations at 25% (APD25), 50% (APD50) and 75% (APD75) repolarization revealed that action potentials are significantly (P < 0.01) longer in septum than in apex cells (Table 2). In contrast, no differences in resting membrane potentials or in action potential amplitudes in apex and septum cells were observed (Table 2).
Figure 1. Action potential waveforms are distinct in apex and septum cells isolated from adult mouse left ventricle.
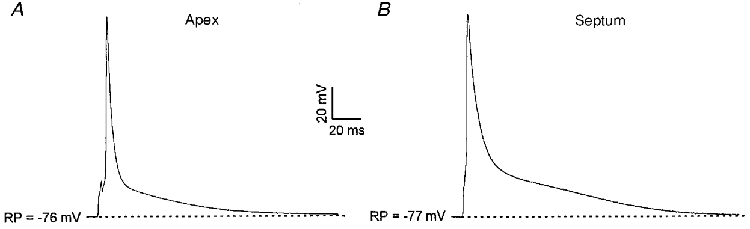
Under current-clamp, action potentials evoked by 3 ms suprathreshold current pulses were recorded from adult mouse left ventricular apex (A) and septum (B) cells. Stimuli were applied at 1.0 Hz and action potentials were recorded after reaching a steady state. Action potentials are significantly broader in the septum cells; similar results were obtained in recordings from 7 apex and 8 septum cells.
Table 2.
Comparison of action potential parameters in adult mouse left ventricular apex and septum cells
| APD25 (ms) | APD50 (ms) | APD75 (ms) | RP (mV) | APA (mV) | |
|---|---|---|---|---|---|
| Apex (n= 7) | 2.3 ± 0.1 | 4.5 ± 0.3 | 10.0 ± 1.2 | −76.2 ± 0.9 | 125.6 ± 2.7 |
| Septum (n= 8) | 4.0 ± 0.2 | 8.5 ± 0.3 | 20.9 ± 1.3 | −77.9 ± 0.7 | 128.2 ± 2.6 |
Mean ±s.e.m. action potential durations (APD) at 25% (APD25), 50% (APD50) and 75% (APD75) of repolarization, resting membrane potentials (RP) and action potential amplitudes (APA) in apex and septum cells are summarized.
Ito,s is eliminated in the ventricles of Kv1.4−/− mice
Although recently it was demonstrated that Kv4 α subunits underlie Ito,f in mouse ventricular myocytes (Barry et al. 1998), the molecular identity of Ito,s has not previously been defined. The slow rates of inactivation and recovery from steady-state inactivation of Ito,s (Xu et al. 1999), however, are similar to the kinetic properties of one of the Kv α subunits, Kv1.4 (Rasmusson et al. 1995; Peterson & Nerbonne, 1999). In addition, Kv1.4 has recently been suggested to underlie the slow transient outward K+ current in rabbit atrial myocytes (Wang et al. 1999), as well as in ferret (Brahmajothi et al. 1999) and human (Näbauer et al. 1998) left ventricular endocardial myocytes. To test directly the hypothesis that Kv1.4 also underlies Ito,s in adult mouse left ventricular septum cells, electrophysiological experiments were completed on left ventricular myocytes isolated from mice with a targeted deletion of the Kv1.4 gene (Kv1.4−/−; London et al. 1998b). As illustrated in Fig. 2B, the waveforms of the outward voltage-gated K+ currents in apex cells from Kv1.4−/− mice are similar to those recorded from wild-type animals (Fig. 2A). Similar results were obtained in eight cells and mean ±s.e.m. peak outward K+ current densities in wild-type and Kv1.4−/− apex cells are indistinguishable (Table 1). There are also no measurable effects of the elimination of Kv1.4 on the densities (Table 1) or the kinetic properties of Ito,f, IK,slow and ISS in apex cells.
Figure 2. The waveforms of the Ca2+-independent, depolarization-activated K+ currents in myocytes isolated from the apex of wild-type (A) and Kv1.4−/− (B) adult mouse left ventricles are indistinguishable.
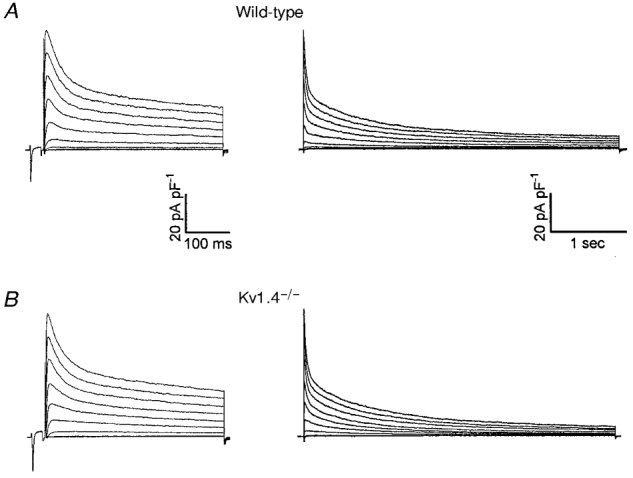
Whole-cell outward K+ currents were evoked during 400 ms (left) and 4 s (right) depolarizing voltage steps to potentials between −40 and +40 mV from a holding potential of −70 mV in 10 mV increments. Each trial was preceded by a short (25 ms) depolarization to −20 mV to eliminate contamination of inward Na+ currents not completely blocked by TTX. The records shown in the left and right panels in A and B were obtained from the same cell.
In contrast to the findings in apex cells, peak outward K+ current densities are significantly (P < 0.05) lower in Kv1.4−/− septum cells than in wild-type septum cells (Table 1). As in wild-type septum cells (Fig. 3A), two populations of septum cells are isolated from Kv1.4−/− animals: cells with Ito,f (Fig. 3Ba) and cells lacking Ito,f (Fig. 3Bb). In approximately ∼20% (6 of 26) of the Kv1.4−/− septum cells studied, outward current waveforms similar to those in Fig. 3Bb were recorded. This subset of cells lacks Ito,f and there is a single component of outward current decay with a mean ±s.e.m.τdecay of 1248 ± 73 ms (Fig. 3Bb). This slowly inactivating K+ current is selectively blocked by micromolar concentrations of 4-aminopyridine (4-AP) similar to IK,slow in wild-type cells (Fiset et al. 1997a; London et al. 1998a; Zhou et al. 1998; Xu et al. 1999). Analysis of the outward K+ currents in the Kv1.4−/− septum cells lacking Ito,f under control conditions and following exposure to 50 μm 4-AP reveals that only IK,slow and ISS are present; there is no evidence for Ito,s in these cells (Fig. 4A).
Figure 3. Ito,s is eliminated in left ventricular septum cells isolated from Kv1.4−/− mice.
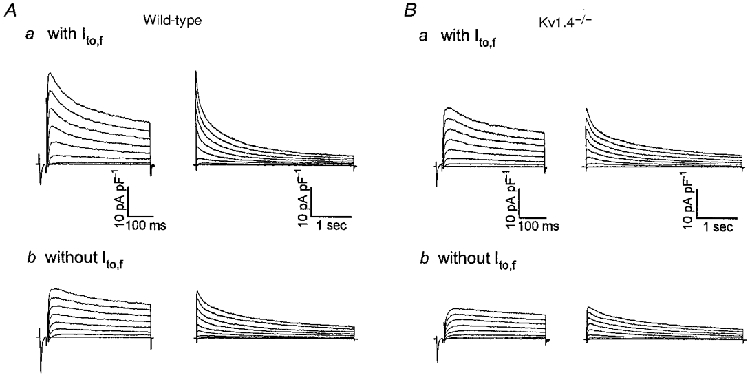
Whole-cell outward K+ currents were recorded as described in the legend to Fig. 2 from left ventricular septum cells isolated from wild-type (A) and Kv1.4−/− (B) mice. In cells isolated from the left ventricular septum of Kv1.4−/− mice, Ito,s is undetectable (B); Kv1.4−/− septum cells express either only IK,slow and ISS (Bb) or Ito,f, IK,slow and ISS (Ba). In addition to the elimination of Ito,s, Ito,f inactivation is slowed in septum cells isolated from Kv1.4−/− mice (see text).
Figure 4. Effects of 50 μm 4-AP and 300 nm HpTx-3 on the outward K+ currents in wild-type and Kv1.4−/− mouse left ventricular septum cells.
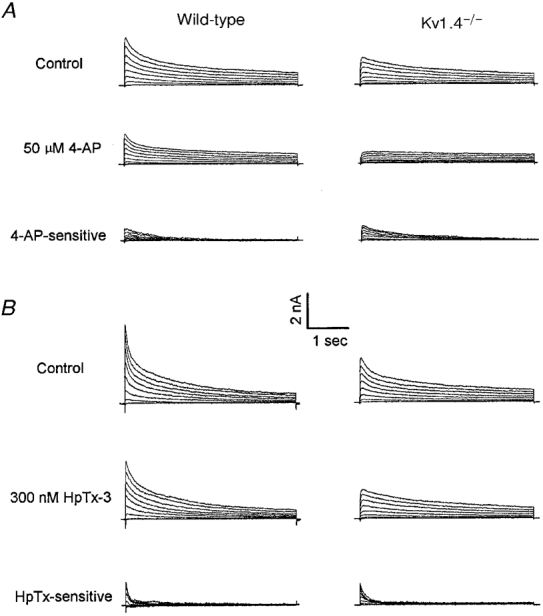
Representative whole-cell outward K+ currents recorded from adult mouse left ventricular septum cells lacking and expressing Ito,f are displayed in A and B, respectively. To determine the effects of 50 μm 4-AP (A) and 300 nm HpTx-3 (B), control currents were recorded before exposure to the drug, and when the effect reached a steady state, the currents were recorded again. The waveforms of the 50 μm 4-AP- and 300 nm HpTx-3-sensitive currents were obtained by offline digital subtraction of the currents in the presence of 50 μm 4-AP or 300 nm HpTx-3 from the controls. Note that Ito,s is clearly evident in wild-type septum cells, but undetectable in Kv1.4−/− septum cells, when IK,slow or Ito,f is selectively blocked by micromolar concentrations of 4-AP (A) or nanomolar concentrations of HpTx-3 (B). Similar results were obtained in experiments on 3 cells of each group.
In the majority (20 of 26) of the Kv1.4−/− septum cells studied, outward currents similar to those shown in Fig. 3Ba were recorded. In these Ito,f-expressing septum cells, the decay phases of the outward K+ currents were well described by two exponentials with mean ±s.e.m.τdecay values of 122 ± 3 and 1336 ± 37 ms; these components contribute 36 and 44% to the peak outward K+ currents, respectively. Although the slower time constant (1336 ± 37 ms) of current decay corresponds to IK,slow, the faster time constant (τdecay= 122 ± 3 ms) is significantly (P < 0.001) slower than Ito,f in wild-type septum cells (τdecay= 53 ± 2 ms). This observation suggests either that a novel current is expressed in Kv1.4−/− septum cells or that the kinetics of Ito,f inactivation is slowed in these animals. Experimental support for the latter hypothesis was provided in pharmacological experiments with Heteropoda toxin-3 (HpTx-3), which reportedly inhibits Ito,f without measurably affecting Ito,s (Xu et al. 1999). As illustrated in Fig. 4B, 300 nm HpTx-3 completely blocks the fast component of outward current decay in wild-type and in Kv1.4−/− septum cells. The mean ±s.e.m.τdecay of the HpTx-3-sensitive currents in the Kv1.4−/− septum cells is 116 ± 17 ms (n= 3), a value that is significantly slower than the τdecay values of the HpTx-3-sensitive current in wild-type cells. This τdecay value (116 ± 17 ms) is, however, similar to the fast component of decay (122 ± 3 ms) derived from the double exponential fits to the decay phases of the outward currents in Kv1.4−/− septum cells. The simplest interpretation of these results is that 300 nm HpTx-3 also blocks Ito,f in Kv1.4−/− septum cells but that the rate of Ito,f decay is slowed in cells lacking Kv1.4 (Ito,s). Consistent with this hypothesis, analysis of the rates of recovery from steady-state inactivation of the outward K+ currents in Kv1.4−/− septum cells revealed that recovery was characterized by the sum of two exponentials with recovery time constants of 42 ms and 1 s (n= 3, data not shown); these values are indistinguishable from those of Ito,f and IK,slow recovery in wild-type septum cells (Xu et al. 1999). Taken together, these results indicate that Ito,s is absent and the kinetics of Ito,f inactivation is altered (slowed) in Kv1.4−/− septum cells, whereas the kinetics of Ito,f recovery are not affected. Further experiments will be necessary to explore the underlying mechanisms involved in mediating the selective modification of Ito,f inactivation kinetics. It is of interest to note that, in spite of the loss of Ito,s in Kv1.4−/− septum cells, the densities of Ito,f (when evident), IK,slow and ISS are not significantly different from those in wild-type septum cells (Table 1).
Elimination of Ito,f and upregulation of Ito,s in Kv4.2W362F-expressing mice
It has been demonstrated that the kinetic and pharmacological properties of Ito,f in myocytes isolated from adult mouse left ventricular apex and septum are indistinguishable, suggesting that the molecular basis of Ito,f in apex and septum cells is the same (Xu et al. 1999). This hypothesis was tested directly in experiments on myocytes isolated from the left ventricular apex and septum of transgenic mice expressing a mutant Kv4.2 α subunit, Kv4.2W362F, that functions as a dominant negative (Barry et al. 1998). In a previous study on myocytes randomly dispersed from the ventricles of Kv4.2W362F-expressing animals, it was reported that Ito (Ito,f) is eliminated and that a ‘novel’ slowly inactivating transient outward K+ current (not detectable in wild-type cells) was evident (Barry et al. 1998). Representative outward K+ current waveforms recorded from left ventricular apex (Fig. 5A) and septum (Fig. 5B) cells isolated from adult Kv4.2W362F transgenic mice are shown. As reported previously (Barry et al. 1998), peak outward K+ current densities in these cells are substantially lower than in wild-type myocytes (Table 1). In all left ventricular septum cells examined (n= 13), the decay phases of the outward K+ currents were well described by the sum of two exponentials with mean ±s.e.m.τdecay values of 230 ± 21 and 1055 ± 67 ms, corresponding to Ito,s and IK,slow, respectively; Ito,f was not detected in any of these cells. In addition, the densities of Ito,s, IK,slow and ISS in Kv4.2W362F-expressing septum cells are not significantly different from the densities of these currents determined in wild-type septum cells (Table 1).
Figure 5. Ito,f is eliminated in ventricular myocytes isolated from both the apex and the septum of adult Kv4.2W362F transgenic mice.
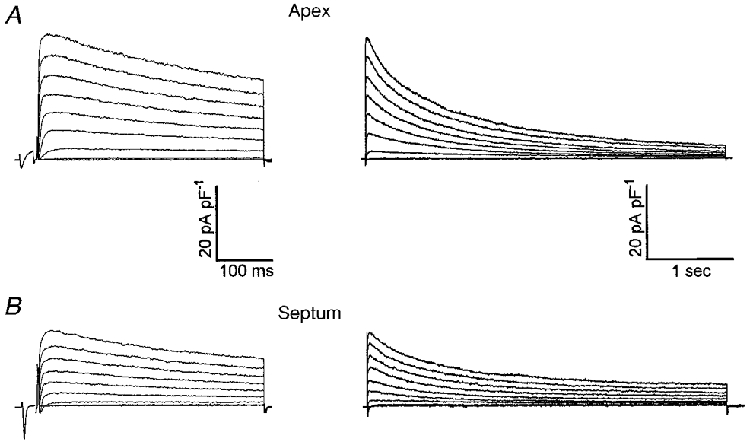
Whole-cell outward K+ currents were recorded from adult mouse left ventricular apex (A) and septum (B) cells as described in the legend to Fig. 2. The records shown in the left and right panels in A and B were obtained from the same cell, and only the durations of the voltage steps are different. As is evident, Ito,f is undetectable in both apex and septum cells. Note that a slowly inactivating transient outward K+ current (Ito,s), which was not detected in wild-type apex cells (see Fig. 2A), is evident in Kv4.2W362F-expressing apex cells.
Analysis of the decay phases of the outward K+ currents in left ventricular apex cells isolated from the Kv4.2W362F transgenics also revealed that Ito,f is undetectable (n= 11, Table 1). In addition, 300 nm HpTx-3 had no measurable effect on the outward K+ currents in these apex cells (Fig. 6A), consistent with the absence of Ito,f. Similar to the findings in randomly dispersed cells (Barry et al. 1998), a slowly inactivating transient outward K+ current with a mean ±s.e.m.τdecay of 209 ± 20 ms was detected in all Kv4.2W362F-expressing apex cells (n= 11). The pharmacological properties of this current are distinct from both Ito,f and IK,slow in wild-type apex cells: the currents are not blocked by nanomolar concentrations of HpTx-3 which block Ito,f (Fig. 6A) or by micromolar concentrations of 4-AP which block IK,slow (Fig. 6B). The τdecay (209 ± 20 ms) for this current, however, is similar to that of Ito,s in wild-type septum cells (231 ± 11 ms, n= 36) and the currents are blocked by higher concentrations (0.5 mm) of 4-AP (data not shown). In addition, the slowly inactivating transient outward K+ current evident in Kv4.2W362F-expressing apex cells recovers slowly from steady-state inactivation with a time constant of 1.0 s (Fig. 7B and C). This recovery rate is significantly slower than the rate of Ito,f recovery (τrec= 28 ms) (Fig. 7A and C), but is similar to the rate of Ito,s recovery (τrec≈ 1.3 s, Xu et al. 1999). Taken together, these observations suggest that the slow transient outward K+ current in Kv4.2W362F apex cells corresponds to Ito,s. If this interpretation is correct, the results further suggest that Ito,s is selectively upregulated in the left ventricular apex, i.e. not in the left ventricular septum in the Kv4.2W362F-expressing transgenics (see Discussion).
Figure 6. Effects of 300 nm HpTx-3 and 30 μm 4-AP on the outward K+ currents in wild-type and Kv4.2W362F-expressing mouse left ventricular apex cells.
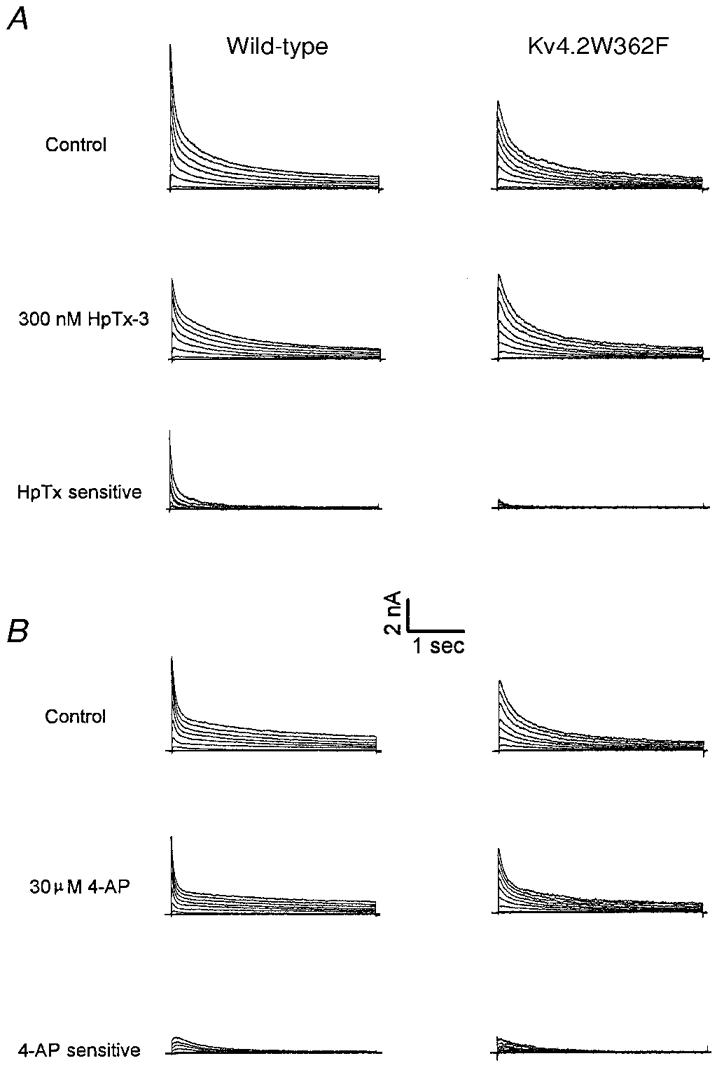
Whole-cell outward K+ currents were recorded as described in the legend to Fig. 2 from left ventricular apex cells isolated from wild-type and Kv4.2W362F transgenic mice. After recording control currents, cells were exposed to either 300 nm HpTx-3 (A) or to 30 μm 4-AP (B), and when the effects reached a steady state, the currents were recorded again. The waveforms of the 300 nm HpTx-3- and 30 μm 4-AP-sensitive currents were obtained by offline digital subtraction of the currents in the presence of HpTx-3 or 4-AP from the controls. Note that the slowly inactivating transient outward K+ current in Kv4.2W362F-expressing apex cells is insensitive to nanomolar concentrations of HpTx-3 and micromolar concentrations of 4-AP. Similar results were obtained in experiments on 4 cells of each group.
Figure 7. The slowly inactivating transient outward K+ current in Kv4.2W362F-expressing mouse left ventricular apex cells recovers slowly from steady-state inactivation.
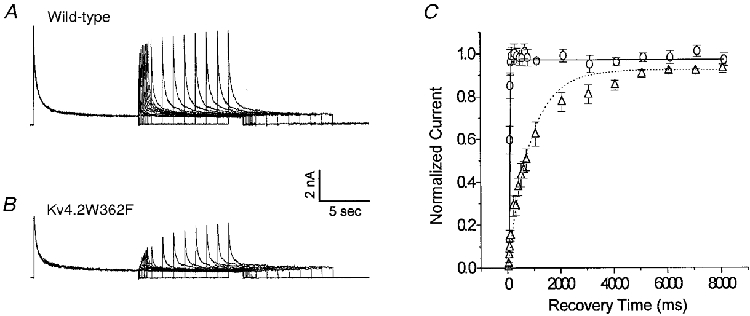
After inactivating the currents by 10 s prepulses to +50 mV, adult mouse left ventricular apex cells were hyperpolarized to −70 mV for varying times (ranging from 10 ms to 8 s) before 10 s test depolarizations to +50 mV to assess the extent of recovery. Typical current waveforms recorded from wild-type and Kv4.2W362F transgenic animals are illustrated in A and B, respectively. The amplitudes of Ito,f in wild-type apex cells and the slow transient outward K+ current (Ito,s) in transgenic apex cells evoked at +50 mV following each recovery period were determined from double exponential fits to the decay phases of the outward currents as described in Methods, and then normalized to the current amplitudes recorded during the 10 s prepulses. Mean ±s.e.m. normalized currents (n= 5) for Ito,f (○) and Ito,s (▵) are plotted as a function of recovery time in C. All recovery data are well described by single exponentials (continuous and dotted lines) although the recovery rates for Ito,f and Ito,s are distinct (see text).
Upregulation of Kv1.4 protein in Kv4.2W362F transgenic mice
The electrophysiological experiments described above revealed the presence of a slowly inactivating transient outward K+ current in Kv4.2W362F-expressing left ventricular apex cells with kinetic and pharmacological properties similar to Ito,s in wild-type left ventricular septum cells. In addition, the experiments on cells isolated from the Kv1.4−/− mice revealed that Ito,s is eliminated thereby demonstrating that Ito,s is encoded by Kv1.4. Based on these experimental observations, it seemed reasonable to speculate that upregulation of Kv1.4 (in apex cells) occurs in Kv4.2W362F animals in which Ito,f channels are reduced/eliminated. Consistent with this hypothesis, Western blot analysis of fractionated mouse ventricular membrane proteins revealed that Kv1.4 protein is increased in Kv4.2W362F-expressing animals relative to wild-type non-transgenic littermates (Fig. 8). Using a polyclonal antibody directed against the N terminal sequence of Kv1.4, a single protein band at 97 kDa was evident in blots of fractionated wild-type mouse ventriclar membrane proteins. This band is undetectable in membrane proteins prepared from the Kv1.4−/− ventricles, consistent with the deletion of the Kv1.4 gene in these animals. As is evident in Fig. 8A, the amount of the Kv1.4 protein is greater in the Kv4.2W362F-expressing mouse ventricles than in wild-type ventricles; similar results were obtained in three separate experiments. In contrast to the findings with Kv1.4, however, the expression levels of two other Kv α subunits, Kv1.2 (Fig. 8B) and Kv2.1 (Fig. 8C), are not measurably different in wild-type and Kv4.2W362F-expressing animals.
Figure 8. Western blots reveal that Kv1.4 protein expression is increased in Kv4.2W362F-expressing transgenic mice.
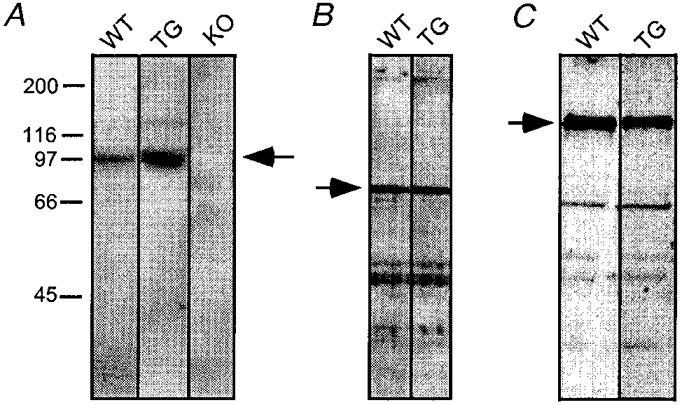
Ventricular membrane proteins (A: 180 μg loaded in each lane; B and C: 50 μg loaded in each lane) were fractionated on SDS-PAGE gels, transferred to PVDF membranes and immunoblotted with the polyclonal anti-Kv1.4 (A), anti-Kv1.2 (B) or anti-Kv2.1 (C) antibodies (see Methods). WT, TG and KO indicate ventricular membrane proteins prepared from adult wild-type, Kv4.2W362F-expressing and Kv1.4−/− mice, respectively. The arrows show the specific bands recognized by the antibodies.
DISCUSSION
Molecular correlates of mouse ventricular Ito,f and Ito,s are distinct
The marked differences in the kinetic and pharmacological properties of mouse ventricular Ito,f and Ito,s described previously (Xu et al. 1999) suggested that the molecular correlates of these two conductance pathways are distinct. Consistent with this hypothesis, the experiments here revealed that Ito,s is absent in cells isolated from the left ventricular septum of Kv1.4−/− mice, thereby documenting a functional role of Kv1.4 in the generation of mouse ventricular septum Ito,s channels. In contrast, no effects on Ito,f, IK,slow or ISS densities were seen in apex or septum cells from Kv1.4−/− animals (see Table 1). In approximately 20% of the Kv1.4−/− septum cells, only IK,slow and ISS were detected. In the other ∼80% of the Kv1.4−/− septum cells studied, two exponentials with mean ±s.e.m.τdecay values of 122 ± 3 and 1336 ± 37 ms were required to fit the decay phases of the outward K+ currents. Although the slower time constant corresponds to IK,slow, the inactivation time constant of the faster component is distinct from that of both Ito,f (τdecay= 53 ± 2 ms) and Ito,s (τdecay= 231 ± 11 ms) in wild-type septum cells. This current is selectively blocked by 300 nm HpTx-3 and recovers rapidly from steady-state inactivation, consistent with Ito,f. Importantly, the properties of Ito,f in wild-type and Kv1.4−/− apex cells are indistinguishable, suggesting that Ito,f modulation is selective for septum cells. Further experiments will be necessary to explore the underlying mechanisms involved in mediating the slowing of Ito,f inactivation kinetics in septum cells.
The observation that the properties of Ito,f in mouse left ventricular apex and septum are indistinguishable suggested that the molecular correlates of Ito,f in these two regions would also be the same (Xu et al. 1999). The findings here that Ito,f is eliminated in both apex and septum cells isolated from Kv4.2W362F-expressing transgenic mice confirm this hypothesis. Since Kv4.2W362F functions as a dominant negative when coexpressed with Kv4.2 or Kv4.3 (Barry et al. 1998), however, further experiments will be necessary to determine if both Kv4.2 and Kv4.3 contribute to mouse ventricular Ito,f in both the apex and the septum and, in addition, to determine if differences in Kv α subunit composition contribute to regional heterogeneity in Ito,f density in mouse left ventricular apex and septum.
In left ventricular apex cells isolated from Kv4.2W362F-expressing mice, however, a slowly inactivating transient outward K+ current undetectable in wild-type apex cells with properties similar to Ito,s in wild-type septum cells is evident. Interestingly, Ito,s in left ventricular septum cells is unaffected by Kv4.2W362F expression. These findings are interpreted as suggesting that Ito,s is upregulated in apex cells in Kv4.2W362F transgenic mice, perhaps as a result of the absence of Ito,f. Consistent with the upregulation of Ito,s, Kv1.4 protein levels are increased in Kv4.2W362F-expressing mouse ventricles. Importantly, the electrophysiological data indicate that this electrical remodelling is only evident in apex cells, which normally lack Ito,s, and is not seen in septum cells, which normally express Ito,s. Further experiments will be necessary to test directly the hypothesis that the molecular identity of the novel current in Kv4.2W362F-expressing apex cells and Ito,s in wild-type septum cells is indeed the same.
Comparison with previous studies
In a previous study of ventricular myocytes isolated from Kv1.4−/− animals, it was reported that the transient outward K+ current, which we now refer to as Ito,f (Xu et al. 1999) was unaffected by the targeted deletion of Kv1.4 (London et al. 1998b). Interestly, however, London and colleagues (1998b) did report a statistically significant decrease in the density of the current remaining 800 ms after the onset of the depolarizing voltage steps in cells isolated from Kv1.4−/− animals relative to the currents in wild-type (control) cells. Based on the present results, it might be suggested that this observation probably reflected the loss of Ito,s in a subset of the cells studied although no attempts were made in the previous study to examine regional differences in K+ channel expression or properties.
Topographic heterogeneity of Ito expression, similar to that described here in adult mouse left ventricle, has been previously reported in other species. In rat ventricle, for example, Ito densities in left ventricular apex and right ventricular free wall are significantly higher than in septum (Bénitah et al. 1993; Gómez et al. 1997; Wickenden et al. 1999). In addition, inactivation of Ito in rat ventricular septum cells is markedly slower than in apex cells (Bénitah et al. 1993). In both human and ferret left ventricles, the density of Ito in endocardial myocytes is significantly lower than in epicardial myocytes. In addition, endocardial Ito inactivates at more negative potentials and recovers more slowly from steady-state inactivation than epicardial Ito (Näbauer et al. 1996, 1998; Brahmajothi et al. 1999). Endocardial Ito in ferret heart is also not blocked by HpTx (Brahmajothi et al. 1999). The properties of human and ferret endocardial Ito, therefore, are similar to Ito,s in mouse left ventricular septum cells. These observations, together with the finding that Kv1.4 is expressed in the endocardium but not in the epicardium of ferret left ventricle (Brahmajothi et al. 1999), suggest that ferret endocardial Ito and mouse Ito,s are similar and that Kv1.4 also probably underlies endocardial Ito in ferret. Further experiments will be necessary to test this hypothesis directly. As noted previously, the properties of the transient outward K+ currents in rabbit atrium and ventricle are distinct from those in other species in that inactivation and recovery from steady-state inactivation are slow (Fedida & Giles, 1991; Wang et al. 1999). The properties of Ito in rabbit heart, therefore, are also similar to Ito,s in mouse left ventricular septum. These observations make it tempting to speculate that Kv1.4 also underlies rabbit Ito and experimental evidence supporting this hypothesis in rabbit atria has been recently provided (Wang et al. 1999).
Physiological and pathological implications
Studies focussed on defining regional differences in the expression and/or the properties of ionic currents in the myocardium are important for a detailed understanding of normal heart functioning, as well as changes in cardiac function that occur in myocardial disease states. Differential expression of Ito,f and Ito,s, for example, is expected to contribute to regional heterogeneity in action potential repolarization in normal ventricle (Antzelevitch et al. 1995; Rosen et al. 1998). In addition, the slow recovery kinetics of Ito,s from steady-state inactivation suggests that, at normal heart rates, particularly in the mouse, Ito,s in the septum (or endocardium) could be largely inactivated. At reduced heart rates, however, Ito,s is expected to have a more pronounced (and frequency-dependent) regulatory effect on action potential durations than the much more rapidly recovering Ito,f in apex (or epicardium).
Defining the molecular basis of Ito (Ito,f and Ito,s) heterogeneity could also have important implications for understanding various myocardial diseases. Reductions in transient outward K+ currents have been found in a variety of cardiac pathologies, including pressure overload-induced myocardial hypertrophy (Tomita et al. 1994), post-myocardial infarction remodelling (Qin et al. 1996), short-term diabetes (Shimoni et al. 1994) and chronic atrial fibrillation (Van Wagoner et al. 1997). In addition, Gómez et al. (1997) recently reported that in pressure overload-induced myocardial hypertrophy in the rat, there is a marked decrease in Ito density in myocytes from the left ventricular apex, whereas there is no significant effect on Ito density in septum cells. It has been reported that Kv4 α subunit mRNA and protein expression are downregulated by a number of myocardial hypertrophic factors, whereas Kv1.4 expression is not affected (Takimoto et al. 1997; Guo et al. 1998). Clearly, further studies aimed at delineating the molecular mechanisms regulating the functional expression of cardiac voltage-gated K+ channels and K+ channel-forming subunits in the normal and diseased myocardium are warranted.
Acknowledgments
The authors thank Ms Janice Delmar and NPS Pharmaceuticals (Salt Lake City, UT, USA) for providing us with Heteropoda toxin-3. In addition, the financial support provided by the National Heart, Lung and Blood Institute of the National Institutes of Health (B.L., J.M.N. and H.X.), the Monsanto/Searle/Washington University Biomedical Agreement (J.M.N.), the American Heart Association National Office (Grant in Aid to B.L.) and the Midwest Affiliate of the American Heart Association (Postdoctoral Fellowship to W.G.) are gratefully acknowledged.
References
- Antzelevitch C, Sicouri S, Lukas A, Nesterenko VV, Liu DW, DiDiego JM. Regional differences in the electrophysiology of ventricular cells: physiological implications. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, PA: W.B. Saunders Co.; 1995. pp. 228–245. [Google Scholar]
- Anumonwo JMB, Freeman LC, Kwok WM, Kass RS. Potassium channels in the heart: electrophysiology and pharmacological regulation. Cardiovascular Drug Reviews. 1991;9:299–316. [Google Scholar]
- Barry DM, Nerbonne JM. Myocardial potassium channels: electrophysiological and molecular diversity. Annual Review of Physiology. 1996;58:363–394. doi: 10.1146/annurev.ph.58.030196.002051. [DOI] [PubMed] [Google Scholar]
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels. Circulation Research. 1995;77:361–369. doi: 10.1161/01.res.77.2.361. [DOI] [PubMed] [Google Scholar]
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 α subunit. Circulation Research. 1998;83:560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- Bénitah J-P, Gomez AM, Bailly P, Da Ponte J-P, Berson G, Delgado C, Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. The Journal of Physiology. 1993;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden PA, Jeck CD. Ion channel function in disease. Cardiovascular Research. 1995;29:312–318. [PubMed] [Google Scholar]
- Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Nerbonne JM, Trimmer JS, Strauss HC. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. Journal of General Physiology. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DL, Rasmusson RL, Comer MB, Strauss HC. The cardiac calcium-independent transient outward potassium current: kinetics, molecular properties, and role in ventricular repolarization. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 2. Philadelphia, PA: W.B. Saunders Co.; 1995. pp. 83–96. [Google Scholar]
- Fedida D, Giles WR. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. The Journal of Physiology. 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset C, Clark RB, Larsen TS, Giles WR. A rapidly activating, sustained current modulates repolarization and excitation-contraction coupling in adult mouse ventricle. The Journal of Physiology. 1997a;504:557–563. doi: 10.1111/j.1469-7793.1997.557bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset C, Clark RB, Shimoni Y, Giles WR. Shal-type channels contribute to the Ca2+-independent transient outward K+ current in rat ventricle. The Journal of Physiology. 1997b;500:51–64. doi: 10.1113/jphysiol.1997.sp021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles WR, Clark RB, Braun AP. Ca2+-independent transient outward currents in mammalian heart. In: Morad M, Kurachi Y, Noma A, Hosada M, editors. Molecular Physiology and Pharmacology of Cardiac Ion Channels and Transporters. Amsterdam: Kluwer Press Ltd; 1996. pp. 141–168. [Google Scholar]
- Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. The Journal of Physiology. 1988;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez AM, Bénitah J-P, Henzel D, Vinet A, Lorente P, Delgado C. Modulation of electrical heterogeneity by compensated hypertrophy in rat left ventricle. American Journal of Physiology. 1997;272:H1078–1086. doi: 10.1152/ajpheart.1997.272.3.H1078. [DOI] [PubMed] [Google Scholar]
- Guo W, Kamiya K, Hojo M, Kodama I, Toyama J. Regulation of Kv4.2 and Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells. Journal of Molecular and Cellular Cardiology. 1998;30:1449–1455. doi: 10.1006/jmcc.1998.0730. [DOI] [PubMed] [Google Scholar]
- Johns DC, Nuss HB, Marban E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4.2 constructs. Journal of Biological Chemistry. 1997;272:31598–31603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated K+ channel. Proceedings of the National Academy of Sciences of the USA. 1998a;95:2926–2931. doi: 10.1073/pnas.95.6.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B, Wang DW, Hill JA, Bennett PB. The transient outward current in targeted mice lacking the potassium channel gene Kv1.4. The Journal of Physiology. 1998b;509:171–182. doi: 10.1111/j.1469-7793.1998.171bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näbauer M, Barth A, Kaab S. A second calcium-independent transient outward current present in human left venntricular myocardium. Circulation. 1998;98:1–231. abstract. [Google Scholar]
- Näbauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- Näbauer M, Kaab M. Potassium channel downregulation in heart failure. Cardiovascular Research. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM. Regulation of voltage-gated K+ channel expression in the developing mammalian myocardium. Journal of Neurobiology. 1998;37:37–59. doi: 10.1002/(sici)1097-4695(199810)37:1<37::aid-neu4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Peterson KR, Nerbonne JM. Expression environment determines K+ current properties: Kv1 and Kv4 α subunit-induced K+ currents in mammalian cell lines and cardiac myocytes. Pflügers Archiv. 1999;437:381–392. doi: 10.1007/s004240050792. [DOI] [PubMed] [Google Scholar]
- Qin D, Zhang Z-H, Caref EB, Boutjdir M, Jain P, El-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circulation Research. 1996;79:461–473. doi: 10.1161/01.res.79.3.461. [DOI] [PubMed] [Google Scholar]
- Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. The Journal of Physiology. 1995;489:709–721. doi: 10.1113/jphysiol.1995.sp021085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MR, Cohen IR, Danilo P, Jr, Steinberg SF. The heart remembers. Cardiovascular Research. 1998;40:469–482. doi: 10.1016/s0008-6363(98)00208-9. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Johnson JH, Hammerland LG, Kelbaugh PR, Volkmann RA, Saccomano NA, Mueller AL. Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels. Molecular Pharmacology. 1997;51:491–498. [PubMed] [Google Scholar]
- Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circulation Research. 1994;74:620–628. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv4.3 K+ channel subunit mRNAs in ventricles of renovascular hypertensive rats. Circulation Research. 1997;81:533–539. doi: 10.1161/01.res.81.4.533. [DOI] [PubMed] [Google Scholar]
- Ten Eick RE, Houser JR, Bassett AL. Cardiac hypertrophy and altered cellular activity of the myocardium. In: Speralakis N, editor. Physiology and Pathophysiology of the Heart. 2. Boston, MA: Martinus Nijhoff; 1989. pp. 573–594. [Google Scholar]
- Tomita F, Bassett AL, Myerburg RJ, Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circulation Research. 1994;75:296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circulation Research. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Wang Z, Feng J, Shi H, Pond AL, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circulation Research. 1999;84:551–561. doi: 10.1161/01.res.84.5.551. [DOI] [PubMed] [Google Scholar]
- Wetzel GT, Klitzner TS. Developmental cardiac electrophysiology: Recent advances in cellular physiology. Cardiovascular Research. 1996;31:E52–60. [PubMed] [Google Scholar]
- Wickenden AD, Jegla TJ, Kaprielian R, Backx PH. Regional contributions of Kv1.4, Kv4.2, and Kv4.3 to transient outward K+ current in rat ventricle. American Journal of Physiology. 1999;276:H1599–1607. doi: 10.1152/ajpheart.1999.276.5.H1599. [DOI] [PubMed] [Google Scholar]
- Xu H, Dixon JE, Barry DM, Trimmer JS, Merlie JP, McKinnon D, Nerbonne JM. Developmental analysis reveals mismatches in the expression of K+ channel α subunits and voltage-gated K+ channel currents in rat ventricular myocytes. Journal of General Physiology. 1996;108:405–419. doi: 10.1085/jgp.108.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated outward K+ currents in adult mouse ventricular myocytes. Journal of General Physiology. 1999;113:661–678. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jeron A, London B, Han X, Koren G. Characterization of a slowly inactivating outward current in adult mouse ventricular myocytes. Circulation Research. 1998;83:806–814. doi: 10.1161/01.res.83.8.806. [DOI] [PubMed] [Google Scholar]


