Abstract
Endogenous nitric oxide has been proposed to play a role in the control of myometrial contractility in pregnancy. In this study, the expression, localisation and regulation of nitric oxide synthase (NOS) isoforms have been examined in human pregnant myometrium and cultured human myometrial smooth muscle cells, by immunoblotting, immunohistochemistry and reverse transcription-polymerase chain reaction.
Immunoblotting of extracts from freshly isolated myometrial tissue, affinity-enriched for NOS proteins by precipitation with ADP-sepharose, revealed expression of endothelial NOS (eNOS or NOS3) in tissues from preterm, term non-labour and active labour at term. Inducible NOS (iNOS or NOS2) and neuronal NOS (nNOS or NOS1) proteins were not detected at any stage of pregnancy.
Immunohistochemical detection showed that expression of eNOS protein was restricted to the endothelium of the myometrial vasculature, with no staining detected in myometrial smooth muscle cells.
Messenger RNA for all three NOS isoforms was detected, although iNOS and nNOS mRNAs were detectable only with high cycle number, implying a low copy number.
NOS isoforms were not detectable in human myometrial smooth muscle cells cultured from term non-labour pregnancies. Cytokine stimulation of cultured myometrial cells did not induce iNOS expression or nitrite accumulation in the culture medium, although both iNOS protein and nitrite release were detected in the human pulmonary epithelial cell line A549.
Levels of eNOS protein and of NOS mRNA expression were not correlated with gestational stage, suggesting that endogenously produced NO is not likely to be a modulator of myometrial tone during human pregnancy.
The initiation of labour in pregnant women is poorly understood. Uterine muscle is responsive both to labour ‘promoting’ and labour ‘inhibitory’ stimuli, and quiescent factors have been suggested to predominate during gestation. The mechanisms maintaining myometrial quiescence are likely to be multifactorial. Exogenous nitric oxide (NO) promotes uterine relaxation and has prompted interest in the use of NO donors as tocolytic agents (Lees et al. 1994). Thus endogenous production of NO may be involved in the regulation of myometrial tone in pregnancy, and a decline in NO production at term could play an important role in the initiation of, or preparation for, parturition (Natuzzi et al. 1993; Sladek et al. 1993; Yallampalli et al. 1994; Bansal et al. 1997).
NO is a short-lived free radical gas involved in the regulation of vascular smooth muscle tone, inflammation, cell-mediated immunity and coagulation (Knowles & Moncada, 1994). The effects of NO on cell function are mediated through a variety of interactions, such as binding to haem-containing enzymes including soluble guanylyl cyclase (sGC) (Moncada et al. 1991). NO-induced activation of sGC stimulates synthesis of intracellular guanosine 3′,5′-cyclic monophosphate (cGMP), which mediates smooth muscle relaxation (Palmer et al. 1987). NO is synthesised from L-arginine via nitric oxide synthases (NOS), of which three distinct isoforms have been identified (Knowles & Moncada, 1994). The two constitutive isoforms, endothelial NOS (eNOS) and neuronal NOS (nNOS), are calcium/calmodulin dependent, whilst the activity of the calcium/calmodulin-insensitive inducible isoform (iNOS) is predominantly regulated at the transcriptional level in response to cytokines and bacterial lipopolysaccharide (Palmer et al. 1987; Nathan & Xie, 1994).
Endogenous production of NO by the pregnant myometrium may play a key role in the maintenance of uterine quiescence in a number of species. NO synthesis is increased in uterine tissues of pregnant rats and rabbits, and declines towards term (Sladek et al. 1993; Natuzzi et al. 1993; Riemer et al. 1997). Consistent with this, expression of iNOS has been demonstrated in rat myometrial smooth muscle and declines at term (Riemer et al. 1997). Cultured rat myometrial smooth muscle cells express eNOS basally and iNOS upon stimulation with cytokines (Gangula et al. 1997). Moreover, expression of calcium-dependent NOS isoforms in a number of tissues appears to be modulated in the guinea-pig by steroid hormones, which are elevated during pregnancy (Weiner et al. 1994).
Two groups have claimed to detect NOS proteins in human myometrial tissue during pregnancy. Using a range of antibodies, Thomson et al. (1997) reported immunohistochemical staining for all three NOS isoforms in term non-labouring and labouring tissue, with no differences observed between the two stages. However, the staining patterns obtained with different iNOS antibodies were quite different. In a subsequent study, the same group reported an elevated level of expression of eNOS and nNOS protein, detected on immunoblots, in preterm non-labouring samples compared with term and non-pregnant tissue (Norman et al. 1999). Bansal et al. (1997) examined the expression of iNOS by immunohistochemistry and immunoblotting and claimed that protein is expressed in preterm non-labouring human myometrium and declines markedly prior to term and in preterm labour. Taken together these studies support the hypothesis that endogenously produced NO maintains human myometrial quiescence early in pregnancy. However, their biochemical evidence is not consistent with the findings from in vitro studies of human myometrial function (Morrison et al. 1996; Jones & Poston, 1997), which demonstrated in muscle strips from preterm and term non-labouring women that the NOS inhibitor NG-nitro-L-argininemethyl ester (L-NAME) had no effect on spontaneous contractility. This is in marked contrast to findings in rat myometrial strips, in which L-NAME increased tension in a concentration-dependent manner (Yallampalli et al. 1994), as would be predicted for a tissue in which NOS activity is regulating muscle tone.
In the light of these conflicting data, we undertook to investigate the expression of all three NOS isoforms in human myometrium. Included in this study were non-labouring tissue from preterm and term deliveries, tissue from women in labour at term and human myometrial smooth muscle cells in culture. Protein expression was analysed by immunoblotting and immunohistochemistry, using characterised antibodies. Messenger RNA was monitored by reverse transcription-polymerase chain reaction (RT-PCR). Preliminary accounts of this work have been presented in abstract form (Bartlett et al. 1998; Campa et al. 1998).
METHODS
Tissue collection
Myometrial tissue was obtained from biopsies at Caesarean section from non-labouring women at term (n= 30, gestation 38.4 ± 0.7 weeks) or preterm (n= 16, gestation 28.9 ± 2.4 weeks). The indications for elective section were breech delivery or previous Caesarean section. The non-labouring group had no evidence of uterine contractions or cervical change. Myometrial tissue from labouring women was collected at emergency Caesarean section (n= 7, gestation 38.4 ± 0.8 weeks). In this group all patients had regular painful contractions with associated cervical dilation greater than 3 cm, and Caesarean section was indicated by fetal distress. There was no evidence of uterine dysfunction. The study was approved by the local Ethics Committee of St Thomas’ Hospital, UK, where all tissues were collected, and all patients gave written, informed consent. Biopsies were obtained from the midline of the upper edge of the lower uterine segment incision.
Drugs and chemicals
Normal sera, biotinylated secondary antibodies and avidin- biotin-peroxidase complex (ABC) were from Dako Ltd (High Wycombe, UK); ADP-sepharose, RNAse inhibitor, enhanced chemiluminescence reagents (ECL+plus) from Amersham Pharmacia Biotech; proteinase K, M-MLV reverse transcriptase, MCDB 131 medium, and Dulbecco's modified Eagle's medium (DMEM) from Gibco Life Technologies; Biotaq polymerase from Biotaq Bioline (London, UK); Nylon transfer membrane from Micron Separations Inc. (Westborough, MA, USA); lipopolysaccharide (LPS) from Difco (Surrey, UK); recombinant human interleukin-1 beta (IL-1β), interferon gamma (IFN-γ), and tumour necrosis factor alpha (TNF-α) from R & D systems (Abingdon, UK); recombinant human nNOS was kindly provided by Dr Valentina Riveros Moreno (King's College London). All other reagents were obtained from Sigma Chemical Co.
NOS antibodies
Rabbit polyclonal antibodies directed against the carboxyl termini of eNOS, iNOS and nNOS were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibodies raised against eNOS and iNOS were purchased from Transduction Laboratories (Lexington, NJ, USA). Control normal rabbit immunoglobulin fractions and purified mouse immunoglobulin (IgG1 and IgG2a) were obtained from Dako.
Isolation and primary culture of human myometrial smooth muscle cells (HMSMCs)
Biopsies from term non-labouring pregnancies were collected in sterile culture medium. In order to obtain rapid outgrowth, cells were initially cultured in MCDB 131 culture medium. Explants (2 mm3, visibly free of blood vessels) were dissected and placed onto the surface of 25 cm2 culture flasks for 2 h (37°C humidified 5 % CO2/95 % air atmosphere), before being submerged in MCDB 131 medium containing 10 % fetal calf serum (FCS), 10 mm L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and supplemented with 2.5 μg ml−1 amphotericin B, 50 μg ml−1 gentamicin, 10 ng ml−1 human recombinant epidermal growth factor (EGF) and 1 μg ml−1 hydrocortisone. After 4 days the medium was replaced with MCDB 131 without added amphotericin B and gentamicin. Primary human myometrial smooth muscle cell (HMSMC) cultures were established within 10 days. After passage 1, cells were cultured in MCDB 131 without EGF or hydrocortisone. Cells exhibited typical smooth muscle morphology by phase contrast microscopy, and cultures stained positively with a monoclonal anti-smooth muscle α-actin antibody (data not shown). Confluent HMSMCs between passages 2 and 5 were used in all experiments.
Cytokine stimulation of cultured myometrial smooth muscle cells
For experiments, cells were seeded into 9 cm diameter culture dishes. In initial experiments, we discovered that cells cultured in MCDB 131 medium showed a much weaker induction of cyclo-oxygenase-2 (COX-2) expression in response to cytokine stimulation than cells cultured in a less enriched medium such as DMEM (data not shown; see Bartlett et al. 1999). Therefore, before addition of agonists, confluent HMSMCs were serum deprived for 16–24 h in DMEM, supplemented with 0.5 % FCS, 4 mm l-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were stimulated for 24 or 48 h with a combination of 100 μg ml−1 LPS and 10 ng ml−1 each of IL-1β, IFN-γ and TNF-α. The human epithelial carcinoma cell line A549, which is known to express iNOS (Watkins et al. 1997), was cultured under the same conditions and treated similarly as a positive control. After cytokine incubation the medium was removed, and cells washed with ice-cold phosphate-buffered saline (PBS) and lysed immediately for RNA or protein extraction.
Affinity extraction of ADP-binding proteins from whole tissue and cultured myometrial cells
NOS enzymes are labile, and for this reason a wide range of protease inhibitors were included in the extraction procedure and samples were heated only for 3 min, and then maintained at 4°C before gel electrophoresis. Initial immunblot analysis of whole-tissue homogenates and cell lysates showed no evidence of expression of any of the NOS isoforms. In order to detect low amounts of protein we exploited the high affinity of these enzymes for ADP which is conferred by the NADPH-cytochrome P450 reductase-like domain common to all NOS isoforms (Knowles & Moncada, 1994). This was achieved by extracting ADP-binding proteins from homogenates and cell lysates with ADP-sepharose beads (Thomsen et al. 1994). The composition of the homogenising/lysis buffer (buffer A) was as follows: 0.2 %[(3-cholamidopropyl)dimethylammonio]-1-propane sulphonate (CHAPS), 50 mm Hepes (pH 7.5), 2 mm EDTA, 1 mm dithiothreitol (DTT), 1 mm phenylmethylsulphonyl fluoride (PMSF), 1 μg ml−1 pepstatin, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, 5 μg ml−1 chymostatin, 100 μg ml−1 antipain and 100 μg ml−1 soybean trypsin inhibitor. Snap-frozen myometrial tissue samples (1 g wet weight) were homogenised with 2 × 20 s bursts of an Ultra-Turrax T50 homogeniser set at 12 000 r.p.m. at 4°C in 5 ml buffer A and centrifuged (15 000 g, 30 min, 4°C). Cultured myometrial cells were lysed in buffer A by three cycles of freezing and thawing (10 min dry ice-hand warming), followed by centrifugation (13 000 g, 5 min, 4°C). Supernatants of homogenates and cell lysates were then adsorbed onto ADP-sepharose beads (45 min, 4°C), washed three times with buffer A containing 0.5 M NaCl, followed by three washes in buffer A, and eluted with electrophoresis sample buffer (62.5 mm Tris HCl pH 6.8, 2.3 % w/v SDS, 5 % v/v glycerol, 5 % v/v 2-mercaptoethanol and 0.02 % w/v Bromophenol Blue).
Immunoblotting
ADP eluates were heated at 95°C for 3 min, and centrifuged (9 000 g for 2 min). ADP-sepharose-extracted proteins equivalent to 400 mg wet weight tissue or 3 × 106 cultured cells were separated by SDS-PAGE on 7.5 % gels (Laemmli, 1970). Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore), blocked overnight at 4°C in blocking solution (3 % w/v BSA in PBS and 0.1 % v/v Tween 20 (PBS-T)), and incubated with agitation at room temperature for 1 h with polyclonal anti-NOS antibodies (each diluted 1:1000 in blocking solution). Secondary antibody was a horseradish peroxidase (HRP) conjugated goat anti-rabbit antibody (diluted 1:10 000), and antibody-bound protein was visualised with ECL+plus. Monoclonal antibodies raised against eNOS and iNOS were used between 1:750 and 1:1000 dilution in blocking solution, with an HRP-conjugated rabbit anti-mouse antibody at 1:10 000 as the secondary antibody. Positive controls for immunoblotting were human umbilical vein endothelial cell (HUVEC) lysates (not shown) and ADP-sepharose-extracted placental tissue (eNOS), lysates of cytokine-stimulated cells of the human epithelial carcinoma cell line A549 (iNOS), and recombinant human nNOS.
Determination of nitrite
Nitrite accumulation in the conditioned medium of cultured cells was determined colorimetrically by a diazotisation reaction using the standard Griess reagent (Green et al. 1982), as described previously (Bogle et al. 1992; Baydoun et al. 1993). Cell cultures from six uteri obtained at term in the absence of labour were used in these experiments, and determinations using cell cultures from individual uteri were performed in quadruplicate.
Immunohistochemistry
Serial cryostat sections (5 μm) were cut and immunohistochemistry performed using the avidin-biotin-peroxidase method. Non-specific binding was prevented by incubation with 10 % normal serum of the same species as the biotin-conjugated secondary antibody. Sections were then incubated for 1 h at room temperature with primary antibodies diluted at either 1:100 (monoclonal) or 1:200 (polyclonal) in PBS containing 5 % FCS. Negative controls were run in parallel, with a non-immune rabbit immunoglobulin fraction for the polyclonals, and purified mouse IgG1 and IgG2a for the monoclonal anti-eNOS and anti-iNOS antibodies, respectively. After washing in PBS, sections were incubated successively for 30 min each with biotinylated secondary antibody (rabbit anti-mouse 1:500, or swine anti-rabbit 1:400) and freshly prepared avidin-biotin-peroxidase complex. Peroxidase activity was developed with diaminobenzidine.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from snap-frozen myometrial tissue and cultured cells (Chirgwin et al. 1979), and samples (1 μg) were denatured (70°C, 5 min) and cooled to 37°C. Reverse transcription (RT) was carried out at 37°C for 1 h in a final volume of 20 μl, with 0.2 μg random hexanucleotide primers, 1 × RT buffer, 10 mm DTT, 1 mm of each dNTP, 40 units M-MLV reverse transcriptase, and 1 unit RNAse inhibitor. The reaction was terminated by heating at 90°C for 5 min. Polymerase chain reaction (PCR) amplification was performed with specifically designed PCR primers for eNOS, iNOS and nNOS. PCR amplification for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was carried out on the same samples as a parallel control. The primer sets used were as follows: eNOS, sense 5′-GCACAGGAAATGTTCACCTAC-3′; antisense 5′-CACGATGGT-GACTTTGGCTAG-3′ (Nadaud et al. 1994): iNOS, sense 5′-GAG-CTTCTACCTCAAGCTATC-3′; antisense 5′-CCTGATGTTGCCA-TTGTTGGT-3′ (Chartrain et al. 1994): nNOS, sense 5′-TGTGTG-GGCAGGATCCAGTG-3′; antisense 5′-GGGACAGGCGCTGAAC-TCCA-3′ (Hall et al. 1994): GAPDH, sense 5′-CCACCCATGGCAA-ATTCCATGGCA-3′; antisense 5′-TCTAGACGGCAGGTCAGGT-CCACC-3′ (Tokunaga et al. 1987).
A 1/40 volume of the RT reaction was used for PCR with 1.5 mm magnesium chloride, 0.2 mm dNTPs, 125 ng of each primer and 1 unit of Biotaq polymerase in a final volume of 25 μl. Reaction cycles were two denaturation steps (94°C, 4 min, 94°C, 30 s), followed by annealing for 30 s at 55°C (eNOS), 62°C (iNOS), 68°C (nNOS) or 58°C (GAPDH), and extension at 72°C for 30 s. Initially 28–40 cycles were carried out, at 2 cycle intervals, in order to determine the cycle profile of linearity for each primer set. The number of cycles used for experiments were as indicated (see Results). Amplification was terminated with a further 5 min extension at 72°C. Aliquots (10 μl) of the PCR product were separated by horizontal gel electrophoresis on a 1 % agarose gel. Identity was confirmed by sequence-verification of the amplified products (Sambrook et al. 1989).
Statistics
All values of nitrite levels are given as means ±s.e.m. of measurements in six cultures. Statistical analyses were performed using Student's unpaired t test.
RESULTS
Detection of NOS isoforms by immunoblotting
Initial immunoblots carried out using whole-tissue homogenates failed to show staining for any of the NOS isoforms (data not shown). Partial enrichment of NOS proteins was achieved by extracting ADP-binding proteins from tissue homogenates and cell lysates with ADP- sepharose beads. With this procedure, all three NOS polyclonal antibodies used in this study (Table 1) were confirmed to be specific for their respective isoforms. Immunoblot analysis of known sources (positive controls) of human eNOS (human umbilical vein endothelial cells and human placental tissue), iNOS (cytokine/LPS-stimulated A549 cells), and of recombinant human nNOS protein showed single protein bands of the correct molecular masses, with no cross-reaction with other NOS isoforms (Fig. 1). When applied to the same samples, the anti-iNOS and anti-eNOS monoclonal antibodies (Table 1) did not stain bands at the appropriate molecular masses in any samples, or in positive controls. They did, however, stain bands at approximately 47 kDa in extracts of myometrial tissue and in all positive controls (Fig. 1D and E).
Table 1.
NOS primary antibodies
| Dilution | |||||
|---|---|---|---|---|---|
| Antibody | Immunogen | Immunoblot | Immuno-histochemistry | Presentation | Source |
| eNOS polyclonal | a. a. 1183–1202 of human eNOS | 1/1000 | 1/200 | Affinity purified | Santa Cruz, sc654 |
| nNOS polyclonal | a. a. 1400–1419 of rat nNOS | 1/1000 | 1/200 | Affinity purified | Santa Cruz, sc648 |
| iNOS polyclonal | a. a. 1135–1153 of human iNOS | 1/1000 | 1/200 | Affinity purified | Santa Cruz, sc649 |
| eNOS monoclonal | a. a. 1030–1209 of human eNOS | 1/750–1000 | 1/100 | Purified IgG1 | Transduction, N30020 |
| iNOS monoclonal | a. a. 961–1144 of human iNOS | 1/750–1000 | 1/100 | Purified IgG2a | Transduction, N32020 |
a. a., amino acid.
Figure 1. Immunodetection of NOS isoforms in ADP-sepharose extracts of human pregnant myometrial tissue (MYO) and human myometrial smooth muscle cells (HMSMCs) cultured from term non-labouring myometrium.
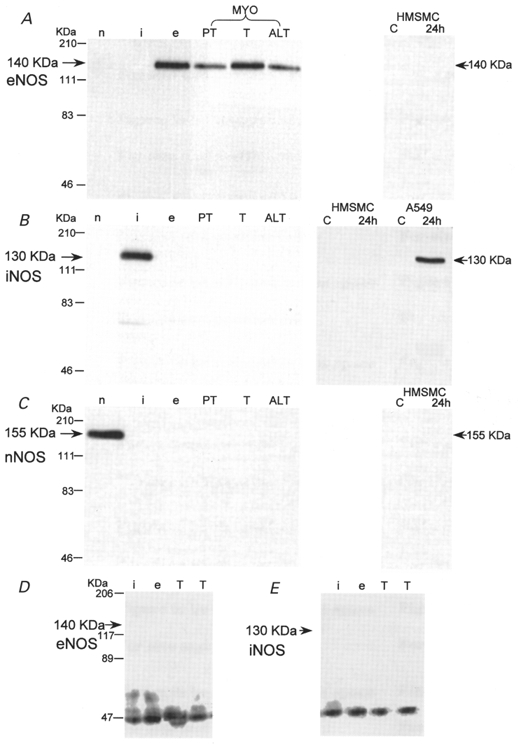
Representative immunoblots for eNOS (A), iNOS (B) and nNOS (C) with rabbit polyclonal antibodies. D and E are representative blots for eNOS and iNOS obtained with mouse monoclonal antibodies. See Methods for details. Lanes n, i and e: positive controls for nNOS, iNOS and eNOS, respectively. MYO (ADP-sepharose extract of ≈400 mg wet weight per lane): PT, preterm non-labour; T, term non-labour; ALT, active labour at term. HMSMC or, for comparison, A549 epithelial cells (ADP-sepharose extract of ≈3 × 106 cells per lane): C, control unstimulated cells; 24h, cells stimulated for 24 h with 100 μg ml−1 LPS and 10 ng ml−1 each of IL-1β, IFN-γ and TNF-α.
The myometrial tissue samples in Fig. 1A–C show representative examples of immunoblots obtained. ADP- sepharose extracts of approximately 400 mg myometrial tissue showed staining of an eNOS protein band (140 kDa; Fig. 1A), present in myometrial tissue from preterm (PT), term non-labour (T) and active labour at term (ALT). No protein bands for iNOS or nNOS were detected at any stage of pregnancy in any of the samples analysed (Fig. 1B and C).
None of the NOS isoforms were detected in ADP-sepharose extracts of non-stimulated cultured myometrial smooth muscle cells (Fig. 1A–C). There was also no evidence of iNOS protein after stimulation of HMSMCs for 24 h (Fig. 1B) and 48 h (data not shown) with a cytokine mix of 100 μg ml−1 LPS and 10 ng ml−1 each of IL-1β, IFN-γ and TNF-α. A549 epithelial cells, cultured under the same conditions and stimulated concurrently, showed detectable protein expression after stimulation for 24 h, which was accompanied by an accumulation of nitrite (an index of NO synthesis) in the culture medium of these cells (Fig. 2), as described previously by Watkins et al. (1997). No accumulation of nitrite was detected in six HMSMC cultures tested. Similar results were obtained from HMSMC cultures that were established without supplementation with hydrocortisone and EGF (data not shown).
Figure 2. Effect of lipopolysaccharide and cytokines on nitrite accumulation in HMSMCs and A549 epithelial cells.
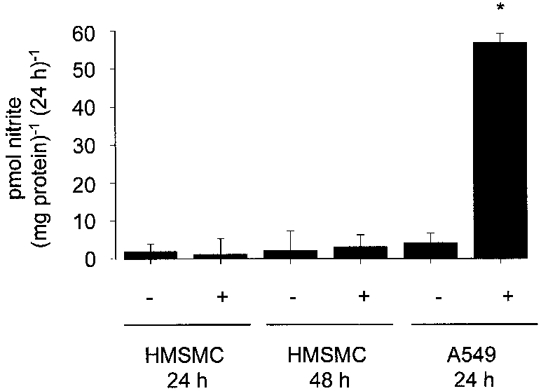
HMSMCs and A549 cells were stimulated with 100 μg ml−1 LPS and 10 ng ml−1 each of IL-1β, IFN-γ and TNF-α. Nitrite accumulation in the culture medium was determined over 24 and 48 h (HMSMCs) and 24 h (A549). +, stimulated cells; -, control unstimulated cells. Determinations on cell cultures from individual uteri were performed in quadruplicate, and values are the means ±s.e.m. of cell cultures from 6 uteri obtained at term in the absence of labour. * P < 0.001 compared with unstimulated HMSMCs and A549 cells.
Localisation of NOS isoforms within human myometrium
Immunostaining of myometrial tissue sections with the polyclonal anti-eNOS antibody (Santa Cruz Biotechnology) revealed immunoreactivity localised to the endothelial cells of the small vessels within the myometrium, but not to myocytes (Fig. 3, top row). The same staining pattern was seen in preterm, term non-labour and term active labour samples. Immunoreactivity for iNOS and nNOS with the Santa Cruz polyclonal antibodies (Fig. 3, rows 2 and 3) was not above background levels obtained with non-immune serum in any of the sections (Fig. 3, row 4).
Figure 3. Immunohistochemical staining of sections of human pregnant myometrial tissue with polyclonal anti-NOS antibodies.
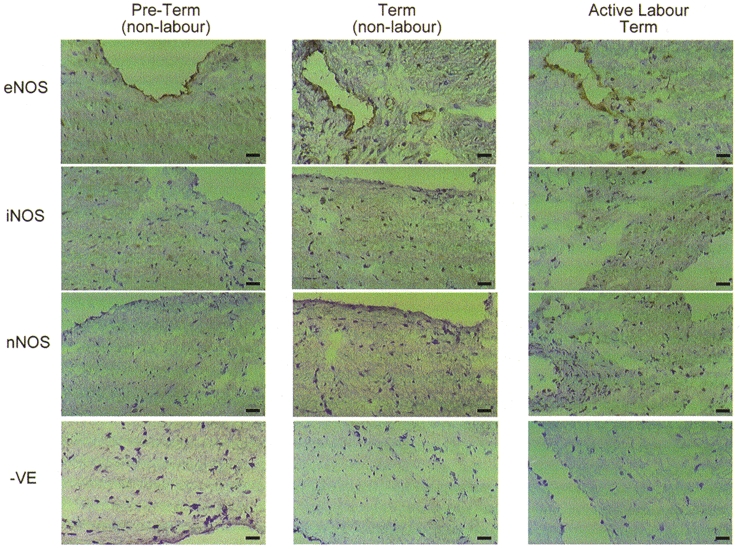
Representative sections are shown from preterm and term non-labouring myometrium and from tissue of women in active labour at term. Cryostat sections 5 μm thick were incubated with polyclonal antibodies raised against eNOS, iNOS and nNOS. Negative controls were incubated with non-immune serum. Staining was by avidin-biotin-peroxidase.
We also examined the immunoreactivity of the two monoclonal antibodies (Transduction Laboratories) used in previous studies (Thomson et al. 1997; Norman et al. 1999) against myometrial tissue sections. In contrast to the staining pattern seen with the polyclonal antibodies, the monoclonal anti-eNOS and iNOS antibodies (both of which failed to detect NOS proteins in immunoblots of positive controls) produced widespread immunoreactivity throughout sections of term non-labouring tissue (Fig. 4).
Figure 4. Immunohistochemical staining of sections of human pregnant myometrial tissue with monoclonal anti-NOS antibodies.
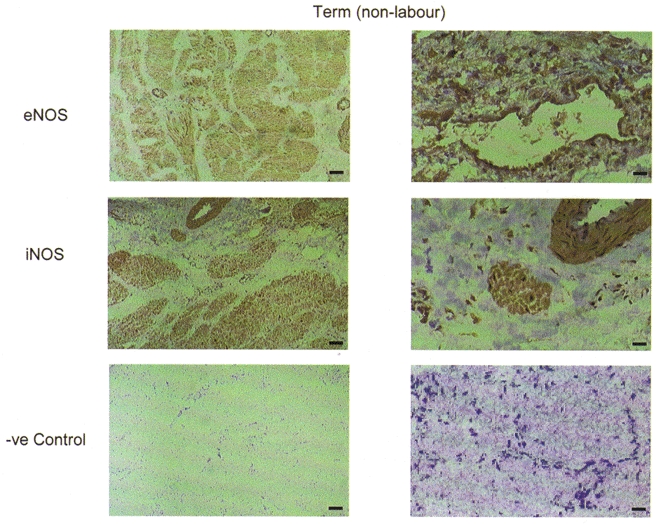
Representative sections are shown of term non-labouring tissue. Cryostat sections 5 μm thick were incubated with monoclonal antibodies raised against eNOS and iNOS. Negative controls were incubated with non-immune mouse IgG. Staining was by avidin-biotin-peroxidase.
Reverse transcription-polymerase chain reaction analysis of human myometrial NOS isoform expression
In a previous study, we demonstrated the expression of NOS mRNA in myometrial tissue throughout gestation (Dennes et al. 1999). In the present study we re-examined NOS mRNA expression by RT-PCR and confirmed that all three NOS isoforms were detected in myometrial tissue from preterm, term non-labour and term active labour (Fig. 5A). Detection of each isoform was achieved within the linear range of amplification cycles. However, iNOS and nNOS were only detectable after 40 cycles, suggesting a low copy number. In HMSMCs, eNOS, nNOS and iNOS mRNAs were not detected (Fig. 5B).
Figure 5. RT-PCR for NOS isoforms from human pregnant myometrial tissue (MYO) (A) and HMSMCs (B).
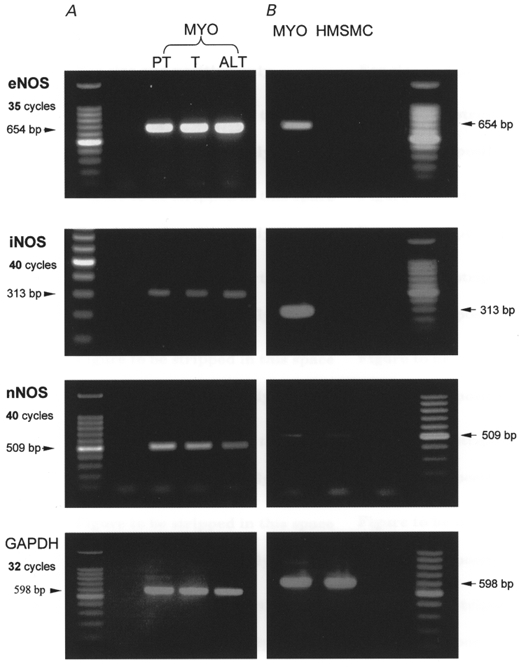
Sequences of eNOS, iNOS and nNOS were amplified from cDNAs derived from preterm non-labour (PT), term non-labour (T) and active labour at term (ALT) myometrial tissue, and from HMSMCs, at the cycle numbers indicated. In B the left-hand lane (MYO) represents pooled cDNAs from myometrial tissue as a positive control. Amplification of a sequence from the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was carried out as a parallel control.
DISCUSSION
This study has examined in detail the expression, localisation and regulation of all three NOS isoforms in human pregnant myometrium. Previous reports that NO release from myometrial cells suppresses myometrial contraction during pregnancy have suggested expression of NOS in amounts that would easily be detectable by the techniques employed here. We have demonstrated with antibodies of proven specificity that eNOS, iNOS and nNOS proteins are not expressed at detectable levels in myocytes of human myometrium at any stage of pregnancy.
To date, investigation into NOS activity and expression in human pregnant myometrium has been inconclusive. Metabolism of radiolabelled L-arginine to L-citrulline has been observed in homogenates of human myometrium (Ramsay et al. 1996; Thomson et al. 1997). This may indicate the presence of NOS enzymes but the contribution of NOS from vascular tissue could not be excluded in these studies. Firm evidence requires the observation of increased contractility of myometrial muscle strips in response to inhibitors of NOS activity and the detection of NOS enzymes in myometrial tissue. Relaxation of murine and human myometrial strips in response to high concentrations of L-arginine has been observed (Izumi et al. 1993). However, at physiological plasma levels, L-arginine is not rate limiting for eNOS or iNOS activity, and it is possible that changes in tone were due to the cationic charge of L-arginine. Subsequent studies have not confirmed L-arginine-induced relaxation of preterm or term non-labouring human myometrial strips and, most significantly, neither have they observed increased contraction upon treatment with inhibitors of NOS activity, as would be predicted if the tissue was expressing functionally active NOS enzymes (Morrison et al. 1996; Jones & Poston, 1997). Claims of NO production, detected with an NO electrode, by human cultured pregnant myometrial smooth muscle cells stimulated with LPS, IL-1α and IL-1β, suggest that iNOS can be induced in this cell type (Nakaya et al. 1996; Yamamoto et al. 1997). However, the presence of NOS protein or mRNA, and inhibition of NOS activity by NOS inhibitors was not confirmed in those studies.
Two groups have examined the expression of NOS proteins in human myometrium during pregnancy. Thomson et al. (1997) reported immunoreactivity for all three isoforms, with no detected changes associated with labour, but the staining presented was not consistent between different anti-iNOS antibodies used, and included staining with the same monoclonal antibody which we have shown in the present study to be non-specific. In a subsequent study (Norman et al. 1999), these authors reported increased expression of eNOS and nNOS protein by immunoblotting and immunohistochemistry in human preterm myometrium in comparison with term and non-pregnant tissue. The expression of iNOS was not reported. Bansal et al. (1997) presented immunohistochemical and immunoblotting evidence suggesting expression of iNOS in preterm, but not term, non-labouring myometrium. Taken together, these observations do not support a role for a decline in NO generation promoting parturition, but favour a model in which NO, generated by NOS isoforms within the uterine smooth muscle, promote uterine quiescence in the early stages of pregnancy. Nevertheless, such a conclusion is not consistent with functional studies (Morrison et al. 1996; Jones & Poston, 1997), which found no effect of NOS inhibitors on contractility of myometrial muscle strips at any stage of pregnancy. In view of the proposal for the use of NO donors in clinical practice, it is important that these apparent paradoxes are clarified.
We sought first to demonstrate the presence of NOS isoforms by immunoblotting. NOS enzymes are susceptible to proteolytic cleavage during extraction for electrophoresis (Lowe et al. 1996). This can reduce or eliminate the signal at the expected molecular mass on an immunoblot and also result in staining of lower molecular mass fragments, which would be difficult to distinguish from non-specific bands. Great care was therefore taken to ensure the preservation of full length NOS polypeptides during extraction and ADP- sepharose purification, and we were able unambiguously to detect all three NOS isoforms in positive controls with the polyclonal antibodies (Santa Cruz) on immunoblots, in the absence of any non-specific bands. We also attempted immunoblots with two monoclonal antibodies directed against eNOS and iNOS, respectively (Transduction Laboratories; see Table 1). When used to analyse the same samples, neither of these antibodies stained bands at the appropriate NOS molecular masses in positive controls or ADP-sepharose-extracted tissue, but did detect bands at approximately 47 kDa (Fig. 1D and E). We therefore conclude that the widespread staining seen with these antibodies on the myometrial sections (Fig. 4) is unreliable and likely to be non-specific.
The data on eNOS expression obtained with the polyclonal antibody, in immunoblots and tissue sections, suggested the presence of this isoform in the vascular endothelium (Figs 1 and 3). This was supported by the failure to detect eNOS mRNA or protein in extracts of cultured myometrial smooth muscle cells. Quantification of the eNOS signal between samples was not attempted, as variable sampling of vascular tissue would influence the levels of the protein detected. However, no gestationally related trends in protein expression were obvious. Based on these findings, we conclude that eNOS is unlikely to play any role in the regulation of myometrial tone during pregnancy. It could, however, be argued that diffusion of NO generated within the endothelium of the myometrial vasculature might mediate uterine relaxation, although the low levels of eNOS detected (after ADP-sepharose purification) argue against this. Moreover, if this were the case, NOS inhibitors should increase myometrial tone and contraction, which has not been found (Morrison et al. 1996; Jones & Poston, 1997).
We did not detect iNOS protein expression in tissues from any gestational stage, which is at odds with the previously reported detection, using the same antibody, of iNOS in preterm samples in the absence of labour (Bansal et al. 1997). In the authors’ published blot the putative iNOS band at 130 kDa is indicated for the positive control cell lysate. However, the authors make no comment about the very much stronger staining of bands at lower molecular weights in all samples (including the positive control). It is thus difficult to reconcile the putative iNOS band in the preterm sample (which is at a different molecular weight from the band indicated in the positive control) with iNOS expression. It is also likely that the prolonged boiling in the homogenisation procedure that was used would have resulted in hydrolytic cleavage of any NOS proteins present. We therefore suggest that our study and that of Bansal et al. (1997) both fail to demonstrate the expression of iNOS in human myometrium throughout pregnancy.
Immunohistochemistry using the polyclonal antibody for iNOS confirmed the lack of iNOS expression in myometrium at all gestational stages. We were unable to detect iNOS staining in preterm sections as reported by Bansal et al. (1997), although this may reflect differences in the fixation procedure. RT-PCR did detect transcripts of iNOS after 40 cycles of amplification, which implies expression of iNOS mRNA at low copy number. A more extensive study of iNOS mRNA expression in human myometrial tissue has been presented elsewhere and confirms the absence of gestationally related changes as described here (Dennes et al. 1999). Expression of iNOS in inflammatory cell types within uterine tissues, including myometrium, has been reported during human pregnancy (Bansal et al. 1997; Eis et al. 1997; Myatt et al. 1997), and the PCR signal we identified is likely to have arisen from leukocytes and macrophages within the tissue. This is supported by the lack of expression of iNOS mRNA in cultured human myometrial smooth muscle cells. We also failed to detect the expression of nNOS protein in tissue homogenates and sections. The apparently low copy number of nNOS mRNA we detected may represent expression within the myocytes or in nerve cells supplying the myometrium.
Our data from the cultured cells were consistent with that from whole tissue. It is intriguing that near-micromolar concentrations of NO have apparently been detected after 24 h in the supernatant of cultured pregnant myometrial cells stimulated by cytokines (Nakaya et al. 1996; Yamamoto et al. 1997). Although this is within the concentration range detectable by the Griess reaction employed in the current study, we could not detect nitrites released from cytokine-stimulated myometrial myocytes, nor could we detect iNOS protein or mRNA in these cells. We have also measured cGMP within HMSMCs, as a more sensitive index of NO production, and found no accumulation in response to cytokine stimulation (data not shown). Other cell types, derived from tissues capable of expressing iNOS, can maintain this capacity in culture (Nussler et al. 1995; Saura et al. 1996; Watkins et al. 1997). If this capacity were reproduced in HMSMCs, a positive finding would provide supporting evidence for a role of iNOS in the function of this tissue. The lack of response we found may be due to a loss of responsiveness under the culture conditions. However, A549 cells exposed to the same conditions express iNOS, and HMSMCs stimulated with the same cytokine mix or with IL-1β alone show a dramatic induction of COX-2 expression and activity (Bartlett et al. 1998, 1999). When taken together with the observations in fresh tissue, we propose that the lack of iNOS expression in cultured cells indicates that this cell type does not have the potential to generate NO in significant quantities via iNOS.
A potential limitation of our study is that tissue samples were obtained from the lower uterine segment which, it is suggested, may be more cervical in character, and less contractile than the fundus (Fuchs et al. 1984; Smith et al. 1998). However, all other published studies that have suggested that NOS is functionally expressed in the human myometrium in pregnancy have used tissue from the same anatomical region (Izumi et al. 1993; Bansal et al. 1997; Thomson et al. 1997; Norman et al. 1999). Moreover, no differences are seen in the sensitivity of human pregnant myometrium from the fundus versus lower segment to oxytocin or potassium chloride (Word et al. 1993), suggesting that at the cellular level, there may be little difference between these two regions.
Low sensitivities of rat and primate myometrium to relaxation by cGMP analogues and NO donors have recently been reported (Hennan & Diamond, 1998; Kuenzli et al. 1998; Word & Cornwell, 1998), and cGMP-dependent protein kinase expression is reduced in the myometrium from pregnant rats (Word & Cornwell, 1998). Similar observations in human pregnant myometrium support these findings (Jones & Poston, 1996; Norman et al. 1997) and are in keeping with the data we present here, suggesting that the human myometrium during pregnancy is not a source of NO production. Published evidence on the expression of NOS isoforms in human myometrium implicating endogenous NO in the regulation of tone early in pregnancy contradicts the data from functional studies. Under the rigorous experimental conditions described in this study, we have been unable to detect protein expression of NOS isoforms in human myometrial smooth muscle during pregnancy. The mRNA we detected for all three NOS isoforms may reflect expression by smooth muscle and/or contributions from other cell types. The presence of mRNA does not confirm translation into protein, but there may be translation into functional NOS proteins at levels below the sensitivity of immunoblotting and immunohistochemical techniques. Such a situation exists in human and rabbit gastrointestinal smooth muscle cells, where eNOS has been identified functionally, and expression of its mRNA detected by RT-PCR and Northern blot in the absence of detectable eNOS protein (Murthy & Makhlouf, 1994; Teng et al. 1998). Similarly, expression of nNOS has also been reported in gastrointestinal smooth muscle cells (Chadker et al. 1998). The levels of NO required to relax smooth muscle are not known, so it is not certain whether such low expression in myometrium would be sufficient to regulate tone. Therefore a role for endogenous NO production by the myometrium cannot be ruled out completely. However, human myometrial contractility is insensitive to L-NAME (Morrison et al. 1996; Jones & Poston, 1997), demonstrating an absence of NOS function, consistent with the lack of detectable NOS protein in the present study. Furthermore, no gestationally related changes in NOS mRNA expression were apparent. We therefore conclude that the weight of experimental evidence indicates that NO produced by the myometrium plays an insignificant role in the regulation of uterine smooth muscle tone during human pregnancy.
Acknowledgments
This research has been conducted by the London Myometrial Group. We gratefully acknowledge the financial support of the Tommy's Campaign, London, UK. We thank the Trustees of St Thomas’ Hospital, London, UK, Dr Valentina Riveros Moreno for advice and assistance with NOS protein purification, and the staff and patients of the Delivery Suites of Guy's and St Thomas’ Hospitals.
References
- Bansal RK, Goldsmith PC, He YP, Zaloudek CJ, Ecker JL, Riemer RK. Decline in myometrial nitric oxide synthase expression is associated with labor and delivery. Journal of Clinical Investigation. 1997;99:2502–2508. doi: 10.1172/JCI119434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett S, Sawdy R, Dennes W, Bennett P, Mann GE. Cytokine-induced regulation of nitric oxide synthase and cyclooxygenase 2 in cultured human myometrial cells. Journal of the Society for Gynecologic Investigation. 1998;5:183A. abstract. [Google Scholar]
- Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1: involvement of p38 mitogen-activated protein kinase. The Journal of Physiology. 1999;520:399–406. doi: 10.1111/j.1469-7793.1999.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun AR, Bogle RG, Pearson JD, Mann GE. Selective inhibition by dexamethasone of induction of NO synthase, but not of induction of L-arginine transport, in murine macrophage J774 cells. British Journal of Pharmacology. 1993;110:1401–1406. doi: 10.1111/j.1476-5381.1993.tb13976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle RG, Baydoun AR, Pearson JD, Moncada S, Mann GE. L-Arginine transport is increased in macrophages generating nitric oxide. Biochemical Journal. 1992;284:15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa JS, Jones GD, Poston R, Poston L. Nitric oxide synthase (eNOS) is localized to the vascular endothelium in term (non-labouring) myometrium of pregnant women. Journal of the Society for Gynecologic Investigation. 1998;5:182A. abstract. [Google Scholar]
- Chakder S, Bandyopadhyay A, Rattan S. Neuronal NOS gene expression in gastrointestinal myenteric neurons and smooth muscle cells. American Journal of Physiology. 1997;273:C1868–1875. doi: 10.1152/ajpcell.1997.273.6.C1868. [DOI] [PubMed] [Google Scholar]
- Chartrain NA, Geller DA, Koty PP, Sitrin NF, Nussler AK, Hoffman EP, Billiar TR, Hutchinson NI, Mudgett JS. Molecular cloning, structure, and chromosomal localization of the human inducible nitric oxide synthase gene. Journal of Biological Chemistry. 1994;269:6765–6772. [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dennes WJ, Slater DM, Poston L, Bennett PR. Myometrial nitric oxide synthase messenger RNA expression does not change throughout gestation or with the onset of labor. American Journal of Obstetrics and Gynecology. 1999;180:387–392. doi: 10.1016/s0002-9378(99)70219-x. [DOI] [PubMed] [Google Scholar]
- Eis ALW, Brockman DE, Myatt L. Immunolocalization of the inducible nitric oxide synthase isoform in human fetal membranes. American Journal of Reproductive Immunology. 1997;38:289–294. doi: 10.1111/j.1600-0897.1997.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. American Journal of Obstetrics and Gynecology. 1984;150:734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Gangula PRR, Dong YL, Yallampalli C. Rat myometrial smooth muscle cells express endothelial nitric oxide synthase. Human Reproduction. 1997;12:561–568. doi: 10.1093/humrep/12.3.561. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Analytical Biochemistry. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. Structural organisation of the human neuronal nitric oxide synthase gene (NOS 1) Journal of Biological Chemistry. 1994;269:33082–33090. [PubMed] [Google Scholar]
- Hennan JK, Diamond J. Evidence that spontaneous contractile activity in the rat myometrium is not inhibited by NO-mediated increases in tissue levels of cyclic GMP. British Journal of Pharmacology. 1998;123:959–967. doi: 10.1038/sj.bjp.0701678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Yallampalli C, Garfield RE. Gestational changes in L-arginine-induced relaxation of pregnant rat and human myometrial smooth muscle. American Journal of Obstetrics and Gynecology. 1993;169:1327–1337. doi: 10.1016/0002-9378(93)90301-x. [DOI] [PubMed] [Google Scholar]
- Jones G, Poston L. The influence of modulators of nitric oxide synthesis on spontaneous human myometrial contractility in vitro. The Journal of Physiology. 1996;491.P:167–168P. [Google Scholar]
- Jones GD, Poston L. The role of endogenous nitric oxide synthesis in contractility of term or preterm human myometrium. British Journal of Obstetrics and Gynaecology. 1997;104:241–245. doi: 10.1111/j.1471-0528.1997.tb11053.x. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric-oxide synthases in mammals. Biochemical Journal. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzli KA, Buxton ILO, Bradley ME. Nitric oxide regulation of monkey myometrial contractility. British Journal of Pharmacology. 1998;124:63–68. doi: 10.1038/sj.bjp.0701799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli EK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees C, Campbell S, Jauniaux E, Brown R, Ramsay B, Gibb D, Moncada S, Martin JF. Arrest of preterm labour and prolongation of gestation with glyceryl trinitrate, a nitric oxide donor. Lancet. 1994;343:1325–1326. doi: 10.1016/s0140-6736(94)92468-6. [DOI] [PubMed] [Google Scholar]
- Lowe PN, Smith D, Stammers DK, Riverosmoreno V, Moncada S, Charles I, Boyhan A. Identification of the domains of neuronal nitric-oxide synthase by limited proteolysis. Biochemical Journal. 1996;314:55–62. doi: 10.1042/bj3140055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric-oxide - physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- Morrison JJ, Perera D, Marshall I, Rodeck CH. Effects of GSNO, SNAP, SIN-1, GTN, L-ARG, L-NOARG, and l-NAME on contractility of isolated human pregnant myometrium. Journal of the Society for Gynecologic Investigation. 1996;3:333A. abstract. [Google Scholar]
- Murthy KS, Makhlouf GM. VIP/PACAP-mediated activation of membrane-bound NO synthase in smooth muscle is mediated by pertussis toxin-sensitive Gi1–2. Journal of Biological Chemistry. 1994;269:15977–15980. [PubMed] [Google Scholar]
- Myatt L, Eis ALW, Brockman DE, Kossenjans W, Greer I, Lyall F. Inducible (type ii) nitric oxide synthase in human placental villous tissue of normotensive, pre-eclamptic and intrauterine growth-restricted pregnancies. Placenta. 1997;18:261–268. doi: 10.1016/s0143-4004(97)80060-4. [DOI] [PubMed] [Google Scholar]
- Nadaud S, Bonnardeaux A, Lathrop M, Soubrier F. Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochemical and Biophysical Research Communications. 1994;198:1027–1033. doi: 10.1006/bbrc.1994.1146. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Yamamoto S, Hamada Y, Kamada M, Aono T, Niwa M. Inducible nitric-oxide synthase in uterine smooth-muscle. Life Sciences. 1996;58:PL249–255. doi: 10.1016/0024-3205(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Regulation of biosynthesis of nitric-oxide. Journal of Biological Chemistry. 1994;269:13725–13728. [PubMed] [Google Scholar]
- Natuzzi ES, Ursell PC, Harrison M, Buscher C, Riemer RK. Nitric-oxide synthase activity in the pregnant uterus decreases at parturition. Biochemical and Biophysical Research Communications. 1993;194:1–8. doi: 10.1006/bbrc.1993.1776. [DOI] [PubMed] [Google Scholar]
- Norman JE, Thomson AJ, Telfer JF, Young A, Greer IA, Cameron IT. Myometrial constitutive nitric oxide synthase expression is increased during human pregnancy. Molecular Human Reproduction. 1999;5:175–181. doi: 10.1093/molehr/5.2.175. [DOI] [PubMed] [Google Scholar]
- Norman JE, Ward LM, Martin W, Cameron AD, Mcgrath JC, Greer IA, Cameron IT. Effects of cGMP and the nitric oxide donors glyceryl trinitrate and sodium nitroprusside on contractions in vitro of isolated myometrial tissue from pregnant women. Journal of Reproduction and Fertility. 1997;110:249–254. doi: 10.1530/jrf.0.1100249. [DOI] [PubMed] [Google Scholar]
- Nussler AK, Disilvio M, Liu ZZ, Geller DA, Freeswick P, Dorko K, Bartoli F, Billiar TR. Further characterization and comparison of inducible nitric-oxide synthase in mouse, rat, and human hepatocytes. Hepatology. 1995;21:1552–1560. [PubMed] [Google Scholar]
- Palmer RMJ, Ferrige AG, Moncada S. Nitric-oxide release accounts for the biological-activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ramsay B, Sooranna SR, Johnson MR. Nitric-oxide synthase activities in human myometrium and villous trophoblast throughout pregnancy. Obstetrics and Gynecology. 1996;87:249–253. doi: 10.1016/0029-7844(95)00391-6. [DOI] [PubMed] [Google Scholar]
- Riemer RK, Buscher C, Bansal RK, Black SM, He YP, Natuzzi ES. Increased expression of nitric oxide synthase in the myometrium of the pregnant rat uterus. American Journal of Physiology. 1997;272:E1008–1015. doi: 10.1152/ajpendo.1997.272.6.E1008. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Plainview, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Saura M, Perezsala D, Canada FJ, Lamas S. Role of tetrahydrobiopterin availability in the regulation of nitric-oxide synthase expression in human mesangial cells. Journal of Biological Chemistry. 1996;271:14290–14295. doi: 10.1074/jbc.271.24.14290. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Regenstein AC, Lykins D, Roberts JM. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. American Journal of Obstetrics and Gynecology. 1993;169:1285–1291. doi: 10.1016/0002-9378(93)90295-t. [DOI] [PubMed] [Google Scholar]
- Smith GCS, Baguma-Nibasheka M, Wu WX, Nathanielsz PW. The reduced contraction of baboon lower uterine segment (LUS) to prostaglandin (PG) E2 is paralleled by increased EP2 and decreased EP3 receptor mRNA compared with the fundus. Journal of the Society for Gynecologic Investigation. 1998;5:64A. abstract. [Google Scholar]
- Teng B-Q, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. American Journal of Physiology. 1998;275:G342–351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Research. 1994;54:1352–1354. [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA, Norman JE. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Human Reproduction. 1997;12:2546–2552. doi: 10.1093/humrep/12.11.2546. [DOI] [PubMed] [Google Scholar]
- Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Research. 1987;47:5616–5619. [PubMed] [Google Scholar]
- Watkins DN, Garlepp MJ, Thompson PJ. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (A549) cells by nitric oxide. British Journal of Pharmacology. 1997;121:1482–1488. doi: 10.1038/sj.bjp.0701283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric-oxide synthases by sex-hormones. Proceedings of the National Academy of Sciences of the USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word RA, Cornwell TL. Regulation of cGMP-induced relaxation and cGMP-dependent protein kinase in rat myometrium during pregnancy. American Journal of Physiology. 1998;274:C748–756. doi: 10.1152/ajpcell.1998.274.3.C748. [DOI] [PubMed] [Google Scholar]
- Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. Journal of Clinical Investigation. 1993;92:29–37. doi: 10.1172/JCI116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallampalli C, Izumi H, Byam-Smith M, Garfield RE. An L-arginine-nitric oxide-cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. American Journal of Obstetrics and Gynecology. 1994;170:175–185. doi: 10.1016/s0002-9378(94)70405-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kamada M, Maegawa M, Takikawa M, Nakaya Y, Niwa M, Aono T. IL-1 alpha induces inducible nitric oxide synthase in uterine smooth muscle. Biochemical and Biophysical Research Communications. 1997;238:12–16. doi: 10.1006/bbrc.1997.7228. [DOI] [PubMed] [Google Scholar]


