Abstract
Withdrawal anxiety is a significant factor contributing to continued alcohol abuse in alcoholics. This anxiety is long lasting, can manifest well after the overt physical symptoms of withdrawal, and is frequently associated with relapse in recovering alcoholics. The neurobiological mechanisms governing these withdrawal-associated increases in anxiety are currently unknown. The basolateral amygdala is a major emotional center in the brain and regulates the expression of both learned-fear and anxiety. Neurotransmitter system alterations within this brain region may therefore contribute to withdrawal-associated anxiety. Since evidence suggests that glutamate-gated neurotransmitter receptors are sensitive to acute ethanol exposure, we examined the effect of chronic intermittent ethanol (CIE) and withdrawal (WD) on glutamatergic synaptic transmission in the basolateral amygdala. We found that slices prepared from CIE and WD animals had significantly increased contributions by synaptic NMDA-receptors. In addition, CIE increased the amplitude of AMPA receptor-mediated spontaneous excitatory postsynaptic currents (sEPSC), while only WD altered the amplitude and kinetics of tetrodotoxin-resistant spontaneous events (mEPSC). Similarly, the frequency of sEPSCs was increased in both CIE and WD neurons; but, only WD increased the frequency of mEPSCs. These data suggest that CIE and WD differentially alter both pre- and post-synaptic properties of BLA glutamatergic synapses. Finally, we show that microinjection of the AMPA receptor antagonist, DNQX, can attenuate withdrawal-related anxiety-like behavior. Together, our results suggest that increased glutamatergic function may contribute to anxiety expressed during withdrawal from chronic ethanol.
Introduction
The amygdala serves as the center for regulation of specific aspects of fear and anxiety behaviors. Highly processed sensory and cognitive information flows into the amygdala from the cortex and thalamus. After additional processing, the amygdala then projects to regions regulating risk assessment (e.g., medial prefrontal cortex, bed nucleus of the stria terminalis) and reward (e.g. nucleus accumbens). More recently, it has been demonstrated that various amygdala subdivisions also modulate drug-seeking behaviors in rodents (Alderson et al. 2000; Di Ciano and Everitt 2004; Fuchs and See 2002; McLaughlin and See 2003), drug cravings in humans (Childress et al. 1999), and the regulation of anxiety-like behaviors during withdrawal from chronic drug exposure (Menzaghi et al. 1994; Rodriguez de Fonseca et al. 1997; Watanabe et al. 2002).
In this context, the lateral and basolateral subdivisions within the amygdala are essential for the development of fear and anxiety-like behaviors. The basolateral amygdala (BLA) is the primary input region for cortical and thalamic afferents. Alterations of neurotransmitter systems within this amygdala region would therefore significantly impact the flow of information both within the amygdala as well as along BLA efferents, including the central amygdala. This point is emphasized by the ability to block fear learning (Walker and Davis 1997a), and the behavioral response to naturally anxiogenic unconditioned stimuli via injection of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist, CNQX, into the BLA (Sajdyk and Shekhar 1997; Walker and Davis 1997a). Additionally, ethanol self administration increases glucose utilization in the BLA (Porrino et al. 1998); and, modulation of BLA neuronal activity can modulate operant ethanol self-administration (Hyytia and Kiianmaa 2001). These data provide a clear rationale for examining the effects of chronic ethanol and withdrawal on BLA glutamatergic neurophysiology.
Given that classic fear-learning behaviors are associated with a N-methyl-D-aspartate (NMDA) receptor-dependent increase in glutamatergic synaptic transmission within the BLA (McKernan and Shinnick-Gallagher 1997), we hypothesize that chronic ethanol-related increases in BLA NMDA receptor function (Floyd et al. 2003) might mediate similar increases in glutamatergic synaptic transmission, ultimately driving increased anxiety during withdrawal. In rats, alcohol withdrawal leads to increased glutamate release in the hippocampus (Dahchour and De Witte 2003). While they are distinctly different regions, NMDA-dependent changes in synaptic plasticity within the BLA and hippocampus are thought to rely upon similar neurophysiological and cellular processes (Chapman et al. 2003). This suggests some parallels between these brain regions. Given that chronic ethanol facilitates NMDA receptor expression and function in isolated BLA neurons (Floyd et al. 2003), it is reasonable to suggest that molecular mechanisms associated with the long-term anxiogenic effects of alcohol exposure are related to alterations in these receptor systems. Indeed, chronic ethanol exposure increases glutamate neurotransmission in the neighboring central nucleus of the amygdala (Roberto et al. 2004b). However, less is known about the effects of chronic ethanol and withdrawal in the BLA, an amygdala subdivision that controls information flow throughout the entire amygdala.
In the present study, we have used a chronic intermittent ethanol inhalation model to examine the effects of chronic ethanol exposure and withdrawal on glutamatergic synaptic transmission in the BLA. Repeated withdrawal from ethanol has been shown to enhance seizure severity in response to convulsants (Pinel 1980). Repeated cycles of exposure/withdrawal also appear to more dramatically alter several different neurotransmitters (Jarvis and Becker 1998; Kang et al. 1998). More importantly, recent findings suggest that the kindling-like effect of repeated ethanol exposure/withdrawal can occlude synaptic plasticity in the amygdala (Stephens et al. 2005). Since plastic synaptic processes in the BLA are induced by NMDA-type glutamate receptors and expressed as increased function of glutamatergic synapses (Huang and Kandel 1998), the current study therefore tested the hypothesis that repeated ethanol exposure/withdrawal cycles would enhance or alter similar glutamatergic mechanisms within the lateral/basolateral amygdala.
Methods
Animals
All animal procedures were performed in accordance with protocols approved by Wake Forest University School of Medicine Animal Care and Use Committee and were consistent with the NIH animal care and use policy. Male Sprague-Dawley rats were obtained from Harlan (Indianapolis IN) and were housed in an animal care facility at 23°C with a 12-hour light/dark cycle and given food and water ad libitum. Rats were weighed daily to ensure that at least 80% of their free-feeding weight was maintained during vapor chamber ethanol exposure.
Chronic Ethanol Exposure
Ethanol exposure was accomplished via ethanol inhalation using a method similar to that used in other studies (Becker and Hale 1993; Roberto et al. 2004b). All animals (100–120g) were housed four-six animals/cage in large, standard polycarbonate cages (Allentown Inc., Allentown NJ) containing wood-chip bedding and with chow/water ad libitum. These home-cages were placed within in large, custom-built plexiglass chambers (Triad Plastics, Winston-Salem NC) that were identical to those described elsewhere (Lopez and Becker 2005) at the beginning of the light-cycle (lights on at 9pm EST). Ethanol-vapor, produced by submerging an air-stone in 95% ethanol, was mixed with room air and was pumped into the chambers at a rate of 16L/min. Ethanol levels were checked daily and were maintained at 25–28mg/L within the chamber. Animals were exposed to the ethanol vapor or room air (CON group) for 12 hours/day over 10 consecutive days. Animals exposed ethanol vapor and sacrificed immediately after the 10th exposure (while still intoxicated) are referred to throughout the manuscript as the CIE (chronic intermittent ethanol) group. Those CIE animals removed from the ethanol exposure for 24hr prior to experimentation are defined here as the WD (withdrawal) group. No supplemental ethanol doses or alcohol dehydrogenase inhibitors were used at any time. Animals were weighed daily at the end of each exposure period (9am); and home-cages were changed every 2–3 days. Tail blood was taken at the end of some exposure periods and then trunk blood was collected on the day of sacrifice for subjects in the CIE group. Blood ethanol concentrations (BEC) were determined using a standard, commercially-available alcohol dehydrogenase/NADH enzymatic assay (Diagnostic Chemicals Limited, Oxford CT). At 3–5 days of exposure, BECs from tail blood were 265±12mg/deciliter. At the time of sacrifice (~30 minutes after removal from ethanol vapor), blood ethanol levels were 186±18 mg/deciliter.
Behavioral Assays
To confirm that the CIE exposure paradigm produced withdrawal anxiety, anxiety-like behaviors were assessed using a two-compartment light/dark box (McCool et al. 2003). Individuals were placed in the ‘light’ side of a Plexiglass arena divided equally into ‘light’ and ‘dark’ sides by an opaque Plexiglass insert (Rat Truscan Activity Arena; Coulborn Inst., Allentown PA). The center of the animal ± 0.8cm was followed for 300 seconds by two infrared sensor rings surrounding the entire apparatus, one in the floor plane and one located ~5cm above the floor to measure rearing behavior. Data were collected and analyzed for general locomotor activity, time spent in the light and dark compartments, number of light-dark transitions, egress latency, re-entry latency, and number of vertical beam breaks. Data was analyzed using one-way ANOVA across the different treatment groups. All variables are reported as the mean±SEM.
Preparation of Brain Tissue
Animals were anesthetized with isoflurane and euthanized by decapitation. 400µm coronal brain slices were prepared as described previously (Floyd et al. 2003). For in vitro slice preparations, 100µM ketamine was added to a modified aCSF (in mM: 180 Sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2•6H2O, 26 NaHCO3, 1.2 NaH2PO4, 10 glucose) during preparation of slices to minimize excitotoxicity. Slices were transferred and stored in 0.5 liter of standard oxygenated aCSF solution (in mM: 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, and 2 CaCl2) at room temperature for at least 1 hour and up to 6 hours before performing electrophysiological analysis.
Electrophysiology
Methods for whole-cell blind patch-clamp recordings from BLA neurons within slices were similar to those reported previously (DuBois et al. 2006). Electrodes were filled with an intracellular pipette solution containing (in mM): 122 CsOH, 17.5 CsCl, 10 HEPES, 1 EGTA, 5 NaCl, 0.1 CaCl2, 4 Mg-ATP, and 0.3 Na-GTP, 2 QX-314 (Cl), pH adjusted to 7.2 with gluconic acid, osmolality ranged from 280–290 mmol/kg with sucrose. Excitatory postsynaptic currents (EPSCs) were electrically evoked every 20 sec by brief (0.2 msec) square-wave stimulations near the boundary between lateral/basolateral amygdala (Fig.1A) using platinum/iridium concentric bipolar stimulating electrodes (FHC, Bowdoinham, ME) with an inner pole diameter of 25 µm and resistance of 8–12MΩ. Stimulation intensities ranged from 5 to 50µA. This varied considerably across different experiments and was a function of the specific stimulating electrode used in that particular experiment. Unless stated otherwise, we used sub-maximal stimulations (just above threshold) that yielded consistent synaptic responses.
Figure 1.
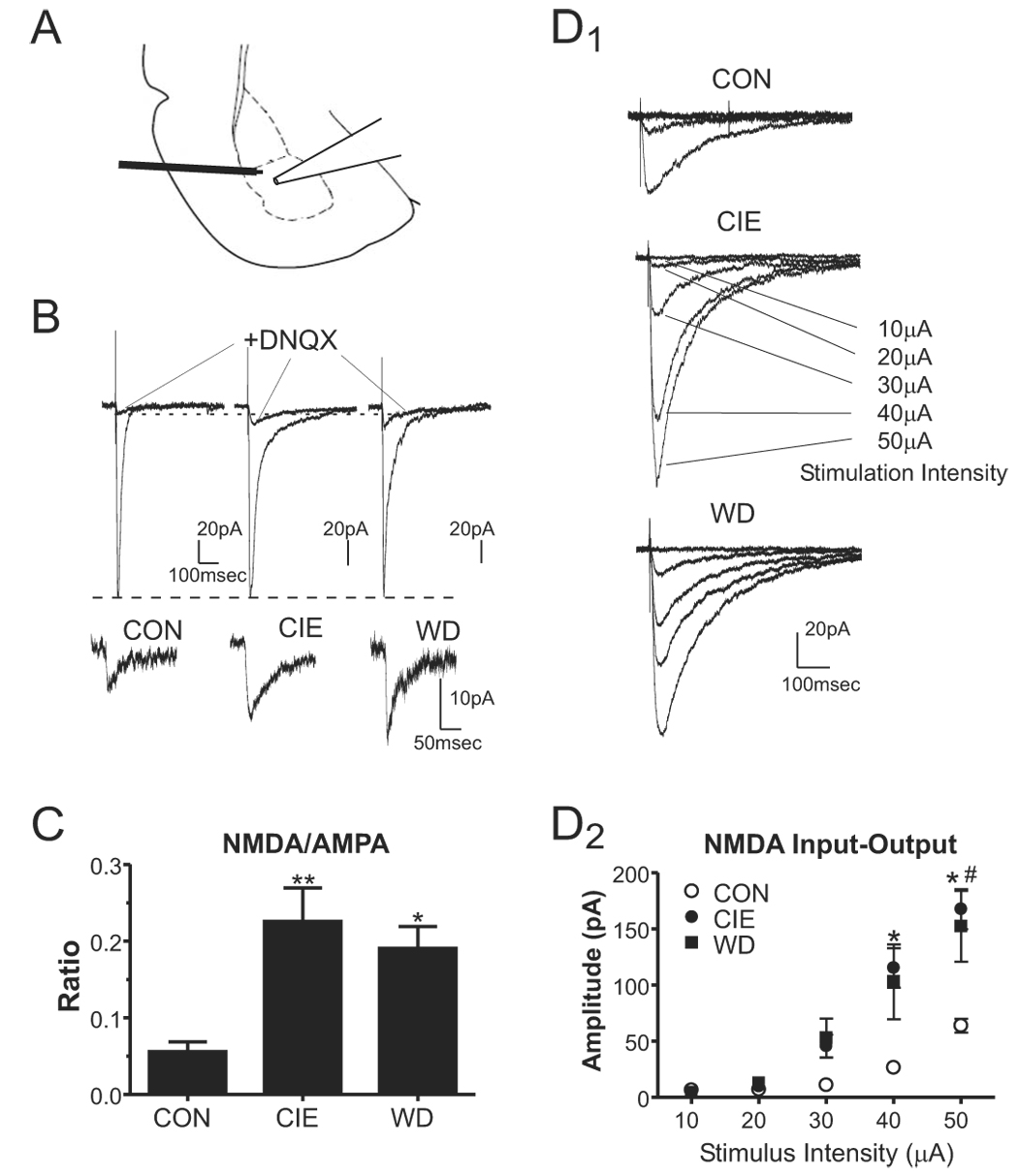
Chronic ethanol and withdrawal increase the NMDA/AMPA current ratio in the BLA measured with low extracellular Mg2+. A, Slices were stimulated locally near the boundary of the lateral/basolateral amygdala and the recording electrode was placed in the basolateral amygdala as shown. B, Average synaptic responses for control and 24hr WD cells, in the presence of 10µM bicuculline, show the compound current consisting of the DNQX-sensitive and –resistant components and the current resistant to 20µM DNQX. The DNQX- resistant currents from these neurons are also enlarged below the compound current traces to aid with visual comparisons. The NMDA receptor antagonist APV inhibited >95% of the DNQX-resistant current (see text). C, The NMDA/AMPA ratio, calculated from the amplitude of the DNQX-resistant component (NMDA) divided by the amplitude of the DNQX-sensitive component (AMPA), show a significant increase of 405% in CIE neurons and 342% following 24hr WD (**, P<0.01 and *, P<0.05 compared to CON, one-way ANOVA with Bonferroni posttest). D, NMDA input-output relationships were measured from CON (D1, top), CIE (D1, middle), and WD (D1, bottom) neurons. Note that the amplitude NMDA-mediated (DNQX-resistant) synaptic responses were dependent on the intensity of the stimulation (in µA) and were significantly larger at the greatest intensities for CIE (n=7 neurons from 3 individuals) and WD neurons (n=6 neurons from three individuals) compared to CON neurons (D2, n=6 neurons from three individuals; *, CIE vs. CON P<0.05, #, WD vs. CON P<0.05, one-way ANOVA with Bonferroni post-test).
All glutamatergic events were pharmacologically isolated using 10µM bicuculline to inhibit fast GABAergic transmission. Recordings were acquired with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA), and digitized with a Digidata 1200B (Axon Instruments). From a holding potential of −60mV, we included square-wave command hyperpolarizations (−5mV) in every sweep to constantly monitor input resistance and capacitance. Neurons with high initial input resistance (>50MΩ; presumptive interneurons (Rainnie 1999)) or whose input resistance increased more than 10% during data collection were excluded from the study.
NMDA Synaptic Responses
To record NMDA-mediated EPSCs, neurons were voltage-clamped at −60mV in the presence of low extracellular magnesium (0.2mM). For the ratio experiment, stimulus strength was adjusted until the amplitude of the compound (AMPA+NMDA) synaptic currents was approximately equivalent across all neurons; and stimulus strength was held constant throughout the remainder of the experiment. After recording a stable baseline of the resulting compound AMPA/NMDA EPSC, the AMPA-component was inhibited with bath application of 20µM 6,7-dinitroquinoxaline-2,3-dione (DNQX). The relative contribution of each component within an individual neuron was calculated by dividing the amplitude of the remaining DNQX-resistant (NMDA receptor-mediated) current by the amplitude of the DNQX-sensitive (AMPA-mediated) component. These ratio data are presented as mean±SEM (averaged across cells) and were analyzed using one-way ANOVA across the different treatment groups.
For the NMDA input-output study, neurons from each treatment group were recorded at −60mV holding potential and incubated with low extracellular Mg2+ and the AMPA receptor antagonist DNQX (20µM). Increasing stimulus intensities (10µA to 50µA) produced graded monosynaptic responses.
Spontaneous Glutamatergic Synaptic Events
Spontaneous excitatory postsynaptic currents (sEPSC) were acquired at 20kHz, and were filtered at 2kHz. For miniature EPSCs (mEPSC), 1µM tetrodotoxin (TTX) was bath applied for >5 minutes before recording spontaneous events. This concentration of TTX inhibited >99% of all electrically-evoked EPSCs (not shown). TTX-sensitive and –resistant spontaneous EPSCs were detected and analyzed using Mini Analysis Program 6.0.3 (Synaptosoft Inc., Fort Lee, NJ). Measures from individual cells were averaged across treatment groups (Van Sickle et al. 2004), reported as mean±SEM, and analyzed using standard one-way ANOVA. In some cases, sEPSC and mEPSC data are expressed as cumulative probability distributions from individual cells representing the median across a treatment group. These data were analyzed using the Kolmogorov-Smirnov (KS) test for different population distributions. Significance was determined at a KS statistic of 0.09 or greater rather than using the P value; this cutoff makes the less-conservative KS test more stringent.
Noise Analysis
Root mean square current noise (Irms) was analyzed in the spontaneous event records. Noise was measured independently in four 50-msec epochs by comparing current amplitudes at each data point to the mean current amplitude across the epoch as previously described (Mtchedlishvili and Kapur 2006). Epochs containing obvious spontaneous EPSCs were avoided in the analysis. These data are reported as a mean±SEM and statistically analyzed using standard one-way ANOVA.
Paired pulse ratio
Paired-pulse facilitation (PP) was measured using pairs of electrical stimuli of equal intensity at 25, 50, or 250 msec interpulse intervals. Ratios of the amplitudes of the evoked EPSCs were calculated as the difference between the amplitude of the second event minus the amplitude of the first, with the result divided by the amplitude of the first synaptic response (Schulz et al. 1995). All values were expressed as mean±SEM, and data were subjected to a one way ANOVA, with P<0.05 considered statistically significant.
Microinjections
We accomplished microinjections into the BLA of male Sprague Dawley rats according to previously published procedures (McCool and Chappell 2007). Briefly, we deeply anesthetized animals with pentobarbital (90mg/kg) and affixed them to a stereotaxic instrument. Chronic guide cannulae were placed bilaterally into the dorsal aspect of the BLA and affixed to the skull with dental cement. We used sterile obturators to maintain the patency of the guide cannulae. During a five day recovery period, animals were handled extensively and habituated to the injection procedure and sound of the syringe pump. On test days, 0.5µl drug in standard aCSF was directly infused into the BLA over a one minute period. Injection cannulae were left in place for 1min; and, animals were placed into the light/dark box 5min after the microinjection. Following the microinjections and behavioral measurements, guide cannulae placement was confirmed post-mortem.
Two separate microinjection experiments were performed in the current study. In the first experiment, adult male Sprague Dawley rats (n=39; 303.9±0.6g at the time of surgery) were used to test the effects of muscimol microinjection into the BLA on light/dark box anxiety-like behavior. For these experiments, guide cannulae were implanted according to the following stereotaxic coordinates (millimeters relative to Bregma, (Paxinos and Watson 1997): −2.8 anterior/posterior; ±5.05 medial/lateral; and −6.20 dorsal/ventral (measured from the top of the brain). Sham, 88nmol (10µg), or 264nmol (30µg) muscimol (in aCSF) was microinjected into separate animals; and, individual animals were exposed to the light/dark box only once. Across two separate cohorts, we misplaced guide cannulae in only three out of 39 animals used in the muscimol study; these are not included in the analysis (not shown, see text).
In the second experiment, male Sprague-Dawley rats (n=19, 138.0±2.6g at the time of surgery) similar to those used for the electrophysiology experiments were used to test the effects of DNQX on withdrawal-related anxiety-like behavior expressed in the light/dark box. For these studies, animals were surgerized 1hr following the fifth ethanol intermittent inhalation. This time point was selected for two reasons. First, animals appeared behaviorally tolerant to the effects of the ethanol inhalation by the fifth day; this may help reduce interactions between the anesthetic and the ethanol during the surgery. Second, surgerizing animals prior to the ethanol exposure proved difficult because of changes in body weight and skull size during the long-term exposure. For the surgery, ethanol exposed animals were deeply anesthetized with pentobarbital (50mg/kg). In three separate cohorts (5–7 animals/cohort), guide cannulae were implanted according to the following stereotaxic coordinates (millimeters relative to Bregma) that were empirically determined in pilot studies on juvenile animals: −2.7 anterior/posterior; +4.5 lateral on the right, +4.75 lateral on the left; −5.8 dorsal/ventral from the top of the brain. Following the surgery, animals recovered for 10hr before being placed back into the inhalation chamber to complete the last 5 days of the chronic inhalation treatment. When not in the inhalation chamber, animals were handled during this period to habituate them to the injection procedure. Following 24hr withdrawal after the 10th ethanol exposure, individual animals were microinjected with either sham or 100pmol DNQX; and, individuals were exposed to the light/dark box only once. In three separate cohorts, we placed guide cannulae outside the BLA in only two out of nineteen animals (see Fig. 6A).
Figure 6.
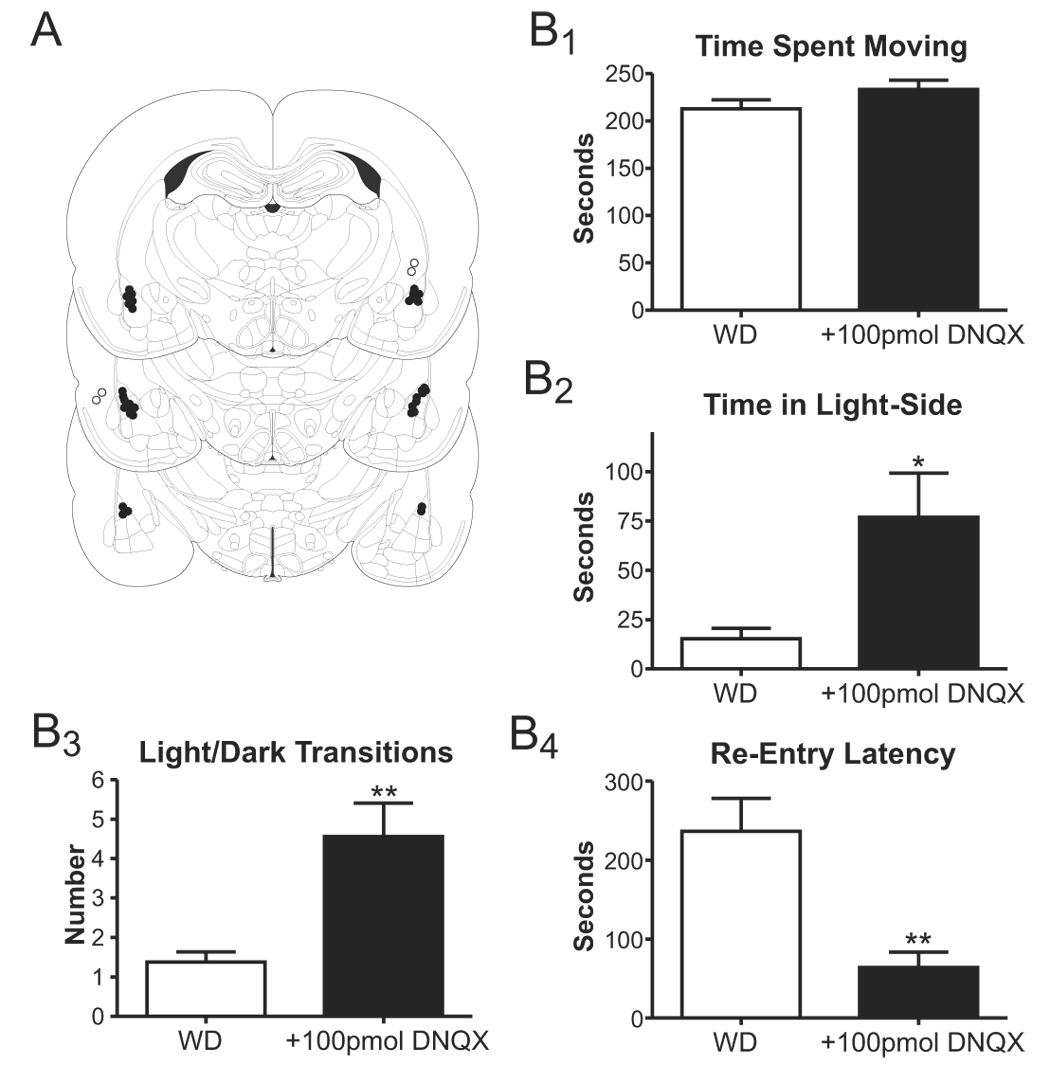
Microinjection of the AMPA-type glutamate receptor antagonist, DNQX, into the lateral/basolateral amygdala attenuates anxiety-like behavior expressed during withdrawal. A, Figure (modified from Paxinos and Watson 1997) illustrating the approximate locations for the guide cannulae tips (correct placements=closed circles). Note that some cannulae were placed outside the BLA (open circles, see text). B, Summary of light/dark box behaviors expressed by sham- (WD) and DNQX-injected animals with guide cannulae place within the BLA. While variables like the time spent moving during the assay were not affected (B1), 100pmol DNQX significantly increased both the total time spent in the light side (B2) and the number of light/dark transitions (B3). Intra-BLA DNQX also significantly decreased the latency to re-enter the light-side following initial egress to the dark side (B4). *P<0.05, **P<0.01, t-test.
Results
Withdrawal from chronic ethanol inhalation increases anxiety-like behaviors
We initially assessed whether our treatment paradigm was relevant for the anxiety-related effects of chronic ethanol and withdrawal. Fifty-seven animals were divided equally into three groups (n=19 each) with two groups receiving 10 days of 12hr/day ethanol vapor while the third control group received room air in an identical chamber. We then measured anxiety-like behavior using the “light/dark” box assay in controls, immediately after the last ethanol exposure while individuals were still intoxicated (as evidenced by blood-ethanol levels; ‘CIE’ animals), or 24hr after withdrawal from ethanol vapors (‘WD’ animals). One CIE animal appeared sedated, was clearly an outlier with regard to low locomotor activity, and was excluded from the behavioral study. Similarly, four animals from the withdrawn group appeared to freeze when initially placed on the light-side of the behavioral apparatus (e.g. reduced locomotion with ≥60sec to initially egress from the light- to the dark-side) and were not analyzed further.
For the remaining animals, 24 hours of withdrawal from 10 days chronic intermittent ethanol exposure caused a significant decrease in time spent in the light side, a significant decrease in the number of light/dark transitions, and a significant increase in the re-entry latency (time to re-enter the light following first egress into the dark; Table 1). Egress latency (initial time leave the light-side and move into the dark side) was not significantly different between the treatment groups. The alterations in most ‘anxiety-related’ variables are consistent with increased expression of anxiety-like behaviors in the WD animals. The total move time was not different between groups indicating that the treatment did not affect the locomotor activity in the animals. Exploratory behaviors, represented by vertical-plane entries (‘rears’), were significantly suppressed in both CIE and WD animals again suggesting that the anxiety-effects were specific to withdrawal.
Recent evidence suggests that anxiety-like behavior expressed in the light/dark box is dependent upon BLA neurotransmitter systems (Bueno et al. 2005; McCool and Chappell 2007). To test this, we microinjected 0 (n=12), 88nmol (10µg; n=14), or 264nmol (30µg; n=13) muscimol into the BLA and measured light/dark box behavior. For several of the anxiety-related dependent variables expressed in this assay, muscimol diminished anxiety-like behavior in a dose-dependent fashion. For example, 264nmol muscimol increased the time spent in the light-side of the apparatus (108±9sec; P<0.05, one-way ANOVA) compared to sham (73±13sec) and 88nmol muscimol (63±15sec). Likewise, the re-entry latency (time to re-enter the light-side following egress into the dark) was significantly less in animals microinjected with 264nmol muscimol (48±14sec; P<0.05, one-way ANOVA) compared to either sham (140±33sec) or 88nmol muscimol (111±22sec). The number of light-dark transitions and the initial egress latency (time to leave the light-side after placement) were not significantly affected by muscimol however. Likewise, locomotor-related behaviors were not altered by the test doses of muscimol examined here. The total distance moved during the assay was 1343±42cm in sham animals, 1241±72cm in animals microinjected with 88nmol muscimol, and 1377±38 with 264nmol muscimol (P>0.05, one-way ANOVA). Similarly, the total time spent moving was 227.3±5.0sec in sham, 209.7±6.6sec with 88nmol muscimol, and 229.8±3.3sec with 264nmol muscimol. Together, these data confirm that manipulation of BLA neural activity can alter anxiety-like behavior expressed in the light dark box.
Chronic ethanol and withdrawal increase the contribution of NMDA-receptors at BLA glutamatergic synapses
Previous studies in our lab have demonstrated that chronic ethanol can lead to increased NMDA receptor function measured in somatic compartments of acutely isolated basolateral amygdala neurons (Floyd et al. 2003). To test whether these alterations are expressed within synaptic compartments, we investigated the effects of CIE and WD on NMDA receptor function at glutamatergic synapses using whole-cell in vitro slice electrophysiology. We first established a stable excitatory synaptic response using both low extracellular magnesium as well as the most minimal stimulation required to produce synaptic consistent monosynaptic responses. For these studies, response amplitudes in the low extracellular magnesium were 127±23pA in CON neurons, 114±15pA in CIE neurons, and 114±13pA in WD neurons; these values were not statistically different from one another (P≫0.05, one-way ANOVA). Within each neuron, we next perfused slices with the AMPA receptor antagonist DNQX (20µM; Fig. 1B) to pharmacologically isolate NMDA receptor-mediated synaptic currents. When expressed as a ratio of NMDA receptor-mediated current amplitude to the DNQX-sensitive, AMPA receptor-specific component, we found significant increases in the DNQX-insensitive NMDA receptor-mediated current (Fig. 1C) in both CIE- (P<0.05, n=6) and WD- (P<0.05, n=7) neurons compared to control (n=7). In a separate set of experiments, greater than 95% of DNQX-insensitive synaptic current in BLA neurons was inhibited by 50µM of the NMDA-receptor antagonist 2-amino-5-phosphonovaleric acid (APV; not shown). This inhibition did not differ between the three treatment groups.
We also examined the effects of CON, CIE, and WD treatments on NMDA-mediated synaptic currents by measuring responses to increasing stimulation intensities (input-output relationship). In all three treatment groups, local electrical stimuli elicited DNQX-insensitive synaptic currents whose amplitude increased in an intensity-dependent fashion (Fig. 1D). At the highest stimulation intensities, the amplitude of the DNQX-insensitive, NMDA-mediated synaptic current was significantly greater in both CIE and WD neurons compared to control (P<0.05, one-way ANOVA). Along with the ratio data, these input-output results clearly suggest that the contributions by synaptic NMDA receptors are increased by chronic ethanol exposure and are maintained during 24hr of withdrawal.
Chronic ethanol and withdrawal increase the amplitude and frequency of spontaneous EPSCs
Since the NMDA/AMPA ratio is also dependent upon the relative contribution by AMPA receptors, a decrease in AMPA receptor-mediated synaptic currents might increase the NMDA/AMPA ratio in slices prepared from CIE and WD animals. We attempted to test this by measuring the AMPA input-output relationship. However, under the current recording conditions (local stimulation and standard aCSF containing 10µM bicuculline), AMPA-mediated synaptic responses had an extremely steep stimulus-response relationship with polysynaptic responses occurring at stimulation intensities >20µA (responses >200pA). This prevented a comparison of AMPA input-output across the treatment groups.
Alternatively, we examined spontaneous excitatory postsynaptic currents (sEPSCs; Fig. 2B). Under standard recording conditions (2mM extracellular magnesium and −60mV holding potential), spontaneous synaptic currents were entirely sensitive to the AMPA receptor-specific antagonist GYKI 53655 (Fig.2A). GYKI 53655 also inhibited electrically-evoked responses by 96±1% in CON neurons (n=4), 98±1% in CIE neurons (n=6), and 96±1% in WD neurons (n=5; P>0.05, one-way ANOVA). These data suggest that AMPA-type glutamate receptors mediate the spontaneous synaptic currents. With respect to the treatment groups, we found the sEPSC amplitude in CIE-, but not WD-, neurons was significantly increased relative to control (Fig. 2B & C). However, the charge carried by these spontaneous events was not significantly different between the treatment groups (Fig. 2D). Analysis of the background current (Irms) was also not significantly different between groups (control, 1.2pA±.01; CIE 1.7pA±0.3; WD 1.3pA±0.1). These results indicate that our ability to detect sEPSCs was approximately equivalent across the treatment groups. In contrast to the modest effects of chronic ethanol and withdrawal on sEPSC amplitude, the frequency of spontaneous events was significantly greater in both treatment groups relative to control. For example, the inter-event interval of the sEPSCs was significantly decreased for CIE- (P<0.01, one-way ANOVA) and WD-neurons (P<0.01) compared to control (Fig. 2E).
Figure 2.
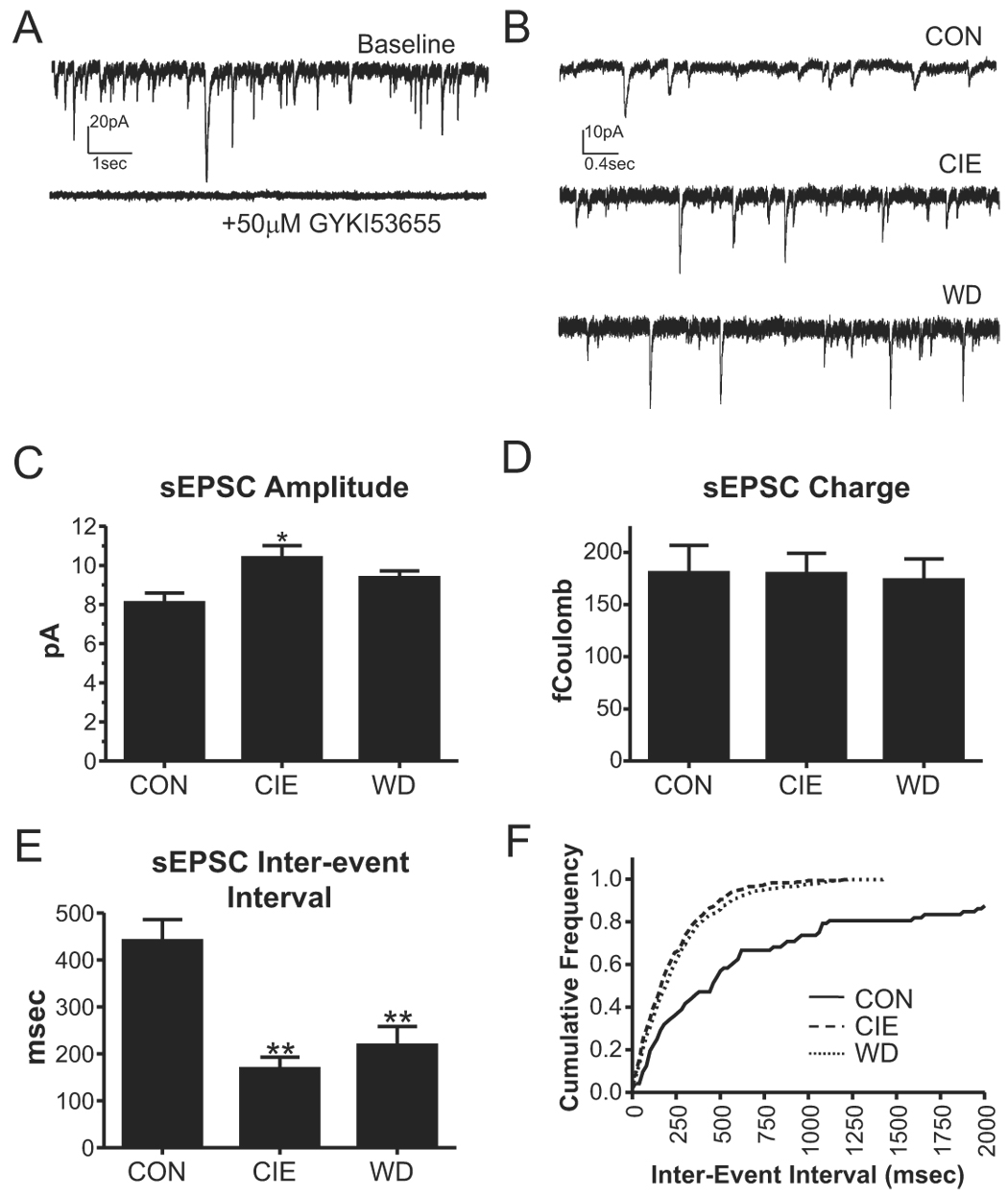
Chronic ethanol and withdrawal increase the frequency of spontaneous excitatory postsynaptic currents (sEPSCs). A, Sample traces illustrate that, under standard conditions (10µM bicuculline; holding potential, −60mV), sEPSCs are mediated by GYKI 53655-sensitive AMPA receptors. B, Sample traces of sEPSCs in the presence of 10µM bicuculline (holding potential, −60mV) are shown for each treatment group. C, The sEPSC amplitude in CIE neurons was significantly larger than CON (*, P<0.05, one-way ANOVA with Bonferroni post-test). Mean±SEM are shown across all cells in a particular treatment group. For these experiments, n=8 neurons for CON (from five rats), n=11 neurons for CIE slices (from four rats), and n=11 for WD neurons (from four animals). D, The charge carried by sEPSCs (amplitude × decay time, expressed in femtoCoulombs) was not significantly affected by CIE or WD. E, The sEPSC inter-event interval was dramatically and significantly decreased (increased frequency) for both CIE and WD neurons (**, P<0.01 compared to CON, one-way ANOVA). F, A cumulative probability plot comparing the inter-event interval from individual CON, CIE, and WD neurons, each neuron represents median values from each treatment group. KS=0.39 for CIE neurons and KS=0.36 for WD neurons (P<0.0001 relative to CON).
Chronic ethanol inhalation and withdrawal decreases the paired pulse ratio
The increased frequency of sEPSCs without substantial changes in amplitude might suggest increased presynaptic function following CIE and WD. To test this, we measured synaptic responses to two closely-juxtaposed electrical stimuli (paired pulses; Fig. 3A). At short interpulse intervals, the ratio of the second synaptic response relative to the first is believed to be inversely related to the probability of release at synapses (Andreasen and Hablitz 1994; Katz et al. 1993). At inter-pulse intervals of 25 and 50 msec (Fig. 3A), CIE and WD both significantly decreased the paired pulse ratios (Fig. 3B). In support of the sEPSC frequency data, the paired-pulse findings suggest that CIE and WD increase presynaptic glutamatergic function, potentially by increasing release probability at the terminal.
Figure 3.
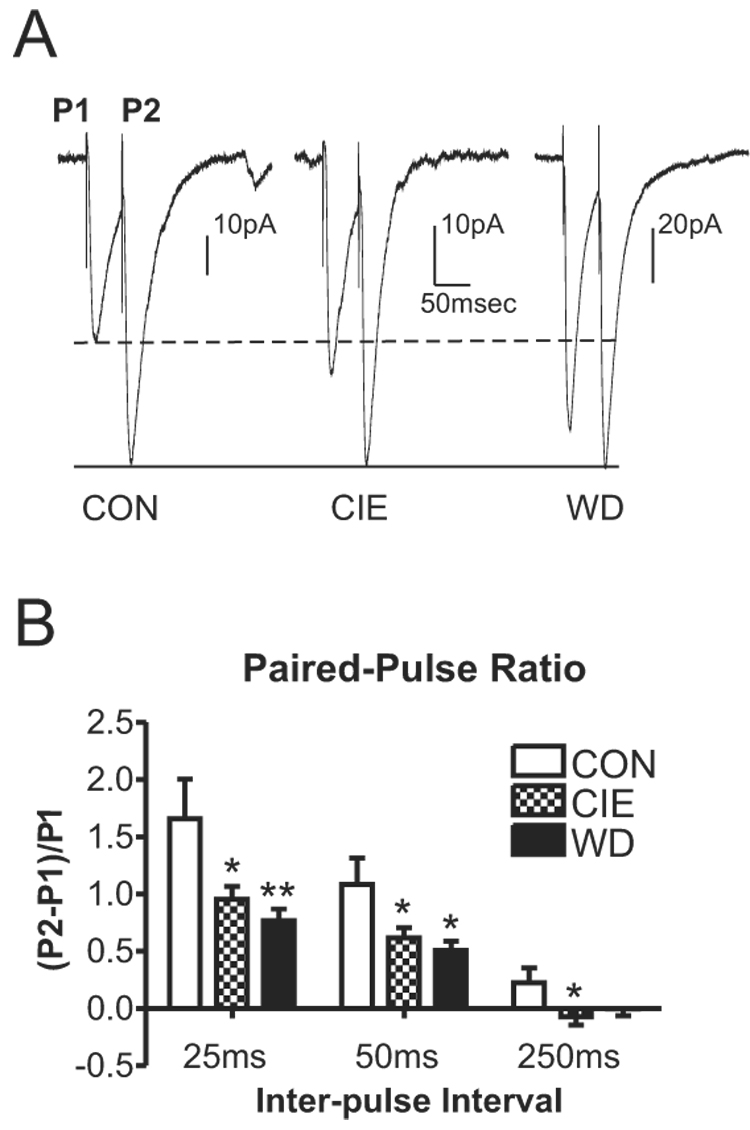
Chronic ethanol and withdrawal decrease paired pulse ratio in the BLA. A, Sample traces of paired pulse EPSCs at 50msec inter-pulse interval in the presence of 10µM bicuculline. The amplitude of the second synaptic response has been normalized across treatment groups to emphasize the relative differences between the first synaptic response and the second. B, Paired pulse ratio is significantly decreased at both the 25 and 50msec interval (*P<0.05, **P<0.01 vs. control, one-way ANOVA with Bonferroni’s post-test). These decreased ratios indicate that chronic ethanol and withdrawal may increase presynaptic release of glutamate.
Withdrawal increases miniature EPSC amplitude, decay, and frequency
To confirm the possible alterations of presynaptic terminal function, we measured the effects of CIE and WD on the amplitude and frequency of TTX-resistant miniature EPSCs under standard recording conditions (2mM extracellular magnesium and −60mV holding potential; Fig. 4A&B). WD robustly increased the frequency of mEPSCs (decreased inter-event interval) compared to control (P<0.01, one-way ANOVA) and CIE (P<0.05; Fig. 4E&F). Importantly, CIE did not alter mEPSC frequency, in contrast to the sEPSC and paired-pulse findings. Because TTX blocks action potentials, these data in the CIE neurons may imply that the presynaptic alterations evident in this treatment group are action potential-dependent. Furthermore, comparisons between the sEPSC and mEPSC data suggest that CIE and WD may alter presynaptic glutamatergic function via distinct mechanisms.
Figure 4.
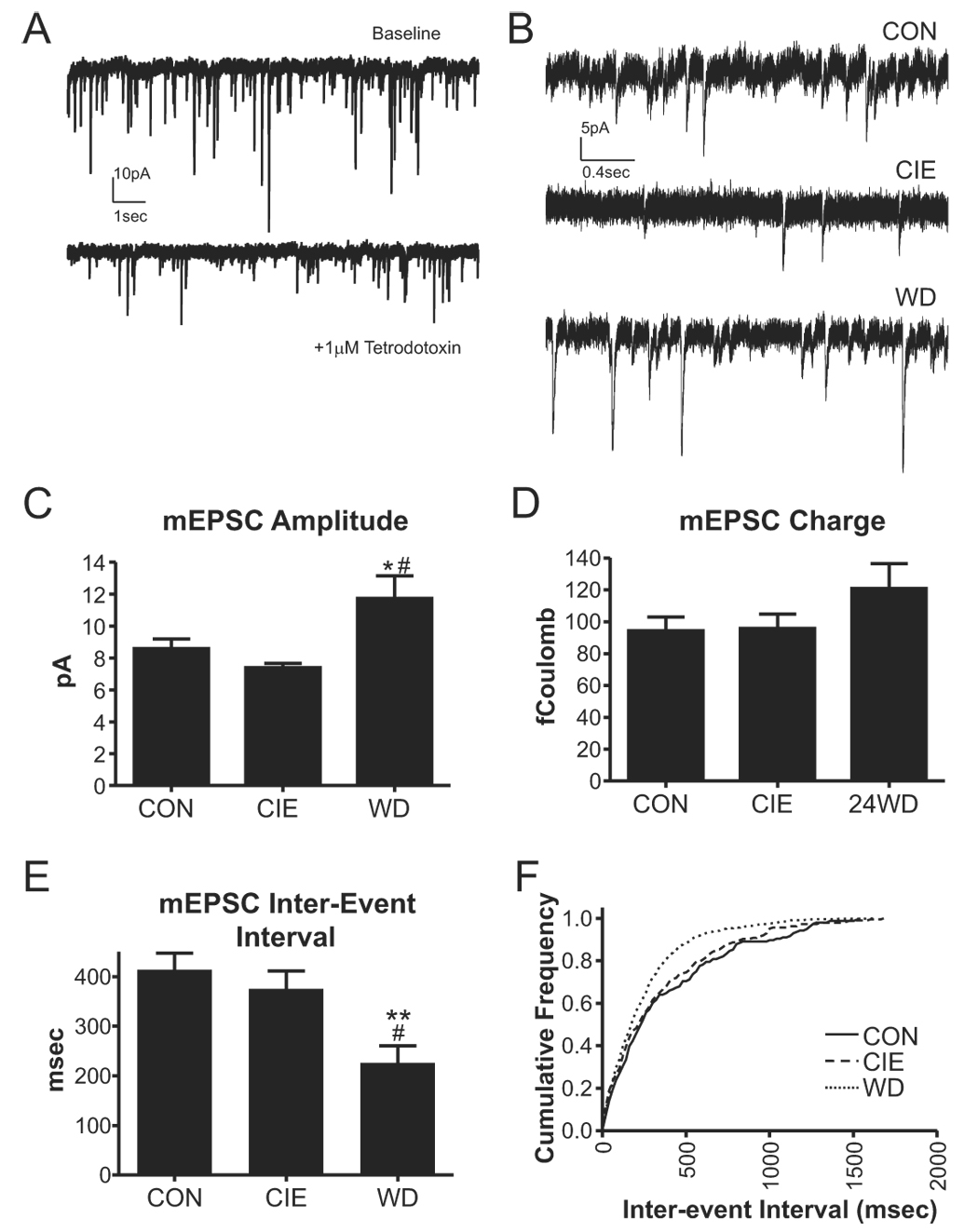
Withdrawal increases the frequency and amplitude of mEPSCs. A, Sample traces demonstrating the efficacy of 1µM tetrodotoxin used in these studies. B, Sample traces of mEPSCs in the presence of 10µM bicuculline and 1µM TTX (holding potential, −60mV) are shown for each treatment group. C, The mEPSC amplitude from WD neurons was significantly greater than CON (*, P<0.05) and CIE neurons (#, P<0.05; one-way ANOVA with Bonferroni’s post-test). D, The charge carried by mEPSCs (amplitude in pA × decay time in milliseconds, expressed as femtoCoulombs) was not significantly different from CON in the CIE and WD treatment groups. E, The inter-event interval of WD mEPSCs was significantly smaller than both control (**, P<0.01) and CIE neurons (#, P<0.05; one-way ANOVA with Bonferroni’s post-test). F, Cumulative probability plot of inter-event interval from individual CON, CIE, and WD neurons; each cell is representative of the median from that particular treatment group. The inter-event interval distribution of the WD neuron (KS=0.19, P<0.0001), but not the CIE neuron (KS=0.06, P≫0.05), was significantly different from the CON neuron.
In addition to these apparent presynaptic changes, WD also increased the amplitude of the mEPSCs (Fig. 4C). However, the charge carried by mEPSCs was not significantly altered by any treatment (Fig. 4D). Together these amplitude and charge data suggest some change in the kinetics of glutamatergic responses in WD neurons. We therefore examined the onset and decay kinetics of the miniature EPSCs (Fig. 5A). Average mEPSC traces were generated by aligning the rising phase of each event in each cell and scaling the amplitude. The resulting average mEPSC from each cell was fit to a 1st-order exponential equation. Rise-times were not significantly different from each other (one-way ANOVA, Fig. 5B). However, the decay of mEPSCs was significantly faster in WD neurons compared to both CIE and CON (P<0.05, one-way ANOVA; Fig. 5C). These data help explain the significant up-regulation of mEPSC amplitude in WD neurons in the absence of any substantial alterations in the amount of charge carried by events in this treatment group.
Figure 5.
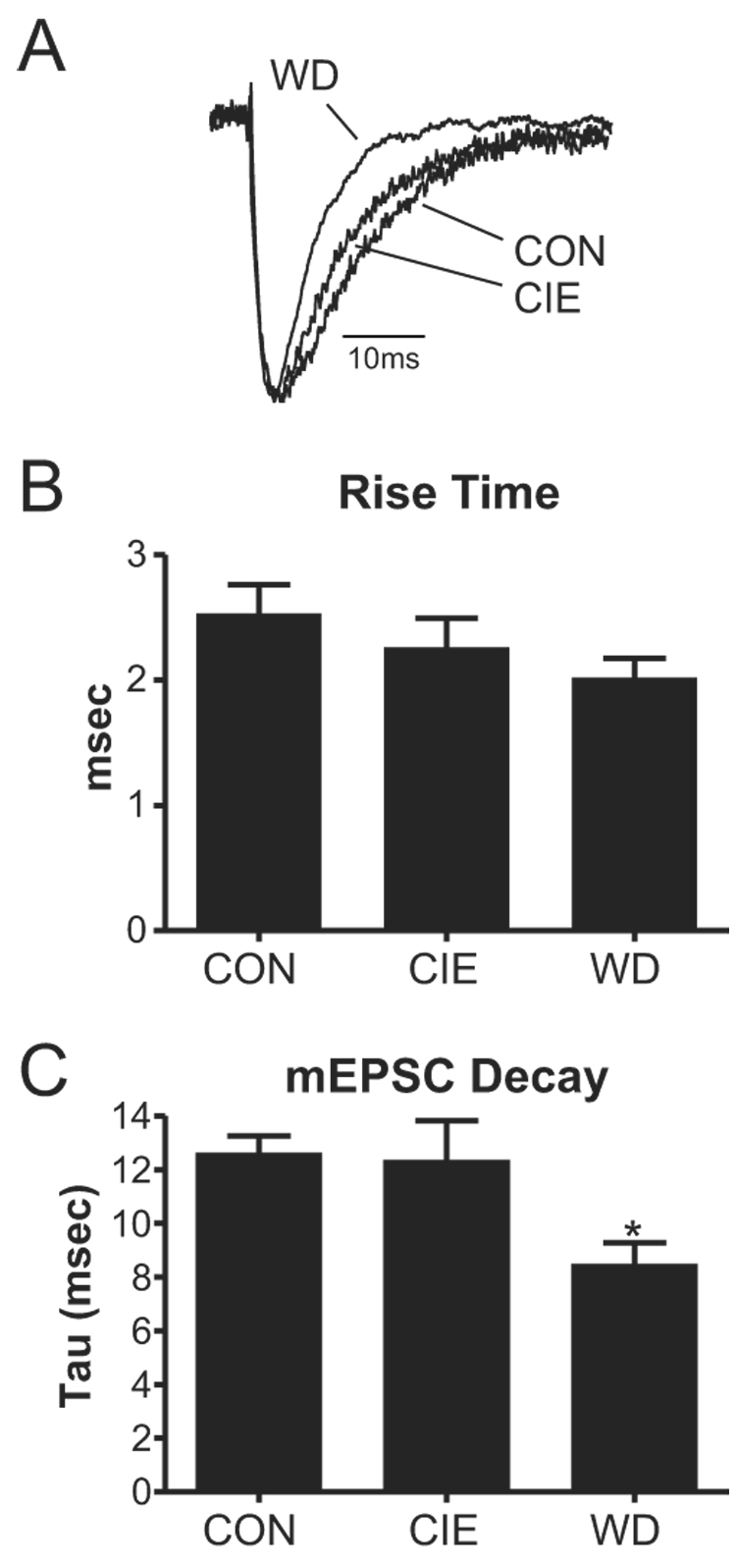
Withdrawal from chronic ethanol decreases the current-decay time constant of miniature events. A, Average traces from each treatment group illustrate the faster decay kinetics of miniature events in withdrawal neurons. B, Rise-times were not significantly different from each other (one-way ANOVA). C, Using a single-exponential fit of miniature EPSCs, the decay time was significantly smaller than CON for WD events (*, P<0.05, one-way ANOVA) but not CIE events. These results suggest possible postsynaptic alterations of the receptor during withdrawal and provide a mechanism for increased amplitude, but not charge, in these neurons.
BLA glutamatergic system and withdrawal-related anxiety-like behavior
Given that the focus of the current work on glutamatergic function in the BLA, we examined the effects of microinjection with the AMPA/kainate receptor antagonist DNQX (100pmol) on anxiety-like behavior in withdrawn (WD) animals (Fig. 6A). Importantly, DNQX substantially diminished the anxiety-like behavior expressed by WD animals in the light/dark box. Both the time spent in the light side (Fig. 6B2) and the number of light-to-dark transitions (Fig. 6B3) were significantly increased (P<0.05 and P<0.01, respectively; t-test) by DNQX microinjection. Likewise, DNQX significantly (P<0.01, t-test) decreased the re-entry latency in WD animals (Fig. 6B4). These effects on anxiety-like behavior appear to be specific to delivery of DNQX to the BLA. In two animals where guide cannulae were placed outside the BLA (open circles, Fig. 6A), neither the time spent in the light-side (6.5±3.5sec; mean ± standard deviation), nor the number of light-dark transitions (3.0±2.8; mean ± standard deviation), nor the reentry latency (293.5±3.5sec; mean ± standard deviation) were significantly affected by the DNQX microinjections. Likewise, microinjection of DNQX into the BLA did not significantly alter locomotor-related behaviors like the total time spent moving (Fig. 6B1) or the total distance moved (934.8±50.4cm in WD animals, 1102.3±72.9cm in DNQX-injected animals; P>0.05, t-test). Together, these data indicate that delivery of the AMPA/kainate receptor antagonist DNQX into the BLA of WD animals can diminish anxiety-like behavior expressed in the light/dark box.
Discussion
Our results suggest that the increased anxiety-like behavior observed during withdrawal may be due to increases in glutamatergic function seen during CIE and WD. This effect appears to have a presynaptic origin, as evidenced by the decreased paired-pulse ratio and increased frequency of mEPSCs during WD. We also found increases in the NMDA/AMPA ratio as well as increases in amplitude and frequency of spontaneous events during CIE and WD and during WD for mEPSCs; these latter data suggest that post-synaptic alterations may result from these treatments as well. Our NMDA findings parallel previous evidence showing an increase in the function and expression of NMDA receptors in isolated BLA neurons following chronic ethanol liquid-diet exposure (Floyd et al. 2003). The most reasonable interpretation of the changes seen during CIE and WD include increased excitability of glutamatergic afferents in the BLA, increased presynaptic terminal function/number, and more modest postsynaptic increases in receptor function.
It should be noted that our findings must be interpreted in the context of the current exposure paradigm. Our intermittent exposure produces robust blood ethanol concentrations during the ten day exposure period. Regardless, CIE rats with ~190mg/dL have little apparent motor in-coordination, at least as it is represented in the light/dark box (Table 1). None-the-less, this same exposure paradigm produces elevated anxiety-like behavior during subsequent withdrawal. Chronic human alcoholics can also have remarkable functional tolerance despite blood levels that would produce coma and death in naïve individuals (Davis and Lipson 1986; Hammond et al. 1973). It is clear then that the intermittent exposure pattern and severity of exposure employed in the current study may model this type of profound ‘tolerance-dependence’.
Chronic Ethanol facilitates synaptic-NMDA and -AMPA receptors in the basolateral amygdala
Our previous work has shown that chronic exposure to an ethanol liquid diet increased NMDA receptor function in the somatic compartments of acutely isolated BLA neurons (Floyd et al. 2003). Here we show that chronic intermittent ethanol inhalation also significantly increased NMDA receptor function, exemplified by the NMDA/AMPA ratio, at BLA glutamatergic synapses. This is similar to findings in the central nucleus after chronic ethanol (Roberto et al. 2006). Together, these results strongly support the hypothesis that CIE up-regulates post-synaptic NMDA receptor number or function in the amygdala. The amygdala therefore joins a growing list of brain regions responding to chronic ethanol by increased expression/function of NMDA receptors.
Chronic ethanol also increases AMPA receptor protein in primary cortical cultures (Chandler et al. 1999) as well as AMPA receptor dependent calcium signaling in cerebellar Purkinje neurons (Netzeband et al. 1999). However, we found no evidence that CIE produces any alteration in AMPA receptor postsynaptic function. Preliminary evidence (J. Weiner, personal communication) suggests that acute ethanol robustly inhibits BLA NMDA-mediated synaptic responses while AMPA-mediated currents are relatively insensitive. This differential acute sensitivity might explain the robust up-regulation of NMDA synaptic currents to chronic ethanol while AMPA-mediated events are more modestly affected.
Chronic ethanol facilitates TTX-sensitive pre-synaptic function in the basolateral amygdala
Our paired-pulse data, specifically the decrease in facilitation, clearly indicate increased presynaptic function with CIE. This is supported by an increased frequency of spontaneous events in these neurons. Our data are further supported by increased presynaptic glutamate release in both the central nucleus of the amygdala using a similar exposure paradigm (Roberto et al. 2004b) as well as in the hippocampus during ethanol consumption (Sabria et al. 2003). However, the increase in BLA sEPSC frequency was entirely TTX-sensitive; CIE did not alter the frequency of miniature, TTX-resistant EPSCs. These data may imply that CIE facilitates action potential-dependent mechanisms within the BLA glutamatergic afferents themselves. In support of this, chronic ethanol can alter a number of action potential-related processes (Scott and Edwards 1981) including decreased calcium-activated potassium channels (Pietrzykowski et al. 2004) and increased TTX-sensitive voltage-gated sodium channels (Brodie and Sampson 1990) or L-type calcium channels (Watson and Little 1999). In fact, dihydropyridine-sensitive voltage-gated calcium channels appear to be recruited to BLA glutamatergic synapses following fear-learning (Shinnick-Gallagher et al. 2003). Changes within the context of action potential-related, TTX-sensitive cellular processes could therefore lead to increased neurotransmitter release at BLA glutamatergic synapses.
Finally, it is worth noting that slices prepared from CIE animals were incubated at room temperature in aCSF for as long as six hours during the data collection. Since we did not include ethanol in the slice incubation media, it is possible that CIE slices were experiencing ‘acute in vitro’ withdrawal during this period. Our data do not support this hypothesis. First, all CIE animals were sacrificed within 30 minutes following removal from the inhalation chamber. Since we know precisely when each data file was collected, we performed a correlation analysis between the various sEPSC and mEPSC parameters and the time elapsed between collection of these data and preparation of the slices. For all CIE neurons, there was no significant correlation between these event-related dependent variables and the time the slice had been stored in vitro. This argues against a significant contribution by ‘acute in vitro’ withdrawal to CIE-related alterations in glutamatergic transmission.
Withdrawal maintains postsynaptic changes seen during chronic exposure
After 24 hours of withdrawal from chronic ethanol, we saw an increase in NMDA/AMPA ratio and the NMDA input-output relationship in BLA neurons. Given that sEPSC data suggests that AMPA receptor function is not down-regulated during WD, the NMD data indicate that the increased receptor function seen with CIE is maintained for at least twenty-four hours after the exposure. There are conflicting reports concerning the effects of ethanol withdrawal, relative to chronic ethanol, on NMDA receptors in other brain regions. NMDA receptor function appears to increase during ethanol withdrawal in the mouse hippocampus (Whittington et al. 1995). However, in the central amygdala, neither chronic ethanol nor withdrawal alter NMDA receptor synaptic function (Roberto et al. 2006). These data indicate that WD-related changes in NMDA receptor function are clearly brain region-dependent, even within the amygdala.
Withdrawal also induced postsynaptic facilitation of AMPA-mediated glutamatergic spontaneous EPSCs in the BLA. Specifically, we found a large increase in the amplitude of TTX-resistant spontaneous EPSCs. This effect appears to share similarities with other drugs of abuse. For example, the AMPA receptor subunit GluR1 has been shown to be up-regulated after 1 and 30 days of withdrawal from cocaine in the BLA of rats (Lu et al. 2005). Similarly, TTX-resistant EPSCs from WD neurons decayed faster than those measured in both control and CIE. Increased AMPA-mediated EPSC decay is associated with increased delivery of GluR1 homomeric channels to glutamatergic synapses in response to sensory-stimulation of barrel cortex (Clem and Barth 2006) and pharmacologic blockade of AMPA receptors in hippocampal neuron cultures (Thiagarajan et al. 2005). The faster decay (Atassi and Glavinovic 1999; Hirasawa et al. 2001; Veruki et al. 2003), along with increased amplitude (Carroll et al. 1998; Thio et al. 1992), may indicate postsynaptic alterations within the AMPA receptor complex expressed by WD neurons. One possible mechanism explaining increased EPSC amplitude/altered kinetics following WD would be increased delivery of specific AMPA-receptor subtypes to BLA glutamatergic synapses. However, this remains to be directly investigated. Regardless, the increase in mEPSC amplitude was not associated with substantial increases in charge carried by individual events. The contribution of postsynaptic alterations to BLA-dependent, WD-related behaviors may therefore be subtle.
Withdrawal Produces Presynaptic, Terminal-Specific Alterations
There is a general lack of data on the effects of ethanol withdrawal on presynaptic mechanisms. In the central nucleus of the amygdala, glutamate release remains elevated during both chronic ethanol exposure and withdrawal (Roberto et al. 2006). The results of our mEPSC and paired pulse data in the BLA complement these findings and suggest that WD specifically alters presynaptic terminal function. Potential mechanisms include altered release machinery (Bacci et al. 2001; Capogna et al. 1997; Herreros et al. 1995; Pang et al. 2006), increased resting calcium levels in the terminal (Cummings et al. 1996; Levesque and Atchison 1988; Li et al. 1998; Nishimura et al. 1990), or increased numbers of synapses (Lauri et al. 2003) following WD. Chronic blockade of glutamate receptors in hippocampal cultures selectively increases AMPA-mediated mEPSC frequency without altering synapse number (Bacci et al. 2001). Conversely, withdrawal from repeated cocaine exposures increases the number of glutamate-containing terminals in the shell of the nucleus accumbens (Kozell and Meshul 2004). Ultimately, the mechanisms regulating increased mEPSC frequency during WD from CIE are likely to be both brain region- and treatment-specific.
Behavioral Implications
Given the association between the BLA glutamatergic system and anxiety (Sajdyk and Shekhar 1997), increases in glutamatergic function like those observed in the current work may contribute to the anxiety-like behavior evident during withdrawal from chronic ethanol. Additional studies that follow a withdrawal time course would be necessary to more robustly illustrate this. However, we have used juvenile animals to facilitate the whole-cell electrophysiology recordings; and this creates confounds between developmental issues and a protracted time-course. Studies in adult animals would perhaps be more appropriate in this respect but are also substantially more challenging.
Regardless, our microinjection results with DNQX clearly suggest that BLA mechanisms associated with AMPA/kainate-type glutamate receptors help regulate the expression of anxiety-like behavior during withdrawal. These findings parallel studies showing that amygdala AMPA receptors are important for the expression of learned fear-potentiated startle (Walker and Davis 1997b) and learned avoidance tasks (Mesches et al. 1996). Importantly, the expression of learned-fear is coincident with increased AMPA receptor-mediated synaptic function at BLA glutamatergic synapses (McKernan and Shinnick-Gallagher 1997) and with increased delivery of AMPA receptor subunits to the cell surface (Yeh et al. 2006). The coincidental increase in BLA glutamatergic synaptic function during withdrawal, the increase in withdrawal-related anxiety-like behavior, and the apparent contribution of BLA AMPA-type glutamate receptors to withdrawal-associated anxiety-like behavior together suggest an intimate relationship between these parameters.
Paradoxically, we found that glutamate function was elevated during the CIE exposure, despite an absence of elevated anxiety-like behavior measured in the light/dark box. One potential explanation is that the BLA contributes minimally to CIE-related behaviors expressed in the light/dark box. Most evidence does not support this hypothesis. Several recent studies (de la Mora et al. 2005; McCool and Chappell 2007; Perez de la Mora et al. 2006; Salome et al. 2006) as well as the muscimol study reported in the current work have demonstrated the contribution of BLA-dependent processes to light/dark box anxiety-like behavior in naïve animals. Therefore, compensatory changes in other BLA/downstream neurotransmitter systems or continued sensitivity of these systems to acute ethanol are more likely to explain the paradoxical increase in BLA glutamatergic function in the absence of significant increases in anxiety-like behavior. This latter ‘continued sensitivity’ hypothesis is particularly relevant since anxiety-like behaviors in CIE animals were measured while the individuals were still intoxicated (based on BEC at sacrifice).
The potential neurotransmitter systems potentially contributing to CIE-related behaviors are quite numerous. Within the amygdala fear/anxiety-circuit, recent reports suggest that GABAergic function is increased (Roberto et al. 2004a) while glutamatergic function is decreased (Roberto et al. 2004b) in the neighboring central amygdala in ethanol-dependent rats. Since the projections from BLA to central amygdala are critical for the expression of learned-fear (Davis 2006), it is possible these central amygdala alterations might offset increased glutamatergic function in the BLA during CIE treatment, ultimately masking anxiety-related behavioral manifestations in these animals. Within the BLA itself, alcohol has well known effects on numerous neurotransmitter systems. For example, acute and chronic ethanol alter GABAergic function in BLA neurons (Floyd et al. 2004; McCool et al. 2003; Zhu and Lovinger 2006). In addition, BLA serotonergic (Gonzalez et al. 1996), dopaminergic (de la Mora et al. 2005), noradrenergic (Schroeder et al. 2003), and various neuropeptide systems (Rupniak et al. 2003; Sajdyk et al. 1999a; Sajdyk et al. 1999b; Wunderlich et al. 2002) all regulate anxiety-like behavior. Disruption or alteration of any of these systems might also contribute to anxiety-related behavior observed in intoxicated rats exposed to chronic ethanol.
Conclusions
In conclusion, we have shown that chronic intermittent ethanol exposure and withdrawal lead to significant increases in glutamatergic synaptic transmission in the basolateral amygdala. Our working hypothesis for the sequence of events that result from this treatment is that chronic ethanol produces both increased postsynaptic NMDA receptor function as well as increased glutamatergic afferent excitability. At some point during the withdrawal process, these alterations trigger significant changes in presynaptic terminal function and more modest alterations of postsynaptic AMPA receptor function. This increased glutamatergic system in the basolateral amygdala was not observed behaviorally until withdrawal because we hypothesize that the additional ethanol-sensitive systems may be engaged while the animal is still intoxicated.
Acknowledgements
Supported by NIH/NIAAA awards AA014445 and AA015179 (BAM) and AA016442 (AKL). A. Läck and M. Diaz contributed equally to this work. D. DuBois current address: Dept. Anesthesiology, Univ. Wisconsin-Madison, Madison WI
References
- Alderson HL, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 2000;153:111–119. doi: 10.1007/s002130000527. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Atassi B, Glavinovic MI. Effect of cyclothiazide on spontaneous miniature excitatory postsynaptic currents in rat hippocampal pyramidal cells. Pflugers Arch. 1999;437:471–478. doi: 10.1007/s004240050803. [DOI] [PubMed] [Google Scholar]
- Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. doi: 10.1523/JNEUROSCI.21-17-06588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Brodie C, Sampson SR. Effects of ethanol on voltage-sensitive Na-channels in cultured skeletal muscle: up-regulation as a result of chronic treatment. J Pharmacol Exp Ther. 1990;255:1195–1201. [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr, Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res. 2005;38:1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- Capogna M, McKinney RA, O'Connor V, Gahwiler BH, Thompson SM. Ca2+or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Chapman PF, Ramsay MF, Krezel W, Knevett SG. Synaptic plasticity in the amygdala: comparisons with hippocampus. Ann N Y Acad Sci. 2003;985:114–124. doi: 10.1111/j.1749-6632.2003.tb07076.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;46:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Cummings DD, Wilcox KS, Dichter MA. Calcium-dependent paired-pulse facilitation of miniature EPSC frequency accompanies depression of EPSCs at hippocampal synapses in culture. J Neurosci. 1996;16:5312–5323. doi: 10.1523/JNEUROSCI.16-17-05312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Davis AR, Lipson AH. Central nervous system tolerance to high blood alcohol levels. Med J Aust. 1986;144:9–12. doi: 10.5694/j.1326-5377.1986.tb113622.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Cardenas-Cachon L, Vazquez-Garcia M, Crespo-Ramirez M, Jacobsen K, Hoistad M, Agnati L, Fuxe K. Anxiolytic effects of intra-amygdaloid injection of the D1 antagonist SCH23390 in the rat. Neurosci Lett. 2005;377:101–105. doi: 10.1016/j.neulet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47 Suppl 1:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Perlegas A, Floyd DW, Weiner JL, McCool BA. Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther. 2006;318:629–640. doi: 10.1124/jpet.105.100552. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Hammond KB, Rumack BH, Rodgerson DO. Blood ethanol. A report of unusually high levels in a living patient. Jama. 1973;226:63–64. doi: 10.1001/jama.226.1.63. [DOI] [PubMed] [Google Scholar]
- Herreros J, Miralles FX, Solsona C, Bizzini B, Blasi J, Marsal J. Tetanus toxin inhibits spontaneous quantal release and cleaves VAMP/synaptobrevin. Brain Res. 1995;699:165–170. doi: 10.1016/0006-8993(95)00739-d. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Shiells R, Yamada M. Blocking AMPA receptor desensitization prolongs spontaneous EPSC decay times and depolarizes H1 horizontal cells in carp retinal slices. Neurosci Res. 2001;40:217–225. doi: 10.1016/s0168-0102(01)00229-2. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Becker HC. Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res. 1998;786:80–88. doi: 10.1016/s0006-8993(97)01413-3. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22:2165–2173. [PubMed] [Google Scholar]
- Katz PS, Kirk MD, Govind CK. Facilitation and depression at different branches of the same motor axon: evidence for presynaptic differences in release. J Neurosci. 1993;13:3075–3089. doi: 10.1523/JNEUROSCI.13-07-03075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell LB, Meshul CK. Nerve terminal glutamate immunoreactivity in the rat nucleus accumbens and ventral tegmental area after a short withdrawal from cocaine. Synapse. 2004;51:224–232. doi: 10.1002/syn.10304. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Lamsa K, Pavlov I, Riekki R, Johnson BE, Molnar E, Rauvala H, Taira T. Activity blockade increases the number of functional synapses in the hippocampus of newborn rats. Mol Cell Neurosci. 2003;22:107–117. doi: 10.1016/s1044-7431(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Effect of alteration of nerve terminal Ca2+ regulation on increased spontaneous quantal release of acetylcholine by methyl mercury. Toxicol Appl Pharmacol. 1988;94:55–65. doi: 10.1016/0041-008x(88)90336-5. [DOI] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson M. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem. 2005;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell A. Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is 'state' dependent. Behav Brain Res. 2007;178:70–81. doi: 10.1016/j.bbr.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Bianchin M, McGaugh JL. The effects of intra-amygdala infusion of the AMPA receptor antagonist CNQX on retention performance following aversive training. Neurobiol Learn Mem. 1996;66:324–340. doi: 10.1006/nlme.1996.0073. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Trotter C, Caguioa JN, Gruol DL. Chronic ethanol exposure enhances AMPA-elicited Ca2+ signals in the somatic and dendritic regions of cerebellar Purkinje neurons. Neurochem Int. 1999;35:163–174. doi: 10.1016/s0197-0186(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Tsubaki K, Yagasaki O, Ito K. Ryanodine facilitates calcium-dependent release of transmitter at mouse neuromuscular junctions. Br J Pharmacol. 1990;100:114–118. doi: 10.1111/j.1476-5381.1990.tb12061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Sudhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Lara-Garcia D, Jacobsen KX, Vazquez-Garcia M, Crespo-Ramirez M, Flores-Gracia C, Escamilla-Marvan E, Fuxe K. Anxiolytic-like effects of the selective metabotropic glutamate receptor 5 antagonist MPEP after its intra-amygdaloid microinjection in three different non-conditioned rat models of anxiety. Eur J Neurosci. 2006;23:2749–2759. doi: 10.1111/j.1460-9568.2006.04798.x. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel JP. Alcohol withdrawal seizures: implications of kindling. Pharmacol Biochem Behav. 1980;13 Suppl 1:225–231. doi: 10.1016/s0091-3057(80)80034-7. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res. 1998;22:176–182. [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Webb JK, Fisher A, Smith D, Boyce S. The substance P (NK1) receptor antagonist L-760735 inhibits fear conditioning in gerbils. Neuropharmacology. 2003;44:516–523. doi: 10.1016/s0028-3908(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Sabria J, Torres D, Pasto M, Peralba JM, Allali-Hassani A, Pares X. Release of neurotransmitters from rat brain nerve terminals after chronic ethanol ingestion: differential effects in cortex and hippocampus. Addict Biol. 2003;8:287–294. doi: 10.1080/13556210310001602194. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999a;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999b;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Using paired-pulse facilitation to probe the mechanisms for long-term potentiation (LTP) J Physiol Paris. 1995;89:3–9. doi: 10.1016/0928-4257(96)80546-8. [DOI] [PubMed] [Google Scholar]
- Scott BS, Edwards BA. Effect of chronic ethanol exposure on the electric membrane properties of DRG neurons in cell culture. J Neurobiol. 1981;12:379–390. doi: 10.1002/neu.480120407. [DOI] [PubMed] [Google Scholar]
- Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F. L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann N Y Acad Sci. 2003;985:135–149. doi: 10.1111/j.1749-6632.2003.tb07078.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thio LL, Clark GD, Clifford DB, Zorumski CF. Wheat germ agglutinin enhances EPSCs in cultured postnatal rat hippocampal neurons by blocking ionotropic quisqualate receptor desensitization. J Neurophysiol. 1992;68:1930–1938. doi: 10.1152/jn.1992.68.6.1930. [DOI] [PubMed] [Google Scholar]
- Van Sickle BJ, Xiang K, Tietz EI. Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal-anxiety. Neuropsychopharmacology. 2004;29:1994–2006. doi: 10.1038/sj.npp.1300531. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. J Physiol. 2003;549:759–774. doi: 10.1113/jphysiol.2003.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Watson WP, Little HJ. Correlation between increases in dihydropyridine binding in vivo and behavioural signs of ethanol withdrawal in mice. Alcohol Alcohol. 1999;34:35–42. doi: 10.1093/alcalc/34.1.35. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Lambert JD, Little HJ. Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol Alcohol. 1995;30:105–114. [PubMed] [Google Scholar]
- Wunderlich GR, Raymond R, DeSousa NJ, Nobrega JN, Vaccarino FJ. Decreased CCK(B) receptor binding in rat amygdala in animals demonstrating greater anxiety-like behavior. Psychopharmacology (Berl) 2002;164:193–199. doi: 10.1007/s00213-002-1181-4. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Mao SC, Lin HC, Gean PW. Synaptic expression of glutamate receptor after encoding of fear memory in the rat amygdala. Mol Pharmacol. 2006;69:299–308. doi: 10.1124/mol.105.017194. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]


