Abstract
The goal of this cross-sectional study was to compare cognitive functioning at age 5 years in prenatal drug-exposed children with nondrug-exposed children from a comparable inner-city environment. Children with prenatal drug exposure scored significantly lower on measures of language, school readiness skills, impulse control, and visual attention span/sequencing than controls matched for age and socioeconomic status. Intelligence, visual-motor, manual dexterity, and sustained attention scores were not significantly different between groups. The total sample scored significantly below the normative mean on standardized measures of intelligence, language, school readiness, visual-motor skills, impulse control, and sustained attention, with 40% scoring at least 1 standard deviation below the mean (IQ <85) on a measure of intelligence. Findings suggest that children with prenatal drug exposure are at increased risk for learning and attention problems and are in need of close developmental surveillance and possible intervention to support school success and improve behavioral outcome.
Keywords: prenatal drug exposure, intelligence, impulsivity, attention
Many questions remain unanswered about the effects of prenatal drug exposure on children’s cognitive functioning and later school performance. As these children reach school age, problems with learning and attention may emerge. Despite the apparent risks that prenatal drug exposure presents to the developing child, research during the past decade has not found convincing evidence of a negative effect of exposure to prenatal cocaine or opiates, or both, on intelligence or on academic skills through early school age.1–8 Recent evidence suggests that exposure to prenatal cocaine may adversely affect children’s language development; however, data remain inconclusive.9–13
Prenatal drug exposure has been frequently associated with behavioral dysregulation in the neonatal period14–16 as well as with problems in attention and impulse control in school-aged children up to age 10 years.1,17–19 Cocaine readily crosses the placenta, with the potential to directly affect the developing fetus.20 It has been assumed that prenatal cocaine exposure can negatively effect the development of the brain monoamine systems that regulate behavior, possibly resulting in reduced impulse control.21
The purpose of this investigation was to compare cognitive functioning at age 5 years in a prenatal drug-exposed group with that of a nondrug-exposed group from the same inner-city environment. An additional aim of this analysis was to assess the effect of prenatal exposure to cocaine or opiates, or both, on attention and impulse control in young school-aged children.
Methods
Study Sample
Between December 1994 and January 1997, 233 infants newly born to mothers who used cocaine or opiates, or both, were recruited at 2 urban university medical centers. Recruitment occurred in the context of a longitudinal study investigating the effectiveness of a home nurse intervention program for drug-exposed infants, as previously reported.22,23 Eligibility of enrollment was based on maternal age between 19 and 40 years and prenatal use of cocaine or opiates, or both.
Mothers were asked about frequency of cocaine or opiate use and use of other substances, including alcohol. Results of maternal and infant urine toxicology screens were used to confirm maternal self-report of drug use. Type of drug exposure was classified into 3 groups: (1) exposure to cocaine only, (2) exposure to opiates only, and (3) exposure to cocaine and opiates. Caregivers who reported excessive alcohol consumption during pregnancy, defined as more than 1 drink per week, were excluded from the present data analysis. Infants were excluded if gestational age was less than 35 weeks or they required neonatal intensive care unit admission for more than 24 hours, were discharged directly into foster care, or were born to mothers with a major psychiatric disorder.
Fifty non-exposed children (controls) were recruited from the same urban hospital settings for comparison with the drug-exposed group. The control group had no documented evidence of prenatal drug exposure and was matched to the study group on socioeconomic status (SES), maternal age, and infant gestational age. Health and sociodemographic data were collected by medical record review and maternal self-report.
Recruitment and retention for this present analysis are diagrammed in Figure 1. The initial study sample consisted of 204 drug-exposed children and 47 controls. After enrollment, children were evaluated annually for measurements of growth, intelligence, language, and behavioral outcomes, and continued maternal drug use was documented. At 5 years of age, 113 drug-exposed and 31 nondrug-exposed children were available for evaluation of cognitive functioning. Children lost to follow-up were not significantly different from the retained children in terms of sex, race, gestational age, and maternal age and educational status.
Figure 1.
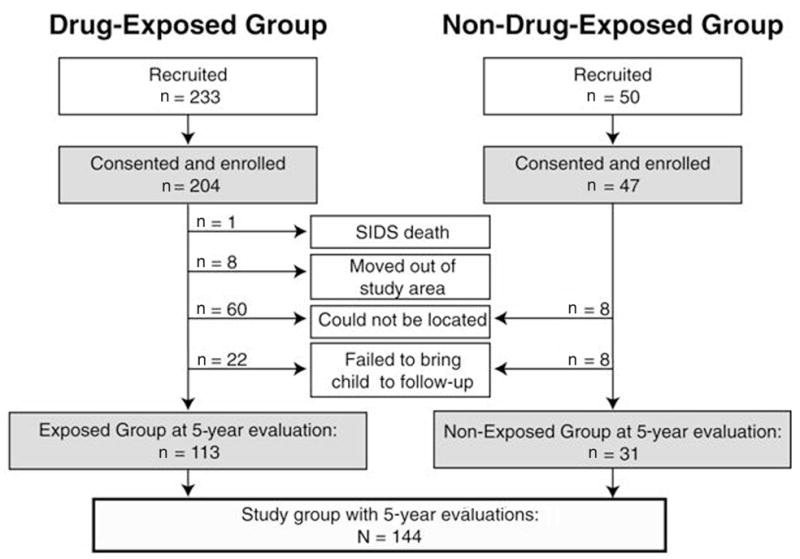
Recruitment and retention status. (SIDS = sudden infant death syndrome.)
The study was approved by the Institutional Review Boards of the Johns Hopkins Medical Institutions, the Bayview Medical Institution, and the Johns Hopkins Medical Systems Corporation, Baltimore, Maryland. Informed consent was obtained from caregivers at the 5-year follow-up evaluation.
Procedure
A comprehensive set of cognitive tests (Table 1)24–32 was administered to the total sample of 144 children at 5 years of age by 2 examiners masked to group status. The test battery assessed 6 areas of cognitive functioning: intelligence, school readiness, language, visual-motor, manual dexterity, and attention. Intelligence, school readiness, language, and visual-motor skills were assessed using standardized measures that yield age-based standard scores (mean, 100; standard deviation, 15).
Table 1.
Cognitive Test Battery
| Area of Functioning | Measure |
|---|---|
| Intelligence | Stanford-Binet Intelligence Scale: Fourth Edition24 |
| School Readiness | Bracken Basic Concept Scale-Revised25 |
| Language | Preschool Language Scale-Third Edition26 |
| Visual-Motor | Developmental Test of Visual–Motor Integration27 |
| Manual Dexterity | Purdue Pegboard Test28 |
| Attention | Gordon Diagnostic System29,30 Knox Cube Test31,32 |
Attention/impulse control was assessed using the Gordon Diagnostic System (GDS), a computerized continuous performance. Normative data for the GDS are based on more than 1300 children aged 4 to 16 years old. For younger children, the GDS consists of 2 tasks, the Delay Task and the Vigilance Task.
The GDS Delay Task measures a child’s ability to inhibit impulsive responding. On this task, the child is instructed to earn as many points as possible by pushing the response button. The child is told to wait before pushing the button again; otherwise, a point will not be earned. Points are displayed on the screen, and the child must determine how long to wait to earn a point. The child earns a point each time he or she waits a minimum of 6 seconds. The Delay Task yields an efficiency ratio (ER) or percentage of correct responses (number of points earned divided by total number of responses) that is converted to age-based percentiles. The ER is considered to be the best indicator of the level of impulsivity demonstrated by a child.29
The GDS Vigilance Task assesses the child’s ability to focus attention for a prolonged period of time without receiving any reinforcement. Numbers flash quickly in the middle of the counter, and the child is told to press the button when a “1” is immediately followed by a “9.” The Vigilance Task yields 2 major scores: Total Correct (the number of correct 1–9 combinations) and Total Commissions (incorrect responses). Raw scores from the Vigilance Tasks are converted to age-based percentiles. Screening of number recognition was conducted using the Bracken Basic Concept Scale-Revised before administration of the GDS to ensure validity of GDS test results.
The Knox Cube Test is a nonverbal measure of visual attention span and sequencing. It consists of 4 stationary wooden blocks placed in a row on a piece of wood. The examiner taps the blocks in prearranged sequences of increasing length and complexity, and the child is asked to imitate the tapping pattern exactly. The number of tapping sequences performed correctly is totaled and raw scores are reported, with a raw score of 6 expected for age 5.
The Purdue Pegboard Test is a measure of finger and hand dexterity. The test consists of three 30-second trials in which the child is asked to place pegs in a pegboard using his or her dominant hand, nondominant hand, and then both hands simultaneously. The total number of pairs of pegs inserted in the bimanual condition is reported here. Normative data indicate that 50% of 5-year-old children place 7 pairs of pegs using both hands.33
Data Analysis
Differences between the drug-exposed and nondrug-exposed groups for the presence of prenatal alcohol exposure, child gender, and race were identified with χ2 analysis. One-way analysis of variance (ANOVA) was conducted to examine differences between drug-exposed and nondrug-exposed groups in birth characteristics, child’s age at follow-up evaluation, and mother’s age at delivery and highest educational level attained. One-way ANOVA was also conducted to determine differences in test scores by drug group status (exposed or not exposed) and by prenatal alcohol exposure (exposed or not exposed). Differences between drug-exposed and nonexposed groups for test scores (standard score ≥85 or <85) on measures of intelligence, expressive language, and school readiness were identified with χ2 analysis. One-way ANOVA was performed to determine if there were differences in test scores by type of prenatal drug use (cocaine only, cocaine plus opiates, opiates only) and by continuation of maternal drug use (yes/no) within the drug-exposed group. Differences between the mean test scores for the total sample and the normative group means were examined using a 1-sample t test. Statistical analyses were performed using SPSS 14.0 (SPSS Inc, Chicago, Ill). Reported P values were computed using two-tailed tests of significance with an α set at 0.05.
Results
Sample Characteristics
Most of the children from the total sample were boys (53%) and African American (95%). Mean gestational age was significantly lower for the drug-exposed group; this group also had a lower mean weight and height at birth. At the 5-year follow-up, there was no significant difference between groups in weight. No significant differences were found between the groups in children’s sex, race, mean head circumference at birth, or mean age at the follow-up evaluation. Child characteristics are summarized in Table 2.
Table 2.
Child Characteristics by Drug Group (N = 144)
| Prenatal Drug Groupa |
||
|---|---|---|
| Characteristic | Exposed (n = 113) | Unexposed (n = 31) |
| Gender (% male) | 54.0 | 48.4 |
| Race (% African American) | 93.8 | 100.0 |
| Gestational age (weeks) | 38.3 ± 1.6 | 40.0 ± 0.0b |
| Birth data | ||
| Head circumference (cm) | 32.9 ± 3.2 | 34.1 ± 1.7 |
| Weight (grams) | 2796.0 ± 486.7 | 3140.4 ± 460.9c |
| Height (cm) | 48.0 ± 3.0 | 50.4 ± 2.2c |
| Age at evaluation (years) | 5.0 ± 0.1 | 5.1 ± 0.1 |
Continuous data are mean ± standard deviation.
P < .05.
P < .01.
Maternal characteristics are summarized in Table 3. Mothers of drug-exposed children were significantly older than the control group at age of delivery. There was no significant difference between the groups in terms of the highest educational level attained, with mothers in both groups reporting, on average, completion of the 11th grade.
Table 3.
Caregiver Characteristics by Prenatal Drug Group Status (N = 144)
| Prenatal Drug Groupa |
||
|---|---|---|
| Characteristic | Exposed (n = 113) | Unexposed (n = 31) |
| Age at delivery (mean years) | 33.9 ± 9.5 | 27.3 ± 5.8b |
| Education: highest year Completed (mean years) | 11.4 ± 1.6 | 11.3 ± 1.7 |
| Prenatal substance exposure | ||
| Cocaine only | 20 (17.7) | — |
| Opiate only | 30 (26.5) | — |
| Cocaine + opiate | 63 (55.8) | — |
| Alcohol (≤1 per week) | 33 (29.2) | 5 (16.1) |
| Postnatal illicit drug use at follow-up | 75 (66.3) | 0c |
Continuous data are presented as mean ± standard deviation; categoric data as number (%).
P < .01.
P < .001.
Most mothers in both groups were from Hollingshead SES class III-V34; 91% of all mothers reported receiving medical assistance, and 93% were unemployed at the time of the evaluation. Few of the caregivers reported a spouse or similar companion in the home: 86% of the mothers from the total sample were never married, and another 8% were widowed, divorced, or separated.
Maternal prenatal drug use consisted of primarily cocaine and opiates (55.8%), followed by opiates only (26.5%), and cocaine only (17.7%). Use during pregnancy of any other illicit drug (eg, marijuana) was reported by less than 10% of mothers in the drug-exposed group. Occasional alcohol use (more than once per month but ≤1 drink per week) was reported by almost one third (29.2%) of mothers from the drug-exposed group and 16.1% of mothers from the non-drug-exposed group. For the total sample, children exposed to occasional alcohol during gestation did not significantly differ on any cognitive measure from those not exposed. About two thirds of mothers (66.3%) in the drug-exposed group continued to use cocaine or opiates, or both, at the 5-year follow-up evaluation.
Cognitive Test Results
As summarized in Table 4, there was no significant difference in child overall intelligence, as measured by the Stanford-Binet, between drug-exposed and control groups. Further, no significant differences were found between groups in the 4 Stanford-Binet Area scores (data not shown). For the combined sample, 40% of children scored at least 1 standard deviation below the mean of the intelligence test (IQ <85); neither group was significantly different in this respect. No significant differences were found by drug exposure group in terms of mean test scores on measures of receptive language, visual-motor skills, or manual dexterity. However, children in the drug-exposed group scored significantly lower than non-exposed children on the Preschool Language Scale-3 scales assessing expressive language and total language skills, with 60% of drug-exposed children earning a standard score of less than 85 on the Expressive Language subscale compared with 33% of the non-exposed children (P = .018). In terms of school readiness skills, the non-exposed group scored significantly higher on the Bracken (P = .05), with 40% of the drug-exposed children achieving a standard score of less than 85.
Table 4.
Test Results between Prenatal Drug Group Status (N = 144)
| Prenatal Drug Groupa |
||
|---|---|---|
| Measure | Exposed (n = 113) | Unexposed (n = 31) |
| Intelligence | ||
| Stanford-Binet Test | 86.7 ± 11.3 | 89.5 ± 13.0 |
| Composite IQ | ||
| School Readiness | ||
| Bracken Basic Concept | 89.3 ± 15.3 | 95.4 ± 15.3b |
| Scale-R SS | ||
| Language | ||
| Preschool Language Scale | ||
| 3 Total SS | 81.4 ± 13.6 | 87.7 ± 13.9c |
| Expressive Language SS | 81.7 ± 13.9 | 89.2 ± 15.2c |
| Receptive Language SS | 83.8 ± 13.5 | 89.3 ± 13.9 |
| Visual-Motor | ||
| Developmental Test of Visual-Motor Integration SS | 88.6 ± 12.5 | 89.1 ± 19.3 |
| Manual Dexterity | ||
| Purdue Pegboard raw score (both hands) | 3.8 ± 1.9 | 4.5 ± 2.6 |
| Attention | ||
| Gordon Diagnostic System | ||
| Delay Task Efficiency Ratio (ER) percentile | 22.6 ± 22.1 | 36.1 ± 30.8c |
| Vigilance Task Commission percentile | 14.0 ± 18.3 | 22.1 ± 22.4 |
| Vigilance Task Total Correct percentile | 13.2 ± 7.3 | 20.1 ± 21.7 |
| Knox Cube Test raw score | 3.0 ± 1.8 | 4.0 ± 2.7c |
Note: SS = standard score (mean, 100; standard deviation, 15).
Data are presented as mean ± standard deviation.
P = .05.
P < .05.
As shown in Figure 2, significant mean group differences were found in impulse control (GDS Delay Task ER percentile), with the drug-exposed group falling well below the mean (50th percentile). No significant group differences were seen for sustained attention (GDS Vigilance Task percentiles); however, both groups scored below average. This suggests that the drug-exposed group had significant difficulty suppressing their responses and exhibited problems with impulsivity, but did not have greater problems with sustained attention relative to the nondrug-exposed group. On an additional measure (Knox Cube), the drug-exposed group was noted to have significant difficulty compared with the control group in visual attention span and sequencing (Table 4).
Figure 2.
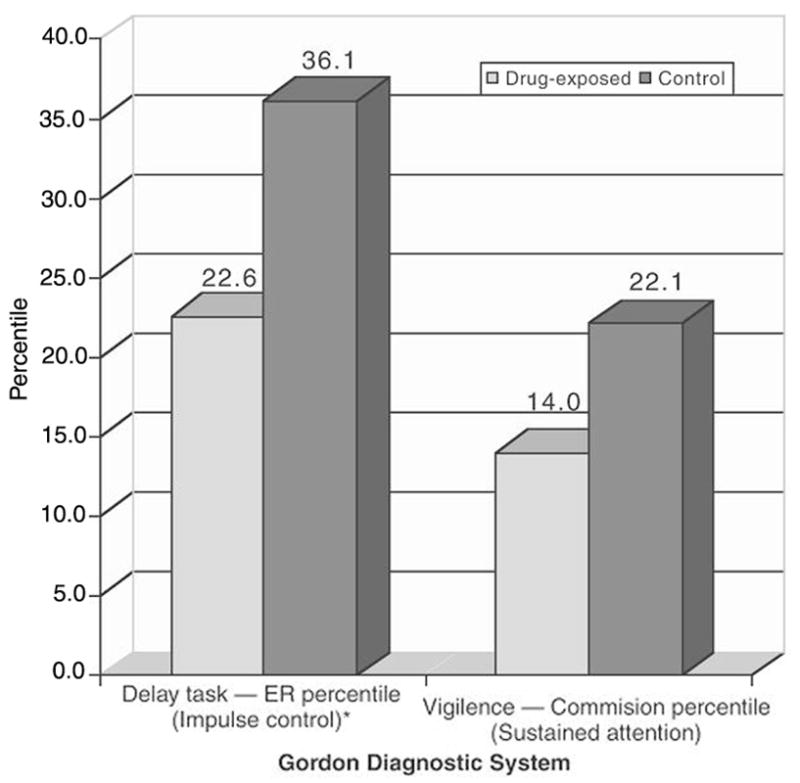
Mean group differences (percentiles) on impulse control and sustained attention as measured by the Gordon Diagnostic System.*P < .05. (ER = efficiency ratio.)
Type of prenatal drug exposure had no appreciable effect on cognitive performance. Specifically, there were no significant differences between the 3 drug-type groups on measures of intelligence, school readiness, language, visual-motor, manual dexterity, or attention span/sequencing. In addition, no significant differences were found between the 3 groups on measures of impulse control or sustained attention.
Of note, the total sample scored significantly below expectation for age compared with normative data on standardized measures of intelligence, language, school readiness, visual-motor skills, impulse control, and sustained attention (P < .01).
Discussion
Children in this study with prenatal drug exposure scored significantly below controls matched for age and SES on measures of expressive and total language, school readiness, impulse control, and visual attention/sequencing. This delay in language skills is consistent with findings of language deficits in early school-aged children with prenatal drug exposure,10,12,35 although such differences have been not been consistently reported in early language development.9
No differences were found in the present study between groups in intelligence, receptive language, visual-motor, and manual dexterity. The lack of difference between groups in intelligence mirrors previous studies,1,2,7,8 most of which have shown that prenatal drug exposure has no significant effect on global intelligence in school-aged children.
The problems with impulse control and inattention revealed in this study are consistent with prior reports.1,18,19,36 Unlike a prior study using the GDS that found cocaine-exposed children performed relatively better than non-exposed children on a measure of impulsivity (Delay Task ER), our data found that drug-exposed children performed significantly lower on this measure. Most importantly, children in both groups in both studies performed equally poorly on measures of sustained attention. Furthermore, both drug-exposed and non-exposed groups in the present study performed below the normative mean on standardized measures of intelligence, language, school readiness, and visual-motor skills. This suggests that study participants share common environmental disadvantages that might be linked to low SES and its related reduced resources and limited developmental benefits.
It is worrisome that two thirds of the caregivers in the drug-exposed group continued to abuse drugs at follow-up after 5 years of the child’s birth, a finding that places these children at even greater risk for learning, attention, and behavior problems. This high rate of continued maternal drug use may result in a home environment that is limited in intellectual stimulation, behavioral inhibition, and stability.
This study has some limitations that may reduce the generalizability of the findings. The retention rate of 57% at the 5-year follow-up, although relatively low, is comparable or exceeds similar studies following drug-exposed children by Hurt et al4 (52.5% retention rate) and Azuma and Chasnoff37 (44% retention rate). Because children lost to follow-up may reside in homes that are less stable, the current results may overestimate the sample’s cognitive functioning and may help to explain the lack of difference in receptive language and visual-motor skills.
Several clinical implications can be derived from these results. Although children with prenatal drug exposure may have a similar level of intelligence compared with their peers matched for age and SES, these children are quite vulnerable to learning disabilities, attention deficits, and impulsive behaviors in part because of environmental disadvantages, including ongoing maternal drug abuse in the home.
Pediatric health care providers can play a critical role in the early identification of cognitive risk factors by conducting close developmental surveillance and providing timely referrals for further evaluation and intervention services. In addition to treatment of the child, pediatric health care providers should recognize the prevalence of ongoing maternal drug use and encourage these mothers to seek medical care for their substance abuse. Addressing the complex needs of the child and parent with substance abuse often requires a team approach, including social workers, educators, speech and language therapists, and behavioral psychologists. Coordinated care by professionals may improve the likelihood of school success and positive behavioral outcome.
Conclusion
Children with prenatal drug exposure scored significantly lower on measures of language, school readiness skills, impulse control, and visual attention span/sequencing than controls matched for age and SES. Intelligence, visual-motor, manual dexterity, and sustained attention scores were not significantly different between groups. The total sample scored significantly below the normative mean on standardized measures of intelligence, language, school readiness, visual-motor skills, impulse control, and sustained attention, with 40% scoring at least 1 standard deviation below the mean (IQ <85) on a measure of intelligence. It is worrisome that 66% of caregivers in the drug-exposed group continue to abuse drugs at the 5-year follow-up. Findings suggest that children with prenatal drug exposure are at increased risk for learning and attention deficits and are in need of close developmental surveillance and coordinated care to support their school success and improve behavioral outcome.
Acknowledgments
This study was funded by the National Institute of Nursing Research, NIH (NR03442).
We thank the families and children who participated in this study.
References
- 1.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school age children. Neurotoxicol Teratol. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 2.Chasnoff IJ, Anson A, Hatcher R, et al. Prenatal exposure to cocaine and other drugs: outcome at four to six years. Ann N Y Acad Sci. 1998;846:314–328. [PubMed] [Google Scholar]
- 3.Kilbride H, Castor C, Hoffman E, Fuger KL. Thirty-six month outcome of prenatal cocaine exposure for near-term infants: impact of early case management. Dev Behav Pediatr. 2000;21:19–26. doi: 10.1097/00004703-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hurt H, Giannetta J, Brodsky NL, Malmud E, Pelham T. Are there neurologic correlates of in utero cocaine exposure at 6 years? J Pediatr. 2001;138:911–913. doi: 10.1067/mpd.2001.113709. [DOI] [PubMed] [Google Scholar]
- 5.Marques PR, Pokorni JL, Teti L, et al. Cognitive capabilities among school-age prenatally exposed to cocaine. Drug Alcohol Depend. 2002;66(suppl 1):S112. [Google Scholar]
- 6.Arendt RE, Short EJ, Singer LT, et al. Children prenatally exposed to cocaine: Developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulsifer MB, Radonovich K, Belcher HM, Butz AM. Intelligence and school readiness in children with prenatal drug exposure. Child Neuropsychol. 2004;10:89–101. doi: 10.1080/09297040490911104. [DOI] [PubMed] [Google Scholar]
- 8.Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicol Teratol. 2005;27:203–211. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta JM. A prospective evaluation of early language development in children with in utero cocaine exposure and in control subjects. J Pediatr. 1997;130:310–312. doi: 10.1016/s0022-3476(97)70361-5. [DOI] [PubMed] [Google Scholar]
- 10.Bandstra ES, Morrow CE, Vogel AL, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 11.Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow CE, Vogel AL, Anthony JC, Ofir AY, Dausa AT, Bandstra ES. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeghly M, Martin B, Rose-Jacobs R, et al. Prenatal cocaine exposure and children’s language functioning at 6 and 9.5 years: moderation effects of child age, birth-weight and gender. J Pediatric Psychol. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayes LC, Granger RH, Frank MA, Schottenfeld R, Bornstein MH. Neurobehavioral profiles of neonates exposed to cocaine prenatally. Pediatrics. 1993;91:778–83. [PubMed] [Google Scholar]
- 15.Delaney-Black V, Covington C, Ostrea E, Jr, et al. Prenatal cocaine and neonatal outcome: evaluation of dose-response relationship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- 16.Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcomes from a prospective, matched study of prenatal crack/cocaine use: II. Interactive and dose effects on neurobehavioral assessment. Pediatrics. 1998;101:237–241. doi: 10.1542/peds.101.2.237. [DOI] [PubMed] [Google Scholar]
- 17.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6 year olds. Neurotoxicol Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 18.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 19.Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–47. [PubMed] [Google Scholar]
- 20.Woods JR, Plessinger MA, Clark K. Effect of cocaine on uterine blood flow and fetal oxygenation. JAMA. 1987;257:957–961. [PubMed] [Google Scholar]
- 21.Bendersky M, Lewis M. Prenatal cocaine exposure and impulse control at two years. Ann N Y Acad Sci. 1998;846:365–367. [PubMed] [Google Scholar]
- 22.Butz AM, Pulsifer M, Marano N, Belcher H, Lears MK, Royall R. Effectiveness of a home intervention for perceived child behavioral problems and parenting stress in children with in utero drug exposure. Arch Pediatr Adolesc Med. 2001;155:1029–1037. doi: 10.1001/archpedi.155.9.1029. [DOI] [PubMed] [Google Scholar]
- 23.Butz AM, Pulsifer MB, Leppert M, Rimrodt S, Belcher H. Comparison of intelligence, school readiness skills and attention in in-utero exposed and non-exposed preschool children. Clin Pediatr (Phila) 2003;42:727–739. doi: 10.1177/000992280304200809. [DOI] [PubMed] [Google Scholar]
- 24.Thorndike RL, Hagen EP, Sattler JM. The Stanford Binet Intelligence Scale (4th ed): Guide for Administering and Scoring. Chicago, IL: Riverside; 1986. [Google Scholar]
- 25.Bracken B. Bracken Basic Concept Scale-Revised. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 26.Zimmerman IL, Steiner V, Pond RE. Preschool Language Scale-Third Edition Examiner’s Manual. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 27.Beery BE, Buktenica NA. The Developmental Test of Visual Motor Integration-Fourth Edition (VMI-4): Administration, Scoring and Teaching Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 28.Tiffin J. Purdue Pegboard: Examiner Manual. Chicago, IL: Science Research Associates; 1968. [Google Scholar]
- 29.Gordon M, McClure FD, Aylward GP. The Gordon Diagnostic System (GDS): Instruction Manual and Interpretive Guide. DeWitt, NY: Gordon Systems, Inc.; 1996. [Google Scholar]
- 30.Gordon M, Mettelman B. Technical Guide to the Gordon Diagnostic System (GDS) DeWitt, NY: Gordon Systems, Inc; 1987. [Google Scholar]
- 31.Knox HA. Mental defectives. N Y Med J. 1914;99:215–222. [Google Scholar]
- 32.Arthur G. A Point Scale of Performance Tests (Rev. Form II) New York, NY: The Psychological Corporation; 1947. [Google Scholar]
- 33.Gardner RA, Broman M. The Purdue Pegboard: normative data on 1,334 school children. J Clin Child Psychol. 1979;8:156–162. [Google Scholar]
- 34.Hollingshead AB. Mimeograph Report. New Haven, CT: Yale University; 1957. Two factor classification of social position. [Google Scholar]
- 35.Lewis BA, Singer LT, Short EJ, et al. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Noland JS, Singer LT, Short EJ, et al. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]


