Abstract
In adults, studies examining the long-lasting cognitive effects of marijuana use demonstrate subtle deficits in attention, executive function, and memory. Because neuromaturation continues through adolescence, these results cannot necessarily generalize to adolescent marijuana users. The goal of this study was to examine neuropsychological functioning in abstinent marijuana using and demographically similar control adolescents. Data were collected from 65 adolescent marijuana users (n = 31, 26% females) and controls (n = 34, 26% females) 16–18 years of age. Extensive exclusionary criteria included independent psychiatric, medical, and neurologic disorders. Neuropsychological assessments were conducted after >23 days of monitored abstinence. After controlling for lifetime alcohol use and depressive symptoms, adolescent marijuana users demonstrated slower psychomotor speed (p < .05), and poorer complex attention (p < .04), story memory (p < .04), and planning and sequencing ability (p < .001) compared with controls. Post hoc analysis revealed that the number of lifetime marijuana use episodes was associated with poorer cognitive function, even after controlling for lifetime alcohol use. The general pattern of results suggested that, even after a month of monitored abstinence, adolescent marijuana users demonstrate subtle neuropsychological deficits compared with nonusers. It is possible that frequent marijuana use during adolescence may negatively influence neuromaturation and cognitive development.
Keywords: Adolescents, Neuropsychology, Cognition, Cannabis, Alcohol, Drug effects
INTRODUCTION
Marijuana is the most widely used illicit intoxicant and a significant public health concern for adolescents. Almost half of 12th graders have tried marijuana, with 5% reporting daily use (Johnston et al., 2005). Early marijuana involvement can be particularly problematic, as use before age 15 is associated with a sevenfold increased risk of developing a substance use disorder in the future (SAMHSA, 2004). Concomitant alcohol and marijuana use is common, as 58% of adolescent drinkers also use marijuana (Agosti et al., 2002; Martin et al., 1996).
Animal studies have demonstrated cellular changes associated with chronic cannabis exposure, especially in pre-frontal, hippocampal, and cerebellar regions among mice (Childers & Breivogel, 1998; Ghozland et al., 2002; Misner & Sullivan, 1999), rats (Carta et al., 1998; Chan et al., 1998; Landfield et al., 1988; Romero et al., 1995; Rubino et al., 1997), and primates (Harper et al., 1977; Heath et al., 1980). Morphometric studies conducted among adult marijuana users have yielded conflicting results. Two studies reported both gray and white matter abnormalities in several brain regions among young adult marijuana users (Aasly et al., 1993; Matochik et al., 2005), although findings reported by Aasly and colleagues may have been attributable to alcohol use. In contrast, Block and colleagues, in a study excluding individuals with histories of heavy drinking, did not find structural brain abnormalities among cannabis users (Block et al., 2000). Recent functional neuroimaging studies on adults have found prefrontal, hippocampal, and cerebellar functioning abnormalities among marijuana users (Block et al., 2000, 2002; Eldreth et al., 2004; Gruber & Yurgelun-Todd, 2005; Loeber & Yurgelun-Todd, 1999; Lundqvist et al., 2001).
The neuropsychological effects of marijuana have been studied in adults for over three decades. However, the long-term effects of chronic cannabis use, as opposed to acute effects, are less characterized. In a meta-analysis examining 11 studies, Grant and colleagues (2003) found that chronic cannabis use was associated with persistent but subtle deficits in learning and memory, but not in other cognitive domains. Other studies have demonstrated persisting deficits in processing speed, attention, working memory, visuospatial skills, and executive functioning (Bolla et al., 2002; Croft et al., 2001; Ehrenreich et al., 1999; Lyons et al., 2004; Pope et al., 1997; Pope & Yurgelun-Todd, 1996; Solowij et al., 2002; Varma et al., 1988). However, some studies found no persisting cognitive deficits among adults with histories of heavy marijuana use (Carlin & Trupin, 1977; Pope et al., 2002; Schaeffer et al., 1981), and one study found that observed neurocognitive deficits normalized within a month of abstinence (Pope et al., 2001).
Because neuromaturation continues through adolescence (Giedd et al., 1996; Sowell et al., 2002), results based on adults cannot necessarily generalize to adolescent marijuana users. White matter develops into the late 20s (Benes et al., 1994; Jernigan & Gamst, 2005; Nagel et al., 2006; Pfefferbaum et al., 1994; Sowell et al., 1999). Concurrently, gray matter volume peaks around ages 12–14 then decreases, due largely to synaptic pruning (Huttenlocher, 1990; Toga et al., 2006) in the striatum and frontal lobe anterior to the motor strip (Jernigan & Tallal, 1990; Jernigan et al., 1991; Sowell et al., 1999), frontal poles, and lastly in the dorsolateral prefrontal cortex (Gogtay et al., 2004; Sowell et al., 2002), which also is late to myelinate (Paus et al., 1999). Furthermore, adolescence may be a period of vulnerability to the neurocognitive effects of drug and alcohol use (Monti et al., 2005; Spear, 2000). For example, CB1 cannabinoid receptor levels in animals peak in early adolescence (Belue et al., 1995), cannabis-exposed adolescent rats are more vulnerable to learning impairments compared with exposed adult rats (Cha et al., 2006; Schneider & Koch, 2003; Stiglick & Kalant, 1982, 1985), and early adolescent onset of use is associated with increased morphometric and cognitive abnormalities in adult marijuana users (Ehrenreich et al., 1999; Pope et al., 2003; Wilson et al., 2000).
Despite the high prevalence of marijuana use, few studies have examined neurocognitive functioning in heavy marijuana using adolescents (Verdejo-García et al., 2004). Recently, we examined hippocampal volume and asymmetry and verbal memory among 63 adolescents (alcohol using, alcohol and marijuana using, and nondrug using; Medina et al., 2007b). Similar to Tzilos and colleagues (2005), we found that marijuana and alcohol using adolescents did not significantly differ from controls in hippocampal volume. However, we did find that the correlations between hippocampal asymmetry and verbal learning were abnormal among the marijuana users compared with the nondrug using controls. More specifically, increased right greater than left hippocampal asymmetry was associated with improved verbal learning among the controls, while no significant correlations between structure and function were found among marijuana users. Consistent with the adult literature (Block et al., 2002; Kanayama et al., 2004), functional neuroimaging studies have found abnormal frontal, temporal, and parietal activation patterns among adolescent marijuana users compared with controls in response to verbal working memory (Jacobsen et al., 2004, 2007) and spatial working memory (Schweinsburg et al., 2005) tasks.
With few exceptions (Teichner et al., 2000), neuropsychological studies focusing on adolescent substance abusers have found persisting cognitive deficits associated with heavy marijuana use. In an inpatient treatment study, marijuana-dependent adolescents demonstrated short-term memory decrements after 6 weeks of abstinence compared with polydrug (nonmarijuana) users and controls (Schwartz et al., 1989). Marijuana using adolescents have also demonstrated increased perseverative responding on a problem solving task compared with control adolescents (Lane et al., 2006). A longitudinal investigation by Tapert and colleagues (2002) followed 47 polysubstance users and 26 normal controls over 8 years, from ages 16 to 24. Cumulative marijuana use over the 8-year follow-up period significantly predicted attention performance above and beyond effects accounted for by baseline attention scores, age, and practice effects. Another longitudinal investigation (Fried et al., 2005) that covaried for baseline functioning before marijuana initiation found that, among individuals with prenatal exposure to cannabis, heavy marijuana users demonstrated poorer overall IQ, processing speed, and immediate and delayed memory compared with controls.
One critique of previous research is that the observed neuropsychological deficits may be due to polysubstance use (Teichner et al., 2000; Tapert et al., 2002), family history of substance use disorders (Tapert et al., 2002; Tapert & Brown, 2000), or comorbid psychiatric disorders (Kruesi et al., 2004; Schwartz et al., 1989). Furthermore, cognitive deficits among marijuana users may be attributable to acute or subacute cannabis withdrawal (Pope et al., 2001). Therefore, the goal of this study was to characterize the neuropsychological effects of adolescent marijuana users without comorbid psychiatric disorders after approximately 1 month of abstinence. It was hypothesized that adolescent marijuana users would demonstrate significantly poorer cognitive function in areas associated with frontal, cerebellar, and hippocampal functioning (Loeber & Yurgelun-Todd, 1999; Lundqvist et al., 2001), including processing speed, complex attention, new learning, and executive function compared with demographically similar control adolescents following at least 23 days of monitored abstinence.
METHODS
Participants
Adolescents were primarily recruited from local high schools and universities via distribution of flyers and ads. To assess for study eligibility, comprehensive telephone screening measures were administered to both adolescents and parents/guardians. Inclusion criteria required that youth were between 16 and 18 years of age, fluent in English, and had a parent or legal guardian available to consent and provide medical and psychiatric history. Exclusionary criteria included history of Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM-IV) Axis I disorder (other than substance use disorder) or use of psycho-active medications; history of chronic medical illness, neurological condition (e.g., meningitis, HIV), or head trauma with loss of consciousness >2 min; significant prenatal alcohol (≥4 drinks in a day or ≥7 drinks in a week) or drug exposure; complicated delivery or premature birth (< 33 weeks gestation); learning disability or mental retardation; first-degree relative with history of bipolar I or psychotic disorders; left-handedness; and noncorrectable vision, colorblindness, or hearing impairments. If at any time during the 28-day abstinence period a teen reported or tested positive for any substance use, he/she was excluded from study and not included in any data analyses (five individuals were excluded for positive toxicology screens).
All participants and their parents/guardians (if teen is a minor) underwent written informed consent (written assent for minors) in accordance with the University of California, San Diego Human Research Protections Program. Teens were classified into two groups: a marijuana using (“MJ-user”) or a drug-free (“control”) group. A priori classification criteria for the MJ-user group included >60 lifetime marijuana experiences; past month marijuana use; < 100 lifetime uses (< 10 in past 3 months) of drugs other than marijuana, alcohol, or nicotine; and not meeting Cahalan criteria for heavy drinking status (Cahalan et al., 1969). Control group classification criteria were < 5 lifetime experiences with marijuana (none in the past month), no previous use of any other drug except nicotine or alcohol, and not meeting criteria for heavy drinking status (see Table 1).
Table 1.
Demographic and substance use information according to group
| Controls (n = 34) M (SD) or % [range] | Marijuana users (n = 31) M (SD) or % [range] | |
|---|---|---|
| Age | 17.86 (0.99) [16.02–18.99] | 18.07 (0.87) [16.53–19.12] |
| % Female | 26% | 26% |
| % Caucasian | 65% | 77% |
| % Family history negative/mild/positivea | 53/29/18% | 45/32/23% |
| Parent annual salary ($thousands) | 134.47 (69.67) [13–275] | 148.70 (113.94) [30–565] |
| WRAT-3 Reading Standard Score | 108.0 (7.9) [85–123] | 105.3 (8.2) [88–119] |
| WAIS-III Vocabulary T score | 57.3 (9.1) [39–75] | 55.7 (8.9) [36–70] |
| Grade point average* | 3.4 (0.58) [1.9–4.0] | 3.0 (0.79) [0.5–4.0] |
| % Experiencing problems in school*** | 0% | 26% |
| Beck Depression Inventory total** | 1.06 (1.82) [0–6] | 4.35 (5.58) [0–20] |
| Spielberger State Anxiety T score | 25.35 (5.93) [20–41] | 28.67 (7.95) [20–51] |
| Years of weekly marijuana use*** | 0 (0) | 2.91 (2.08) [0.75–9.90] |
| Marijuana hits/month, past 3 months*** | 0 (0) | 170.72 (234.03) [0–1125] |
| Lifetime marijuana use*** | 0.68 (1.36) [0–5] | 540.64 (380.24) [60–1800] |
| Marijuana abuse/dependence symptoms, past 3 months*** | 0 (0) | 3.19 (2.18) [0–9] |
| Days since last alcohol useb ** | 169.3 (234.14) [17–998] | 47.77 (63.08) [5–270] |
| Lifetime alcohol use episodes*** | 26.47 (45.51) [0–196] | 184.45 (145.53) [14–450] |
| Days of drinking, past month *** | 0.59 (1.18) [0–6] | 4.03 (3.82) [0–17] |
| Alcohol abuse/dependence symptoms, past 3 months*** | 0.18 (0.72) [0–4] | 1.87 (1.68) [0–6] |
| Years of weekly drinking*** | 0.10 (0.41) [0–1.90] | 1.22 (1.50) [0–4.83] |
| Drinks per month, past 3 months*** | 6.44 (12.47) [0–53] | 44.06 (39.09) [0–179] |
| Alcohol withdrawal symptoms, past 3 months*** | 0.08 (0.38) [0–2] | 0.71 (1.32) [0–4] |
| % Smoked cigarette in past month*** | 6% | 52% |
| Average cigarettes smoked per week in past month* | 0.58 (3.43) [0–20] | 13.37 (32.00) [0–150] |
| Days since last use of any drug (besides alcohol or nicotine)b ** | 0 (0) | 490.80 (458.18) [30–998] |
| Lifetime other drug use episodes*** | 0.06 (0.34) [0–2] | 8.60 (10.73) [0–33] |
Note. WAIS-III = Wechsler Adult Intelligence Scale-Third Edition; WRAT-3 = Wide Range Achievement Test-Third Edition.
Family history was calculated as Negative = no relatives with substance use disorder (SUD); Mild = one second-degree relative or two second-degree relatives on different sides with SUD; Positive = one or more first-degree relative or two second-degree relatives on the same side with SUD.
Lengths of abstinence only include those who had used in their lifetime; maximum value is 998 days.
Group difference p < .05.
Group difference p < .01.
Group difference p < .001.
Study Protocol
All participants from the current study completed the larger ongoing study (e.g., Medina et al., in press). Initial youth and parent/guardian screening interviews were administered separately by trained laboratory assistants to assess eligibility criteria. Participants were informed of the purpose of the study, procedures, potential risks and benefits, and confidentiality. Both parents and youth were informed that all study data are confidential (including group classification and toxicology results). If eligible after the initial screens, teens and parents were administered detailed interviews assessing demographic and psychosocial functioning, Axis I psychiatric disorders, and substance use history. To facilitate open disclosure, parents and youths were interviewed by different lab assistants, and confidentiality was guaranteed within ethical and legal limits. Adolescents who remained eligible were scheduled to begin the monitored abstinence protocol.
Youths were monitored with supervised urine and breath samples every 3–4 days for 4 weeks. Youths with positive urine samples or breath alcohol concentrations (Intoximeter AlcoSensor IV) or who appeared intoxicated were offered the option of restarting the toxicology screen procedure at a later time or to discontinue the study. If toxicology results indicated cessation and maintenance of abstinence, the adolescent received an evaluation between Day 23 and 27. Of MJ using youth who initiated monitored abstinence, 5 individuals had data suggesting substance use during the 4-week period, leaving 31 abstinent MJ users for this study. Youth who did not maintain abstinence were discontinued and compensated for their time. Upon completion of the study, youth and parents/guardians received financial compensation for participation.
Screening Inventories and Questionnaires
The detailed screening interview included the Structured Clinical Interview (SCI) measuring psychosocial functioning, activities, estimated pubertal stage, last menstruation (for females), health history, and handedness, and the computerized NIMH Diagnostic Interview Schedule for Children (C-DISC-4.0; Shaffer et al., 2000) excluded participants with major psychiatric disorders, including DSM-IV Axis I mood, anxiety, attention deficit hyperactivity disorder, and conduct disorders. Parallel modules of the computerized Diagnostic Interview Schedule (C-DIS-IV; Robins et al., 1996) were used for 18-year-olds who lived independently. Family history of psychiatric and substance use disorders was also assessed (Rice et al., 1995).
Youth were then administered the Customary Drinking and Drug Use Record (CDDR) to assess lifetime and past 3-month use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related life problems (Brown et al., 1998; Stewart and Brown, 1995). Youth were administered the modified Time-Line Followback (TLFB; Sobell and Sobell, 1992) to obtain detailed information regarding type, quantity, and frequency of drug use during the past month. The TLFB provides a detailed substance use pattern using a calendar format with temporal cues to aid recall. Teens were asked how much they used each of the following drugs: marijuana, alcohol, nicotine, stimulants (cocaine, amphetamine, methamphetamine, MDMA/ecstasy), opiates (heroin, narcotic pain relievers other than as prescribed), dissociatives/hallucinogens (PCP, mushrooms, LSD, ketamine), sedatives (GHB, barbiturates, benzodiazepines), and misuse of other prescription or over-the-counter medications.
If the youth continued to be eligible, a parent or guardian underwent a detailed screening interview using the parent version of the SCI, including information on prenatal/infant development, childhood behavior, age of developmental milestones, parental socioeconomic status (SES; Hollingshead, 1965), family history of psychiatric and substance use disorders (Rice et al., 1995) and youth and family medical and psychiatric history. Parents/guardians were also administered the parent version of the C-DISC-4.0 and the TLFB to improve the reliability of the youth diagnostic and substance use reports. At the neuropsychological session, youth were administered the Beck Depression Inventory (BDI; Beck, 1978) and the Spielberger State–Trait Anxiety Inventory (STAI) to assess mood (Spielberger et al., 1970).
Neuropsychological Battery and Composite Scores
A battery of standardized neuropsychological tests was administered to all participants. To reduce the number of dependent variables, a hybrid method using composite scores was used (for discussion, see Delis et al., 2003). This approach considered both the established categorization of cognitive domains (Lezak et al., 2004) as well as the results of reliability analyses. This strategy ensured that, for both subject groups, the individual tasks in each theoretical category were significantly correlated. After developing the composite categories, each individual neuropsychological variable was converted to a Z score based on the whole sample of adolescents (n = 65). The individual test Z scores were then averaged to form the final composite Z score for each cognitive domain. Internal consistency of the composite scores was assessed by standardized Cronbach’s α coefficients. Composite scores were reevaluated if α coefficient levels were < .50. As indicated in Table 2, this approach resulted in eight composite scores: (1) Psychomotor Speed: Delis–Kaplan Executive Function System (D-KEFS; Delis & Kaplan, 2000) Trail Making Test (TMT) Number Sequencing and Letter Sequencing subtest scores. (2) Complex Attention: California Verbal Learning Test-II (CVLT-II; Delis et al., 2001) List A Trial 1 recall; D-KEFS Letter Fluency total score; Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997b) Digit Symbol total score, Arithmetic total score, and Digit Span backwards score; and the Paced Auditory Serial Addition Test (PASAT) 2-second trial total score (Gronwall, 1974). (3) Sequencing Ability: D-KEFS TMT switching score and total errors. (4) Verbal Story Memory: The first recall, immediate recall, delayed recall, and recognition scores from Wechsler Memory Scale-Third Edition (WMS-III; Wechsler, 1997a) Logical Memory. (5) Verbal List Learning: Total recall, short delay free recall, long delay free recall, and recognition discriminability from the CVLT-II. (6) Visuo-spatial Function and Memory: Rey Osterrieth Complex Figure (Rey & Osterrieth, 1993) copy and delay accuracy; Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) Block Design subtest. (7) Verbal Accuracy: D-KEFS Verbal Fluency repetition errors; CVLT-II total repetitions and total intrusion errors. (8) Planning and Problem Solving: D-KEFS Towers achievement total score, final item score, and accuracy score.
Table 2.
Descriptive statistics of neuropsychological composite variables by group
| Controls (n = 34)
|
Marijuana users (n = 31)
|
||||
|---|---|---|---|---|---|
| Composite variablesa Testsb | M (SD) | Range | M (SD) | Range | Cronbach’s αc |
| Psychomotor Speedd | 0.06 (0.89) | −1.98–1.49 | −0.09 (0.78) | −2.59–1.02 | .57 |
| D-KEFS Trails Number Sequencing (SS) | 11.2 (2.8) | 1–15 | 10.8 (2.5) | 1–14 | |
| D-KEFS Trails Letter Sequencing (SS) | 11.2 (2.8) | 3–15 | 10.7 (2.6) | 3–14 | |
| Visuospatial Function and Memory | −0.09 (0.76) | −1.31–1.58 | 0.12 (0.78) | −1.71–1.53 | .65 |
| Rey-Osterrieth Copy Accuracy (raw) | 29.3 (3.4) | 23.0–34.0 | 30.9 (2.9) | 24.5–35.0 | |
| Rey-Osterrieth Delay Accuracy (raw) | 18.4 (4.1) | 9.0–29.0 | 19.5 (5.7) | 5.0–29.0 | |
| WASI Block Design (SS) | 58.1 (5.7) | 44–67 | 57.9 (6.3) | 47–67 | |
| Complex Attentiond | 0.04 (0.63) | −1.31–0.83 | −0.04 (0.68) | −1.44–1.43 | .73 |
| CVLT-II Trial 1 (Z score) | −0.18 (0.09) | −2.0–3.0 | −0.55 (0.6) | −2.0–2.0 | |
| D-KEFS Letter Fluency Total Correct (SS) | 12.2 (2.2) | 8–17 | 13.2 (3.3) | 7–19 | |
| WAIS-III Digit Symbol (SS) | 10.9 (2.2) | 6–15 | 10.4 (2.9) | 5–16 | |
| WAIS-III Arithmetic (SS) | 11.5 (2.5) | 6–15 | 11.1 (2.3) | 5–14 | |
| WAIS-III Digit Span Backward (raw) | (6.92.1) | 3–11 | 7.1 (2.2) | 4–14 | |
| PASAT-2 (raw) | 36.0 (9.1) | 16–53 | 34.9 (10.8) | 15–60 | |
| Verbal Story Memoryd | 0.04 (0.94) | −1.59–1.72 | −0.07 (0.93) | −2.20–1.52 | .94 |
| WMS-III Logical Memory 1st Recall (raw) | 26.6 (6.3) | 16–38 | 25.3 (6.1) | 14–36 | |
| Logical Memory I–Total (SS) | 9.7 (2.9) | 5–15 | 9.4 (2.6) | 4–14 | |
| Logical Memory II–Total (SS) | 10.5 (2.7) | 5–16 | 10.5 (2.7) | 5–16 | |
| Logical Memory Recognition (raw) | 26.5 (2.7) | 19–30 | 26.1 (3.0) | 17–30 | |
| Verbal List Learning | 0.08 (1.00) | −1.81–1.63 | −0.12 (0.82) | −2.22–1.54 | .94 |
| CVLT-II Total Recall (T score) | 53.8 (9.4) | 35–69 | 51.7 (8.5) | 28–66 | |
| CVLT-II Short Delay Free Recall(Z score) | 0.09 (1.1) | −2.0–2.0 | 0.12 (0.8) | −2.0–2.0 | |
| CVLT-II Long Delay Free Recall (Z score) | 0.07 (1.2) | −2.5–1.5 | −0.13 (1.0) | −2.5–1.5 | |
| CVLT-II Recognition Discrim. (Z score) | −0.12 (0.9) | −2.0–1.0 | 0.15 (0.9) | −2.5–1.0 | |
| Verbal Accuracy | 0.05 (0.84) | −3.05–0.91 | −0.04 (0.66) | −1.55–0.91 | .61 |
| D-KEFS Verbal Fluency Rep. Errors (raw) | 0.6 (1.2) | 0–5 | 0.7 (0.8) | 0–2 | |
| CVLT-II Total Repetitions (Z score) | 0.34 (1.0) | −1.0–4.0 | 0.23 (1.3) | −1.0–5.0 | |
| CVLT-II Total Intrusions (Z score) | 0.0 (0.9) | −1.0–2.5 | 0.29 (0.9) | −1.0–2.5 | |
| Sequencing Abilityd | 0.31 (0.73) | −2.02–1.18 | −0.31 (0.87) | −2.49–0.86 | .60 |
| D-KEFS Trails Switching (SS)e | 11.1 (1.8) | 6–14 | 9.9 (2.1) | 4–13 | |
| D-KEFS Trails Total Errors (SS)e | 11.4 (1.3) | 6–12 | 10.3 (1.3) | 7–12 | |
| Planning & Problem Solving | −0.15 (0.57) | −1.04–1.19 | 0.20 (0.79) | −1.55–2.56 | .65 |
| D-KEFS Towers Achievement (SS) | 9.6 (1.9) | 6–15 | 10.8 (2.8) | 6–19 | |
| D-KEFS Towers Item #9 Score (raw) | 0.9 (.7) | 0–3 | 1.2 (.9) | 0–4 | |
| D-KEFS Towers Accuracy (SS) | 8.1 (2.4) | 2–12 | 9.4 (2.2) | 6–14 | |
Note. SS = Scaled Score; CVLT-II = California Verbal Learning Test-Second Edition; D-KEFS = Delis–Kaplan Executive Function System; PASAT = Paced Auditory Serial Addition Test; WASI = Wechsler Abbreviated Scale of Intelligence; WAIS-III = Wechsler Adult Intelligence Scale-Third Edition; WMS-III = Wechsler Memory Scale-Third Edition, LM = Logical Memory subtest.
All composite variables used converted Z scores based on control and marijuana-user participants (N = 65).
Age-adjusted norm-based scaled scores and Z scores (when available) and raw scores for individual neuropsychological measures are provided for descriptive purposes only; sample-based Z scores were used in statistical analysis.
Composite variable reliability analysis used Cronbach’s standardized α.
Group status significantly (p < .05) predicted composite score after controlling for gender and lifetime alcohol use. Lifetime marijuana use significantly (p < .05) predicted composite score after controlling for lifetime alcohol use.
Follow-up analysis; group status predicted individual subtest (α = .05/number of subtests within a composite score).
Data Analysis
Demographic comparisons
To explore any potential group differences, ANOVAs and χ2 tests were run to compare groups on important demographic and drug use variables. Interpretations of statistical significance were made if p < .05.
Primary analysis: Group and composite scores
To assess the relationships between group status and neuropsychological performance, after controlling for depressive symptoms and lifetime alcohol use, ordinary least squares multiple regressions were run (n = 65) with each of the eight neuropsychological composite scores as dependent variables. The first step entered the following independent variables: group status (MJ-user vs. control), BDI score, and lifetime alcohol use. An interaction between group and lifetime alcohol use was entered on the second step. If the interaction term did not significantly contribute to the model, only results from the first step were reported.
Post hoc analyses: Individual neuropsychological tasks
For each significant composite score, we ran regressions to determine which individual neuropsychological tasks were predicted by group status after controlling for depressive symptoms and lifetime alcohol use. To reduce Type I error, domain-specific α levels were used (α = .05/number of subtests within a composite score).
Secondary analyses: Substance use patterns
To examine whether a dose-dependent relationship exists between lifetime marijuana consumption and neuropsychological function, multiple regressions were run (n = 65) in which the dependent variables were the eight neuropsychological composite scores and the independent variables were lifetime marijuana and alcohol consumption.
RESULTS
Demographic and Mood Information
ANOVAs and χ2s tested whether MJ-users and controls differed demographically (see Table 1). The MJ-users and controls did not differ in age [F(1,64) = .82; p = .37], grades completed [F(1,64) = .01; p = .92], WRAT-3 Reading standard score [F(1,64) = 2.1; p = .17] (Wilkinson, 1993), Vocabulary T score [F(1,64) = .71; p = .40] (Wechsler, 1999), gender composition [17 females, 48 males; χ2(1) = .004; p = .95], parental SES (Hollingshead, 1965) [F(1,64) = .01; p = .93], family history of substance use disorders (none, mild, or positive) [χ2(2) = .44; p <.80], STAI state anxiety T score [F(1,64) = 3.69; p = .06], racial identification (71% Caucasian, 12% multiple ethnicities, 9% Asian, 3% African American, 2% Pacific Islander, and 3% “other”) [χ2(5) = 6.96; p = .22], or percent reporting Hispanic or Latino ethnicity (14%) [χ2(2) = 0.50; p = .98]. The MJ-users reported more depressive symptoms on the BDI [F(1,64) = 10.62; p = .002] and lower grade point averages [F(1,64) = 4.5; p = .04], and they were more likely to report problems in school within the past 2 years [χ2(1) = 10.00; p = .002] than controls (although on the BDI, MJ-users were still within the nondepressed range).
Drug Use Information
As described above, monitored abstinence with urine toxicology occurred for a minimum of 23 days; based on youth self-report, participants were abstinent from all drugs for at least 30 days (light to moderate alcohol use may have occurred; participants with self-reported binge drinking or biological evidence of alcohol use during this time were excluded). The average length of abstinence from any alcohol use for MJ-users was 48 days (±63; range, 5–270 days) and 169 days for controls (±234; range,17–998 days) [F(1,64) = 13.77; p < .001]. Average length of abstinence from all other drugs for the MJ-users with such histories was 490 days (±458; range, 30–998 days). As expected, MJ-users reported more lifetime marijuana use episodes [F(1,64) = 68.7; p < .001], past 3-month marijuana use [F(1,64) = 18.1; p < .001], and symptoms of marijuana dependence [F(1,64) = 79.9; p < .001] than controls. MJ-users also had more lifetime [F(1,64) = 36.2; p < .001] and recent [F(1,64) = 20.7; p < .001] experience with alcohol than controls. Heavy nicotine use rates were low in both groups; however, more MJ-users than controls had smoked in the past month [χ2(1) = 16.9; p < .001]. Although MJ-users divulged more intake of other drugs than controls [F(1,64) = 21.4; p < .001], such use was limited to 33 lifetime experiences, most commonly recreational use of narcotic pain medications or hallucinogens (see Table 1).
Neuropsychological Functioning
See Table 2 for mean composite Z scores, Cronbach’s α coefficients, and standardized scores (when available) on the individual neuropsychological tests.
Bivariate Relationships
See Table 3 for bivariate relationships between marijuana and alcohol use variables and the neuropsychological composite scores according to group. In general, increased lifetime marijuana use was associated with poorer Complex Attention and Verbal Story Memory (p’s < .05), and marginally associated with poorer Verbal List Learning (p < .10). In contrast, more lifetime alcohol use episodes was associated with better Psychomotor Speed and Complex Attention scores (p’s < .05).
Table 3.
Bivariate correlations between neuropsychological composite scores and drug use variables by group
| Marijuana users (n = 31)
|
||||||
|---|---|---|---|---|---|---|
| Years of MJ use | Lifetime MJ use | # MJ symptoms | Years of Alc use | Lifetime Alc use | # Alc symptoms | |
| Psychomotor Speed | −.14 | −.27 | −.10 | .21 | .44* | .16 |
| Visuospatial Function and Memory | .04 | .01 | .09 | .31 | .14 | .15 |
| Complex Attention | −.36 | −.46** | .15 | .26 | .43* | .37* |
| Verbal Story Memory | −.28 | −.41* | .10 | .16 | .28 | .22 |
| Verbal List Learning | −.23 | −.31 | .21 | −.07 | .16 | −.06 |
| Verbal Accuracy | .14 | .29 | .16 | .01 | −.06 | .07 |
| Sequencing Ability | −.18 | −.07 | .04 | .15 | .29 | .33 |
| Planning and Problem Solving | −.10 | −.26 | .05 | .34 | .29 | .29 |
|
| ||||||
| Controls (n = 34) | ||||||
| Years of MJ use | Lifetime MJ use | # MJ symptoms | Years of Alc use | Lifetime Alc use | # Alc symptoms | |
|
| ||||||
| Psychomotor Speed | n/a | n/a | n/a | .04 | .14 | .12 |
| Visuospatial Function and Memory | n/a | n/a | n/a | .19 | .12 | .33 |
| Complex Attention | n/a | n/a | n/a | .21 | .31 | .14 |
| Verbal Story Memory | n/a | n/a | n/a | .36* | .28 | .31 |
| Verbal List Learning | n/a | n/a | n/a | .23 | .13 | .19 |
| Verbal Accuracy | n/a | n/a | n/a | .09 | .08 | .14 |
| Sequencing Ability | n/a | n/a | n/a | .15 | .14 | .19 |
| Planning and Problem Solving | n/a | n/a | n/a | −.13 | −.24 | .16 |
Note. # MJ/Alc Symptoms = denotes the number of DSM-IV marijuana or alcohol abuse or dependence symptoms met.
p < .05.
p < .01
Multivariate Relationships
Primary regression analysis: Group
After controlling for depressive symptoms and lifetime alcohol use, MJ-group status was associated with poorer Psy-chomotor Speed (β = −.32, p < .05), Complex Attention (β = −.33, p < .04), Sequencing Ability (β = −.53, p < .001), and Verbal Story Memory (β = −.34, p < .04). MJ-group status did not predict performance on the Verbal List Learning, Visuospatial Function and Memory, Verbal Accuracy, or Planning & Problem Solving composite scores.
Individual Test Follow-Up
The follow-up α levels corrected for multiple comparisons within each cognitive domain were as follows: Psychomotor Speed, .025 (.05/2); Complex Attention, .008 (.05/6); Sequencing Ability, .025 (.05/2); and Verbal Story Memory, .0125 (.05/4). Follow-up analysis revealed that MJ-users performed significantly poorer than controls on the two subtests that comprised the Sequencing Ability score, the D-KEFS TMT switching (β = −.44, p < .006) and total errors (β = −.45, p < .005) scores. The MJ-users performed marginally poorer (p < .10) than controls on the following individual tests: D-KEFS TMT Number Sequencing (p < .09; Psychomotor Speed subtest); CVLT-II Trial 1 recall (p < .02), Digit Symbol (p < .10), Digit Span backwards (p < .05), PASAT 2-second trial (p < .02; Complex Attention subtests); WMS-III Logical Memory first recall (p < .06), immediate recall (p < .06), delayed recall (p < .09), and recognition scores (p < .03; Verbal Story Memory subtests). Regarding covariates, unexpectedly, increased lifetime alcohol consumption was associated with better Psychomotor Speed (β = .37; p < .02) and Complex Attention (β = .45; p < .004) scores, but no significant Group × Alcohol use interactions were found. Higher BDI scores were associated with poorer Verbal Story Memory (β = .27; p < .05).
Secondary regression analysis: Substance use patterns
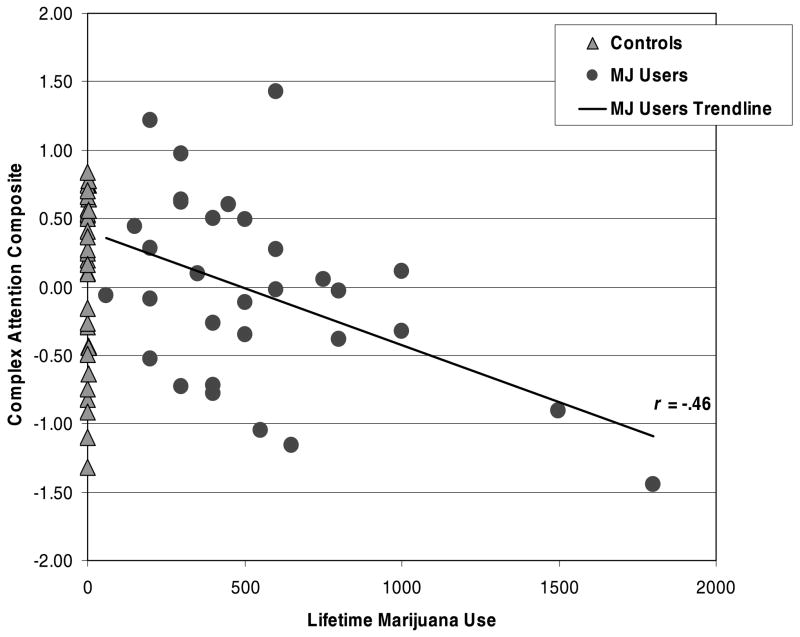
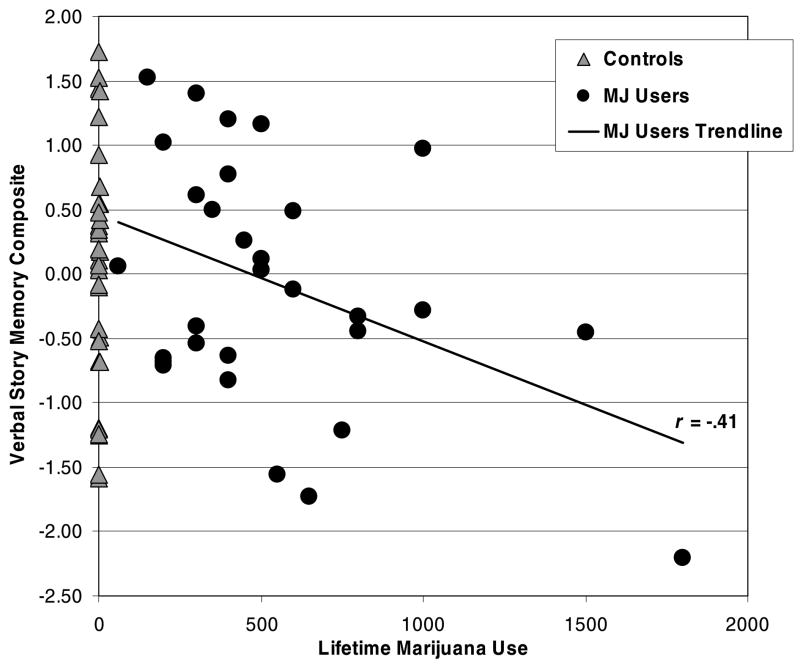
Given the bivariate results, a series of multiple regressions was run to examine the influence of lifetime marijuana use on neuropsychological performance after controlling for lifetime alcohol consumption. Similar to the group findings, increased lifetime marijuana use was associated with poorer Psychomotor Speed (β = −.27, p < .04), Complex Attention (β = −.40, p < .001), Verbal Story Memory (β = −.32, p < .02), and Sequencing Ability (β = −.32, p < .02). Unexpectedly, higher lifetime alcohol use episodes was associated with better Psychomotor Speed (β = .28, p < .04), Complex Attention (β = .38, p < .002), Verbal Story Memory (β = .26, p < .05), and Planning and Problem Solving (β = .31, p < .02). These relationships within the entire sample (Table 3) were primarily driven by the MJ-users. Figures 1 and 2 display the bivariate scatterplot between the Complex Attention and Verbal Story Memory scores, respectively, and lifetime marijuana use by group.
Fig. 1.
Bivariate scatterplot between the Complex Attention composite score and lifetime marijuana (MJ) use by group.
Fig. 2.
Bivariate scatterplot between the Verbal Story Memory composite score and lifetime marijuana (MJ) use by group.
DISCUSSION
The intent of the current study was to examine whether group status or extent of marijuana use was associated with neuropsychological functioning in a sample of adolescents who demonstrated approximately 1 month of abstinence. The primary finding was that, after controlling for alcohol use and depressive symptoms, adolescent marijuana users demonstrated poorer Complex Attention, Sequencing Ability, and Verbal Story Memory, and slower Psychomotor Speed compared with nondrug using control adolescents. Furthermore, dose-dependent relationships were observed between lifetime marijuana use and poorer cognitive performance in these same cognitive domains, even after controlling for lifetime frequency of alcohol use.
In general, post hoc analysis revealed that composite score differences were primarily driven by a pattern of slightly poorer performance among the MJ-users across several individual subtests within a cognitive domain. More specifically, after correcting for multiple comparisons, MJ-users significantly differed from controls on both sequencing and error subtest scores from the Sequencing Ability composite score (p’s < .006). MJ-users performed marginally poorer (p’s < .10) than controls on several other subtests, including the TMT Number Sequencing (Psychomotor domain); CVLT-II trial 1 recall, Digit Symbol, Digit Span backwards, PASAT 2-second trial (Complex Attention domain); and WMS-III Logical Memory first recall, immediate recall, delayed recall, and recognition (Verbal Story Memory domain) scores. This finding is consistent with longitudinal research following adolescents with substance use disorders over 8 years, also finding dose-dependent relationships between cumulative marijuana use and attentional and executive functioning (which concur with the current study’s complex attention and sequencing ability composite scores; Tapert et al., 2002). These findings lend further evidence to the literature that marijuana use during adolescence is associated with poorer attention, memory, and executive functioning (e.g., sequencing ability; Ehrenreich et al., 1999; Fried et al., 2005; Schwartz et al., 1989). This neuropsychological profile is consistent with the hypothesis, based on adult studies, that marijuana is primarily associated with frontal, hippocampal, and cerebellar dysfunction (Block et al., 2000, 2002; Eldreth et al., 2004; Gruber & Yurgelun-Todd, 2005; Loeber & Yurgelun-Todd, 1999; Lundqvist et al., 2001). Additional structural and functional neuroimaging research focused on abstinent adolescent marijuana users is necessary to confirm this hypothesis.
The current neuropsychological findings differ from those of Pope and colleagues (2001), who found that deficits in attention, short-term memory, and psychomotor speed were no longer measurable among adult marijuana users following 28 days of abstinence. One possible explanation for this discrepancy is that marijuana use during adolescence may negatively impact neuromaturation and cognitive development, resulting in more severe cognitive consequences compared with use during adulthood. For example, introduction of cannabis during adolescence may interrupt pruning of gray matter or disruption of white matter myelination, especially in the prefrontal cortex (Block et al., 2002; Egerton et al., 2006; Lundqvist et al., 2001), which continues to develop into early adulthood (Gogtay et al., 2004; Lenroot & Giedd, 2006; Nagel et al., 2006; Sowell et al., 2004). The current findings are consistent with animal studies that found more severe cannabis-induced learning impairments among adolescents compared with adults (Cha et al., 2006; Schneider & Koch, 2003; Stiglick & Kalant, 1982, 1985) and findings that early onset use is associated with increased morphometric, electrophysiological, and cognitive abnormalities among adult marijuana users (Ehrenreich et al., 1999; Kempel et al., 2003; Pope et al., 2003; Wilson et al., 2000). It is unknown whether continued abstinence from marijuana results in neurocognitive recovery or subsequent healthy neurodevelopment among adolescents. Therefore, longitudinal studies are necessary to investigate the long-term trajectory of cognitive and brain functioning in adolescent marijuana users.
Greater lifetime alcohol use was unexpectedly related to better performance on psychomotor speed and complex attention, primarily among the marijuana users. Of note, individuals who met Cahalan and colleagues’(Cahalan et al., 1969) criteria for Heavy Drinker were excluded, so adolescents with regular heavy binge drinking histories were not included in the current study. Still, this finding is in conflict with previous studies demonstrating dose-dependent relationship between increased alcohol use and poorer attention and sequencing ability (Tapert et al., 2002). One possible explanation is that some other unknown moderating factors (e.g., complex use variables, family functioning, or health behaviors) may explain the relationship between increased moderate alcohol use and improved cognitive function in this sample. Another possible explanation is that marijuana use could be somewhat neuroprotective in combination with moderate alcohol use during adolescence. For example, we have found that alcohol using adolescents demonstrated significantly smaller left hippocampal volumes, while combined marijuana and alcohol using adolescents had volumes similar to nonusers (Medina et al., 2007a). However, the combined users had significantly weaker correlations between hippocampal morphometry and verbal learning compared with healthy control adolescents, suggesting abnormal memory system functioning. Among adults, simultaneous use of cannabidiol and alcohol actually reduced blood alcohol levels compared with an alcohol-only condition (Consroe et al., 1979), and combined marijuana and alcohol dependent adults have performed better than alcohol-only dependent adults on an overall mean efficiency score derived from a computerized battery of cognitive tasks (Nixon, 1999). Thus, there is some evidence in the adult literature that the combined effects of marijuana and alcohol may not be as damaging as alcohol alone. Due to high rates of concurrent alcohol and marijuana use (Agosti et al., 2002; Martin et al., 1996; SAMHSA, 2004), we were unable to recruit a sizable sample of heavy marijuana users with no history of drinking for the current study, hindering our ability to tease apart the independent contributions of each substance. Additional animal and human research is necessary to further examine the independent and interactive effects of alcohol and marijuana use on neurocognitive function in adolescents.
As with any neuropsychological study, it is important to consider the clinical implications of these findings. Marijuana users performed 0.62 standard deviations poorer than controls on the Sequencing Ability composite, but less than half a standard deviation worse on other composite scores. However, considering that almost half of high school seniors have tried marijuana and 5% use it daily (Johnston et al., 2005), any observed differences in cognitive functioning is of concern. Notably, these group differences and dose-dependent relationships were observed among adolescent marijuana users who may be considered high functioning, with high SES and parental income (see Table 1), good physical and neurologic health, above average intelligence and reading ability, and the ability to abstain from substances for at least 1 month. Furthermore, the marijuana users in this sample did not have comorbid conditions associated with neurocognitive impairments, such as conduct disorder or attention deficit hyperactivity disorder (Aronowitz et al., 1994; Kruesi et al., 2004), groups were similar on family history of substance use disorders (Tapert & Brown, 2000), and abnormalities were observed after nearly a month of monitored abstinence. Thus, the current results may underestimate cognitive difficulties among the general population of adolescent marijuana users, who are more likely to be current users with comorbid psychiatric conditions. Still, even subtle cognitive difficulty may result in negative consequences in school and work (Lynskey & Hall, 2000). Students may miss information presented in class due to poorer processing speed, initial learning, and complex attention and working memory. Indeed, although their verbal intelligence and reading ability were comparable, the marijuana users obtained significantly lower grade point averages (3.0 vs. 3.4) and were more likely to demonstrate behavioral problems in school (26% vs. 0%) compared with controls. This finding may be a direct result of subtle cognitive difficulties, or due to effects of intoxication, sleep alterations, poor mood, withdrawal effects, and preexisting neurobehavioral problems (Tarter et al., 2006) for which the marijuana users are at increased risk.
Some methodological limitations should be considered. First, preexisting differences in neurocognition, which may increase risk for substance use (Nigg et al., 2004), cannot be ruled out in this cross-sectional study. Second, given the studies suggesting decreased motivation associated with marijuana use (Cherek et al., 2002; Lane et al., 2005), the observed cognitive differences may be due to amotivational influences on test performance. Third, we used composite scores for data reduction purposes, and although common practice, they may not reproduce in other samples. Fourth, results may not generalize to other samples with different lengths of abstinence, patterns of substance use (including nicotine; Jacobsen et al., 2007), gender or ethnic distribution, or SES/parental income.
In conclusion, the general pattern of results suggested that even after a month of abstinence, adolescent marijuana users demonstrate subtle deficits in psychomotor speed, complex attention, planning and sequencing, and verbal story memory compared with nonmarijuana using teens. Increased frequency of lifetime marijuana use was also associated with decreased performance in these areas. Implications include the need for psychoeducation aimed at informing adolescents and parents of the potential long-term cognitive consequences of heavy marijuana use. Longitudinal studies are critical to help rule out premorbid influences on cognitive function and to assess the developmental trajectory of neuropsychological functioning among adolescent marijuana users over time.
Acknowledgments
We thank the research associates in the Laboratory of Cognitive Imaging at UCSD, as well as the adolescent participants and their families. Funding was provided by grants from the National Institute on Drug Abuse (P.I.: Tapert, R21 DA15228 and R01 DA021182; P.I.: Medina, F32 DA020206), the National Institute of Neurological Disorders and Stroke (P.I.: Nagel, 7K08 NS052147), the National Institute on Alcohol Abuse and Alcoholism funded Fellowship (Hanson; P.I.: Riley, 5T32 AA1352505), and the National Institute on Mental Health funded Fellowship (Cohen-Zion; P.I.: Turner, 2T32 MH018399). We acknowledge that the information in this manuscript and the manuscript itself is new and original, that it is not currently under review by any other publication, and that it has never been published either electronically or in print. Portions of this study are presented at the 2007 annual meeting of the International Neuropsychological Society. We acknowledge that any human data included in this manuscript was obtained in compliance with the University of California, San Diego’s IRB regulations and the guidelines of the Helsinki Declaration. We do not have any financial or other relationships to disclose. All sources of financial support for this study have been disclosed.
References
- Aasly J, Storsaeter O, Nilsen G, Smevik O, Rinck P. Minor structural brain changes in young drug abusers. Acta Neurologica Scandinavica. 1993;87:210–214. doi: 10.1111/j.1600-0404.1993.tb04103.x. [DOI] [PubMed] [Google Scholar]
- Agosti V, Edward N, Frances L. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal of Drug and Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Aronowitz B, Liebowitz MR, Hollander E, Fazzini E, Durlach-Misteli C, Frenkel M, Mosovich S, Garfinkel R, Saoud J, DelBene D. Neuropsychiatric and neuropsychological findings in conduct disorder and attention-deficit hyper-activity disorder. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:245–249. doi: 10.1176/jnp.6.3.245. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corp; 1978. [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory related regional cerebral blood flow. Pharmacology, Biochemistry, and Behavior. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol 1998. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Carlin AS, Trupin EW. The effect of long-term chronic marijuana use on neuropsychological functioning. The International Journal of the Addictions. 1977;12:617–624. doi: 10.3109/10826087709022166. [DOI] [PubMed] [Google Scholar]
- Carta G, Nava F, Gessa GL. Inhibition of hippocampal acetylcholine release after acute and repeated Δ9-tetrahydrocannabinol in rats. Brain Research. 1998;809:1–4. doi: 10.1016/s0006-8993(98)00738-0. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta(9)-THC on learning in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. Journal of Neuroscience. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Lane SD, Dougherty DM. Possible amotivational effects following marijuana smoking under laboratory conditions. Experimental and Clinical Psychopharmacology. 2002;10:26–38. doi: 10.1037//1064-1297.10.1.26. [DOI] [PubMed] [Google Scholar]
- Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug and Alcohol Dependence. 1998;51:173–187. doi: 10.1016/s0376-8716(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology (Berlin) 1979;66:45–50. doi: 10.1007/BF00431988. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology. 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E. Delis-Kaplan Executive Functioning Scale Manual. San Antonio, Texas: Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition. San Antonio, Texas: The Psychological Corporation; 2001. [Google Scholar]
- Egerton A, Allison C, Brett RB, Pratt JA. Cannabinoids and prefrontal cortical function: Insights from pre-clinical studies. Neuroscience and Biobehavioral Reviews. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marijuana: A comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Aguado F, Espinosa-Parrilla JF, Soriano E, Maldonado R. Spontaneous network activity of cerebellar granule neurons: Impairment by in vivo chronic cannabinoid administration. European Journal of Neuroscience. 2002;16:641–651. doi: 10.1046/j.1460-9568.2002.02112.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Nonacute (residual) neurocognitive effects of cannabis use: A meta analytic study. Journal of the International Neuropsychological Society. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1974;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Brain Research, Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Harper JW, Health RG, Myers WA. Effects of cannabis sativa on ultrastructure of the synapse in monkey brain. Journal of Neuroscience Research. 1977;3:87–93. doi: 10.1002/jnr.490030202. [DOI] [PubMed] [Google Scholar]
- Heath RG, Fitzjarrell AT, Fontana CJ. Cannabis sativa: Effects of brain function and ultrastructure in rhesus monkeys. Biological Psychiatry. 1980;15:657–690. [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of the New York Academy of Science. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Gamst A. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Developmental Medicine and Child Neurology. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use, 1975–2004: Vol. 1. Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2005. (NIH Publication No. 05-5727) [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kempel P, Lampe K, Parnefjord R, Hennig J, Kunert HJ. Auditory-evoked potentials and selective attention: Different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research: Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: Possible mediation by glucocorticoid systems. Brain Research. 1988;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Steinberg JL. Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addictive Behaviors. 2005;30:815–828. doi: 10.1016/j.addbeh.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behaviors. 2006;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Loeber RT, Yurgelun-Todd DA. Human neuroimaging of acute and chronic marijuana use: Implications for frontocerebellar dysfunction. Human Psychopharmacology-Clinical and Experimental. 1999;14:291–304. [Google Scholar]
- Lundqvist T, Jönsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology. 2001;23:437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: A twin study. Psychological Medicine. 2004;34:1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, Tarter RE. Polydrug use in adolescent drinkers with and without DSM-IV alcohol abuse and dependence. Alcoholism: Clinical & Experimental Research. 1996;20:1099–1108. doi: 10.1111/j.1530-0277.1996.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, McQueeny T, Park A, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal asymmetry. Neurotoxicology and Teratology. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. The Journal of Neuroscience. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: Booze, brains, and behavior. Alcoholism: Clinical and Experimental Research. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitz-gerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. Journal of Abnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Neurocognitive performance in alcoholics: Is polysubstance abuse important? American Psychological Society. 1999;10:181–185. [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. Journal of Clinical Pharmacology. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychotherapy and Psychosomatics. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275:521–527. [PubMed] [Google Scholar]
- Rey A, Osterrieth PA. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and P.A. Osterrieth’s “The complex figure copy test”. In: Corwin J, Bylsma FW, translators. The Clinical Neuropsychologist. Vol. 7. 1993. pp. 3–21. [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Numberger JI, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0. (DIS 4.0) St. Louis, MO: Washington University of Medicine; 1996. [Google Scholar]
- Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira J, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to Δ9-Tetrahydrocannabinol. Pharmacology, Biochemistry, and Behavior. 1995;51:731–737. doi: 10.1016/0091-3057(95)00023-p. [DOI] [PubMed] [Google Scholar]
- Rubino T, Patrini G, Perenti M, Massi P, Paroloro D. Chronic treatment with a synthetic cannabinoid CP-55, 940 alters G-protein expression in the rat central nervous system. Molecular Brain Research. 1997;44:191–197. [PubMed] [Google Scholar]
- SAMHSA. Results from the 2003 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies, DHHS; 2004. [Google Scholar]
- Schaeffer J, Andrysiak T, Ungerleider JT. Cognition and long-term use of ganja (Cannabis) Science. 1981;213:465–466. doi: 10.1126/science.6972600. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory and performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1790. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases in Children. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BN, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Raye Z, Litten JPA, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press, Inc; 1992. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Learning impairment in radial-arm maze following cannabis treatment in rats. Psychopharmacology. 1982;77:117–123. doi: 10.1007/BF00431932. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology. 1985;85:436–439. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence, and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB. Predictors of marijuana use in adolescents before and after licit drug use: Examination of the gateway hypothesis. American Journal of Psychiatry. 2006;163:2134–2140. doi: 10.1176/ajp.2006.163.12.2134. [DOI] [PubMed] [Google Scholar]
- Teichner G, Donohue B, Crum TA, Azrin NH, Golden CJ. The relationship of neuropsychological functioning to measures of substance use in an adolescent drug abusing sample. International Journal of Neuroscience. 2000;104:113–124. doi: 10.3109/00207450009035012. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Yurgelun-Todd D. Lack of hippocampal volume change in long-term heavy cannabis users. American Journal of Addiction. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Varma VK, Malhotra AK, Dang R, Das K, Nehra R. Cannabis and cognitive functions: A prospective study. Drug and Alcohol Dependence. 1988;21:147–152. doi: 10.1016/0376-8716(88)90061-0. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, López-Torrecillas F, Giménez CO, Pérrez-García M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychology Review. 2004;14:1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-3rd Edition. New York: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. WAIS-III Manual. New York: Psychological Corporation; 1997b. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilkinson G. Wide Range Achievement Test. 3. Wilmington, DE: Wide Range, Inc; 1993. (WRAT-3) Manual. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]