Abstract
Background
Marijuana intoxication appears to impair response inhibition, but it is unclear if impaired inhibition and associated brain abnormalities persist after prolonged abstinence among adolescent users. We hypothesized that brain activation during a go/no-go task would show persistent abnormalities in adolescent marijuana users after 28 days of abstinence.
Methods
Adolescents with (n=16) and without (n=17) histories of marijuana use were compared on blood oxygen level dependent (BOLD) response to a go/no-go task during functional magnetic resonance imaging (fMRI) after 28 days of monitored abstinence. Participants had no neurological problems or Axis I diagnoses other than cannabis abuse/dependence.
Results
Marijuana users did not differ from non-users on task performance but showed more BOLD response than non-users during inhibition trials in right dorsolateral prefrontal, bilateral medial frontal, bilateral inferior and superior parietal lobules, and right occipital gyri, as well as during “go” trials in right prefrontal, insular, and parietal cortices (p<0.05, clusters>943 μl). Differences remained significant even after controlling for lifetime and recent alcohol use.
Conclusions
Adolescent marijuana users relative to non-users showed increased brain processing effort during an inhibition task in the presence of similar task performance, even after 28 days of abstinence. Thus, increased brain processing effort to achieve inhibition may predate the onset of regular use or result from it. Future investigations will need to determine whether increased brain processing effort is associated with risk to use.
Keywords: Marijuana, Cannabis, Functional magnetic resonance imaging, Adolescence, Response inhibition, Abstinence
Introduction
Many individuals first experiment with alcohol and drugs during adolescence (SAMHSA 2003), and marijuana is the most commonly used illicit substance among teenagers (Johnston et al. 2005). Nearly half of 12th graders have tried marijuana, and 6% disclose daily use (Johnston et al. 2005). Adolescence is also a period of continued neuro-development, including frontal lobe myelination and synaptic pruning that subserve improved executive functioning (Gogtay et al. 2004; Sowell et al. 2002), including abilities such as decision making, inhibitory processing, and impulse control. The influence of marijuana use on these maturational processes is unknown, although executive dysfunction may affect the development and maintenance of substance use and problems during adolescence (Tapert et al. 2002a).
Adults with heavy marijuana use histories have demonstrated abnormalities in executive functioning (Fletcher et al. 1996; Pope and Yurgelun Todd 1996; Solowij et al. 2002) and indices of frontal lobe operation (Block et al. 2000; Kanayama et al. 2004; Lundqvist et al. 2001; Solowij et al. 1991, 1995; Struve et al. 1998). To determine if cognitive deficits in marijuana users are transient or persistent, Pope et al. (2001) tested 63 current marijuana users (>5,000 lifetime uses), 45 former users (>5,000 lifetime uses), and 72 normal controls (1–50 lifetime uses), all ages 30–55. Participants were abstinent for 28 days, verified by urine drug screens and given neuropsychological tests on days 0, 1, 7, and 28 of abstinence. Current users showed some deficits relative to controls on days 0, 1, and 7, but groups did not differ on any test by day 28. In contrast, a study (Bolla et al. 2002) that hospitalized 22 marijuana-using adults (>2 years of marijuana use) for 28 days to assure abstinence found greater marijuana intake linked to poorer performance on tests of inhibition, problem solving, learning, and reaction time.
Disinhibition, i.e., the impaired ability to inhibit a potentially inappropriate response, is a common feature of substance misuse (Chen et al. 2007; Kamarajan et al. 2006; Kirisci et al. 2006; Verdejo-Garcia et al. 2006). Neuroimaging studies have reported both prefrontal and posterior parietal cortical involvement during inhibitory processing in healthy volunteers (Liu et al. 2004), which may be overactive in populations with impulse control problems (Schulz et al. 2004).
Few studies have examined inhibitory processing in marijuana users. Functional magnetic resonance imaging (fMRI) characterized the neural correlates of inhibition during a Stroop task, in which heavy marijuana-using adults and non-users were asked to inhibit the automatic process of reading words and, instead, name the colors of words printed in incongruent ink (Gruber and Yurgelun-Todd 2005). Marijuana users not only showed greater mid-cingulate and reduced anterior cingulate blood oxygen level dependent (BOLD) response but also more widespread bilateral dorsolateral prefrontal activation during inhibition relative to color naming trials. The investigators speculated that the altered activation pattern in marijuana users reflects compensation and use of alternate strategies. The same investigators had shown in a previous fMRI study using a working memory task before and after a 28-day abstinence period (Yurgelun-Todd et al. 1998) that adult users demonstrated less prefrontal and more anterior cingulate response than controls after just 24 h of non-use. Although users showed some normalization of prefrontal functioning after 28 days of abstinence, they still showed anterior cingulate dysfunction, which is supportive of persisting neural processing abnormalities. These results support the hypothesis that adult marijuana users show abnormal neural inhibitory processing, compensatory hyper-activity, and altered neural processing differences in the absence of the intoxicating agent.
Few studies have examined neurocognitive effects of marijuana use among adolescents, but some reports identified decrements in attention (Tapert et al. 2002b), and learning and memory (Millsaps et al. 1994; Schwartz et al. 1989) associated with early marijuana use. We previously demonstrated increased dorsolateral prefrontal and decreased inferior frontal fMRI response during a spatial working memory task among teens with comorbid alcohol and marijuana use disorders compared to those with alcohol use disorders alone and to non-using controls, suggesting a marijuana use-related increase in dorsolateral prefrontal effort (Schweinsburg et al. 2005b). However, most studies of adolescent marijuana users were conducted after several days of abstinence, so effects could be transitory. Jacobsen et al. (2004) addressed this concern by evaluating seven adolescent marijuana users, seven demographically similar tobacco smokers, and seven non-users with a working memory task during fMRI acquisition after about a month of abstinence. Marijuana users were less accurate on the task and showed increased BOLD response in the right hippocampus relative to other groups. The authors suggested that marijuana users might have failed to inhibit hippocampal activity during the task, perhaps due to cannabis-induced changes in inhibitory neurotransmission or apoptosis in the hippocampus. These researchers then compared 15 adolescents with and 18 without histories of cannabis use during nicotine withdrawal. Cannabis users, but not non-users, showed nicotine withdrawal-precipitated increases in parietal activation and disruptions in fronto-parietal connectivity during a verbal working memory task (Jacobsen et al. 2007). Collectively, existing data indicate some executive impairment and neural dysfunction associated with adolescent marijuana use, although the neural substrates specific to inhibitory processing have not yet been examined in teenage marijuana users.
In the current study, we performed BOLD fMRI during a go/no-go task to characterize response inhibition among adolescent marijuana users and non-using controls. Because studies of marijuana-using adults have suggested improvements in cognitive and neural functioning after 28 days of abstinence (Pope et al. 2001), we required participants to complete 28 days of monitored abstinence before scanning to ensure that any group differences were not due to recent use. The go/no-go task (Anderson et al. 2005; Schweinsburg et al. 2004b) was designed to assess inhibitory processing by asking participants to withhold a response to an infrequently occurring stimulus. Previous studies have observed activation in dorsolateral prefrontal, inferior frontal, anterior cingulate, and posterior parietal regions among adolescents using this task (Anderson et al. 2005; Schweinsburg et al. 2004a) and similar inhibition paradigms (Adleman et al. 2002; Luna et al. 2001; Tamm et al. 2002). Based on findings of response inhibition among teenagers and marijuana-using adults, we predicted that abstinent marijuana-using adolescents would demonstrate increased prefrontal and decreased cingulate response compared to non-using controls during inhibition trials.
Materials and methods
Participants
Participants were 16- to 18-year-olds enrolled in an ongoing study of adolescents recruited from local high schools and colleges (Medina et al. 2007a, b; Nagel et al. 2006; Schweinsburg et al. 2005a). Sixteen adolescents were marijuana users (MJ) with at least 60 lifetime episodes of cannabis use and limited histories of other drug use, and 17 adolescents were demographically similar non-using controls (see Table 1 for demographic characteristics) with less than five lifetime episodes of cannabis use. Written assent and consent were obtained from adolescents and their parent/legal guardians in accordance with the University of California San Diego Human Research Protections Program. Eligibility was ascertained through separate, private telephone screening interviews with the youth and the parent. Exclusionary criteria included left handedness; history of head injury with loss of consciousness >2 min, learning disabilities, medical or neurological problem, DSM-IV (APA 1994) Axis I psychiatric disorder (i.e., mood, anxiety, psychotic, attention, and conduct disorders) as assessed by the computerized NIMH Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b; Lucas et al. 2001; Shaffer et al. 2000) Youth and Parent versions, and use of any psychotropic medication; significant maternal drinking (more that two drinks on an occasion or more than four drinks in a week) or other drug use during pregnancy; family history of bipolar I or psychotic disorders determined by the Family History Assessment Module (Rice et al. 1995); and MRI contraindications.
Table 1.
Demographic and substance use characteristics of participants
| Marijuana users (n=16), M (SD) or % | Controls (n=17), M (SD) or % | |
|---|---|---|
| Age (range, 16.0–18.9) | 18.1 (0.7) | 17.9 (1.0) |
| Grades completed in school | 11.4 (0.8) | 11.2 (1.1) |
| % Female | 25% | 29% |
| % White | 75% | 59% |
| % Family history negative for substance use disorder a | 63% | 76% |
| Parent annual salary (*$ thousands) | 131.4 (88.9) | 131.7 (65.3) |
| Grade-point average | 3.3 (0.4) | 3.1 (0.6) |
| WRAT3 reading standard score | 106.9 (6.4) | 105.8 (8.3) |
| WASI vocabulary T score | 54.9 (9.1) | 55.8 (7.6) |
| Child Behavior Checklist Externalizing T score | 48.1 (6.0) | 44.9 (7.1) |
| Child Behavior Checklist Internalizing T score | 47.6 (7.3) | 46.8 (9.0) |
| Spielberger State Trait Anxiety T score | 39.2 (8.1) | 39.7 (9.8) |
| Beck Depression Inventory total | 4.6 (7.0) | 1.2 (2.0) |
| Age of first marijuana use | 14.0 (1.6) | 14.7 (0.6)b |
| Age of first weekly marijuana use | 15.4 (1.7) | – |
| Lifetime marijuana use episodes** | 475.6 (268.5) | 0.5 (1.3) |
| Marijuana use days per month before abstinence** | 14.3 (11.6) | 0.0 (0.0) |
| Days between scan and last marijuana use** | 58.4 (52.8) | 608.0 (210.7)b |
| Lifetime total drinks** | 194.50 (136.81) | 15.06 (38.81) |
| Drinks per month before monitored abstinence** | 34.8 (21.5) | 2.7 (9.4) |
| Alcohol hangover symptoms, past 3 months | 0.4 (1.2) | 0.0 (0.0) |
| Cigarettes per smoking day | 1.4 (2.7) | 0.2 (0.0) |
| Fagerstrom Test for Nicotine Dependence score | 0.3 (0.8) | 0.1 (0.2) |
| Lifetime other drug use episodes* | 6.9 (8.6) | 0.0 (0.0) |
No first-degree biological relative with alcohol or drug abuse or dependence
Figure includes only the 3 controls with history of use
p<.05
p<.001
MJ youth disclosed using marijuana an average of ~500 times in their lives (see Table 1), and three control youth had experience with marijuana (less than five times). MJ youth used marijuana every other day on average, before being asked to remain abstinent for the 28 days before scanning. Most MJ youth were current users, as all but three used in the month before monitored abstinence. MJ users typically consumed 35 drinks per month, one cigarette per day, and had used alcohol roughly 100 times and other drugs approximately seven times (range, 0 to 25 times; primarily misuse of pain medications) in their lives. Users and controls did not differ on age, household income, general intellect, externalizing and internalizing behaviors, and other demographic variables (see Table 1).
Measures
Substance use assessment
Substance involvement was ascertained using the Customary Drinking and Drug Use Record (Brown et al. 1998), an interview that obtains information on lifetime and past 3-month use of marijuana, alcohol, nicotine, other illicit drugs, and misuse of prescription and over-the-counter medications, and negative consequences associated with substance use. Strong internal consistency, test–retest, and inter-rater reliability have been demonstrated with adolescents (Brown et al. 1998; Stewart and Brown 1995). The Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al. 1991) indicated that no participant was dependent on nicotine. The Timeline Followback (Sobell and Sobell 1992) collected details on substance use patterns for the month before and during the 28-day abstinence period.
Mood
The Beck Depression Inventory (BDI; Beck 1978) and state scale of the Spielberger State Trait Anxiety Inventory (STAI; Spielberger et al. 1970) assessed mood 30 min before scanning.
Psychopathological syndromes
Continuous indices of level of internalizing and externalizing psychopathological syndromes were assessed by the 113-item Child Behavior Checklist (CBCL; Achenbach and Rescorla 2001), administered to parents (typically biological mothers) covering youth behaviors in the past 6 months. Eight-factor T scores (rule-breaking behavior, aggressive behavior, withdrawn/depressed, anxious/depressed, somatic complaints, attention problems, social problems, and thought problems) and summary T scores (externalizing, internalizing) are provided.
Personality
Disinhibition and substance use may relate to personality traits. Thus, all participants completed the NEO Five-Factor Inventory (NEO-FFI; Costa and McCrae 1992), a 60-item self-report personality questionnaire that assesses five domains of personality: neuroticism, extraversion, openness, agreeableness, and conscientiousness. This factor structure has been replicated in adolescent samples (Fruyt et al. 2000).
Neurocognition
Executive processing was assessed using the Delis–Kaplan Executive Function System (D-KEFS; Delis and Kaplan 2001) Trail Making, Towers, and Verbal Fluency subtests. Premorbid IQ was assessed with the Wide Range Achievement Test-3 (WRAT3; Wilkinson 1993) Reading subtest and Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999) Vocabulary subtest.
Go/no-go task
Participants performed a go/no-go task during fMRI scanning (Anderson et al. 2005; Schweinsburg et al. 2004b). The task alternated between active blocks and resting blocks. During resting blocks, participants viewed a “+” in the center of the screen. During active blocks, a large square, small square, large circle, and small circle were presented one at a time for 200 ms every 1,500 ms. Participants were instructed to press a button as fast as possible every time they saw a shape (go stimuli) except for the small square (no-go stimulus), thus requiring response inhibition. The task lasted 6 min and 24 s. Participants additionally performed the entire task before scanning to ensure adequate comprehension of instructions and to resolve potential practice effects (Drummond et al. 2006).
Procedures
To confirm that participants did not use substances in the 4 weeks before scanning, all participants (controls and marijuana users) provided at least nine urine toxicology screens (two to three times per week) during these 28 days. Urine toxicology procedures can reliably detect evidence of marijuana use 4 to 25 days after last use (Fraser et al. 2002; Huestis and Cone 1998), sometimes longer (Lafolie et al. 1991), depending on pattern of use, body lipid content, and metabolic features. Each urine sample was evaluated for metabolites indicating recent use of cannabis, amphetamines, methamphetamines, barbiturates, benzodiazepines, cocaine, codeine, morphine, phencyclidine, and ethanol using cloned enzyme donor immunoassay (CEDIA DAU, Microgenics, Fremont, CA, USA). Observed urine sample collection procedures reduced the possibility of participant tampering. Tetrahydrocannabinol (THC) values were monitored over the 28-day period to ensure that levels decreased. Initial samples testing positive for THC metabolites were assumed to reflect residual use if values decreased with each subsequent sample collected. If levels increased or a positive screen was obtained after a negative screen, the participant was given the option to restart the 28-day screening process or be dropped from the study. Breathalyzers (AlcoSensor IV, Intoximeter, St. Louis, MO, USA) were conducted at each visit. To confirm urine results, a hair sample collected on the scan day was analyzed for THC and other drug metabolites suggestive of past month use (Psychemedics, Culver City, CA, USA). Seven users, not described in this paper, were unable to complete the 28-day abstinence protocol.
In preparation for scanning, each participant lay on the scanner bed. Foam pads were arranged around the head to minimize motion. A mirror above the participant’s eyes enabled viewing of a screen at the foot of the scanner bed, on which the task was rear-projected from a laptop. Behavioral responses during the task were collected through a fiber-optic button box (LumiTouch, Vancouver, BC, Canada). Imaging data were acquired on a 1.5 Tesla Signa LX (General Electric, Madison, WI, USA) system. A structural image was collected in the sagittal plane using an inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (repetition time 2,000 ms, echo time 16 ms, field of view 240 mm, 128 continuous slices, resolution 0.9375×0.9375× 1.328 mm, acquisition time 8:36; Wong et al. 2000). During the go/no-go task, functional imaging was acquired in the axial plane using T2*-weighted spiral gradient recall echo imaging (repetition time 3,000 ms, echo time 40 ms, flip angle 90°, field of view 240 mm, 20 slices covering the whole brain, slice thickness 7 mm, in-plane resolution 1.875×1.875 mm, 128 repetitions, acquisition time 6:24).
Data analyses
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI; http://www.afni.nimh.nih.gov; Cox 1996). First, an automated motion correction algorithm was applied to the time series datasets (Cox and Jesmanowicz 1999). Two raters examined the time series data and removed repetitions containing residual visible head motions. If >18% of repetitions were removed, the participant was not included (n=3 not described in this paper). Groups did not differ in the number of repetitions removed (p=0.87) or absolute mean rotational or translational adjustments applied. Average rotational (roll, pitch, and yaw) and translational (superior, left, and posterior) movement, respectively, was 0.04, 0.11, and 0.05° and 0.07, 0.04, and 0.04 mm for MJ users, and 0.06, 0.12, 0.06° and 0.10, 0.04, and 0.06 mm for controls. No significant differences between groups were found for task-correlated motion (rs for the correlation between the task reference function and each motion parameter were −0.08, −0.10, −0.02, −0.11, −0.07, and −0.07 for MJ users and −0.04, −0.06, −0.04, −0.10, −0.08, and −0.06 for controls).
Next, time series datasets were deconvolved with a reference function representing the timing of go, no-go, and rest stimuli presentation over the course of the task (Ward 2002) while accounting for hemodynamic delays (Bandettini et al. 1993; Boynton et al. 1996) and covarying for motion adjustments applied and linear trends. This yielded fit coefficients representing the BOLD response for (1) inhibition (no-go) trials relative to baseline and (2) go trials relative to baseline in each voxel of the brain for each subject. Data were transformed to standard space (Lancaster et al. 2000; Talairach and Tournoux 1988), resampled into isotropic 3.5-mm voxels, and spatially smoothed with a Gaussian filter (3.5 mm full width half maximum) to account for anatomic variability between subjects.
We determined regions of significant BOLD response differences between MJ and control adolescents using independent samples t tests in each voxel of the brain, performed separately on the BOLD response contrast for no-go and for go trials. To control for type I error, we used a combination of t statistic magnitude and cluster volume thresholding (Forman et al. 1995; Ward 1997) by only interpreting clusters comprised of at least 22 contiguously activated voxels at a<.05 (≥943 μl in volume). Anatomic localization was confirmed using the Talairach Daemon (Lancaster et al. 2000; Ward 1997) and AFNI (Ward 1997).
Normality of distribution was evaluated for key variables (Tabachnick and Fidell 2007). No outliers or non-normal distributions were found for BOLD response contrast in clusters showing group differences. However, five variables (marijuana hits per month, lifetime other drug use, cigarettes smoked per month, FTND total, and BDI total) were positively skewed and kurtotic, so were inverse transformed [i.e., 1/(1 + the skewed variable); Tabachnick and Fidell 2007]. Group comparison results were further examined in analyses of covariance (ANCOVAs) that controlled for potential confound variables that differed between groups. Follow-up regression analyses were conducted among the MJ users (n=16) to examine BOLD response contrast to no-go trials, averaged across the cluster, in the regions that continued to differentiate users from controls in the above ANCOVAs. BOLD response contrast to no-go trials was the dependent variable, and variables indexing marijuana use, other substance use, and risk factors for substance involvement were the predictors. Behavioral data were compared between groups in one-way ANOVAs (a=0.05).
Results
Behavioral measures
Groups did not differ significantly on mood state measures (see Table 1), but MJ users showed a trend for higher BDI scores (p=0.08). Groups were similar and in the normal range on all CBCL factor T scores, except that the MJ group was higher on Rule-Breaking Behavior (53.00±8.23 MJ, 45.21±5.19 controls, p<0.005). On the NEO-FFI, MJ youth demonstrated greater scores on Openness (44.25± 3.11 MJ, 41.18±4.88 controls, p<0.05) but were statistically equivalent on other personality scores. MJ youth committed more errors than controls on several cognitive tests: more sequencing errors across all DKEFS Trails conditions (p<0.01), more intrusion errors on a word list learning task (p<0.05), and more repetition errors on DKEFS Verbal Fluency (p<0.05).
Task performance data were available for 15 MJ and 15 control adolescents. No group differences or group by condition interactions were found. As expected, participants responded more accurately (p<.001) to go trials (98.97± 1.88% MJ, 98.70±1.62% controls) than no-go trials (82.92± 9.57% MJ, 81.64±10.73% controls). Groups were equivalent on reaction time to go trials (588.11±40.44 MJ, 606.13± 74.35 controls, p=.38). D′, a signal detection index that considers the probability of correct and incorrect responses (i.e., the ability to separate go stimuli from the no-go stimulus), was also similar between groups (3.30±0.49 MJ, 3.04±0.84 controls, p=0.54). Exit interview data showed that groups were equivalent on task effort, motivation, and perceived errors, but MJ users indicated use of more perceptual strategies (e.g., “look for the small square to show up”), while controls tended to endorse cognitive strategies (“remember to hold off when I see that shape” or “concentrate”).
fMRI response
Whole brain analyses showed that during inhibitory trials relative to baseline, control adolescents showed significantly increased activation in superior medial frontal cortex and the left parahippocampal gyrus, and decreased activation in bilateral posterior parietal and inferior frontal regions. MJ users also showed increased inhibition-related activation in superior frontal areas, as well as in additional regions compared to controls, including numerous left and medial prefrontal clusters, left posterior parietal cortex, and multiple bilateral cerebellar regions. MJ users showed decreased BOLD response during no-go trials relative to baseline in right posterior parietal, inferior frontal, and middle temporal cortices.
Between-group comparisons revealed several clusters in which MJ users showed significantly more BOLD response during inhibition trials than controls: right anterior superior and middle frontal gyri (BA 10, 46), right superior middle frontal gyrus extending down to the anterior insula (BA 6, 13), left anterior middle and superior frontal gyri (BA 10), medial prefrontal cortex (BA 6), right and left posterior parietal cortex (BA 7, 40), and right lingual gyrus (BA 18; see Table 2 and Fig. 1). There were no clusters in which MJ users showed significantly less inhibitory brain response than controls.
Table 2.
Inhibition (no-go) trial differences in BOLD response contrast between marijuana using and non-using adolescents
| Anatomic region | Brodmann area | Volume (μl) | Talairach coordinatesa |
Effect size, Cohen’s d | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Marijuana users > controlsb | ||||||
| R superior and middle frontal gyri | 10, 46 | 2,015 | 37R | 48A | 14S | 2.84 |
| R middle frontal gyrus and insula | 6, 13 | 2,358 | 44R | 3A | 42S | 2.33 |
| L middle and superior frontal gyri | 10 | 1,200 | 33L | 55A | 3S | 3.03 |
| Bilateral medial frontal cortex | 6 | 1,544 | 2L | 3A | 52S | 3.00 |
| R inferior and superior parietal lobules | 40, 7 | 1,372 | 40R | 57P | 45S | 2.16 |
| L inferior and superior parietal lobules | 40, 7 | 3,473 | 40L | 54P | 52S | 2.35 |
| R lingual and middle occipital gyri | 18 | 1,929 | 23R | 89P | 3S | 1.99 |
R Right; L left; A anterior; P posterior; S superior; I inferior
Talairach coordinates refer to maximum signal intensity group difference within the cluster.
Marijuana users did not show less BOLD response contrast than controls in any region.
Fig. 1.
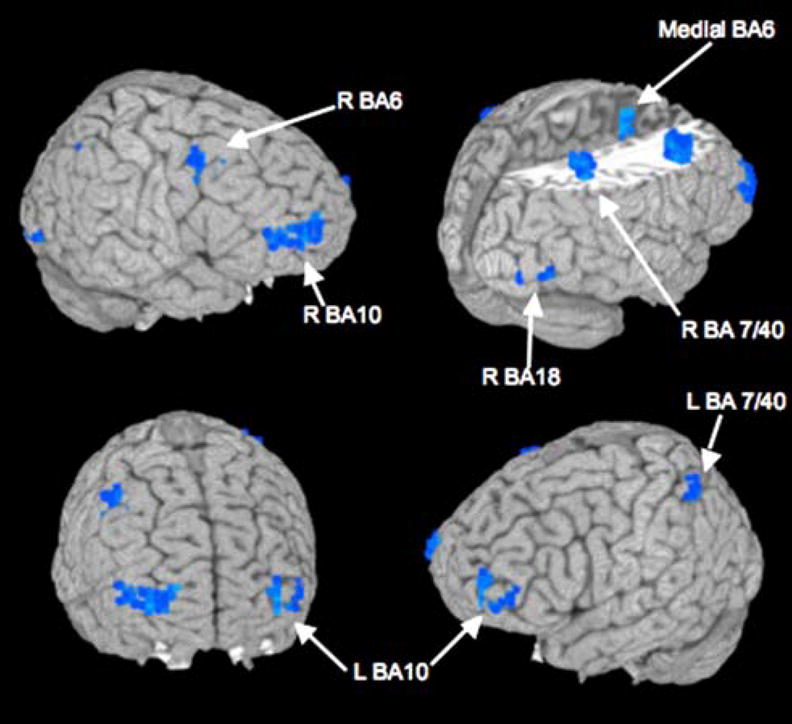
Inhibition trial differences in BOLD response contrast between marijuana-using (n=16) and non-using (n=17) adolescents after 28 days of monitored abstinence. Blue areas show where users had more significantly BOLD response during inhibition (no-go) trials relative to baseline than non-users (p<0.05, clusters >943 μl); no clusters in which users showed significantly less activation than controls were found (see Table 2)
For go trials relative to baseline, controls showed significant BOLD response in the bilateral ventral prefrontal cortex, bilateral medial posterior parietal cortex and cuneus, anterior cingulate, precentral gyrus, right anterior insula, and medial cerebellum. MJ users had multiple clusters of significant response to go trials relative to baseline, including increased activation in substantial portions of bilateral ventral prefrontal cortex, bilateral medial superior frontal cortex, right posterior parietal cortex, bilateral temporal cortex, anterior cingulate, pre-central gyrus, left insula, and bilateral cerebellum.
Between-group comparisons indicated that MJ users had significantly more BOLD response during go trials relative to baseline than did controls in the right inferior frontal gyrus and anterior insula (BA 44, 13), right superior frontal gyrus (BA 9, 10), right superior parietal lobule (BA 7), right inferior parietal lobule (BA 40), and medial precuneus (BA 7; see Table 3). There were no clusters in which MJ users had significantly less go response than controls.
Table 3.
“Go” trial differences in BOLD response contrast between marijuana using and non-using adolescents
| Anatomic region | Brodmann area | Volume (μl) | Talairach coordinatesa |
Effect size, Cohen’s d | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Marijuana users > controlsb | ||||||
| R inferior frontal gyrus, insula | 44, 13 | 4,588 | 54R | 13A | 14S | 2.58 |
| R superior and middle frontal gyrus | 9, 10 | 1715 | 23R | 52A | 31S | 2.55 |
| R superior parietal lobule | 7 | 1,544 | 30R | 57P | 56S | 3.11 |
| R inferior parietal lobule | 40 | 1,372 | 47R | 43P | 49S | 2.92 |
| R medial precuneus | 7 | 1,243 | 5R | 64P | 59S | 3.47 |
R Right; L left; A anterior; P posterior; S superior; I inferior
Talairach coordinates refer to maximum signal intensity group difference within the cluster.
Marijuana users did not show less BOLD response contrast than controls in any region
To determine whether the group differences reported above were due to differences in other substance use or factors that could predate marijuana use, secondary analyses using ANCOVAs separately (due to limited power) controlled for lifetime alcohol consumption, days since last drink, drinks per month, years of regular drinking, time since last cigarette, past week cigarettes per smoking day, cigarettes per month, FTND total, number of lifetime other drug use episodes, family history of substance use disorders, family history of psychiatric disorders, each NEO-FFI score, and BDI total. For all analyses, groups continued to differ on BOLD response in all clusters (p range, 0.001 to 0.0001).
To determine whether differences between groups were due to duration and intensity of exposure to THC or related to the recency of marijuana use, we conducted a series of exploratory regression analyses among MJ users (n=16). Duration of regular marijuana use was negatively related to no-go brain response in the right anterior superior frontal gyrus (BA 10; β=−0.73, p<0.001; plotted in Fig. 2), right superior middle frontal gyrus (BA 6; β=−0.58, p<0.025), and left anterior superior frontal gyrus (BA 10; β=−0.58, p< 0.025), suggesting that youth with longer durations of marijuana involvement had less no-go brain response. Similarly, early age of onset of regular marijuana use (β= 0.66,p<0.01) and more lifetime marijuana use episodes (β=−.62, p<0.025) related to less inhibitory response in the right anterior superior frontal gyrus (BA 10). Typical number of marijuana hits per month also was negatively related to brain response in the right anterior superior frontal gyrus (BA 10; β=−0.73, p<.005), right superior middle frontal gyrus (BA 6; β=−0.71, p<0.005), left anterior superior frontal gyrus (BA 10; β=−0.68, p<0.01), and left posterior parietal cortex (BA 40; β=−0.62, p< 0.025). Days since last marijuana use (range, 28 to 240), age, and neuropsychological test performance were not associated with brain response in any region demonstrating a group difference. Number of lifetime other drug use episodes (primarily pain medications) was positively associated with BOLD response contrast to no-go trials in the right anterior superior frontal gyrus (BA 10; β=0.57, p< 0.025) and right middle frontal gyrus (BA 6, β=0.56, p< 0.025). All analyses were re-run controlling for lifetime other drug use and remained unchanged except that hits per month no longer significantly predicted BOLD response.
Fig. 2.
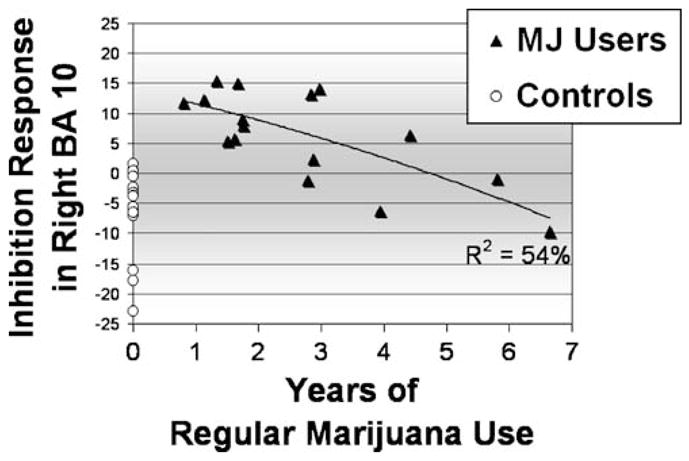
Marijuana using adolescents (n=16) show more BOLD response (y-axis displays fit coefficients) during inhibition trials relative to baseline than control adolescents (n=17) in right dorsolateral prefrontal cortex, largely accounted for by users with 1 to 3 years of regular (weekly) marijuana use
Discussion
In this study, marijuana-using adolescents showed increased BOLD response during both inhibitory and non-inhibitory trials of a go/no-go task, even after a 28-day washout period of abstinence from cannabis, as compared to control adolescents with limited substance use histories. Differences were prominent in dorsolateral prefrontal and parietal areas. Our findings with adolescent marijuana users extend those of others (Gruber and Yurgelun-Todd 2005), who showed that adult marijuana users exhibited increased prefrontal response during inhibition tasks. The present results thus suggest that the effects of cannabis use on brain function in adolescents may persist after a period of abstinence. A pattern of increased activation yet comparable performance is consistent with functional compensation and dedifferentiation (Rajah and D’Esposito 2005), which supposes that loci of functional activity are spread to more and larger regions. Therefore, adolescent marijuana users appear to recruit more neural tissue in executive control areas to adequately perform the task, which is consistent with other studies showing increased dorsolateral prefrontal activation in MJ users during inhibition (Gruber and Yurgelun-Todd 2005) and other (Schweinsburg et al. 2005b) cognitive tasks. Repeated cannabinoid use may alter neuromaturation in regions with high CB1 densities, such as prefrontal cortex (Eggan and Lewis 2007). Synaptic pruning and gray matter sculpting occurs late in some of the regions showing increased BOLD signal among users, such as bilateral dorsolateral prefrontal cortex, where cortical thickness peaks at age 11.5 on average (Gogtay et al. 2004; Sowell et al. 2003). If prefrontal synaptic pruning is slowed by adolescent marijuana use, greater activation may reflect excess neural firing caused by a larger number of synaptic links.
In their study of heavy marijuana-using adults, Gruber and Yurgelun-Todd (2005) reported that heavy users had increased prefrontal response to an inhibition task, as seen in this study of adolescents, but also showed reduced anterior cingulate response, which we did not observe in this study. One possible explanation for this difference is the duration of marijuana use in our cohort. It may take a longer period of use to evidence such changes in cingulate responding. The increased parietal activation among marijuana-using adolescents is consistent with findings from Jacobsen et al. (2007) of heightened parietal response in marijuana-involved youth during a verbal working memory task while undergoing nicotine withdrawal, although here, nicotine use rates were relatively low. As right dorsolateral prefrontal and parietal regions have been implicated in sustained attention (Drummond et al. 2005), perhaps, users had a greater cognitive load associated with attending to the stimuli in general and in identifying the no-go stimulus in particular. Both go and no-go trials require sustained attention, and overlap was observed for the right prefrontal/insular and parietal regions where users showed greater BOLD response than controls for both conditions.
Adolescents with later onsets (age, 16–18) and briefer durations (1–2 years) of regular marijuana use showed the greatest divergence from normal controls in BOLD response to the inhibition trials. Similarly, Chang et al. (2006) reported that adults who started marijuana use in late adolescence had more frontal activation during a visual attention task than adults who started in early adolescence. Although our data do not address the molecular mechanism of this difference, we can speculate about the nature of this finding. For example, increased prefrontal activation during an inhibitory task in adolescent users with a more recent onset may reflect a stage of neural and behavioral compensation. This compensatory response may occur within the first few years of regular adolescent marijuana use and may later be followed by neuroadaptation or other processes, possibly related to downregulation of cannabinoid (CB1) receptors. Therefore, an inverted U-shaped response pattern may emerge over time, which eventually results in brain activation patterns that are indistinguishable from non-users (suggested in Fig. 2).
BOLD response is influenced by cerebral blood flow, often abnormal in marijuana users acutely and subacutely. Up to 36 h since last use, THC intake is associated with increased cerebral blood flow (Mathew et al. 2002) and volume (Sneider et al. 2006) in frontal, insular, anterior cingulate, cerebellum, temporal, and paralimbic regions; reductions in auditory and visual cortex, parietal lobe, and thalamus; and no change in nucleus accumbens, basal ganglia, or the hippocampus (O’Leary et al. 2002). Although increases in blood flow velocity and cerebrovascular resistance dissipate from 3 to 28 days of abstinence in adults with marijuana use levels consistent with this sample (i.e., 15.9 days per month), very heavy users remained abnormally elevated (Herning et al. 2005). Thus, increased BOLD response in adolescent marijuana users could reflect residual cerebrovasculature abnormalities, although increased blood flow or volume would likely yield lower BOLD response (Brown et al. 2003), not greater, as seen here.
This study had several limitations. While groups were equivalent on many demographic variables, intelligence, and task performance, marijuana users had greater alcohol and other substance histories and a trend for more depressed symptomatology. Although activation patterns remained after controlling for these factors, substance and affective differences pose the possibility of synergistic effects, and we have previously found adolescent heavy drinking to be related to abnormal activation patterns on a spatial working memory task (Caldwell et al. 2005; Schweinsburg et al. 2005b; Tapert et al. 2001, 2004). Reduced educational engagement or altered sleep quality related to marijuana use might account for the differences. Critically, the current data cannot ascertain if marijuana use during adolescence produces the observed differences or if abnormalities existed before the onset of regular use. Groups did not differ on proportion with a family history of substance use disorder, and youths with psychiatric diagnosis (e.g., conduct disorder, attention-deficit/hyperactivity disorder) were excluded from the study. Groups were similar with regard to most personality and psychopathological syndrome dimensions but did differ on openness to new experiences and parent report of delinquent behaviors, which also may predate or result from adolescent marijuana use, but did not account for group differences in brain response.
On the other hand, the adolescents who diverged most from normal controls may simply differ on unmeasured risk factors related to the initiation and escalation of substance use. Altered inhibitory processing may make adolescents vulnerable to substance use initiation and escalation. The indication of extra neural effort required to inhibit responses has important implications for adolescent development and future substance involvement. Demands for inhibitory control increases substantially over the course of late adolescence, particularly in school and work environments as well as social and intimate relationships. Consequently, youth required to exert extra neurocognitive effort may be disadvantaged or become less successful in maneuvering the normal transitions of adolescence and young adulthood. Additionally, it may be harder for these youth to inhibit the use of substances, particularly in situations where cognitive demands are strained due to conflicting goals, distractions, peers, substance-relevant cues, or low mood states. Longitudinal studies are needed to see if changes in marijuana use among youth are associated with corresponding changes in brain functions shown abnormal in adolescent marijuana users.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants R21 DA015228-03 and R01 DA021182-01 to S. F. Tapert, and R37 AA07033-19 to S. A. Brown. Appreciation is expressed to the following people for their assistance with this project: Valerie Barlett, Christina Burke, Lisa Caldwell, Mairav Cohen-Zion, Lawrence Frank, Krista Lisdahl Medina, Tim McQueeny, MJ Meloy, Bonnie Nagel, Ann Park, Claudia Padula, Brian Schweinsburg, Rebecca Theilmann, and Jennifer Winward. We would also like to acknowledge the support and advice of Dr. Robert Fitzgerald and the VA Laboratory Service.
Portions of this research were presented at the Annual Scientific Meeting of the College on Problems of Drug Dependence, June 18-23 2005, Orlando, FL, USA.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Schweinsburg AD, Paulus MP, Brown SA, Tapert SF. Examining personality and alcohol expectancies using fMRI with adolescents. J Stud Alcohol. 2005;66(3):323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC, USA: 1994. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck depression inventory (BDI) Psychological Corporation; San Antonio, TX, USA: 1978. [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypo-activity in frequent marijuana users. NeuroReport. 2000;11(4):749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: Differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(Pt 5):1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31(1):156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Costa PTJ, McCrae RR. Professional manual: revised NEO personality inventory (NEO PI-R) and NEO five-factor inventory (NEO-FFI) Psychological Assessment Resources; Lutz, FL, USA: 1992. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E. Manual for the Delis–Kaplan executive function system. Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Drummond SPA, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick CM, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Drummond SPA, Paulus MP, Tapert SF. Effects of 2 nights sleep deprivation and 2 nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Morris R, Krauskopf D, Satz P. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53(11):1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fraser AD, Coffin L, Worth D. Drug and chemical metabolites in clinical toxicology investigations: the importance of ethylene glycol, methanol and cannabinoid metabolite analyses. Clin Biochem. 2002;35(7):501–511. doi: 10.1016/s0009-9120(02)00325-9. [DOI] [PubMed] [Google Scholar]
- Fruyt FD, Mervielde I, Hoekstra HA, Rolland J-P. Assessing adolescents’ personality with the NEO-PI-R. Assessment. 2000;7:329–345. doi: 10.1177/107319110000700403. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;64(3):488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22(6):445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use 1975–2004: vol 1, secondary school students (NIH Publication No. 05-5727) National Institute on Drug Abuse; Bethesda, MD, USA: 2005. [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibi-tion as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176(3–4):239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: a prospective study. Addict Behav. 2006;31(4):686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Lafolie P, Beck O, Blennow G, Boreus L, Borg S, Elwin CE, Karlsson L, Odelius G, Hjemdahl P. Importance of creatinine analyses of urine when screening for abused drugs. Clin Chem. 1991;37 (11):1927–1931. [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22(3):1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio Stipec M, Lahey BB, Friman P. The DISC predictive scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40 (4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol. 2001;23(5):437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, Provenzale J. Time course of tetrahydrocannabinol induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res. 2002;116(3):173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsaps CL, Azrin RL, Mittenberg W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescents. J Child Adolesc Subst Abuse. 1994;3(1):47–55. [Google Scholar]
- Nagel B, Medina K, Yoshii J, Schweinsburg A, Moadab I, Tapert S. Age-related changes in prefrontal white matter volume across adolescence. NeuroReport. 2006;17:1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26(6):802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun Todd D. Neuropsychological performance in long term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HGJ, Yurgelun Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275(7):521–527. [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128(Pt 9):1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19 (4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2002 national survey on drug use and health: national findings (No. DHHS Publication No. SMA 03-3836) Office of Applied Studies; Rockville, MD, USA: 2003. [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event related FMRI study. Am J Psychiatry. 2004;161 (9):1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child. 1989;143(10):1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Barlett VC, Killeen LA, Caldwell LC, Pulido CP, Brown SA, Paulus MP, Tapert SF. fMRI of response inhibition across adolescent development. Paper presented at the Annual Meeting of the International Neuropsychological Society; Baltimore, MD, USA. 2004a. [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido CP, Brown SA, Tapert SF. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann NY Acad Sci. 2004b;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005a;11(5):631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005b;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psych. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Pope HG, Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Altered regional blood volume in chronic cannabis smokers. Exp Clin Psychopharmacol. 2006;14(4):422–428. doi: 10.1037/1064-1297.14.4.422. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa, NJ, US: 1992. pp. 41–72. [Google Scholar]
- Solowij N, Michie PT, Fox AM. Effects of long-term cannabis use on selective attention: an event-related potential study. Pharmacol Biochem Behav. 1991;40(3):683–688. doi: 10.1016/0091-3057(91)90382-c. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. 1995;37(10):731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Struve FA, Patrick G, Straumanis JJ, Fitz-Gerald MJ, Manno J. Possible EEG sequelae of very long duration marihuana use: pilot findings from topographic quantitative EEG analyses of subjects with 15 to 24 years of cumulative daily exposure to THC. Clin Electroencephalogr. 1998;29(1):31–36. doi: 10.1177/155005949802900110. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Pearson; Boston: 2007. [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: an approach to cerebral imaging. Thieme; New York: 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psych. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann S, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psych. 2002a;41(6):680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002b;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12(3):405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI, USA: 1997. [Google Scholar]
- Ward BD. Deconvolution analysis of FMRI time series data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI, USA: 2002. [Google Scholar]
- Wechsler D. Manual for the Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX, USA: 1999. [Google Scholar]
- Wilkinson GS. The wide range achievement test-3 administration manual. Jastak Associates; Wilmington, DE, USA: 1993. [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank LR. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc Int Soc Magn Reson Med. 2000;8:683. [Google Scholar]
- Yurgelun-Todd D, Gruber AJ, Hanson RA, Baird AA, Renshaw PF, Pope HG., Jr Residual effects of marijuana use: an fMRI study. In: Harris LS, editor. Problems of drug dependence 1998: proceedings of the 60th annual scientific meeting, the college on problems of drug dependence. NIDA Research Monograph 179, National Institute on Drug Abuse; Bethesda, MD, USA. 1998. p. 78. [Google Scholar]


