Abstract
Objective
To determine the effectiveness of a home-based asthma education intervention in increasing appropriate nebulizer use and reducing symptom frequency, emergency department (ED) visits, and hospitalizations over 12 months.
Design
A randomized clinical trial.
Settings
Pediatric primary care, pulmonary/allergy, and ED practices associated with the University of Maryland Medical System and The Johns Hopkins Hospital, Baltimore.
Participants
Children with persistent asthma, aged 2 to 9 years, with regular nebulizer use and an ED visit or hospitalization within the past 12 months. Children were randomized into the intervention (n=110) or control (n=111) group. Follow-up data were available for 95 intervention and 86 control children.
Intervention
Home-based asthma education, including symptom recognition, home treatment of acute symptoms, appropriate asthma medication, and nebulizer practice.
Main Outcome Measures
Estimates of mean differences in asthma symptom frequency, number of ED visits and hospitalizations and appropriate quick relief, controller medication, and nebulizer practice over 12 months.
Results
Of the 221 children, 181 (81.9%) completed the study. There were no significant differences in home nebulizer practice, asthma morbidity, ED visits, or hospitalizations between groups (P range, .11–.79). Although most children received appropriate nonurgent asthma care (mean, 2 visits per 6 months), more than one third of all children received at least 6 quick-relief medication prescriptions during 12 months, with no difference by group.
Conclusions
A nebulizer education intervention had no effect on asthma severity or health care use. Of concern is the high quick-relief and low controller medication use in young children with asthma seen nearly every 3 months for nonurgent care.
Asthma, the leading chronic illness among US children, affects approximately 6.2 million children younger than 18 years,1 with the highest prevalence in African American boys from birth to the age of 4 years.2,3 Management of acute asthma symptoms in children begins at home. Decisions of when and how to treat home acute asthma episodes rely on the parent’s and child’s ability to accurately identify symptoms and to implement timely and appropriate medication therapy. Yet, one third of caregivers make significant errors in accurately perceiving their child’s asthma symptom severity4 that may result in overuse or underuse of short-acting β-agonist or quick-relief asthma medications. In young children, nebulizers are used to deliver quick-relief medications for symptom relief and to provide some controller medications, but are only effective if properly administered. However, many families fail to properly use nebulizers.5,6 Nebulizer use in young children is common, ranging from 33% to 71% in children younger than 12 years.5–7 Although metered-dose inhalers (MDIs) are as effective as nebulized medications for asthma in young children,8–11 many families prefer nebulizers to dispense medication to their child because of the parental lack of confidence in administering MDIs to young children and the difficulty of some children in coordinating respiration with MDIs.12 Physician preference for nebulizer administration of asthma medications is low, but those who favor nebulizer delivery usually do so because of the perceived direct aerosolization of medication into the respiratory system vs MDIs.13
Most asthma educational programs and national guidelines14,15 lack specific content addressing appropriate nebulizer practice and warnings of overdependence on quick-relief medications for asthma control. Furthermore, overuse of short-acting β-agonist medications delivered via MDI or nebulizer has been associated with fatal asthma.16,17 The present study determines the effectiveness of a home-based asthma educational intervention (INT) targeting symptom recognition and nebulizer use in young children with persistent asthma in reducing symptoms and health care use and in increasing appropriate home asthma medication use. We hypothesized that children receiving the intervention would demonstrate a decrease in asthma symptoms, improvement in appropriate nebulizer and asthma medication use, and a decrease in emergency department (ED) visits and hospitalizations.
METHODS
STUDY DESIGN
This was a 2-group randomized clinical trial conducted to evaluate the effectiveness of a home-based INT vs basic or standard asthma education (CON) on symptom frequency, appropriate nebulizer and asthma medication use, and ED visits and hospitalizations in children aged 2 to 9 years who had persistent asthma. The institutional review boards of The Johns Hopkins University Medical Institutions and the University of Maryland School of Medicine, Baltimore, approved the study protocols. We obtained informed consent from all participating parents, including consent for electronic monitoring of the child’s nebulizer and collection of the child’s pharmacy records. Assent was obtained from children 7 years and older. Data collection included telephone-administered questionnaires, pharmacy records, and electronic monitoring of home nebulizer use.
STUDY SUBJECTS
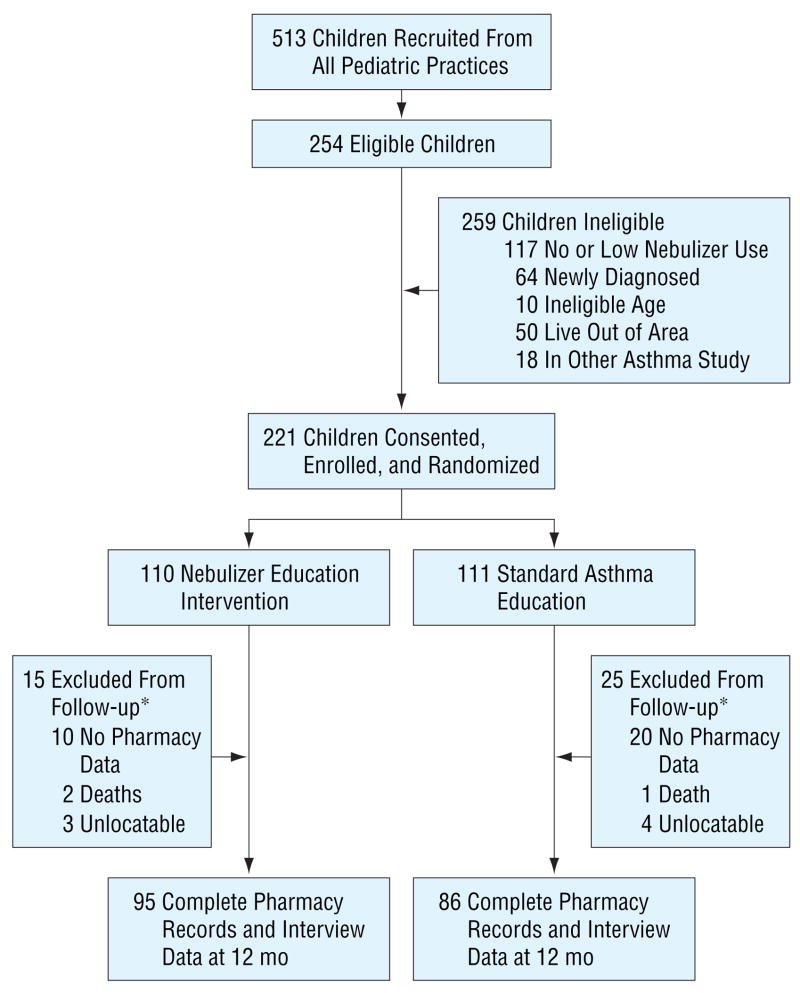
Children were recruited from pediatric practices affiliated with 2 large urban university hospitals, including pediatric primary care (29.9%), pulmonary/allergy specialty (50.2%), and ED (19.9%) practices between October 1, 2001, and December 31, 2003, and were followed up for 12 months. Eligibility criteria were as follows: aged 2 to 9 years, previous medical diagnosis of asthma, daytime asthma symptom frequency at least 2 or more times a week within the past 30 days, nighttime asthma symptom frequency at least 2 or more times a month for the past 30 days, use of a nebulizer to administer asthma medication within the past 30 days, resident of Baltimore, and 1 or more ED visits for asthma within the past 12 months or hospitalization for asthma in the past 12 months. A total of 513 children were recruited, of whom 254 (49.5%) were eligible; 221 (85.3%) provided consent and were randomized into the 2 study groups (INT or CON) (Figure). Ineligibility was primarily due to low or no nebulizer use in the prior 30 days and to children newly diagnosed as having asthma. At the 12-month follow-up, 181 (81.9%) of the children had complete interview and pharmacy record data to be included in the analysis (Figure). Incomplete pharmacy record data (n=30) were the primary reason for exclusion in final analysis. Three children died of asthma complications during the follow-up study, as described later. Children excluded from follow-up (n=40) did not significantly differ from children with complete data (n=181) by group status, age, sex, race/ethnicity, parental educational level, specialty care, days of nebulizer use in the past 30 days, or baseline asthma severity.
Figure.
Flow diagram of subject progress through a randomized trial. The asterisk indicates that P=.09 for the differences by group for those excluded from follow-up.
INT PROGRAM
The 6-month home-based asthma/nebulizer educational intervention was based on the Wee Wheezers Program,18 the A+ Asthma Club Program,19 an asthma symptom identification program, or children4,20 and nebulizer therapy recommendations.12,13 Parents of children in the INT group received 6 home visits of 1-hour sessions. The intervention curricula are detailed elsewhere.21
Parents of children randomized into the CON group received basic asthma education comparable to asthma education received during nonurgent care visits, during 3 home visits addressing dose and frequency of current asthma medications in the home, use of a peak flow meter, importance of an asthma action plan, and importance of regular asthma care. Specific quick-relief and controller medication use, symptom identification, and nebulizer use technique were not included in the educational content for CON families.
All home education visits were delivered by 3 community health nurses with pediatric asthma training and were supervised monthly by a pediatric nurse asthma specialist (A.M.B.). Families of both groups were randomly assigned to each nurse to ensure equal exposure to any differences in nurse teaching style. Most families completed all assigned educational home visits (CON group, mean [SD] of 2.9 (0.5) of 3 assigned visits; and INT group, mean [SD] of 5.6 (1.2) of 6 assigned visits).21
ELECTRONIC MONITOR OF NEBULIZER USE
Electronic nebulizer monitors, fitting all brands of nebulizer devices and developed by Hill-Rom Company, Inc (St Paul, Minn), were installed on all children’s nebulizers to record the date, time, and total length of each nebulizer use event, as described elsewhere.22 Electronic monitor data were downloaded at 3-month intervals over the 6-month intervention period. Monitors with no recorded use in the 12-week monitoring period were considered monitor failures (n=17) and were excluded from the analysis because of lack of objective validation of nebulizer use. Electronic data were used to measure the length and frequency of nebulizer sessions.
CHILD HEALTH OUTCOMES
Child health outcome and nebulizer practice measures were collected by face-to-face and telephone interviews conducted with the parent or caregiver at baseline and at the 12-month follow-up.
Asthma Severity
Children were assigned an asthma severity level based on national guidelines14,15,23 using frequency of child day and night symptoms based on caregiver responses to 2 specific questions, including “During the past 6 months on average, how many days per week did your child cough, wheeze or experience shortness of breath to limit exercise, ability to play sports or play with friends?” and “During the past 6 months on average, how many nights per month did your child wake up at night with cough, wheeze, shortness of breath, or tightness in chest?” Children who met the National Asthma Education and Prevention Program criteria for mild, moderate, or severe persistent asthma of daytime symptoms (>2 times per week) or nighttime symptoms (>2 times per month)14,15 were included in the study. Children classified as having mild intermittent asthma based on symptom frequency, and reporting daily anti-inflammatory medication use, were reclassified as having mild persistent asthma based on premedication severity.23
Asthma Morbidity and Health Care Use
Symptom frequency, number of ED visits, nonurgent or specialty care visits, and hospitalizations in the past 6 months were based on self-report. Specialty care visits were verified by medical record review, and nonurgent care visits were verified with the child’s primary care provider.
NEBULIZER USE PRACTICE
Nebulizer use practices, including sharing the nebulizer with family members, frequency of changing the nebulizer tubing and cleaning the nebulizer device, and home management of acute asthma exacerbations using a nebulizer, were ascertained by self-report.
MEDICATION PRESCRIPTION PRACTICE OUTCOMES
Pharmacy records were obtained from all pharmacies used by each child, as reported by the parent or caregiver over the 12-month follow-up. Change in pharmacy use was ascertained every 6 months. Pharmacies were contacted for complete lists of all medications dispensed for each child. A database was created to capture the dispensing date, product name, strength, dosage form, quantity, and days supplied (if available). Before double entry in the database, all pharmacy records were reviewed by an asthma nurse specialist (K.E.M.) to ensure complete data retrieval. Discrepancies in data entries were adjudicated. Quick-relief medications were defined as short-acting β-agonist and oral cortico-steroids, while controllers included inhaled corticosteroids, leukotriene modifiers, and mast cell stabilizers.
STATISTICAL ANALYSIS
Standard χ2 tests for categorical variables and t tests for continuous variables were used to test for differences between groups (INT vs CON) at baseline and at the 12-month follow-up. To analyze differences between groups for variables with nonnor-mal distributions, we used nonparametric tests, including the Wilcoxon rank sum test. For comparison of asthma medication regimens by asthma severity and study group, children were categorized into 3 groups based on their prescribed asthma medication regimens over the 12-month follow-up: (1) no asthma medications dispensed, (2) quick-relief medications dispensed only, and (3) anti-inflammatory controller use and quick-relief medications dispensed. Statistical significance was accepted as P≤.05. All analyses were performed using SAS statistical software (SAS V.8.0)24 and Stata V.7.025 software.
RESULTS
SAMPLE CHARACTERISTICS
Children were primarily African American, male, Medicaid insured, and living in households with parents reporting at least a high school level of education and annual incomes of less than $20 000 (Table 1). Asthma severity and health care use were high at baseline (moderate or severe asthma severity, 34.8%; and mean [SD] ED visits in the past 6 months, 2.0 [2.7]). Most parents and caregivers (75.9%) correctly reported cleaning the nebulizer after every use at baseline, but fewer correctly reported the recommended frequency of changing the tubing at 1 time per month (27.9%) compared with when it looks dirty (28.8%). Almost all caregivers (93.6%) reported trying nebulizer treatments at home before taking their child to the ED, and most (63.4%) preferred a nebulizer to an MDI (20.3%) for home administration of asthma medications. Sharing the nebulizer with other family members by the child was reported by almost 1 in 5 (19.0%) parents and caregivers.
Table 1.
Baseline Sociodemographic and Health Characteristics for 221 Children by Group Status*
| Characteristic | CON Group
(n = 111) |
INT Group
(n = 110) |
Total
(N = 221) |
|---|---|---|---|
| Race/ethnicity | |||
| African American | 103 (92.8) | 94 (85.5) | 197 (89.1) |
| White or other | 8 (7.2) | 16 (14.5) | 24 (10.9) |
| Male sex | 70 (63.1) | 75 (68.2) | 145 (65.6) |
| Child age (range, 2–9 y) † | 4.5 (2.0) | 4.6 (2.1) | 4.5 (2.1) |
| Medicaid health insurance | 87 (78.4) | 89 (80.9) | 176 (79.6) |
| Parent or guardian‡ | |||
| Mother | 99 (89.2) | 101 (91.8) | 200 (90.5) |
| Legal guardian or other relative | 11 (9.9) | 9 (8.2) | 20 (9.0) |
| Parent or guardian educational level | |||
| Some HS | 23 (20.7) | 30 (27.3) | 53 (24.0) |
| HS graduate or GED | 44 (39.6) | 41 (37.3) | 85 (38.5) |
| Some college or trade school or college graduate | 44 (39.6) | 39 (35.5) | 83 (37.6) |
| Mother employed outside the home | 72 (64.9) | 55 (50.0) | 127 (57.5) |
| Annual household income, $ | |||
| <20 000 | 56 (50.5) | 50 (45.5) | 106 (48.0) |
| ≥20 000 | 47 (42.3) | 42 (38.2) | 89 (40.3) |
| Asthma severity | |||
| Mild intermittent | 5 (4.5) | 5 (4.5) | 10 (4.5) |
| Mild persistent | 65 (58.6) | 69 (62.7) | 134 (60.6) |
| Moderate persistent | 27 (24.3) | 19 (17.3) | 46 (20.8) |
| Severe persistent | 14 (12.6) | 17 (15.5) | 31 (14.0) |
| Daytime symptom frequency | |||
| <2 times per week | 43 (38.7) | 46 (41.8) | 89 (40.3) |
| ≥3 times per week | 43 (38.7) | 44 (40.0) | 87 (39.4) |
| Daily, not continual | 20 (18.0) | 13 (11.8) | 33 (14.9) |
| Continual | 5 (4.5) | 7 (6.4) | 12 (5.4) |
| Night symptom frequency | |||
| <2 times per month | 41 (36.9) | 41 (37.3) | 82 (37.1) |
| ≥3 times per month | 38 (34.2) | 41 (37.3) | 79 (35.7) |
| >1 time per week | 21 (18.9) | 16 (14.5) | 37 (16.7) |
| Daily symptoms | 11 (9.9) | 12 (10.9) | 23 (10.4) |
| Health care use | |||
| No. of hospitalizations for asthma† | 0.41 (0.8) | 0.34 (0.6) | 0.38 (0.7) |
| No. of ED visits for asthma† | 2.0 (2.9) | 1.9 (2.5) | 2.0 (2.7) |
| No. of regular or nonurgent visits for asthma† | 2.8 (2.5) | 3.3 (3.0) | 3.0 (2.8) |
| Specialty care for asthma in the past 2 y | 57 (51.4) | 50 (45.5) | 107 (48.4) |
| Asthma medication use in the past 6 mo (pharmacy data) † | |||
| No. of short-acting 3-agonist prescriptions | 1.7 (1.8) | 1.8 (2.0) | 1.8 (1.9) |
| No. of oral corticosteroid prescriptions | 0.6 (0.9) | 0.6 (0.8) | 0.6 (0.9) |
| No. of inhaled corticosteroid prescriptions | 1.0 (1.5) | 0.8 (1.3) | 0.9 (1.4) |
Abbreviations: CON, standard asthma education; ED, emergency department; GED, general equivalency diploma; HS, high school; INT, asthma educational intervention.
Data are given as number (percentage) of each group unless otherwise indicated. Percentages may not total 100 because of rounding or because the number of children was fewer than the denominator given. At baseline, pharmacy data were available for 6 months before enrollment.
Data are given as mean (SD).
N = 220.
NEBULIZER USE PRACTICE
Baseline and follow-up nebulized and MDI quick-relief medication was predominantly albuterol (baseline, 95%; and follow-up, 93%). Controller medications were nebulized less often (ie, budesonide, 20.8%; and cromolyn, 1.8%). The mean (SD) duration of all nebulizer sessions was 12.9 (8.5) minutes (range, 5–96 minutes), the number of sessions ranged from 0 to 8 times per day, and neither variable differed by group. The mean number of short-acting β-agonist and budesonide prescriptions did not differ by group for delivery device (ie, MDI, dry powder inhaler, or nebulizer). The accuracy for nebulizer cleaning frequency increased from 75.9% to 86.2%, and there was a 18.8% increase in the accuracy of changing nebulizer tubing once a month, yet these nebulizer use behavior items did not differ by group. Sharing of nebulizers decreased to 11.0% at follow-up from baseline, yet this change did not differ between groups.
INTERVENTION EFFECTS AT 12 MONTHS IN 181 CHILDREN
Severity remained stable at follow-up (Table 2). Children in the CON group were more likely than those in the INT group to have had 1 or more hospitalizations and/or 1 or more ED visits for asthma during the past 6 months (P<.05 for both); however, the size of these effects remains uncertain. The mean number of non-urgent care visits over the past 6 months decreased from 3 to 2 from baseline to follow-up, with no differences by group.
Table 2.
Health Characteristics by Group for 181 Children at Follow-up
| Characteristic | CON Group
(n = 86)* |
INT Group
(n = 95)* |
Total
(N = 181)* |
Statistic† |
|---|---|---|---|---|
| Asthma morbidity and mortality over 12-mo follow-up | ||||
| Severity at 12 mo‡ | ||||
| Mild persistent | 57 (66.3) | 60 (63.2) | 117 (64.6) | 1.07 (0.93–1.22) |
| Moderate persistent | 7 (8.1) | 13 (13.7) | 20 (11.0) | 0.96 (0.50–1.83) |
| Severe persistent | 15 (17.4) | 10 (10.5) | 25 (13.8) | 1.50 (0.87–2.57) |
| Death | 1 (1.2) | 2 (2.1) | 3 (1.7) | 0.50 (0.05–5.39) |
| Health care use over 12-mo follow-up | ||||
| Health insurance, medical assistance | 70 (81.4) | 78 (82.1) | 148 (81.8) | 0.98 (0.86–1.12) |
| Specialty care in the past 12 mo | 43 (50.0) | 37 (38.9) | 80 (44.2) | 1.28 (0.92–1.78) |
| Hospitalized in the past 6 mo (≥1 time) | 11 (12.8) | 4 (4.2) | 15 (8.3) | 3.03 (1.00–9.18) |
| ED visit in the past 6 mo (≥1 time) | 40 (46.5) | 27 (28.4) | 67 (37.0) | 1.60 (1.08–2.37) |
| No. of nonurgent clinic visits for asthma in the past 6 mo | 1.85 (1.20–2.49)§ | 2.36 (1.26–3.46) § | 2.11 (4.40) || | t Test = 0.78, P =.44 |
| Asthma medication prescriptions over 12-mo follow-up | ||||
| No. of short-acting 3-agonist prescriptions (quick relief ) (range, 0–26) | 3.88 (2.94–4.82) § | 3.40 (2.74–4.06) § | 3.65 (3.83) || | z = 0.50, P =.61¶ |
| No. of oral corticosteroid prescriptions (quick relief ) (range, 0–8) | 1.20 (0.88–1.52) § | 1.16 (0.84–1.48) § | 1.20 (1.50) || | z = 0.48, P =.63¶ |
| No. of inhaled corticosteroid prescriptions (range, 0–14) | 3.30 (2.64–3.96) § | 2.47 (1.84–3.11) § | 2.90 (3.10) || | z = 2.38, P =.02¶ |
Abbreviations: See Table 1.
Data are given as number (percentage) of each group unless otherwise indicated.
Data are given as relative risk (95% confidence interval) unless otherwise indicated.
Reference includes mild intermittent severity.
Data are given as mean (95% confidence interval).
Data are given as mean (SD).
Two-sided Wilcoxon rank sum test.
Almost all children obtained inhaled quick-relief medications (93.4%), and most (59.1%) also received courses of oral corticosteroids. Most children (88.9%) had at least 1 albuterol prescription (range, 0–26 prescriptions) over the 12-month follow-up. More than one third of the children received 6 or more short-acting β-agonist prescriptions during the same period. The number of nebulizer and MDI quick-relief prescriptions did not differ by group over the follow-up. Although almost three quarters (71.8%) of the children obtained at least 1 prescription for an inhaled corticosteroid medication over the follow-up (Table 3), both groups had low exposure to inhaled corticosteroids, with a mean of 2.9 prescriptions over 12 months. The mean number of inhaled cortico-steroid prescriptions was significantly higher for the CON vs the INT children at follow-up (3.30 vs 2.47 prescriptions; P=.02). The frequency of inhaled corticosteroid refills was low for all children, with only 19.9% of children obtaining 6 or more inhaled corticosteroid prescriptions over the 12-month follow-up. Use of cromolyn was low (n=3).
Table 3.
Specific Asthma Medication Regimens Over Follow-up by Study Group and Severity Level in 174 Children*
| CON Group Severity† |
INT Group Severity† |
||||
|---|---|---|---|---|---|
| Asthma Medication Regimen | Mild Persistent
(n = 60) |
Moderate or Severe Persistent
(n = 32) |
Mild Persistent
(n = 54) |
Moderate or Severe Persistent
(n = 28) |
Total
(N = 174) |
| No medication | 2 (3.3) | 2 (6.3) | 4 (7.4) | 4 (14.3) | 12 (6.9) |
| Quick-relief medication only | |||||
| Short-acting β-agonist only | 7 (11.7) | 3 (9.4) | 11 (20.4) | 6 (21.4) | 27 (15.5) |
| Anti-inflammatory controller plus quick-relief use | |||||
| Inhaled corticosteroid plus quick-relief use‡ | 16 (26.7) | 9 (28.1) | 10 (18.5) | 8 (28.6) | 43 (24.7) |
| Leukotriene modifier plus quick-relief use | 2 (3.3) | 0 | 7 (13.0) | 1 (3.6) | 10 (5.7) |
| Inhaled corticosteroid plus leukotriene modifier or mast cell stabilizer plus quick relief‡ | 27 (45.0) | 14 (43.8) | 28 (51.9) | 13 (46.4) | 82 (47.1) |
| Oral corticosteroid use | |||||
| Oral corticosteroid bursts (≥1) | 34 (56.7) | 14 (43.8) | 38 (70.4) | 16 (57.1) | 102 (58.6) |
Abbreviations: See Table 1.
Data are given as number (percentage) for those who received at least 1 prescription over 12 months. Data exclude 7 children with incomplete severity data at follow-up.
All comparisons between groups and by severity were nonsignificant (P range, .13 to .94).
For any inhaled corticosteroid use alone or in combination, there were 125 (71.8%) of 174 children.
MORTALITY
Of the 3 deaths, 2 occurred in subjects assigned to the INT group, yet 1 INT child died before receiving any intervention education. The deaths occurred in children (mean [SD] age, 6.3 [2.5] years) with severe persistent asthma (66.6%), Medicaid insurance (100.0%), and low specialty care (33.3%). Health care use was also high in these children, with a mean (SD) of 2.0 (2.6) hospitalizations and a mean (SD) of 3.0 (1.7) ED visits over the past 6 months. Excessive quick-relief medication use (9 refills over 6 months) was noted in 1 child, yet only 1 child had adequate anti-inflammatory medication refills (4 refills) over 6 months. Neither asthma severity nor specialty care was associated with group assignment in the child deaths (severity: relative risk, 2.00 [95% confidence interval, 0.50–8.00]; and specialty care: relative risk, 4.50 [95% confidence interval, 0.32–63.94]).
COMMENT
Our data indicate that most parents and caregivers preferred using a nebulizer device for home medication administration to their children even when they had a choice of medication delivery device. Preference for nebulizer use has been noted in more symptomatic high-risk adults with asthma,26 suggesting that disease severity may be related to increased nebulizer use. We are unable to determine if preferential nebulizer use by parents was associated with the perception of more severe manifestations of disease in this sample; however, more than one third of the children had moderate or severe asthma.
Our results show that an INT targeting symptom recognition and appropriate nebulizer behavior had little effect on home nebulizer practice, asthma morbidity, or health care use outcomes over a 12-month follow-up. This lack of intervention effect may be due to not measuring other risks associated with nebulizer use, such as adequate storage of the device. Contamination of nebulizer equipment with cockroach allergen, resulting in antigen deposit directly into the small airways associated with severe asthma exacerbations, has been reported.27 We did not ascertain nebulizer storage practice, yet it is plausible in these inner-city homes that some nebulizers were contaminated with mouse, cockroach, or mold antigen27 and this may have indirectly contributed to the high asthma morbidity.
More important, this study indicates a high level of undertreatment and asthma mortality (1.4%) in this group of young inner-city children compared with the national asthma mortality rate of 0.7 per 100 000 in children younger than 18 years.2 This study confirms the overreliance of young children with persistent asthma on quick-relief medication only (short-acting β-agonist and oral corticosteroid use) for asthma control, as previously reported in children with asthma-related ED visits.28 Misuse of quick-relief medications has been associated with increased morbidity and mortality.17,29 Although almost three quarters of the children in this study received some inhaled corticosteroids over the 12-month follow-up, a mean of 3 inhaled corticosteroid prescriptions over 12 months is inadequate for preventive asthma care. Patterns of inappropriate asthma medication use may result from parental misunderstanding of the role of inhaled anti-inflammatory medicines in asthma,30 costly and overly complex medication regimens, worry about adverse effects,30 lack of follow-up care,31–33 underdiagnosis of asthma severity,34 lack of specialty care,35,36 psychosocial factors,37 and the increased risk of violence, parental substance abuse, and poor housing associated with inner-city residence contributing to nonadherence to asthma management regimens.38,39
Most children in this sample had at least 2 opportunities (ie, nonurgent care visits) for assessment of symptoms, asthma control, and appropriate nebulizer and medication use by the child’s health care providers (physicians, nurse practitioners, etc). The identification of young children requiring controller medication may decrease asthma morbidity40,41 but relies on an accurate diagnosis of asthma severity. Spirometry measurement is impractical in young children, and parental symptom reports may be inaccurate. The public health implications of this study are to include standard assessment of the frequency of quick-relief medication use or review of pharmacy refill records to improve identification of children with “uncontrolled” asthma. In fact, pediatricians who received training in accurate diagnosis of asthma and patient communication not only had shorter patient-physician interactions compared with patients of physicians not receiving the training (22.8 vs 27.1 minutes) but also increased their rate of anti-inflammatory prescriptions.42
There are several limitations to be considered with this study. Although we were able to obtain pharmacy records regarding the number of prescriptions dispensed over a 12-month period, we are limited in determining actual medication use by the child or sharing of medication among household members. Prescription records do indicate drug availability and have been shown to be a reliable source of drug exposure.43 Parent self-report indicates that quick-relief medication was the predominant nebulizer medication administered. Asthma severity may not be precise in that we based severity on parent recall of symptoms and report of anti-inflammatory medication use at 1 point. However, the severity classifications were consistent at baseline and 12 months. Last, some families assigned to the CON group may have been contaminated with content from the INT group in that nurses delivered the INT and CON to all families.
In summary, despite preference by most caregivers to use a nebulizer device for home asthma medication administration, an INT designed to teach symptom recognition and appropriate nebulizer use had little effect on asthma morbidity or health care use. Although nebulizer use remains prevalent in young children, the trend is toward increased use of MDIs. More important, this study reveals a high level of undertreatment and asthma mortality in a group of young children with persistent asthma despite most children being seen almost every 3 months for nonurgent care. We believe that a more mul-tifaceted intervention may be necessary to improve health outcomes in this high-risk group of children.
Acknowledgments
Funding/Support: This study was supported by grant NR05060 from the National Institute of Nursing Research.
We thank the pharmacists for retrieving the pharmacy data and the families for their willingness to participate in this study.
Footnotes
Role of the Sponsor: The funding body had no role in data extraction and analyses, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- 1.American Lung Association Epidemiology and Statistics Unit. Trends in Asthma Morbidity and Mortality. New York, NY: American Lung Association; 2005. pp. 14–16. [Google Scholar]
- 2.National Center for Health Statistics, Centers for Disease Control and Prevention. [Accessed October 20, 2005];Health data for all ages. Available at: http://www.cdc.gov/NCHS/Healthdataforallages.htm.
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma: United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 4.Yoos HL, Kitzman H, McMullen A, Sidora K. Symptom perception in childhood asthma: how accurate are children and parents? J Asthma. 2003;40:27–39. doi: 10.1081/jas-120017204. [DOI] [PubMed] [Google Scholar]
- 5.Zach MS, Karner U. Sudden death in asthma. Arch Dis Child. 1989;64:1446–1451. doi: 10.1136/adc.64.10.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butz AM, Eggleston P, Huss K, Kolodner K, Rand C. Nebulizer use in inner-city children with asthma: morbidity, medication use, and asthma management practices. Arch Pediatr Adolesc Med. 2000;154:984–990. doi: 10.1001/archpedi.154.10.984. [DOI] [PubMed] [Google Scholar]
- 7.Dawson KP, Jandera E, Penna AC. The prehospital management of children with acute asthma. J Paediatr Child Health. 1992;28:321–322. doi: 10.1111/j.1440-1754.1992.tb02677.x. [DOI] [PubMed] [Google Scholar]
- 8.Chou KJ, Cunningham SJ, Crain EF. Metered-dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med. 1995;149:201–205. doi: 10.1001/archpedi.1995.02170140083015. [DOI] [PubMed] [Google Scholar]
- 9.Schuh S, Johnson DW, Stephens D, Callahan S, Winders P, Canny GJ. Comparison of albuterol delivered by metered-dose inhaler with spacer versus a nebulizer in children with mild acute asthma. J Pediatr. 1999;135:22–25. doi: 10.1016/s0022-3476(99)70322-7. [DOI] [PubMed] [Google Scholar]
- 10.Lewis RA, Fleming SS. Fractional deposition from a jet nebulizer: how it differs from a metered dose inhaler. Br J Dis Chest. 1985;79:361–367. doi: 10.1016/0007-0971(85)90069-5. [DOI] [PubMed] [Google Scholar]
- 11.Kerem E, Levison H, Schuh S. Efficacy of albuterol administered by nebulizer versus spacer device in children with acute asthma. J Pediatr. 1993;123:313–317. doi: 10.1016/s0022-3476(05)81710-x. [DOI] [PubMed] [Google Scholar]
- 12.Canny GJ, Levison H. Aersols: therapeutic use and delivery in childhood asthma. Ann Allergy. 1988;60:11–20. [PubMed] [Google Scholar]
- 13.Barry PW, O’Callaghan CO. Nebuliser therapy in childhood. Thorax. 1997;52(suppl 2):S78–S88. doi: 10.1136/thx.52.2008.s78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Heart, Lung, and Blood Institute. National Institutes of Health publication. Bethesda, Md: National Institutes of Health, National Heart, Lung, and Blood Institute; 1997. National Asthma Education and Prevention Program: Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma; pp. 97–4051. [Google Scholar]
- 15.National Heart, Lung, and Blood Institute. Expert Panel Report: guidelines for the diagnosis and management of asthma: update on selected topics—2002. Bethesda, Md: US Dept of Health and Human Services; 2002. [Google Scholar]
- 16.Hannaway PJ. Demographic characteristics of patients experiencing near-fatal and fatal asthma: results of a regional survey of 400 asthma specialists. Ann Allergy Asthma Immunol. 2000;84:587–593. doi: 10.1016/s1081-1206(10)62408-8. [DOI] [PubMed] [Google Scholar]
- 17.Sears MR, Rea HH, Fenwick J, et al. 75 deaths in asthmatics prescribed home nebulisers. BMJ. 1987;294:477–480. doi: 10.1136/bmj.294.6570.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson SR, Latini D, Starr NJ, et al. Education of parents of infants and very young children with asthma: a developmental evaluation of the Wee Wheezers Program. J Asthma. 1996;33:239–254. doi: 10.3109/02770909609055365. [DOI] [PubMed] [Google Scholar]
- 19.Schneider SL, Richard M, Huss K, et al. Moving health care education into the community. Nurs Manage. 1997;28:40–43. [PubMed] [Google Scholar]
- 20.Yoos HL, McMullen A. Symptom perception and evaluation in childhood asthma. Nurs Res. 1999;48:2–8. doi: 10.1097/00006199-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Butz AM, Syron L, Johnson B, Spaulding J, Walker M, Bollinger ME. Home-based asthma self-management education for inner city children. Public Health Nurs. 2005;22:189–199. doi: 10.1111/j.0737-1209.2005.220302.x. [DOI] [PubMed] [Google Scholar]
- 22.Butz AM, Donithan M, Bollinger ME, Rand C, Thompson RE. Monitoring nebulizer use in children: comparison of electronic and asthma diary data. Ann Allergy Asthma Immunol. 2005;94:360–365. doi: 10.1016/S1081-1206(10)60988-X. [DOI] [PubMed] [Google Scholar]
- 23.Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention: Revised 2002. Bethesda, Md: National Institutes of Health, National Heart, Lung, and Blood Institute; 2002. [Google Scholar]
- 24.SAS Institute Inc. SAS/STAT User’s Guide: Version 8.01 Ed. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 25.Stata Statistical Software: Release 7.0. College Station, Tex: Stata Corp; 2001. [Google Scholar]
- 26.Krishnan JA, Demott M, McCoy JV, Chanmugam A, Rand CS. Nebulized β2-agonist use in high-risk inner-city adults with asthma. J Asthma. 2003;40:367–373. doi: 10.1081/jas-120018636. [DOI] [PubMed] [Google Scholar]
- 27.Bollinger ME, Wolf B, Schwindt C, Hamilton RG. Contamination of nebulizer equipment with cockroach allergen: there’s a bug in the system! Ann Allergy Asthma Immunol. 2004;92:475–477. doi: 10.1016/S1081-1206(10)61786-3. [DOI] [PubMed] [Google Scholar]
- 28.Stempel DA, McLaughlin TP, Stanford RH. Treatment patterns for pediatric asthma prior to and after emergency department events. Pediatr Pulmonol. 2005;40:310–315. doi: 10.1002/ppul.20264. [DOI] [PubMed] [Google Scholar]
- 29.Stempel DA, McLaughin TP, Stanford RH, Fuhlbrigge AL. Patterns of asthma control: a 3-year analysis of patient claims. J Allergy Clin Immunol. 2005;115:935–939. doi: 10.1016/j.jaci.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 30.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25:107–130. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Farber HJ, Capra AM, Finkelstein JA, et al. Misunderstanding of asthma controller medications: association with nonadherence. J Asthma. 2003;40:17–25. doi: 10.1081/jas-120017203. [DOI] [PubMed] [Google Scholar]
- 32.Bender B, Milgrom H. Compliance with asthma therapy: a case for shared responsibility. J Asthma. 1996;33:199–202. doi: 10.3109/02770909609055360. [DOI] [PubMed] [Google Scholar]
- 33.David C. Preventive therapy for asthmatic children under Florida Medicaid: changes during the 1990s. J Asthma. 2004;41:655–661. doi: 10.1081/jas-200026432. [DOI] [PubMed] [Google Scholar]
- 34.Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the United States. Pediatrics. 2000;105:272–276. [PubMed] [Google Scholar]
- 35.Vollmer WM, O’Hollaren M, Ettinger KM, et al. Specialty differences in the management of asthma: a cross-sectional assessment of allergists’ patients and generalists’ patients in a large HMO. Arch Intern Med. 1997;157:1201–1208. [PubMed] [Google Scholar]
- 36.Mudd K, Bollinger ME, Donithan M, Butz AM. The impact of asthma action plans on quality of life and other asthma outcomes [abstract] J Allergy Clin Immunol. 2004;113:S103. [Google Scholar]
- 37.Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med. 2001;155:347–353. doi: 10.1001/archpedi.155.3.347. [DOI] [PubMed] [Google Scholar]
- 38.Wade S, Weil C, Holden G, et al. Psychosocial characteristics of inner-city children with asthma: a description of the NCICAS psychosocial protocol. Pediatr Pulmonol. 1997;24:263–276. doi: 10.1002/(sici)1099-0496(199710)24:4<263::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 40.Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 41.Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long-term prevention of hospitalization for asthma. Thorax. 2002;57:880–884. doi: 10.1136/thorax.57.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark NM, Gong M, Schork A, et al. Impact of education for physicians on patient outcomes. Pediatrics. 1998;101:831–836. doi: 10.1542/peds.101.5.831. [DOI] [PubMed] [Google Scholar]
- 43.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50:619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]