Abstract
Background
Wound infections have been a problem in the field of surgery for a long time. Advances in control of infections have not completely eradicated this problem because of development of drug resistance. Antimicrobial resistance can increase complications and costs associated with procedures and treatment.
Objective
A study was carried out on drug sensitivity patterns of bacterial isolates from septic postoperative wounds in Jinja hospital, Uganda. This study was designed to determine the distribution of bacterial pathogens isolated from septic post-operative wounds and their antimicrobial susceptibility patterns.
Method
Specimens of pus swabs were collected aseptically and analysed in the laboratory. Colony characteristics and Grams technique were used to differentiate the organisms. Biochemical tests were done to confirm the species of the organisms. Sensitivity testing was done on the isolates using the disk diffusion method.
Results
Pathogenic bacteria were recovered from 58.5% of the specimens. The isolates were: S.aureus (45.1%), Coliforms (16.9%), Proteus mirabilis (11.3%), P.aeruginosa (9.9%), Klebsiella pneumoniae (7.0%) and Enterobacter spp (2.82%). Most of the organisms were sensitive to gentamicin, ciprofloxacin and ceftazidime. There was resistance to ampicillin, amoxycillin and chloramphenicol. Staphylococcus aureus was generally sensitive to gentamicin (87.5%), ciprofloxacin (68.7%) and methicillin (75%), but resistant to erythromycin (56.2%) and ampicillin (97%). Most of the gram-negative bacteria isolated (Coliforms, P.aeruginosa, E.coli, Proteus mirabilis, and Klebsiella pneumoniae) were sensitive to Ciprofloxacin, Gentamicin and Ceftazidime but resistant to Ampicillin, Amoxycillin and Chloramphenicol. Methicillin-resistant Staphylococcus aureus (MRSA) strains formed 25% of this species. Pseudomonas aeruginosa was sensitive to gentamicin (87.5%) and ceftazidime (85.7%) but showed resistance to ciprofloxacin (57.2%). Some organisms e.g. S.aureus, Pseudomonas aeruginosa and Proteus mirabilis exhibited multi-drug resistance to the antibiotics tested.
Conclusion
Since a high proportion of samples had positive cultures, infection control is recommended as a strategy to minimise spread of resistant organisms. It is recommended that gentamicin, ciprofloxacin and ceftazidime be used in preference to ampicillin and amoxycillin for treatment of septic wounds.There is need to develop national surveillance of antibiotic- resistant organisms.
Introduction
Wound infections have been a problem in the field of surgery for a long time. Advances in control of infections have not completely eradicated the problem because of development of resistance (Thomas, 1981). Antimicrobial resistance can increase complications and costs associated with procedures and treatment. An infected wound complicates the postoperative course and results in prolonged stay in the hospital and delayed recovery (Marjorie and Dudas, 1977). Most bacteria live on our skin, in the nasopharynx, gastrointestinal tract and other parts of the body with little potential for causing disease because of first line defence within the body. Surgical operation, trauma, burns, diseases, nutrition and other factors affect these defences. The skin barrier is disrupted by every skin incision, and microbial contamination is inevitable despite the best skin preparation (Howard et al., 1980).
The widespread use of antibiotics, together with the length of time over which they have been available have led to major problems of resistant organisms, contributing to morbidity and mortality. Pathogens that infect surgical wounds can be part of the patient's normal flora (endogenous source) or acquired from the hospital environment or other infected patients (exogenous source). The skin bacteria comprise commensals, transients and pathogens. The transient organisms include S.aureus, the hospital acquired methicillin-resistant forms (MRSA) and coliforms. Identification of a microbe that has been recovered from a clinical specimen is beneficial to the patient and assists in selection of chemotherapy (Elmer et al., 1997).
Here we report the findings from a study conducted in Jinja hospital, a national referral hospital in Uganda. It was designed to determine the distribution of bacterial pathogens isolated from septic post-operative wounds and their antimicrobial susceptibility patterns.
Materials and methods
Study area and study population: This study was conducted in Jinja hospital. The hospital has a bed capacity of three hundred. A total of ninety-four specimens obtained from patients who had undergone surgical operations were analysed.
Sample size and selection criteria: All patients who had septic wounds during the 3 month study period were included in the study. Patients who had sub clinical infection were excluded from the study. Patients were enrolled after obtaining informed consent from them or guardians/attendants.
Sampling procedure: A questionnaire was used to obtain data from the patient after obtaining an informed consent from the patient. Pus swabs were aseptically obtained from surgical sites before the wound was cleaned using an antiseptic solution. The specimen was collected on sterile cotton swab without contaminating them with skin commensals. The samples were transported to the laboratory soon after being obtained. In the laboratory, the specimens were registered and macroscopically examined for their appearances. The swabs were cultured and smears made on clean slides for Gram-staining techniques.
Microscopic examination and Culture: Smears were air-dried, heat fixed and stained by Gram's technique. This technique helps to group the bacterial pathogens into Gram positive or Gram negative by the ability of bacterial cells to retain primary stain.The stained slides were examined microscopically under oil immersion lens for pus cells, bacterial cells and quantified as No. of cells/High power field (Hpf). The specimens were inoculated on both differential and enriched media (MacConkey agar and 7% blood agar respectively). The culture plates were incubated aerobically for 24–48 hours before colonial morphologies were interpreted.
Identification of bacterial pathogens: Preliminary identification of bacteria was based on colony characteristics of the organisms i.e. haemolysis on blood agar, changes in physical appearance in differential media and enzyme activities of the organisms (Elmer et al., 1997). Biochemical tests were performed on colonies from primary cultures for final identification of the isolates.
Antibacterial susceptibility testing: Susceptibility testing was performed by Kirby-Bauer technique (Bauer et al., 1966). The test organism was uniformly seeded over the Mueller-Hinton agar surface and exposed to a concentration gradient of antibiotic diffusing from antibiotic-impregnated paper disk into the agar medium. The isolate was then incubated at 37°C for 16–18 hours (Barry, 1986). Organisms sensitive to the antibiotic were inhibited from growing in a circular zone around the antibiotic impregnated paper disk. A comparison of the inhibition zone diameter that was produced by a control strain was used to interpret the antimicrobial sensitivity (Collee et al, 1989). Grades of sensitivity recognised are sensitive, intermediate and resistant by comparison of zone of inhibition as indicated. Drugs tested against Gram-positive cocci were: methicillin, erythromycin, ampicillin, ciprofloxacin and gentamicin. For Gram-negative rods, the drugs were gentamicin, ceftazidime, ciprofloxacin, chloromphenicol, amoxycillin and ampicillin. The drugs tested for gram-negative oxidase positive isolates were gentamicin, ciprofloxacin and ceftazidime.
Inoculum preparation: The inoculum was prepared by picking parts of similar test organisms with a sterile wire loop. This was suspended in sterile peptone water (broth) and incubated up to two hours to allow organisms reach their log-phase in growth. The density of suspension to be inoculated was determined by comparison with opacity standard on McFarland 0.5 Barium sulphate solution (Elmer et al., 1997). A sterile swab was dipped into the suspension of the isolate in peptone water, squeezed free from excess fluid against the side of bottle and then spread over the agar plate.
Results
Patients' profiles: A total of 94 patients presenting with septic wounds were enrolled in the study between February and April 2003. Out of 94 patients studied 59.6% were male and 38 (40.4%) were female. The ages of study groups ranged from 1–77 years. The modal age group was 11–20 years with frequency of 22.3. The pre operative stay in hospital ranged from 1–42 days with the distribution lowest above 35 days. Majority of the patients (78.7%) stayed in the hospitalised for 1–7 days before operations. The predominant operation was incision and drainage 32 (34.0%), followed by caesarean section 13 (13.8%) and herniorrhaphy 12 (12.8%).
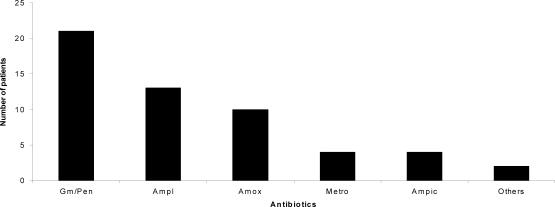
Antibiotic use: Pre-operative antibiotics administered. The majority of the study patients 54 (57.5 %) were treated with antibiotics before surgical operation while 40 (42.5 %) were not given any antibiotics (Figure 1). The antibiotics used were gentamicin/benzyl -penicillin 21(38.9%), ampiclox 13 (24.1%), amoxycillin 10 (18.5%), ampicillin 4 (7.4%), metronidazole 4 (7.4%) and others 2 (3.7%).
Figure 1.
Antibiotics administered before surgical operation
Key:
Gm/Pen=Gentamicin/Crystalline-penicillin; Ampl=Ampiclox; Amox=Amoxycillin
Metro=Metronidazole; Ampic=Ampicillin
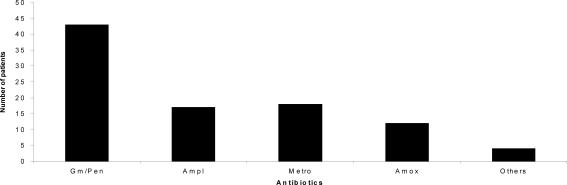
Postoperative antibiotics administered: All the 94 patients were treated with antibiotics after the surgical operation (Figure 2). The antibiotics used were gentamicin/crystalline penicillin 43 (45.7%) amoxycillin 12 (12.8%) metronidazole 18 (19.1%) and others 4 (4.3%).
Figure 2.
Antibiotics administered after surgical operation
Key: Gm/Pen=Gentamicin/Crystalline-penicillin; Ampl = Ampiclox; Amox =Amoxycillin; Metro=Metronidazole
Bacteriological findings
Majority of the pus swabs were purulent 31 (33%) blood stained were 29 (30.8%) moist 26 (27.7%) and dry 8 (8.5%).
The majority of slides as shown in table 1 revealed no pus cells 49 (52.1%) while 14 (14.9%) had 1–4 pus cells/Hpf, 11 had 5–9 pus cells /Hpf and 20 (21.3%) had greater than 10 pus cells / Hpf.
Table 1.
Number of pus cells /Hpf in Gram stain
| Pus cells | Number/Hpf | Number of specimens | Percentage |
| None | 0 | 49 | 52.1 |
| A few | 1–4 | 14 | 14.9% |
| Moderate | 5–9 | 11 | 11.7% |
| Very many | >10 | 20 | 21.3% |
| Total | 94 | 100% |
Most of the Gram- stained smears (48.9%) had a few bacterial cells/Hpf, 13.8% had moderate number of bacterial cells and 5.3% had very many bacterial cells/Hpf.
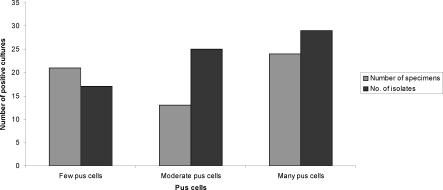
In the group of patients where few pus cells were seen on the Gram stain 17(24%) isolates were recovered (Figure 3). While in those patients who had moderate pus cells/Hpf 20 (35.2%) isolates were recovered. In the category of patients with very many pus cells/Hpf 29 (40.8%) of the isolates were recovered.
Figure 3.
Pus cells/Hpf in relation to positive cultures
Culture results
The majority of cultured specimens 56 (59.6%) had bacterial growth within 48-hours of incubation. Fifteen out of fifty five (27.3%) had mixed growth while 40 (72.7%) had pure bacterial growth. The rest had no bacterial growth. The bacteria isolated from the wounds are as indicated in table 3 below.
Table 3.
Characterisation of organisms isolated
| Isolate | Frequency | Percentage |
| Staphylococcus aureus | 32 | 45.1 % |
| Coliforms | 12 | 16.9 % |
| Proteus mirabilis | 8 | 11.3 % |
| Pseudomonas aeruginosa | 7 | 9.9 % |
| Klebsiella pneumoniae | 5 | 7.0 % |
| Escherichia coli | 5 | 7.0 % |
| Enterobacter species | 2 | 2.8 % |
| Total | 71 | 100 % |
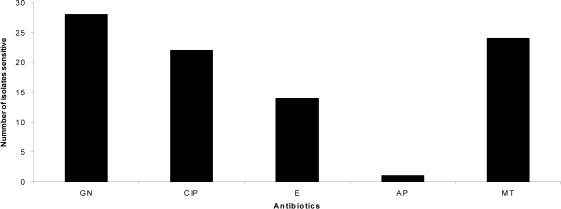
All the Gram-positive isolates were Staphylococcus aureus (Figure 4). The majority of them 28 (87.5%) were sensitive to gentamicin, 24 were sensitive to methicillin and 22 (68.7%) were sensitive to ciprofloxacin. Only 1 (3%) was sensitive to ampicillin. Vancomycin sensitivity was not tested because the antibiotic disc was not available. Among the S.aureus isolates 8 (25%) were Methicillin- Resistant Staphylococcus aureus (MRSA).
Figure 4.
Antimicrobial sen sitivity pattern for S.aureus (n=32)
Key:
GN = Gentamicin; CIP = Ciprofloxacin; E = Erythromycin; AP = Ampicillin MT = Methicillin
The commonest organism isolated (Table 3) was Staphylococcus aureus 32 (45.1%) while the least isolated organism was Enterobacter species 2 (2.8%). The majority of the P. aeruginosa isolated (85.7%) were sensitive to both gentamicin and ceftazidime while 42.8% were sensitive to ciprofloxacin.
Out of the thirty- two Gram-negative and oxidase negative isolates 20 (62.5%) were sensitive to gentamicin, 19 (59.4%) were sensitive to ciprofloxacin, 27 (84.4%) were sensitive to ceftazidime while only 3 (9.4%) and 1 (3.1%) were sensitive to ampicillin and chloramphenicol respectively. No Gram-negative isolate was sensitive to chloramphenicol (Table 4).
Table 4.
Antibacterial sensitivity pattern of Gram-negative and oxidase-negative isolates (n = 32)
| Organism | GN | CIP | CAZ | AP | C | A |
| Esch.coli n=5 | 4(80%) | 3(60%) | 3(60%) | 1(20%) | 0(0%) | 0(0%) |
| Proteus mirabilis n=8 | 3(37.5%) | 6(75%) | 7(87.5%) | 2(25%) | 0(0%) | 1(12.5%) |
| Kleb.pneumoniae n=5 | 5(100%) | 2(40%) | 5(100%) | 0(0%) | 0(0%) | 0(%) |
| Entero. spp n=2 | 2(100%) | 1(50%) | 2(100%) | 0(0%) | 0(0%) | 0(%) |
| Unidentified Coliforms | 6(50%) | 7(58.3%) | 10(83.3%) | 0(0%) | 0(0%) | 0(%) |
| n=12 |
Key:
GN = Gentamicin; C = Chloramphenicol; CIP = Ciprofloxacin; A = Amoxycillin; CAZ = Ceftazidime; AP =Ampicillin; Kleb. = Klebsiella; Entero. spp= Enterobacter species
Comparison of type operation with micro organisms isolated
The majority of organisms 26(36.6%) were isolated from surgical sites after incision and drainage (Table 5). The commonest organism isolated after this operation was S.aureus (53.8%). From caesarean sections, 70% of the isolates were S.aureus while 40% of the organisms isolated after O.R.I.F operations were Coliforms. The predominant organism isolated after appendicectomy was E.coli (33%).
Table 5.
Organisms isolated from the various operation sites
| Type of | E.coli | Pseudo. | S.aureus | Proteus | Entero-spp | Coliform | Kleb. | Total |
| operation | aeruginosa | mirabilis | pneumoniae | |||||
| I/D | - | 4(15.4%) | 14(53.8%) | 3(11.5%) | - | 3(11.5%) | 2(7.7%) | 26 |
| C/S | 1(10%) | - | 7(70%) | 2(20%) | - | - | - | 10 |
| Hern. | - | - | 2(66.7%) | - | - | - | 1(33.3%) | 3 |
| Append. | 2(33.3%) | - | 1(16.7%) | 1(16.7%) | - | 1(16.7%) | 1(16.7%) | 6 |
| O.R.I.F | - | 3(30%) | 3(30%) | - | - | 4(40%) | - | 10 |
| Lap. | 1(25%) | - | 1(25%) | - | 1(25%) | 1(25%) | - | 4 |
| Others | 1(8.3%) | - | 4(33.3%) | 2(16.7%) | 1(8.3%) | 3(25%) | 1(8.3%) | 12 |
| Total | 5 | 7 | 32 | 8 | 2 | 12 | 5 | 71 |
Key
I/D - Incision and drainage; Hern.- Herniorraphy; Append. -Appendicectomy.
O.R.I.F- Open reduction internal fixation; Lap. - Laparatomy.
Discussion
This study revealed that forty-five out of ninety four patients studied (47.87%) showed varying degrees of sepsis indicated by pus cells in the gram stain. Twenty of the ninety- four samples (21.3%) had >10 WBC/Hpf. These findings are in agreement with those done by others in Mulago (Buwembo, 1990) that put the rate of infections of potentially contaminated wounds at 25%. The presence of 10 or more WBC/Hpf is due to contamination of surgical site by pathogenic bacteria. This response was confirmed by recovery of 40.8% of the isolates (Figure 3) from category of patients with very many pus cells (>10 pus cells/Hpf). As a natural defence mechanism by the host, white blood cells are drawn to the inflamed area. The purulent exudates (33%) are a result of large numbers of WBCs in response to bacterial infection of the surgical site. The number of WBC/Hpf is an indicator of the extent of bacterial contamination of the wound (Marjorie and Dudas, 1997).
The organisms associated with the infections were Staphylococcus aureus (45.1%) Coliforms (16.9%), Proteus mirabilis (11.3%) Pseudomonas aeruginosa (9.9%), Klebsiella pneumoniae (7.0%) Escherichia coli (7.0%) and Enterobacter Spp (2.8%). These findings agree with those reported by Taylor (1992) on surgical site infections where the most common wound contaminant was Staphylococcus aureus (50.32%). The findings also agree with those of Buwembo (1990) who identified Staphylococcus aureus as the commonest causative agent of potentially contaminated wounds in Mulago hospital.
The high prevalence of Staphylococcus aureus infection may be because it is an endogenous source of infection. Nasal carriage of S. aureus is an important risk factor for infection of surgical site as the organism is a normal flora in the nostrils. Infection with this organism may also be due to contamination from the environment e.g. contamination of surgical instruments. With the disruption of natural skin barrier S.aureus, which is a common bacterium on surfaces, easily find their way into surgical sites (Brown, 1990).
Thirty-nine out of ninety-four swabs (41.5%) had no bacterial growth. This could be due to normal healing process where the bacteria have been overpowered by body's defence mechanism, antimicrobial activity in patients circulation since all of them had been on antibiotic therapy post operatively at time of collecting the samples or adequate nursing care e.g. use of antiseptics for cleaning the wounds. It is also possible that some organisms could have been anaerobic bacteria that were missed as cultures were incubated aerobically. This condition could not therefore support growth of such organisms.
The pre operative antibiotics that the patients received were gentamicin/crystalline penicillin, ampiclox, amoxycillin, ampicillin, metronidazole and others. The most probable reason for their choice being that these antibiotics have been on market for long, they are readily available and relatively cheap (WHO, 1991).
The majority of gram-negative bacteria isolated were sensitive to gentamicin, ceftazidime and ciprofloxacin. However, most of the gram-negative bacteria isolated were resistant to ampicillin, chloramphenicol and amoxycillin (Table 4). Resistance to chloramphenicol was 100% while only 12.5% of the Proteus mirabilis was sensitive to amoxycillin. The resistance shown to amoxycillin, ampicillin and chloramphenicol may be due to the antibiotics having been in use for much longer time and their oral route of administration that affects their rate of absorption into blood stream. Some of them were used as prophylaxis therefore increasing their use in patients. Over-use of antibiotics contributes to organisms developing resistance (Seppala et al., 1992).
Ceftazidine and Ciprofloxacin are third-generation cephalosporins that are relatively rare in the hospitals and are expensive. Their high cost and being less readily available to patients means these drugs have not been misused and hence are more effective compared to those that have been in use for quite a long time.
The majority of Staphylococcus aureus (87.5%) were sensitive to gentamicin, 75% sensitive to methicillin and 68% sensitive to ciprofloxacin. All the Staphylococci aureus isolates were resistant to ampicillin; about 55% resistant to erythromycin and 25% were resistant to methicillin (MRSA). This finding is similar to the study done in Mulago hospital by Wewedru et al., (2001) where the sensitivity of S.aureus to gentamicin was 70%, methicillin 73% and erythromycin 37%. The multiple resistances in MRSA could be due to the mec A gene that encodes for protein PBP-2a that binds to available â-lactams. Another reason is the production of Staphylococcal penicillinase and other enzymatic deactivators. Presence of other resistance factors e.g. Plasmids could have been involved as well (Elmer et al., 1997). The resistance observed in S.aureus could also be attributed to irrational use of antibiotics for conditions that may not clinically indicate their use, over-the-counter sell of antibiotics in pharmacies without prescription by authorised practitioners, some new drug formulations which may be of poor quality and dumping of banned products into the market where the public may get access to them. In view of the resistance observed, infections caused by MRSA can be expensive in terms of costs of treatment, morbidity and prolonged hospitalisation (Hiramatsu et al., 1997).
Most of the isolates (39.4%) were from the abdomen where the most common isolate from the surgical site was Staphylococcus aureus (46.4%). This may be due to surface contamination by organisms on the skin and environment causing nosocomial infections. The presence of Coliforms (14.3%), Proteus mirabilis (14.3%), Escherichia Coli (10.7%) and Enterobacter spp (7.1%) can be due to contamination of wounds with patient's endogenous flora. Escherichia coli and Coliforms are normal flora of gastro-intestinal tract (Brown, 1990).
Conclusions
Since a high proportion of samples had positive cultures, infection control is recommended as a strategy to minimise spread of resistant organisms. Future studies should be extended to include cultures under anaerobic conditions to establish presence of other organisms that require such environment for growth. It is recommended that gentamicin, ciprofloxacin and ceftazidime be used in preference to ampicillin and amoxycillin for treatment of septic wounds. Finally, there is need to develop national surveillance of antibiotic- resistant organisms.
Table 2.
Number of bacterial cells/Hpf in Gram stain
| Bacterial cells | Number/Hpf | Number of specimens | Percentage |
| None | 0 | 30 | 32% |
| A few | 1–9 | 46 | 48.9% |
| Moderate | 10–20 | 13 | 13.8% |
| Very many | >20 | 5 | 5.3% |
| Total | 94 | 100% |
Acknowledgements
We would like to acknowledge the assistance and guidance provided by Dr. Anyama of Jinja Hospital and the Medical superintendent (Jinja Hospital) for permission to do the work in Jinja Hospital.
References
- 1.Barry AL. Procedure for testing antimicrobial agents in agar medium, in Theoretical consideration in laboratory medicine. 5th edition. Larian: 1986. pp. 1–26. [Google Scholar]
- 2.Bauer AW, Kirby M, Sheris JD, Turch M. Antibiotic susceptibility testing by standard single disc method. American Journal of Clinical pathology. 1966;45:493–496. [PubMed] [Google Scholar]
- 3.Brown AD. Bacteriology of wound infections in surgical ward of teaching hospitals. West African Journal of Medicine. 1990;9:4285–4290. [PubMed] [Google Scholar]
- 4.Buwembo KBM. Post operative wound infection. Kampala: Makerere University; 1990. pp. 1–45. [Google Scholar]
- 5.Collee JG, Duguid JP, Fraser AG, Marmion BP. Mackie and McCartney Practical Medical Microbiology. 5th edition. Churchill Livingstone: Longman groups U.KLtd.; 1989. [Google Scholar]
- 6.Elmer WK, Stephen DA, William MJ, Schreckenberger Paul C, Winn Washingtone C., Jr . Antimicrobial susceptibility testing in, Colour Atlas and text Book of Diagnostic microbiology. 5th edition. 227 East Washington Square, Philadelphia: Raven Publisher; 1997. pp. 69–120. [Google Scholar]
- 7.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. Journal of Antimicrobial Chemotherapy. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Howard RJ, Ravitch MM, Steichen FM. Host against infections. Current problems in surgery. New English Journal of Medicine. 1980;12:1823–1830. [Google Scholar]
- 9.Beyers Marjorie, Dudas Susan. The clinical practice of Medical-Surgical Nursing. Boston: Publisher Little, Brown and Company; 1977. [Google Scholar]
- 10.Seppala H, Nissinen A, Jarvinen H. Resistance to Erythromycin in group A Streptococcus. The New England Journal of Medicine. 1992;326:292–297. doi: 10.1056/NEJM199201303260503. [DOI] [PubMed] [Google Scholar]
- 11.Thomas KH. Surgical wound infection, an over view. American Journal of Medicine. 1981;70:712–718. doi: 10.1016/0002-9343(81)90602-1. [DOI] [PubMed] [Google Scholar]
- 12.Wewedru Izale C, Godfrey Pimundu, Fred Kironde. Staphylococcus aureus antibiotypes at Mulago hospital. Mulago Hospital Bulletin. 2001;4:62–64. [Google Scholar]
- 13.WHO, author. Basic Laboratory Procedure in Clinical Bacteriology. 1991:62–64. [Google Scholar]