Abstract
Background
Helicobacter pylori has become recognized as a major cause of gastroduodenal diseases in man. Evidence indicates that once acquired, H. pylori persists, usually for life unless eradicated by antimicrobial therapy. Over the past few years, we have accumulated some knowledge of the epidemiology of H. pylori in Ile-Ife, South-West Nigeria. In one collaborative study, we detected H. pylori in 195 (73%) patients referred for endoscopy at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC). Furthermore we have observed a variegated gastric inflammatory response and atrophy including atrophic pangastritis but are yet to demonstrate MALToma in any of our patients. In addition we have demonstrated that dental plaque is a possible source of gastric H. pylori infection and such an endogenous source could account for difficulty in eradication leading to re-infection. Presently, infected patients are treated with standard combination therapy made up of amoxycilin and ciprofloxacin with a proton pump inhibitor /bismuth. Reports however have shown that the incidence of antimicrobial resistance in Helicobacter pylori is a growing problem and which has been linked with failures in treatment and eradication. Given this situation it has become necessary to have information about the susceptibility of isolates to particular antimicrobial agents before the selection of an appropriate treatment regimen.
Objectives
More recently, we sought to study antimicrobial susceptibility of locally isolated H. pylori strains.
Methods
We subjected 32 isolates to antimicrobial susceptibility testing against seven agents.
Results
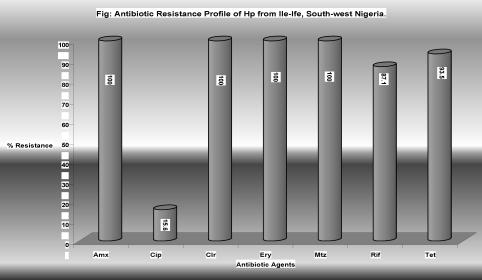
All the isolates showed multiple acquired antimicrobial resistance as they were all resistant to amoxicillin, clarithromycin, metronidazole, while 29/31, 27/31 showed resistance to rifampicin and tetracycline respectively. Five (15.6%) of these isolates showed resistance to ciprofloxacin.
Conclusions
Our findings suggest that H. pylori strains isolated within our study environment have acquired resistance to all the commonly prescribed antibiotics. On the basis of the findings it would be necessary to re-evaluate the eradication treatment regime in our setting.
Introduction
Helicobacter pylori (H. pylori) is a ubiquitous gram-negative, microaerophilic spiral bacterium infecting half the world's population and causing chronic active gastritis in virtually all infected individuals 1. Apart from gastritis however, this organism has also been associated with gastric and duodenal ulcers, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (MALToma).2 Evidence indicates that once acquired, H. pylori persists, usually for life unless eradicated by antimicrobial therapy. Treatment regimens for H. pylori infection have been evolving since the early 1990s. Antimicrobial therapy for this infection is a complex issue, and the following drugs are currently used in combination regimens: proton-pump inhibitors and or bismuth, metronidazole, clarithromycin, and amoxicillin3. Tetracycline is used in rescue therapy4.
Guidelines on the management of H. pylori have been developed in a number of regions around the world. The European Helicobacter Pylori Study Group (EHSG) produced the first Maastricht Concensus Report in 1996 which has undergone two revisions until the current Maastricht 3 - 2005 report5. The report gave directions on who to treat, how to treat and the need for H. pylori eradication with a view to reducing the risk for gastric cancer development. Although H. pylori is sensitive to many antibiotics in vitro, the in vivo eradication rate is often disappointing a major reason being the quick appearance of resistant strains. Strains resistant to metronidazole6 and clarithromycin7 have been well documented, while reports from Asia indicate the prevalence of resistance to amoxicillin and tetracycline.8 9 The resistance problem with H. pylori shows that treatment regimens will continue to evolve as the search continues for effective treatment eradication protocols. What is required is a simple and more efficacious strategy for the treatment of H. pylori infection10.
Over the past few years, we have accumulated some knowledge of the epidemiology of H. pylori in Ile-Ife, South-West Nigeria. Much of this has been in the area of endoscopy in adult patients suffering from dyspepsia. In those studied, a strong association exists between H. pylori infection and duodenal ulcer as well as severe erosive gastritis11. In a study in which 268 patients at the OAUTHC were investigated for H. pylori infection, Ndububa et al.12 reported that 195 (73%) were infected with the organism. Furthermore a variegated gastric inflammatory response and atrophy including atrophic pangastritis have been observed in infected subjects. It is noteworthy however that none of the patients studied had developed MALToma (Unpublished report). In a different study carried out in the same hospital, dental plaque was shown to be a possible source of H. pylori infection and presence of this endogenous source of infection in the patients could account for incidences of re-infection13.
Having established the prevalence of H. pylori infection, patients are currently treated (with a view to eradication) with combination therapy (amoxicillin, ciprofloxacin and a proton pump inhibitor/bismuth). The relationship between antimicrobial resistance and the successful treatment of H. pylori infection has dictated that a study of the incidence of acquired antimicrobial resistance among H. pylori isolates obtained within our environment be determined hence the importance of this study.
Methods
Gastric biopsies were collected from consecutive patients who had oesophagogastroduodenoscopy at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC) from July 2002 to January 2003 and from September 2005 to March 2006. These were dyspeptic patients referred from the Surgical and Medical outpatient departments of the OAUTHC. Initially biopsies were transported using Stuart's transport medium in ice packs. Later specimens were transported to the laboratory in screwtop bottles with 0.1–0.5ml of 0.9% saline14. Specimens were inoculated onto Dent's medium and then cultured under a microaerophilic atmosphere in a candle extinction jar. Incorporating NaHCO3 and citric acid or tartaric acid in the environment generated the required CO2. High humidity was achieved by placing a moistened towel at the bottom of the jar. Plates were incubated at 37°C for 3–7 days. Bacterial colonies were identified as H. pylori on the basis of colonial morphology, positive urease, catalase and oxidase tests and a Gram stain15.
The susceptibility of isolates to antibiotics was tested using the disc diffusion assay, according to the methodology described by Lopez Brea and Alarcon16. Frozen vials containing the different strains were inoculated on blood agar and incubated in a microaerophilic environment for five days. The colonies obtained were suspended in 1ml tryptic soy broth (™106CFU/ml). 0.5ml of the inoculum was flooded to a non-selective blood agar plate and allowed to dry for 5–10minutes. Seven different discs were tested using amoxycillin (25µg), ciprofloxacin (5µg), clarithromycin (15µg), erythromycin (15µg), metronidazole (5µg), rifampicin (5µg) and tetracycline (30µg) (Oxoid, England). They were then incubated under microaerophilic conditions for 72hours. Quality control was performed with E. coli K-12 C600. The breakpoint for resistance was as defined by National Committee for Clinical Laboratory Standards17. An interpretative correlate (susceptible or resistant) was provided by reference to published guidelines. Intermediate zones between susceptible and resistant were recorded as resistant.
Results
We subjected 32 isolates to antimicrobial susceptibility testing. The 32 isolates were from 16 females and 16 males with an age range of 20–73 years and mean of 48.6±16.23 years. The resistance profile to 7 different antibiotics as determined by disc diffusion test is as shown
A total of 5 distinct antibiograms were encountered in all the H. pylori strains and the patterns varied from resistance to 4 antimicrobial agents to 6 antimicrobial agents (Table 1). Most frequently encountered antibiograms were ampicillin, clarithromycin, erythromycin, tetracycline, metronidazole and rifampicin.
Table 1.
Resistance antibiograms of Helicobacter pylori isolates
| Resistance antibiogram | Number |
| AML, CLR, ERM, RFC | 1 |
| AML, CLR, ERM, MTZ | 1 |
| AML, CLR, ERM, MTZ, TET | 2 |
| AML, CLR, ERM, TET, CIP, RFC | 3 |
| AML, CLR, ERM, TET, MET, RFC | 25 |
| 32 |
AML-ampicillin, CLR-clarithromycin, ERM-erythromycin,TET-tetracycline, MET-metronidazole, CIP-ciprofloxacin, RFC-rifampicin
Discussion
The geographical variation in the resistance of H. pylori to antimicrobial agents is thought to be related to the level of use of the agents in different communities18. In Nigeria, evidence has shown that antimicrobials especially ampicillin, tetracycline, metronidazole and erythromycin are in wide use19. In addition antibiotics self-medication is encouraged by free access and over the counter purchase and by ineffective drug control policy20. This could be a contributing factor for the very high level of resistance of H. pylori to amoxycillin (100%), clarithromycin (100%), metronidazole (100%), and tetracycline (93.5%) observed in this report.
Previous use of any macrolides and metronidazole has been correlated for resistance to clarithromycin and metronidazole respectively21. The prevalence of H. pylori resistance to metronidazole varies from 20% to 40% in Europe and the USA, with one exception in Northern Italy22,23. It is well known that the prevalence is much higher in developing countries (50–90%) for example in Mexico7. It must be emphasized that different resistance testing methods may be discrepant in 10–20% when testing for metronidazole. Furthermore, reproducibility using a given method is also not good24. Nevertheless, although the exact prevalence rate obtained must be interpreted with caution, the trends of high, medium, or low resistance seem real.
When risk factors are studied, past use of metronidazole, which is very common in tropical countries for parasitic diseases is involved. In developed countries, most studies have reported a higher resistance rate in women than in men, probably due to the use of nitroimidazole drugs to treat gynaecological infections25. The use of metronidazole for dental infections may also add to selection pressure.
Although macrolides have been found to be useful therapeutic agents in treating H. pylori infections, our findings negate this as all our strains were shown to be resistant to erythromycin and clarithromycin. This observation corroborates findings of 92.6% erythromycin resistance rates reported by Quintana-Guzmam et al.26. We expected clarithromycin which has been reported to be acid stable and clinically more effective than erythromycin in the treatment of H. pylori27 to be highly inhibitory, but it is remarkable that our strains were not inhibited by this agent. The finding is surprising because, relative to erythromycin, clarithromycin is new in the Nigerian market. One possible explanation for the finding is that acquired resistance to clarithromycin28 could be as a result of previous exposure to erythromycin as documented by some workers23, 29. Kato et al.28 have also demonstrated similar cross-resistance with azithromycin in clarithromycin resistant strains. The cross-resistance may explain resistance to clarithromycin even when it is introduced newly as observed among our strains.
Midolo and colleagues were first to report tetracycline resistance in H. pylori in 199630, while in the following year Piccolomini et al.31 reported that 6% of strains were resistant in 1997 in Italy. The prevalence of tetracycline resistance was 58.8% in China8. The finding showing very poor inhibitory effect of tetracyclines to H. pylori in the present study was in keeping with experience of others26. In the Costa Rican26 report 80.4% of strains are resistant to tetracycline. In fact, the pattern of multiresistance seen in our study is similar to that from Costa Rica where resistance to erythromycin, metronidazole and amoxycilin was 92.6%, 95.1% and 52.2% respectively. On the other hand, resistance to ciprofloxacin and another common agent nitrofurantoin was only 7.3% and 9.8% respectively similar to our report (though we did not include nitrofurantion). With tetracycline, as with other antibiotics, resistance increases with the use of the drugs due to selection pressure.
Amoxycillin resistance was not considered important until when amoxycillin resistance in H. pylori isolates were identified in the USA, Canada and Italy. Resistance rates of 31%32 and 45%33 have been reported from Italy. Wu et al.8 reported a rate of 71.9%. All our isolates were resistant in vitro to amoxicillin. In the China study reported by Wu et al.8, though the MICs for many resistant strains were high, no strain produced β-lactamase. It was assumed that amoxycillin resistance was probably a result of alterations in penicillin-binding proteins (PBP).
15.6% of our isolates were resistant to the fluoroquinolone, ciprofloxacin. The prevalence of fluoroquinolone has been determined only in a limited number of studies23. However, Portugal has reported a high resistance rate of 20.9% in 110 adult patients34. Resistance to fluoroquinolones mirrors the use of these drugs. Ciprofloxacin is relatively new in Nigeria hence the comparatively low resistance to it by the isolates. Studies23 that have examined resistant H. pylori isolates from various geographic locations have identified point mutations in different genes which confer resistance. However the resistance mechanisms of our isolates remain to be investigated.
The most common resistance antibiograms were those combinations containing amoxicillin, clarithromycin, erythromycin, tetracycline, metronidazole and rifampicin. H. pylori isolates resistant to multiple drugs have been reported elsewhere8. The high frequency of multiple antibiotic resistant H.pylori isolates observed in this study most probably reflects the ease of access and extensive use of antibiotics in Nigeria20.
In the present report, the widespread resistance to the antimicrobial agents documented justified a critical need to monitor local variations in H. pylori sensitivity patterns, and resistance rates in order to devise therapeutic guidelines. While the principles of treatment should be based on simplicity, effectiveness, affordability, drug safety and efficacy, the in vitro resistance of H. pylori isolates to commonly used antimicrobials in this report has resulted in limited therapeutic options. On the basis of the findings it would be necessary to investigate ciprofloxacin in any eradication treatment regime in our setting, since it appears to be the only active antibiotic in eradicating H. pylori in this environment.
Furthermore, there is the need to continue the evaluation of new treatment agents such as NE-200110, older agents such as nitrofurantoin26 or introduction of herbal management (we are investigating the promising effects of herbal drugs in the management of H. pylori infection) in order to eradicate Helicobacter pylori.
Future case-control studies employing larger sample size are needed to demonstrate the effectiveness or otherwise of therapy in the different patient groups as has been stated in the EHSG Maastricht guidelines5. A long-term follow-up of our patients will also contribute to the development of guidelines on the issues of referral, diagnostic methods and treatment of H. pylori.
Figure 1.
All the isolates were resistant to amoxicillin, clarithromycin, metronidazole, while 29/31, 27/31 were resistant to rifampicin and tetracycline respectively. Five (15.6%) of the isolates were resistant to ciprofloxacin.
Acknowledgements
We appreciate the cooperation of nurses in the endoscopy unit, OAUTHC during the endoscopy sessions for biopsy collections.
References
- 1.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G European Helicobacter pylori Study Group (EHPSG), author Current concepts in the management of Helicobacter pylori infection - the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Therap. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, Pajares JM. Helicobacter pylori therapy: first line options and rescue regimen. Dig Dis. 2001;19:134–143. doi: 10.1159/000050668. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers E J. Current concepts in the management of Helicobacter pylori infection- The Maastricht III Consensus Report. Gut. 2006 Dec 14; doi: 10.1136/gut.2006.101634. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenks PJ, Edwards DI. Metronidazole resistance in Helicobacter pylori. Int J Antimicrob Agents. 2002;19:1–7. doi: 10.1016/s0924-8579(01)00468-x. [DOI] [PubMed] [Google Scholar]
- 7.Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161:1217–1220. doi: 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Shi XD, Wang HT, Liu JX. Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxycillin. J Antimicrobial Chemother. 2000;46:121–123. doi: 10.1093/jac/46.1.121. [DOI] [PubMed] [Google Scholar]
- 9.Kwon DH, Kim JJ, Lee M, et al. Isolation and characterization of tetracycline-resistant clinical isolates of Helicobacter pylori. Antimicro Agents Chemother. 2000;44:3203–3205. doi: 10.1128/aac.44.11.3203-3205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai G, Cheng N, Dong L, et al. Bactericidal and morphological effects of NE-2001, a novel synthetic agent directed against Helicobacter pylori. Antimicro Agents Chemother. 2005;49:3468–3473. doi: 10.1128/AAC.49.8.3468-3473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawal OO, Fadiran OA, Oluwole SF, Campbell B. Clinical pattern of prepyloric and duodenal ulcer at Ile-Ife, Nigeria. Trop Doct. 1998;28(3):152–155. doi: 10.1177/004947559802800309. [DOI] [PubMed] [Google Scholar]
- 12.Ndububa DA, Agbakwuru AE, Adebayo RA, et al. Upper gastrointestinal findings and incidence of Helicobacter pylori infection among Nigerian patients with dyspepsia. West Afr J Med. 2001;20(2):140–145. [PubMed] [Google Scholar]
- 13.Ogunbodede EO, Lawal OO, Lamikanra A, Okeke IN, Rotimi O, Rasheed AA. Helicobacter pylori in the dental plaque and gastric mucosa of dyspeptic Nigerian patients. Trop Gastroenterol. 2002;23(3):127–133. [PubMed] [Google Scholar]
- 14.Soltesz V, Zeeberg B, Wadstrom T. Optimal survival of Helicobacter pylori under various transport conditions. J Clin Microbiol. 1992;30(6):1453–1456. doi: 10.1128/jcm.30.6.1453-1456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koneman EW, Allen SD, Janda WM, Schreckenberger W, editors. Color atlas and textbook of diagnostic Microbiology. 5th edition. Lippincott: 1997. pp. 321–361. [Google Scholar]
- 16.Lopez Brea M, Alarcon T. Sensibilidad antimicrobiana en la infeccion por Helicobacter pylori. In: Lopez Brea M, editor. Helicobacter pylori microbiologia, clinica y tratamiento. Madrib: Morsby/Doyma Libros; 1995. pp. 32–53. [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards, author. Approved Standard M7-A5: Methods/or Dilution Antimicrobial Susceptibility Tests/or Bacteria That Grow Aerobically. Wayne, PA: National Committee for Clinical laboratory Standards; 2000. [Google Scholar]
- 18.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterol. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 19.Okeke IN, Fayinka ST, Lamikanra A. Antibiotic resistance in Escherichia coli from Nigerian students, 1986–1998. Emerg Infect Dis. 2000;6(4):393–396. doi: 10.3201/eid0604.009913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5(1):18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon BJ, Hennessy TD, Bensler JM, et al. The relationship among antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Int Med. 2003;139:463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 22.Pilotto A, Rassu M, Leandro G, et al. Prevalence of Helicobacter pylori resistance to antibiotics in Northeast Italy: a multicentre study. GISU. Interdisciplinary Group for the Study of Ulcer. Dig Liver Dis. 2000;32:763–768. doi: 10.1016/s1590-8658(00)80352-7. [DOI] [PubMed] [Google Scholar]
- 23.Megraud F. H. pylori antibiotic resistance: prevalence, importance and advances in testing. Gut. 2004;53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres J, Camorlinga-Ponce M, Perez-Perez G, et al. Increasing multidrug resistance in Helicobacter pylori strains isolated from children and adults in Mexico. J Clin Microbiol. 2001;39:2677–2680. doi: 10.1128/JCM.39.7.2677-2680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glupczynski Y, Megraud F, Lopez-Brea M, Anderson LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Euro J Clin Mirobiol Infect Dis. 2000;11:820–823. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 26.Quintana-Guzman EM, Arias-Echandi ML, Salas-Chaves P, Davidovich-Rose H, Schosinsky-Neverman K. Helicobacter pylori: susceptibility to amoxycillin, erythromycin, tetracycline, ciprofloxacin, nitrofurantoin and metronidazole in Costa Rica. Rev Biomed. 1998;9:92–96. [Google Scholar]
- 27.Graham DY, Hepps KS, Ramirez FC, Lew GM, Saeed ZA. Treatment of Helicobacter pylori reduces the rate of re-bleeding in peptic ulcer disease. Scand J Gastroenterol. 1993;28:939–942. doi: 10.3109/00365529309098288. [DOI] [PubMed] [Google Scholar]
- 28.Kato S, Fujimura S, Udagawa H, et al. Antibiotic resistance of Helicobacter pylori strains in Japanese children. J Clin Microbiol. 2002;40:649–653. doi: 10.1128/JCM.40.2.649-653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia HX, Buckley M, Keane Ct, O'Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicroibial Chemother. 1996;37:473–481. doi: 10.1093/jac/37.3.473. [DOI] [PubMed] [Google Scholar]
- 30.Midolo PD, Korman MG, Turnidge JD, Lambart JR. Helicobacter pylori resistance to tetracycline. Lancet. 1996;347:1194–1195. [PubMed] [Google Scholar]
- 31.Piccolomini R, Bonaventura GD, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth dilution for testing susceptibilities of Helicobacter pylori to 20 antimicrobial agents. J Clin Microbiol. 1997;135:1842–1846. doi: 10.1128/jcm.35.7.1842-1846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dore MP, Piana A, Carta M, et al. Amoxycillin resistance is one reason for failure of amoxycillin-omeprazole treatment of Helicobacter pylori infection. Aliment Pharmacol Therap. 1998;12:635–639. doi: 10.1046/j.1365-2036.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 33.Dore MP, Sepulveda AR, Mura I, Realdi G, Osato MS, Graham DY. Explanation for variability of omeprazole amoxycillin therapy? Tolerance of H. pylori to amoxicillin. Gastroenterol. 1997;112:A105. (Abstract) [Google Scholar]
- 34.Cabrita J, Oleastro M, Matos R, et al. Features and trends in Helicobacter pylori antibiotic resistance in Lisborn area, Portugal (1990–1999) J Antmicrob Chemother. 2000;46(6):1029–1031. doi: 10.1093/jac/46.6.1029. [DOI] [PubMed] [Google Scholar]