Abstract
The actions of group II muscle afferents projecting to the lower-lumbar (L6 and L7) segments of the cat spinal cord were investigated by recording the cord dorsum and focal synaptic field potentials evoked by electrical stimulation of hindlimb muscle nerves.
Cord dorsum potentials recorded over the lower-lumbar segments were generally much smaller than those produced by group II afferents terminating within the midlumbar and sacral segments. Only group II afferents of tibialis posterior produced potentials with an amplitude (mean maximal amplitude 39 μV, n = 7) approaching that of potentials over other segments.
Focal synaptic potentials (mean maximal amplitudes 135–200 μV) were evoked by group II afferents of the following muscle nerves, listed in order of effectiveness: quadriceps, tibialis posterior (throughout L6 and L7), gastrocnemius soleus, flexor digitorum longus, posterior biceps-semitendinosus and popliteus (mainly within L7).
Field potentials were recorded in the dorsal horn (laminae IV–V) and also more ventrally in a region which included the lateral part of the intermediate zone (lateral to the large group I intermediate field potentials) and often extended into the ventral horn (laminae V–VII). The latencies of the group II potentials are considered compatible with the monosynaptic actions of the fastest conducting group II muscle afferents.
The results are compared with morphological evidence on the pattern of termination of group II muscle afferents in the lower-lumbar segments and with previous descriptions of the actions of group II muscle afferents in midlumbar and sacral segments.
Group II muscle afferents (which carry signals largely from the secondary ending of the muscle spindle; Matthews, 1972) influence the activity in α-motoneurones via multiple reflex pathways. In the spinal animal the dominating reflex pattern is excitation of flexor motoneurones and inhibition of extensor motoneurones (the flexor reflex pattern) but alternative pathways with the opposite effects also exist (see Lundberg et al. 1987a; Hongo & Pettersson, 1988). There are also crossed group II pathways contributing to inter-limb co-ordination (Arya et al. 1991) and there is evidence that group II muscle afferents may contribute to the stretch reflex of certain muscle groups (e.g. Corna et al. 1995). In addition, various descending systems concerned with voluntary and postural aspects of motor control interact with group II neuronal circuits (Lundberg & Voorhoeve, 1962; Yates et al. 1989; Davies & Edgley, 1994) as does the spinal pattern generator for stepping (Shefchyk et al. 1990). Group II reflex circuits are therefore part of a highly integrated system which is likely to contribute to the control of a range of motor behaviours.
Group II reflex pathways involved in the control of the cat hindlimb appear to be distributed throughout the lumbosacral enlargement (see Jankowska, 1992), but detailed investigations have focused mainly on the circuitry in the midlumbar and sacral segments, at either end of the enlargement. The midlumbar segments contain neurones receiving a powerful monosynaptic input from group II afferents of hip muscles, knee extensor and pretibial flexor muscles (Edgley & Jankowska, 1987b; Aggelopoulos et al. 1996). These neurones include both excitatory and inhibitory interneurones connecting directly with motoneurones of hindlimb muscles (Cavallari et al. 1987). The sacral segments also contain neurones monosynaptically activated by group II muscle afferents but in this case the most powerful input is from afferents of the gastrocnemius and posterior biceps-semitendinosus muscles (Jankowska & Riddell, 1993, 1994). While these interneurones may be interposed in pathways to motoneurones, they do not appear to contact motoneurones directly (Jankowska et al. 1993; Jankowska & Riddell, 1994). It therefore appears that different regions of the lumbosacral enlargement are involved in mediating the group II reflex actions of particular muscle groups and that neurones within these regions may show differences in connectivity and function.
Much less is known about the actions of group II muscle afferents in the lower-lumbar segments but several sets of observations suggest that further group II reflex circuits are located here. Firstly, small group II field potentials have been reported to occur in the dorsal and ventral grey matter of the lower-lumbar segments following activation of afferents of triceps surae (Fu et al. 1974). Secondly, a small number of group II muscle spindle afferents have been examined morphologically after intra-axonal injection of horseradish peroxidase and shown to arborize within the grey matter of lower-lumbar segments (Fyffe, 1979; Mannen et al. 1981; Hongo, 1992). Finally, there are several brief reports of interneurones in the lower-lumbar segments with excitatory input from group II afferents of a variety of hindlimb muscle nerves (Lundberg et al. 1987b; Harrison & Riddell, 1989; Harrison et al. 1994).
The aims of the investigations reported here and in the companion paper (Riddell & Hadian, 2000) were firstly to systematically assess the extent to which processing of information from group II muscle afferents occurs within neuronal circuits in the lower-lumbar segments, and secondly to obtain information on the likely location of interneurones in reflex pathways from group II afferents, in particular, those originating from distal hindlimb muscles. This paper describes the central actions of group II afferents of a range of muscle nerves within the lower-lumbar segments, studied using extracellular recording of focal synaptic potentials (Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993). The accompanying paper (Riddell & Hadian, 2000) describes the response characteristics of interneurones excited by group II muscle afferents which were encountered in those regions of the lower-lumbar segments where field potentials were recorded.
METHODS
Preparation
Experiments were performed on 10 cats under deep general anaesthesia in accordance with Home Office guidelines. Anaesthesia was induced with halothane (4% in a 50:50 O2 and N2O mixture). Following cannulation of a carotid artery and radial vein, anaesthesia was switched to chloralose (70 mg kg−1i.v.) and maintained with further doses of chloralose (10 mg kg−1i.v.) as required. The adequacy of the anaesthesia was verified throughout surgery by monitoring withdrawal reflexes, corneal reflexes, blood pressure and ECG. During recording, when the animals were paralysed with gallamine triethiodide (2–3 mg kg−1i.v., every 40 min) and artificially ventilated, the adequacy of anaesthesia was verified by monitoring the diameter of the pupils and continuously recording blood pressure. Regular tests (at least every 40 min) were made of the stability of blood pressure and heart rate when noxious stimuli were applied to the forepaws.
Mean blood pressure was kept above 90 mmHg (usually 110–130 mmHg) and the end-tidal CO2 was maintained close to 4% by adjusting the parameters of artificial respiration and the rate of infusion of a solution of 100 mM sodium bicarbonate containing 5% glucose. Core temperature was maintained at 37–38°C and the temperature of paraffin pools formed from skin of the back and left hindlimb at 35–37°C. At the end of the experiment, the animal was killed by administration of an overdose of sodium pentobarbital anaesthetic, immediately prior to intra-arterial injection of a fixative solution (see below).
Surgery
A range of hindlimb peripheral nerves were dissected and mounted on electrodes. These included: quadriceps (all branches, Q), posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius soleus (GS), plantaris (Pl), the caudal branch of sural, flexor digitorum and halucis longus, dissected free from the interosseous nerve (FDL), tibialis posterior (TP) and popliteus (Pop). The spinal cord was exposed by laminectomy from the fourth lumbar (L4) to sacral segments and the dura was opened over the full length of the exposed spinal cord.
Recording and stimulating procedures
Peripheral nerves were mounted on bipolar electrodes and stimulated electrically with 0.1 ms rectangular current pulses, applied singly, in pairs or as trains. Stimulus strengths are expressed relative to threshold (T) for the most excitable afferents in the nerve, as determined from recordings of the afferent volley. Afferent volleys and cord dorsum potentials were recorded using silver ball electrodes placed on the surface of the dorsal columns close to the root entry zone. When mapping the rostro-caudal distribution of cord dorsum potentials these electrodes were moved along the dorsal columns, between the sacral and midlumbar segments (S1-L5), at intervals of 2 mm. Field potentials were recorded using glass microelectrodes filled with 2 M NaCl (2–3 μm tip diameter, 2–4 MΩ resistance) introduced into the spinal cord through small tears in the pial membrane over the dorsal columns. Because the border between the L7 and S1 segments can be difficult to identify without dissection, recordings of cord dorsum potentials evoked by group II afferents of PBST were used as an additional guide; the most caudal electrode tracks were made 3–5 mm rostral to the location where the largest of these potentials could be recorded (see Jankowska & Riddell, 1993). Cord dorsum and field potential recordings were digitized and stored on a PC at a sampling rate of 24–40 kHz, without filtering. Averaging (16–64 individual sweeps) and latency measurements were performed using a signal averaging program (Cambridge Electronic Design).
Histology
Marking electrodes were left in situ in the last track of each recording site and at the end of experiments animals were perfused with 4% formal-buffered saline introduced through the arterial cannula. After measuring conduction distances, the precise locations of all recording sites in relation to the lumbar spinal segments were confirmed by dissection of the spinal roots. Blocks of spinal cord containing marking electrodes were sectioned on a vibratome (50 μm transverse sections) and stained with Cresol Violet. Sections were viewed under a light microscope and camera lucida drawings made of the positions of marking electrodes and boundaries of the grey matter. Maps of the areas of grey matter within which field potentials were recorded were constructed from information on the angle of electrode tracks in relation to the marking electrode and the depth of recordings along each track.
RESULTS
Cord dorsum potentials
Electrical activation of group II afferents in nerves from hindlimb muscles produces distinct negative potentials (cord dorsum potentials) at the surface of midlumbar and sacral segments of the spinal cord (Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993). These cord dorsum potentials reflect activity at synapses between group II afferents and neurones in the grey matter, where field potentials with a similar time course to the cord dorsum potentials can be recorded.
Systematic recordings from the surface of the lower-lumbar segments following stimulation of muscle nerves were made in seven animals. Group II afferents of those nerves that very effectively evoked cord dorsum potentials at the midlumbar (Q; Edgley & Jankowska, 1987a) and sacral (PBST, GS; Jankowska & Riddell, 1993) levels produced potentials of small or negligible amplitude at lower-lumbar levels, confirming previous reports. Attention was therefore focused on the actions of group II afferents in those muscle branches of the tibial nerve (FDL, TP and Pop) which recent evidence suggests may have a strong projection to the lower-lumbar segments (Harrison & Riddell, 1989; Harrison et al. 1994). Figure 1 shows examples of surface recordings made at the lower-lumbar level following stimulation of these nerves at different stimulus strengths. When stimuli were increased to a strength (5T) sufficient to activate the majority of group II muscle afferents, negative potentials could be seen following afferent volleys of each of the nerves, but only those evoked by stimulation of TP were of an amplitude comparable to those evoked by group II afferents at midlumbar and sacral levels. As illustrated by the records shown in Fig. 1a, the cord dorsum potentials evoked on stimulation of TP appeared at stimulus intensities close to twice nerve threshold, grew in amplitude as the stimulus intensity was raised and reached a maximal amplitude at about 4–5T. This range of stimulus strengths (2–5T) corresponds to that over which the majority of group II afferents are recruited (Jack, 1978; Ellaway et al. 1982; Lundberg et al. 1987a). Stronger stimuli (10T) did not increase the size of the group II potential but did evoke a later potential, the onset of which can be seen in the lower-most record of Fig. 1A.
Figure 1. Cord dorsum potentials evoked by group II muscle afferents.
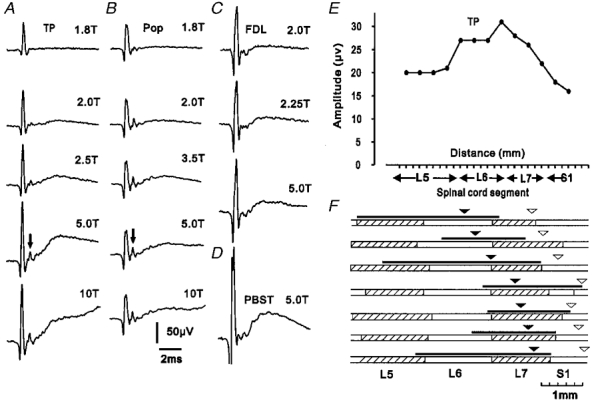
A-C, cord dorsum potentials recorded from the surface of the L7 segment following stimuli of different strengths applied to the tibialis posterior (TP, A), popliteus (Pop, B) and flexor digitorum longus (FDL, C) nerves. For comparison, a cord dorsum potential evoked by group II afferents of posterior biceps-semitendinosus (PBST) and recorded more caudally (rostral S1) is shown in D. All recordings are averages of 32 sweeps (negativity upwards). Time and voltage calibrations apply to all records. Arrows indicate group II afferent volleys. E and F, rostro-caudal distribution of potentials. E, plot of amplitude of cord dorsum potentials evoked by 5T stimuli applied to the TP nerve, recorded at 2 mm intervals along the surface of the lumbosacral cord in one experiment. F, location of potentials evoked by 5T stimuli applied to TP in seven animals. Filled arrow heads indicate the positions where the largest potentials of group II origin were recorded and the black lines above the alternately hatched and open bars, representing spinal segments, indicate the length over which potentials were 75% or more of peak amplitude. For comparison, open arrow heads indicate the positions at which the largest group II potentials were evoked by stimuli applied to PBST nerves.
The mean maximal amplitude of cord dorsum potentials evoked by group II afferents of TP was 39 μV (range 17–57 μV, n = 7); slightly smaller than the cord dorsum potentials evoked by group II afferents of PBST at sacral segments in the same experiments (27–66 μV, mean 49 μV, n = 7; see example in Fig. 1D). The onset latencies of the group II potentials (1.3-1.5 ms with respect to group I volleys) were the same or slightly shorter than those for sacral group II cord dorsum potentials (1.5-2.2 ms for PBST; Jankowska & Riddell, 1993). In two experiments in which group II afferent volleys could be identified (see arrows in Fig. 1a and B), the cord dorsum potentials followed these volleys at latencies of 0.5 and 0.6 ms.
The rostro-caudal distribution of potentials evoked by group II afferents of TP was determined by recording their amplitudes at 2 mm intervals along the surface of the spinal cord. Figure 1E shows a plot from one experiment while Fig. 1F summarizes the results from all seven experiments. The locations at which group II afferents of TP induced the largest potentials varied from caudal L6 to caudal L7, though most occurred within the L7 segment (see filled arrow heads in Fig. 1F). The largest group II potentials evoked by afferents of PBST show a similar variation with respect to segmental topography but show a consistant relationship to the internal organization of the spinal cord (using the rostral ends of the pudendal motor columns as a landmark; see Jankowska & Riddell, 1993). In the present investigation, cord dorsum potentials evoked by group II afferents of both TP and PBST were mapped in the same experiments and their locations could therefore be compared. As can be seen from Fig. 1F, the largest potentials produced by TP were consistently located between 6 and 8 mm rostral of those evoked by PBST (open arrow heads in Fig. 1F). The potentials evoked by afferents of TP were distributed over a comparatively long length of the spinal cord, with potentials three-quarters of maximum size being recorded over 10–20 mm, sometimes as far rostrally as L5 (see black lines above bars representing spinal segments in Fig. 1F).
Figure 1b and C shows examples of the cord dorsum potentials produced when stimuli applied to the FDL and Pop nerves were increased from 2T to 5T. The potentials evoked by group II afferents of these nerves were generally small in comparison to those evoked by TP (FDL 21–30 μV, mean 27 μV, n = 7; Pop 13–33 μV, mean 22 μV, n = 7).
Field potentials
The central actions of group II muscle afferents within the grey matter of lower-lumbar segments were investigated by recording focal synaptic potentials (field potentials) in 10 animals. As shown schematically in Fig. 2a, the grey matter was explored systematically by recording along 10–12 electrode tracks sharing the same entry point on the dorsal columns but covering a range of angles in the transverse plane, separated by 5 deg. Recordings were made at 200 μm intervals along each track, to depths of up to 4 mm, so that each transverse mapping involved 100–130 recordings throughout the dorsal horn, intermediate region and more dorsal parts of the ventral horn (laminae I-VII).
Figure 2. Recording sites, frequency of occurrence and amplitude of group II field potentials.
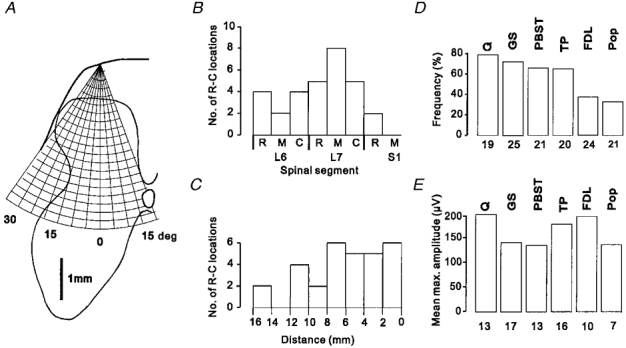
A, schematic diagram of the procedure used to systematically explore the grey matter in the transverse plane. A grid representing 200 μm steps along a series of electrode tracks made from the same entry point on the cord dorsum but at different angles is superimposed on a representative outline of the grey matter of the lower-lumbar segments (for further details see text). B and C, distribution of 30 rostro-caudal locations at which a systematic exploration of the grey matter in the transverse plane was performed. B, rostro-caudal locations of transverse mapping sites in relation to segmental boundaries; rostral (R), middle (M) and caudal (C) third of the L6-S1 segments. C, rostro-caudal locations of transverse mapping sites in relation to the location of the largest cord dorsum potential evoked by group II afferents of PBST (0 mm). D, relative frequency of occurrence of group II field potentials of different nerves (identified above each bar). Bars represent the percentage of rostro-caudal locations at which field potentials (> 50 μV in amplitude) were detected in any electrode track of the transverse mapping; the number of rostro-caudal locations where the actions of each nerve were investigated is shown underneath each bar. E, mean maximal amplitudes of field potentials evoked by group II afferents of each nerve. These values are calculated from the largest field potentials recorded at each rostro-caudal location, i.e. each transverse mapping site; numbers of potentials from which means were calculated are shown underneath each bar. Data for ABSM have been omitted because no field potentials were detected at any location. (NB, the numbers beneath the histograms in E and D therefore represent, for each nerve, the number of potentials detected (E) following transverse mapping at the number of sites shown in D.)
Because of the restricted access over the lower-lumbar segments (due to blood vessels and dorsal roots) a similar mapping in the rostro-caudal plane is difficult to perform. The transverse mapping process described above was therefore repeated at various rostro-caudal locations throughout the lower-lumbar segments (usually at 3 locations in each of the 10 animals). Figure 2b shows the distribution within the L6-S1 segments of 30 rostro-caudal locations where systematic transverse explorations of the grey matter, as illustrated schematically in Fig. 2a, were performed. In view of the variation in the segmental distribution of cord dorsum potentials, these rostro-caudal locations are also shown in relation to the location of the largest cord dorsum potentials evoked by group II afferents of PBST (Fig. 2C). The plots show that all levels of the L6 and L7 segments were sampled, though with some bias towards the L7 segment.
The effects of stimulation of several muscle nerves was generally investigated during the course of each transverse mapping procedure, i.e. several nerves were stimulated in turn, and if necessary at different stimulus intensities, at each of the 100–130 recording sites on the transverse grid. Following stimulation of most of the muscle nerves tested, distinct field potentials attributable to the actions of group II afferents could be recorded within the grey matter. These field potentials were induced not only by afferents of TP, but also by other nerves producing only minimal potentials at the cord dorsum. Examples of potentials evoked by group II afferents are shown in Fig. 3a (TP and Pop), Fig. 3C (FDL) and Fig. 4a (Q). As illustrated in Fig. 3a and C, the appearance and amplitude of the field potentials were clearly related to the range of stimulus strengths expected to recruit increasing proportions of group II muscle afferents (Jack, 1978; Ellaway et al. 1982; Lundberg et al. 1987a). At stimulus intensities of 1.8-2.0T (top records of Fig. 3a and C) very small group I field potentials could sometimes be observed (as in Fig. 3C) while the group II potentials were small or absent. With increasing stimulus intensities, the group II potentials grew in amplitude, reaching a maximum at 4–5T. Further evidence that these potentials originate from group II muscle afferents is provided by their latencies (Table 1), which are consistent with the actions of afferents conducting at velocities in the group II range (see below) and were always longer than those of potentials evoked at lower stimulus intensities (i.e. < 2T) by group I muscle afferents.
Figure 3. Field potentials evoked by group II muscle afferents.
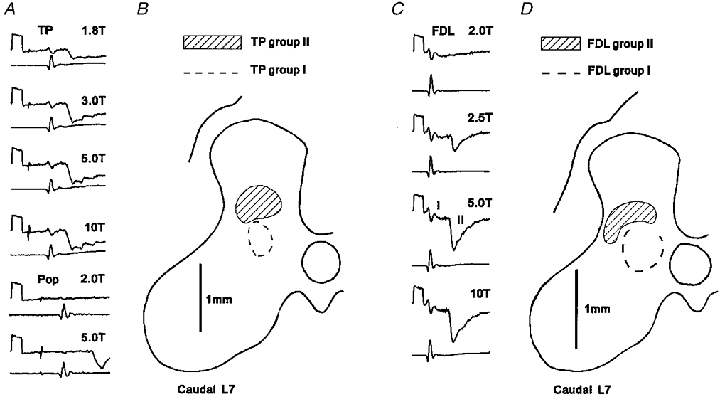
A, field potentials evoked in the caudal L7 segment by group II afferents of TP and Pop. The intensity of electrical stimuli used to evoke the potentials is shown above each record. The top record of each pair is a field potential recording (negativity downwards) and the bottom record the afferent volley (negativity upwards). All records are averages of 32 sweeps. Calibration pulse; 1 ms, 200 μV. Both TP and Pop field potentials were recorded at the same location. B, outline of grey matter on which are indicated the areas in which field potentials > 200 μV evoked by group II afferents (hatched region) and field potentials > 150 μV evoked by group I afferents (dashed line) of the TP nerve could be recorded. The recordings in A were made within the shaded region. C, examples of field potentials evoked by stimulation of FDL (same format as for A). Note that a stimulus at 2T was sub-threshold for the group II potential and produced only a small group I field (I) while a stimulus of 5T was close to maximal for the group II potential (II). D, outline of grey matter on which is indicated the areas in which field potentials > 100 μV evoked by group II afferents (hatched region) and field potentials > 200 μV evoked by group I afferents (dashed line) of the FDL nerve, including those shown in C, could be recorded.
Figure 4. Field potentials evoked by afferents of the quadriceps nerve.
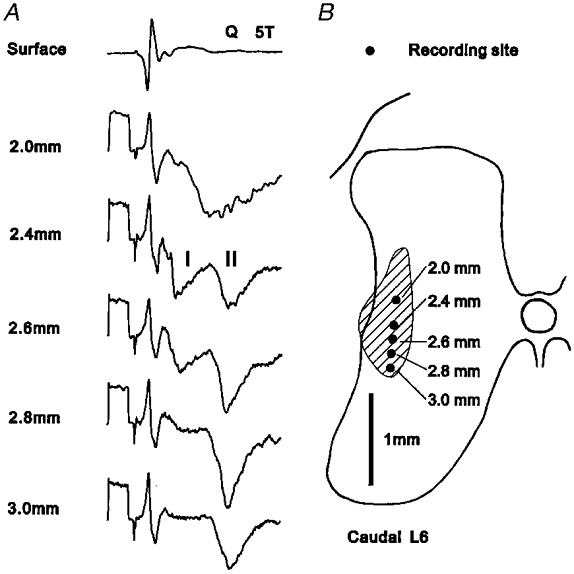
A, potentials recorded at the surface and at different depths within the grey matter following stimulation of the Q nerve at 5T. Field potentials evoked by group I and group II afferents are denoted I and II, respectively. Records are averages of 32 sweeps. Calibration pulse; 1 ms, 200 μV. B, outline of grey matter at caudal L6 on which are indicated the recording sites of the potentials shown in A. The hatched area represents the region within which group II fields of at least 200 μV were evoked.
Table 1.
Latencies of field potentials
| Group I FPs | Dorsal horn group II FPs | Intermediate group II FPs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nerve | Mean (ms) | Range (ms) | n | Mean (ms) | Range (ms) | n | Mean (ms) | Range (ms) | n | |
| A. | PBST | 0.80 | 0.57–0.97 | 8 | 1.50 | 1.10–2.45 | 5 | 2.40 | 2.10–2.95 | 8 |
| GS | 0.63 | 0.42–1.22 | 8 | 1.55 | 1.32–2.05 | 4 | 3.04 | 2.40–3.35 | 8 | |
| Q | 0.95 | 0.70–1.21 | 10 | — | — | — | 2.64 | 1.90–3.37 | 13 | |
| FDL | 0.49 | 0.40–0.57 | 8 | — | — | — | 2.10 | 1.37–2.95 | 8 | |
| TP | 0.56 | 0.55–0.57 | 3 | 1.43 | 1.02–1.82 | 8 | 2.30 | 1.60–2.92 | 7 | |
| Pop | — | — | — | 2.07 | 1.42–2.80 | 5 | — | — | — | |
| B. | PBST | 1.84 | 1.65–1.95 | 8 | 2.40 | 2.10–2.95 | 5 | 3.44 | 3.10–4.02 | 8 |
| GS | 2.23 | 2.00–2.75 | 8 | 3.30 | 2.0–2.75 | 4 | 2.23 | 3.07–3.70 | 8 | |
| Q | 1.59 | 1.35–1.82 | 10 | — | — | — | 3.34 | 2.52–4.15 | 13 | |
| FDL | 2.38 | 2.20–2.55 | 8 | — | — | — | 3.98 | 3.20–4.95 | 8 | |
| TP | 2.37 | 2.35–2.40 | 3 | 3.32 | 2.97–3.77 | 8 | 4.13 | 3.45–4.70 | 7 | |
| Pop | — | — | — | 3.95 | 3.17–4.70 | 5 | — | — | — | |
Minimal latencies of field potentials (FPs) measured from the onset of the group I volley (A) or from the stimulus (B). Main columns show, from left to right, nerve of origin, data for field potentials evoked by group I muscle afferents and data for field potentials evoked by group II muscle afferents in the dorsal and intermediate grey matter. Values shown are derived from the shortest of many latency measurements made during systematic transverse mapping of the grey matter. Latencies of group I field potentials are for recordings made at the same locations as group II field potentials.
Figure 2D shows the proportion of transverse mapping locations at which field potentials were evoked by each of the muscle nerves tested. Only field potentials which exceeded 50 μV in amplitude have been considered and data for ABSM, which failed to evoke field potentials at any location, are omitted. Group II afferents of Q, GS, PBST and TP were the most frequently effective, evoking field potentials at 60–80% of the rostro-caudal locations where transverse mapping was performed, while field potentials evoked by group II afferents of FDL and Pop were recorded at 30–40% of the locations. As shown in Fig. 2E, the mean maximal amplitudes of field potentials evoked by each nerve were in the range 130–200 μV.
The information shown in the histograms of Fig. 2D and E on the frequency of occurrence and mean amplitude of the field potentials provides an indication of the overall effectiveness of group II afferents of each of the muscle nerves tested, as a source of input to neurones in the L6 and L7 segments. There was, however, some variation in both the rostro-caudal and transverse distributions of field potentials evoked by different nerves and in the amplitudes of field potentials evoked by the same nerve in different regions of the grey matter.
Rostro-caudal distribution
Field potentials evoked by group II afferents of Q and TP were encountered with similar frequency throughout the L6 and L7 segments and also more caudally in the first sacral segment, where potentials are evoked in the dorsal horn by group II afferents (Jankowska & Riddell, 1993). Potentials evoked by TP were of a similar amplitude at all levels but those evoked by Q tended to be larger in L6 (mean 231 μV, n = 8) than in L7 (mean 144 μV, n = 5), a tendency that was clearer still when recording sites were related to the position of the largest PBST cord dorsum potential (9–16 mm, mean 262 μV, n = 7; 0–8 mm, mean 122 μV, n = 6).
Group II potentials evoked by afferents of the remaining nerves were concentrated within caudal regions of the lower-lumbar segments. Field potentials evoked by afferents of GS, although occasionally encountered in L6, were seen with greater frequency in L7; a distribution that is in agreement with an earlier report (Fu et al. 1974). Field potentials evoked by group II afferents of PBST were evoked almost exclusively in L7 or within 8 mm rostral of the largest PBST cord dorsum potential.
Field potentials evoked by group II afferents of FDL and Pop were less frequently encountered than those evoked by other muscle nerves but were seen with greatest frequency within L7 (4–8 mm rostral of the largest PBST potential).
Distribution in the transverse plane
In midlumbar segments, group II field potentials are evoked with two clear foci, one in the dorsal horn and the other in the intermediate nucleus (Edgley & Jankowska, 1987a). In the sacral segments large dorsal horn fields analogous to those of the midlumbar segments can be seen but intermediate potentials are mainly absent (Jankowska & Riddell, 1993). In the lower lumbar segments the distinction between dorsal and intermediate potentials was less marked and the two types of potential were rarely evident at the same mapping location. Nevertheless, group II fields in the lower-lumbar segments were encountered in broadly two main areas.
Dorsal group II potentials
One area in which field potentials evoked by group II afferents were commonly encountered was at the base of the dorsal horn (lamina IV and V), overlying the region where group I afferents evoked large group I field potentials (group I intermediate nucleus; Eccles et al. 1954). Examples of the area occupied by fields of this type are shown in Fig. 3b and D. Distinct group II potentials (> 100 μV) were generally encountered within a relatively small area, rarely more than 0.8-1.0 mm in medio-lateral or dorso-ventral extent. The field potentials usually occupied an area within the middle two-thirds of the dorsal horn (e.g. Fig. 3b) but sometimes extended almost to the lateral border of the grey matter (e.g. Fig. 3D). The fields recorded in this area were usually not preceded by potentials evoked by group I afferents. However, occasionally, as in the records shown in Fig. 3C, small, indistinct group I potentials could just be discerned.
Intermediate group II potentials
The second location at which group II field potentials were commonly encountered was in the area lying immediately lateral to the group I intermediate nucleus. Potentials recorded here could often be mapped over an area extending from the base of the dorsal horn through to the most dorsal aspect of the ventral horn; that is, from the lateral part of lamina V through to the lateral part of lamina VII. An example of a group II potential with a distribution of this type is shown in Fig. 4b. As in the dorsal horn, the area within which distinct group II potentials were recorded was rarely more than 0.8-1.0 mm across and was often more restricted than this in the medio-lateral plane. Most of the group II intermediate field potentials were preceded by shorter latency potentials which grew to a maximal amplitude in parallel with the earliest component of the afferent volley. These potentials, which are attributed to the actions of group I muscle afferents, were of equal or smaller amplitude than the group II components of the fields (see Fig. 4a).
In general, group II afferents of a given nerve evoked potentials either of the dorsal type or of the intermediate type but rarely within both areas at the same mapping site. An exception to this was that group II afferents of Q occasionally showed a distribution of the type illustrated in Fig. 4, which although lying predominantly within the intermediate region also extends into the dorsal horn. As can be seen from the records in Fig. 4a, the group II potentials recorded in the dorsal region were of shorter latency than those recorded more ventrally and were not preceded by a group I potential. However, potentials of this type were seen only within the L6 segment in some animals and they presumably reflect a gradual transition towards the type of field potential distribution that is commonly seen in the midlumbar segments. For the purposes of the analysis which follows, potentials which lay predominantly level with or ventral to the group I intermediate nucleus have been treated as a single field potential and categorized as intermediate.
Not all nerves produced dorsal group II field potentials and intermediate group II potentials with equal frequency and effectiveness. Pop produced only dorsal group II potentials while Q and FDL produced group II potentials almost exclusively in the intermediate region (but see above). GS produced intermediate group II potentials with twice the frequency of dorsal potentials (dorsal 7/25 mappings; intermediate 15/25 mappings) while group II afferents of PBST (dorsal 8/21 mappings; intermediate 10/21 mappings) and TP (dorsal 9/20 mappings; intermediate 7/20 mappings) produced both types of potentials with about equal frequency. Those nerves which were effective at both sites generally produced potentials of similar magnitude in the dorsal horn and the intermediate region. However, TP was an exception to this, evoking field potentials in the dorsal horn (mean maximal amplitude 220 μV, n = 9) which were nearly twice the amplitude of those recorded more ventrally (mean maximal amplitude 120 μV, n = 7).
It was quite common for field potentials evoked by group II afferents of more than one nerve to be recorded at the same transverse mapping location. The effects of stimulation of five or six different nerves were investigated at 28 of the transverse mapping locations, and field potentials evoked by two or more nerves (up to 6) were recorded at 20 (71%) locations while field potentials evoked by three or more nerves were recorded at 12 (43%) locations. There was a greater tendency for potentials evoked by two or more nerves to be seen at locations in the L7 segment (83%) than in L6 (50%). Field potentials evoked by two or more nerves at the same transverse mapping location often occurred within the same or overlapping regions of the grey matter. Of the 20 transverse mapping locations where field potentials were evoked by two or more nerves, group II potentials were recorded in the same area (dorsal horn or intermediate area) at 17. This observation implies that spinal neurones located within such regions are likely to receive convergent input from group II afferents of more than one muscle nerve.
Latency and synaptic linkage
The minimal latencies of onset of field potentials evoked by muscle afferents of different nerves in the dorsal horn and in the intermediate region are shown in Table 1. Table 1A shows the central latencies of the field potentials, which were measured with respect to the onset of the group I volley since group II components of afferent volleys were only rarely detectable. Table 1B shows the latencies of the same potentials with respect to application of the stimulus to the peripheral nerve.
The central latencies of the potentials evoked by group II afferents of PBST and GS in the dorsal horn were very similar to those of potentials evoked monosynaptically by these afferents in the dorsal horn of the sacral segments (PBST mean 1.55 ms; GS mean 1.87 ms; Jankowska & Riddell, 1993). This is to be expected if the potentials at both locations are evoked monosynaptically since the conduction paths to the two regions are comparable. Group II afferents of PBST and GS also evoked field potentials in more ventral regions of the grey matter and the central delays of these intermediate potentials were generally of longer latency (by about 1.0 ms, see Table 1) than the potentials evoked in the dorsal horn. This is in keeping with previous observations on field potentials evoked in these segments by group II afferents of GS (Fu et al. 1974) and with the actions of group II afferents of other nerves in midlumbar and sacral segments (Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993). Although the longer delay could theoretically be explained by the addition of an interneurone in the pathway (i.e. a disynaptic linkage), it is more likely to be due to the additional time required for conduction along the ventrally directed collaterals of group II afferents. For example, Fu & Schomburg (1974) have shown that there is a difference of up to 0.8 ms in the conduction time for group II afferent fibres when they are activated by intraspinal stimuli applied in the dorsal horn compared to the ventral horn, indicating that branching of their intraspinal terminals is accompanied by an appreciable slowing in conduction velocity. The latencies of the intermediate group II potentials, though longer than those in the dorsal horn, are therefore also compatible with monosynaptic actions.
The central latencies of field potentials evoked by group II afferents of FDL and TP were on average slightly shorter (0.1-0.3 ms) than those of potentials evoked by group II afferents of PBST and GS despite the longer conduction distances of the former. This reflects the fact that the dorsal root entry of TP and FDL afferents is mainly at the L6/L7 level (as judged by the largest afferent volleys) and conduction within the dorsal columns is therefore minimal for these afferents.
The central latencies of the intermediate field potentials evoked by group II afferents of the Q nerve were longer than those for TP and FDL (on average by 0.34 and 0.55 ms, respectively), despite the fact that the impulses in group II afferents of the Q nerve should be less delayed with respect to group I afferents because of the shorter peripheral conduction path. The explanation for this is that while TP and FDL afferents enter the spinal cord at the level at which recordings were made, Q afferents enter at caudal L5 and rostral L6, up to 15 mm from the more caudal recording locations. The additional conduction time within the dorsal columns may be up to 0.5 ms since the conduction velocity of the intraspinal collaterals of group II afferents is 30–60% of that in the peripheral nerves (e.g. Fern et al. 1988) and is thought to be lower for descending collaterals. In comparison to previous reports, the range of latencies of the Q group II potentials in the lower-lumbar segments was similar to that of EPSPs evoked by Q group II afferents in dorsal horn neurones of the S1 segment (Jankowska & Riddell, 1994) and the average latency was less than 0.2 ms longer than that for field potentials evoked monosynaptically by Q group II afferents in the intermediate region of the midlumbar segments (2.46 ms; Edgley & Jankowska, 1987a). The latencies of fields evoked by group II afferents of Q in the intermediate region of the lower-lumbar segments are also therefore considered compatible with a monosynaptic action.
The latencies of group I field potentials recorded at the same locations as intermediate group II potentials (see Table 1) are typical of those previously recorded in the group I intermediate nucleus in the lower-lumbar segments (Eccles et al. 1954).
Further evidence that the field potentials were evoked monosynaptically was sought by verifying that at least the main components (up to the peak) of potentials followed a train of stimuli (200–300 Hz) without increasing in amplitude. None of the field potentials tested in this way (5 dorsal and 8 intermediate) showed any evidence of the temporal summation that would be expected if one or more interneurones were interposed in the pathway (not illustrated).
DISCUSSION
Distribution of field potentials in the grey matter
Since field potentials reflect the flow of current at synapses formed by afferent fibres with their target spinal neurones (see Eccles et al. 1954; Coombs et al. 1956), the relatively small field potentials recorded within the dorsal horn of the lower-lumbar segments (generally < 200 μV) indicate a less powerful projection from group II muscle afferents to this region than to the equivalent regions of the midlumbar and sacral segments, where potentials of up to 1 mV can be recorded (Edgley & Jankowska, 1987a; Jankowska & Riddell, 1993). This implies that there may be differences in the functional organization of neurones processing information from group II muscle afferents in these two regions of the lumbosacral enlargement. Previous observations on the sources of input to ascending tract neurones located in the dorsal horn provide evidence consistent with this. Ascending tract neurones in the lower-lumbar segments, including neurones contributing to the spinocervical tract (Harrison & Jankowska, 1984), neurones of the postsynaptic dorsal column system (Jankowska et al. 1979) and neurones of the dorsal spinocerebellar tract (Aoyama et al. 1988), appear to receive little if any input from group II muscle afferents. In contrast, neurones in the dorsal horn of the midlumbar and sacral segments appear to provide an important route for the transmission of information signalled by group II muscle afferents to supraspinal structures. Neurones contributing to the spinocervical tract in both the midlumbar and sacral segments are strongly excited by group II muscle afferents in addition to their powerful input from cutaneous afferents (Hammer et al. 1994; Riddell et al. 1994), as are neurones of the dorsal spinocerebellar tract in the midlumbar segments (Edgley & Jankowska, 1988). It could be argued that previous studies may not have sampled the most lateral aspects of the grey matter in the lower lumbar segments where group II field potentials were frequently located in the present study. However, this is unlikely because, as we report in the accompanying paper (Riddell & Hadian, 2000), when neurones in the lateral grey matter of lower lumbar segments were systematically investigated, only seven of 81 neurones excited by group II afferents (8%) had axons which projected rostral to the lumbar segments.
In addition to neurones of ascending tracts, the dorsal horn of the midlumbar and sacral segments contains a functionally heterogenous population of group II interneurones, some of which appear to be excitatory and others inhibitory (Edgley & Jankowska, 1987b; Jankowska & Riddell, 1994; Maxwell et al. 1997). These interneurones appear to have mainly local actions (Jankowska et al. 1993) and there is evidence that some are involved in the presynaptic inhibition of group II muscle afferents (Riddell et al. 1995; Jankowska & Riddell, 1995). The accompanying paper (Riddell & Hadian, 2000) shows that some interneurones excited by group II muscle afferents are located within the dorsal horn of the lower-lumbar segments but it remains to be determined whether any of these interneurones perform functions analogous to the dorsal horn neurones studied in the midlumbar and sacral segments.
Intermediate group II potentials in the lower-lumbar segments were if anything larger (maximally up to 400 μV) than those recorded in the dorsal horn and although potentials evoked by a given nerve tended to be restricted in their distribution, group II afferents of one or more of the muscle nerves tested evoked potentials at virtually all of the rostro-caudal locations where systematic tracking was performed. The intermediate potentials are therefore of a comparable amplitude to those seen in the intermediate region of the midlumbar segments (Edgley & Jankowska, 1987a) and are much larger and more widespread than those seen in the sacral segments (see Fig. 4 of Jankowska & Riddell, 1993). Indeed, since the later occurred only close to the L7-S1 border it is probable that they represent the caudal extent of the field potentials described here.
In previous studies, combined stimulation of the nerves to FDL, TP and Pop together with a small branch innervating the interosseal membrane (FDL et al.) suggested that these nerves are among those with synaptic actions within the lower-lumbar segments (Harrison & Riddell, 1989; Harrison et al. 1994). In the present experiments, the actions of the muscle nerve branches were assessed individually and the nerve to the interosseous membrane was removed in order that the actions of group II afferents innervating Pacinian corpuscles in the interosseal membrane could be excluded. The results confirm that group II afferents of TP and FDL have significant synaptic actions in the lower-lumbar segments, in particular those of TP, which evoke field potentials in both the dorsal horn and intermediate area throughout both the L6 and L7 segments.
The actions of group II afferents of Q have been extensively investigated in the midlumbar segments where they produce a powerful excitation of spinal neurones. However, field potentials produced by these afferents have been reported to decline rapidly in amplitude caudal of mid-L5 (Edgley & Jankowska, 1987a; see their Fig. 5). In the present study, although stimulation of Q only rarely evoked field potentials in the dorsal horn of the L6-L7 segments, distinct group II potentials were frequently observed in the intermediate region. These observations are in agreement with a report that the reflex actions of Q group II muscle afferents on motoneurones (at least, excitation of flexor motoneurones) are mediated in part by interneurones located caudal of the L5-L6 border (Cavallari & Pettersson, 1991).
Distribution of field potentials in relation to morphological studies of group II muscle afferents
The present observations are based on the use of electrical stimulation which activates muscle spindle and non-spindle afferents in muscle nerves indiscriminately. However, there have been several morphological studies of the pattern of termination of functionally identified group II muscle spindle afferents (mainly of ankle extensors) terminating in the lower-lumbar segments (Fyffe, 1979; see also Brown, 1981; Mannen et al. 1981; Ishizuka et al. 1984; Hongo, 1992) and Fu & Schomburg (1974) used intraspinal stimulation of the central terminals of GS group II muscle spindle afferents to map their area of termination. Both approaches showed that group II muscle spindle afferents terminate in the dorsal horn and in the intermediate region of the lower-lumbar segments where group II field potentials were recorded in the present study. This evidence, together with other evidence which has been considered in detail elsewhere (see Discussion of Edgley & Jankowska, 1987a, and Jankowska & Riddell, 1993) favours the proposal that group II muscle spindle afferents at least contribute to the field potentials described here.
The finding that group II afferents have a less powerful action in the dorsal horn of lower-lumbar segments than in sacral segments but a stronger action in the intermediate zone of the lower-lumbar segments than sacral segments (Jankowska & Riddell, 1993) is entirely consistent with morphological evidence from intra-axonally labelled group II afferent fibres. According to a brief description by Ishizuka et al. (1984), unfortunately never published in full, the pattern of termination of collaterals from the same group II afferent fibre varies depending upon its location relative to the homonymous motor column. At the level of the motor column, each collateral gives rise to about 150 boutons which are mainly distributed in the intermediate grey matter (laminae V-VII), while caudal to the motor column the number of boutons per collateral increases dramatically (to 350–750) and the great majority of boutons are distributed within the dorsal horn (laminae IV and V). For the afferents which were the subject of this morphological study (medial gastrocnemius and flexor digitorum brevis), the caudal end of the homonymous motor column occurs close to the border between the L7 and S1 segments (Romanes, 1951; Vanderhorst & Holstege, 1997). The change in distribution of the terminal boutons of group II muscle afferents seen morphologically therefore correlates with the transition from the large amplitude group II field potentials seen in the dorsal horn of the sacral segments to the smaller more ventrally distributed potentials seen in the lower-lumbar segments.
Other aspects of the morphology of group II afferent fibres may explain the more patchy and restricted nature of the field potentials recorded in the lower-lumbar segments compared to the dorsal horn of the sacral segments. Although axon collaterals originate at similar intervals (800–1000 μm) along the length of the ascending and descending branches of group II fibres, their rostro-caudal development varies. At the level of the homonymous motor column (L6 and L7) the collaterals show a very limited development in the rostro-caudal plane (about 200 μm), whereas beyond the motor column (L7/S1) the dorsal horn collaterals of the same group II fibre show a high degree of rostro-caudal development, extending for 500–1000 μm (Fyffe, 1979; see also Brown, 1981; Mannen et al. 1981).
Acknowledgments
We wish to thank Anne Ward for excellent technical assistance. We gratefully acknowledge the support of the Wellcome Trust (grant ref. no. 043630/Z/91/Z/1.4U) and the McNaught Bequest. M.H. was supported on an Iranian Government studentship.
References
- Aggelopoulos NC, Bawa P, Edgley SA. Activation of midlumbar neurons by afferents from anterior hindlimb muscles in the cat. The Journal of Physiology. 1996;497:795–802. doi: 10.1113/jphysiol.1996.sp021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama M, Hongo T, Kudo N. Sensory input to cells of origin of uncrossed spinocerebellar tract located below Clarke's column in the cat. The Journal of Physiology. 1988;398:233–257. doi: 10.1113/jphysiol.1988.sp017040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. The Journal of Physiology. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG. The Anatomy and Physiology of Identified Neurones. Berlin, Heidelberg and New York: Springer Verlag; 1981. Organisation in the spinal cord. [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hindlimb muscles in the cat. The Journal of Physiology. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Petterson LG. Synaptic effects in lumbar motoneurones evoked from group II muscle afferents via two different interneuronal pathways in the cat. Neuroscience Letters. 1991;129:225–228. doi: 10.1016/0304-3940(91)90467-8. [DOI] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Landgren S. Spinal cord potentials generated by impulses in muscle and cutaneous afferent fibres. Journal of Neurophysiology. 1956;19:452–467. doi: 10.1152/jn.1956.19.5.452. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. The Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumber interneurones from descending motor pathways in the cat. The Journal of Physiology. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Landgren S, Winsebury GJ. Spinal cord potentials generated by volleys in the large muscle afferents. The Journal of Physiology. 1954;125:590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group I and II muscle afferents in the middle lumbar segments of the cat spinal cord. The Journal of Physiology. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987b;389:675–690. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones. The Journal of Physiology. 1988;397:81–97. doi: 10.1113/jphysiol.1988.sp016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Murphy PR, Tripathi A. Closely coupled excitation of gamma motoneurones by group III muscle afferents with low mechanical threshold in the cat. The Journal of Physiology. 1982;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R, Harrison PJ, Riddell JS. The dorsal column projection of muscle afferent fibres from the cat hindlimb. The Journal of Physiology. 1988;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T-C, Santini M, Schomburg ED. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiologica Scandinavica. 1974;91:298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Fu T-C, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiologica Scandinavica. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Fyffe REW. The morphology of group II muscle afferent fibre collaterals. The Journal of Physiology. 1979;296:39–40P. [PubMed] [Google Scholar]
- Hammer I, Szabo-Läckberg Z, Jankowska E. New observations on input to spinocervical tract neurones from muscle afferents. Experimental Brain Research. 1994;100:1–6. doi: 10.1007/BF00227273. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Connolly G, Guzman-Villalba JM. Characteristics of input to the group II interneurones in the caudal lumbar segments of the cat spinal cord. The Journal of Physiology. 1994;480.P:46P. [Google Scholar]
- Harrison PJ, Jankowska E. An intracellular study of descending and non-cutaneous afferent input to spinocervical tract neurones in the cat. The Journal of Physiology. 1984;356:245–261. doi: 10.1113/jphysiol.1984.sp015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Riddell JS. Group II activated lumbosacral interneurones with an ascending projection to midlumbar segments of the cat spinal cord. The Journal of Physiology. 1989;408:561–570. doi: 10.1113/jphysiol.1989.sp017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 389–394. IBRO series no. 1. [Google Scholar]
- Hongo T, Pettersson L-G. Comments on group II excitations in high and low spinal cats. Neuroscience Research. 1988;5:563–566. doi: 10.1016/0168-0102(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Hongo T, Kudo N, Sasaki S, Yamashita M, Mannen H. Distribution pattern of boutons of muscle spindle group II afferents in relation to the homonymous motor column in the cat. Neuroscience Research. 1984;(suppl. 1):551. [Google Scholar]
- Jack JJB. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology. Cambridge University Press; 1978. pp. 155–176. [Google Scholar]
- Jankowska E. Interneuronal relays in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Rastad J, Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal column. The Journal of Physiology. 1979;290:185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. A relay for group II muscle afferents in sacral segments of the cat spinal cord. The Journal of Physiology. 1993;465:561–578. doi: 10.1113/jphysiol.1993.sp019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones in pathways from group II muscle afferents in sacral segments of the feline spinal cord. The Journal of Physiology. 1994;475:455–468. doi: 10.1113/jphysiol.1994.sp020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones mediating presynaptic inhibition of group II muscle afferents in the cat spinal cord. The Journal of Physiology. 1995;483:461–471. doi: 10.1113/jphysiol.1995.sp020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Szabo-Läckberg Z, Hammer I. Morphology of interneurones in pathways from group II muscle afferents in sacral segments of the cat spinal cord. Journal of Comparative Neurology. 1993;336:1–11. doi: 10.1002/cne.903370312. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Experimental Brain Research. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Voorhoeve P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiologica Scandinavica. 1962;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Mannen H, Ishizuka N, Hongo T, Kudo N, Sasaki S, Yamashita M. Quantitative analysis of the morphology of group II fibres in the spinal cord of the cat. Neuroscience Letters. 1981;(suppl. 6):596. [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold publishers Ltd; 1972. [Google Scholar]
- Maxwell DJ, Kerr R, Jankowska E, Riddell J. Synaptic connections in dorsal horn group II spinal interneurones: synapses formed with the interneurones and by their axon collaterals. Journal of Comparative Neurology. 1997;380:51–69. [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Interneurones in pathways from group II muscle afferents in the lower-lumbar segments of the feline spinal cord. The Journal of Physiology. 2000;522:109–123. doi: 10.1111/j.1469-7793.2000.t01-2-00109.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Hammer I, Szabo-Läckberg Z. Ascending tract neurones processing information from group II muscle afferents in sacral segments of the feline spinal cord. The Journal of Physiology. 1994;475:469–481. doi: 10.1113/jphysiol.1994.sp020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organisation of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the spinal cord of the anaesthetised cat. The Journal of Physiology. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanes GJ. The motor cell columns of the lumbosacral spinal cord of the cat. Journal of Comparative Neurology. 1951;94:313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, Mccrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Experimental Brain Research. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VGJM, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. Journal of Comparative Neurology. 1997;382:46–76. [PubMed] [Google Scholar]
- Yates BJ, Kasper J, Wilson VJ. Effects of muscle and cutaneous hindlimb afferents on L4 neurons whose activity is modulated by neck rotation. Experimental Brain Research. 1989;77:48–56. doi: 10.1007/BF00250566. [DOI] [PubMed] [Google Scholar]


