Abstract
Ca2+ sensitization of smooth muscle contraction involves the small GTPase RhoA, inhibition of myosin light chain phosphatase (MLCP) and enhanced myosin regulatory light chain (LC20) phosphorylation. A potential effector of RhoA is Rho-associated kinase (ROK).
The role of ROK in Ca2+ sensitization was investigated in guinea-pig ileum.
Contraction of permeabilized muscle strips induced by GTPγS at pCa 6.5 was inhibited by the kinase inhibitors Y-27632, HA1077 and H-7 with IC50 values that correlated with the known Ki values for inhibition of ROK. GTPγS also increased LC20 phosphorylation and this was prevented by HA1077. Contraction and LC20 phosphorylation elicited at pCa 5.75 were, however, unaffected by HA1077.
Pre-treatment of intact tissue strips with HA1077 abolished the tonic component of carbachol-induced contraction and the sustained elevation of LC20 phosphorylation, but had no effect on the transient or sustained increase in [Ca2+]i induced by carbachol.
LC20 phosphorylation and contraction dynamics suggest that the ROK-mediated increase in LC20 phosphorylation is due to MLCP inhibition, not myosin light chain kinase activation.
In the absence of Ca2+, GTPγS stimulated 35S incorporation from [35S]ATPγS into the myosin targeting subunit of MLCP (MYPT). The enhanced thiophosphorylation was inhibited by HA1077. No thiophosphorylation of LC20 was detected.
These results indicate that ROK mediates agonist-induced increases in myosin phosphorylation and force by inhibiting MLCP activity through phosphorylation of MYPT. Under Ca2+-free conditions, ROK does not appear to phosphorylate LC20in situ, in contrast to its ability to phosphorylate myosin in vitro. In particular, ROK activation is essential for the tonic phase of agonist-induced contraction.
Contractile stimulation of smooth muscle with physiological agonists leads to a rise in intracellular free Ca2+ concentration ([Ca2+]i), calmodulin-dependent activation of myosin light chain kinase (MLCK), myosin regulatory light chain (LC20) phosphorylation, crossbridge cycling and force development. Potential for regulation of contraction by agonists is found at all levels. Recently, much attention has been focused on regulation of force that is independent of changes in [Ca2+]i, referred to as Ca2+ sensitization (Somlyo & Somlyo, 1994). Ca2+ sensitization occurs in response to stimulation of G protein-coupled receptors and can be mimicked by the non-hydrolysable GTP analogue GTPγS or antagonized by GDPβS (Nishimura et al. 1988; Kitazawa et al. 1991a). Cellular signalling pathways involved in Ca2+ sensitization converge on an increase in LC20 phosphorylation and analyses of kinase and phosphatase activities in situ have indicated that LC20 dephosphorylation is selectively reduced in this process, i.e. Ca2+ sensitization is mediated via inhibition of myosin light chain phosphatase (MLCP) (Kitazawa et al. 1991b; Kubota et al. 1992).
Several different mechanisms have been proposed to account for this inhibition of MLCP (for reviews see Somlyo & Somlyo, 1994; Hartshorne et al. 1998). These include dissociation of the subunits of the holoenzyme by arachidonic acid (Gong et al. 1992), phosphorylation of the myosin targeting subunit (MYPT) of MLCP by an unknown kinase (Ichikawa et al. 1996) or through the action of protein kinase C (PKC) (Masuo et al. 1994; Buus et al. 1998). The small GTPase RhoA (either the GTPγS-bound form or the constitutively active mutant, RhoAV14-GTP) induced Ca2+ sensitization when added to permeabilized smooth muscle (Hirata et al. 1992; Noda et al. 1995; Gong et al. 1996; Otto et al. 1996). Conversely, inhibition of RhoA function (by ADP-ribosylation catalysed by exoenzyme C3 from Clostridium botulinum or by glucosylation catalysed by Clostridium difficile toxin B) reduced Ca2+ sensitization (Otto et al. 1996; Gong et al. 1996; Akopov et al. 1998) and inhibited sustained contraction in intact smooth muscle (Fujihara et al. 1997; Lucius et al. 1998) indicating that RhoA plays an important role in this process. Ca2+ sensitization requires translocation of RhoA to the plasma membrane where it interacts with an effector (Fujihara et al. 1997; Gong et al. 1997).
Several RhoA effectors, including Rho-associated kinase (ROK) (Leung et al. 1995; Ishizaki et al. 1996; Matsui et al. 1996), have been identified. Based on the observations that ROK phosphorylates myosin (Amano et al. 1996) and MYPT (causing MLCP inhibition) (Kimura et al. 1996), that recombinant constitutively-active ROK causes contraction in Triton X-100-skinned tissue (Kureishi et al. 1997) and that the selective ROK inhibitor Y-27632 inhibits phenylephrine-induced contraction of intact arterial smooth muscle (Uehata et al. 1997) and GTPγS-induced contraction of permeabilized arterial smooth muscle (Uehata et al. 1997; Fu et al. 1998), ROK has emerged as a candidate constituent of a signalling pathway leading to Ca2+ sensitization.
It is interesting to note that longitudinal smooth muscle from the guinea-pig ileum, unlike other smooth muscles, demonstrates almost complete dependence on RhoA for Ca2+ sensitization (Otto et al. 1996). It is, therefore, unlikely that Ca2+ sensitization is a composite of various parallel pathways in this tissue. Guinea-pig ileum longitudinal smooth muscle therefore provides a suitable tissue for investigating the role of ROK in Ca2+ sensitization of smooth muscle contraction.
METHODS
Animals
Guinea-pigs (250–400 g) were killed either by halothane inhalation or cervical dislocation as approved by the local Animal Care Committees at the University of Calgary and the University of Lund, respectively. A 20–30 cm long segment of the intestine just proximal to the ileocaecal valve was removed and washed in nominally Ca2+-free N-(2-hydroxyethyl)piperazine-N’-ethanesulphonic acid (Hepes)-buffered Krebs solution (for composition see ‘Solutions’). Strips were prepared by tearing with fine forceps along natural lines of cleavage from the outer layer of ileal longitudinal smooth muscle. Taeniae coli were dissected from the colon and washed in Ca2+-free Hepes-buffered Krebs solution. Preparations with a width of 100–200 μm, a thickness of 50–100 μm and a length of 5–10 mm were thus obtained.
Force determination and permeabilization
Muscle strips were attached with cellulose acetate glue to a force transducer (AME 801, SensoNor A/S, Horten, Norway) at one end and a carbon fibre support at the other end. They were stretched shortly after mounting to ∼0.2 mN and thereafter the passive tension, determined in Ca2+-free solution, declined to a steady level of 40 ± 5 μN (2.4 % of the maximum active plus passive force, n (no. of strips) = 47). Solution changes were rapidly effected by lowering a small platform supporting a Perspex cup and changing the cup for one containing the desired fresh solution. After equilibration in Hepes-buffered Krebs solution, the strips were contracted twice by immersion in high-K+ solution (for composition see ‘Solutions’) at 22°C then relaxed by immersion in Ca2+-free Hepes-buffered Krebs solution followed by intracellular substitution solution of pCa (-log[Ca2+]) = 9. Strips were permeabilized with 0.05 mg ml−1 (ileum) or 0.1 mg ml−1 (taenia coli) β-escin in pCa 6.0 solution for 20–40 min at room temperature (20–22°C), until force reached a plateau. The contractions elicited in pCa 4.5 solution immediately following permeabilization were used to normalize subsequent responses and these amounted to 111.7 ± 5.3 % (n = 47) of the peak of the second contraction obtained in high-K+ solution prior to permeabilization. The incubation volume was 200 μl and agents or vehicle were added directly to the bath prior to change.
Solutions
The Hepes-buffered Krebs solution used for intact strips had the following composition (mM): 135.5 NaCl, 5.9 KCl, 1.2 MgCl2, 11.6 glucose, 11.6 Hepes, 2.5 CaCl2, pH 7.35 at room temperature (20–22°C). High-K+ solution was obtained by replacing NaCl with equimolar KCl.
Solutions for permeabilized smooth muscle contained (standard solution): 3.2 mM MgATP; 2 mM free Mg2+, 12 mM phosphocreatine, 0.5 mM NaN3, 30 mM N-tris(hydroxymethyl)methyl-2-amino-ethanesulphonic acid, 0.5 mM dithioerythritol, 15 U ml−1 creatine kinase, and 0.5 μM calmodulin, pH 6.9 at room temperature. The desired free [Ca2+] for different solutions was obtained by mixing potassium ethyleneglycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetra-acetic acid (EGTA) and K2CaEGTA, and the ionic strength was adjusted to 150 mM with potassium propionate. Rigor solution had the same composition as standard solution except it contained no ATP, phosphocreatine or creatine kinase; free [Mg2+] and ionic strength were maintained at 2 mM and 150 mM, respectively.
Thiophosphorylation experiments
For mechanical experiments in which ATPγS was co-incubated with carbachol, GTP and ROK inhibitors, permeabilized muscle strips were first contracted in pCa 4.5 solution. After relaxation in pCa 9 solution, strips were washed (2 × 5 min) in pCa 9 rigor solution before incubation with ATPγS (10 μM) and additions as specified in the text. This was followed by three 5 min washes in pCa 9 solution containing ATP which ensured washout of carbachol and GTP, as determined in separate experiments. Strips were then contracted in pCa 6.5, 6.25 or 6.0 solution as specified. After a plateau response in pCa 4.5 solution was recorded, the chart recorder speed was increased to obtain relaxation times (t½) in pCa 9 solution. In some experiments, strips were finally exposed to 10 μM microcystin-LR. The responses to microcystin-LR after 10 min incubation in the absence and presence of ATPγS were 1.8 ± 0.6 and 2.0 ± 0.3 mN (P > 0.4, n = 8), respectively. The corresponding values after 30 min incubation were 1.5 ± 0.5 and 1.6 ± 0.5 mN (P > 0.5, n = 6), respectively, suggesting that changes in maximal contractility were time dependent (i.e. a 20 % reduction in 20 min) and not affected by the presence of the ATPγS during pre-incubations.
Measurement of [Ca2+]i
Muscle strips were mounted in a bath staged on top of an inverted microscope for simultaneous recording of force and epifluorescence from 340 nm/380 nm excitation of the acetoxymethyl ester (AM) form of fura-2 with an emission wavelength of 510 nm using an IonOptix imaging system as described by Lindqvist et al. (1997). The tissue was loaded with 8 μM fura-2 AM (Molecular Probes) in normal Hepes-buffered Krebs solution (loading solution) for 4 h at room temperature. Once every hour the strips were made to contract once by immersion in high-K+ solution and then reimmersed in fresh loading solution. This loading protocol has previously been shown not to affect the mechanical performance of the tissue (Gomez & Swärd, 1997). After loading, each strip was allowed to equilibrate with perfusion for 30 min at 22°C before the excitation light was turned on and data acquisition was started. Force and fluorescence signals were sampled at 10 Hz.
Strips were first contracted with carbachol (100 μM) which was then washed out in normal Hepes-buffered Krebs solution (≥15 min) during which time the lamp was turned off. After washout, strips were treated with 1-(5-isoquinolinesulphonyl)-homopiperazine (HA1077, 30 μM) for 2–5 min before the lamp was turned on and the strips were contracted with carbachol in the presence of HA1077. Each experiment was concluded with an in situ calibration as described by Himpens et al. (1988). After background subtraction, fluorescence from excitation at 340 and 380 nm was used to calculate [Ca2+]i as described by Grynkiewicz et al. (1985). The dissociation constant of fura-2 for Ca2+ was taken to be 224 nM but is not known with certainty under the present intracellular conditions. The absolute values of [Ca2+]i reported should therefore be considered estimates.
Quantification of LC20 phosphorylation
For determination of LC20 phosphorylation levels, strips were tied as loops on stainless steel holders. The strips were then frozen at the desired times during contraction by rapidly (∼1 s) submerging the entire holder in acetone mixed with 10 % (w/v) trichloroacetic acid (TCA) and 20 mM dithiothreitol (DTT) that had been precooled with dry ice. After lyophilization, protein extraction and urea- glycerol gel electrophoresis were performed as described by Weber et al. (1999). Separated proteins were blotted onto 0.2 μm nitrocellulose (16 h, 30 V) and immunostained as described by Mita & Walsh (1997). LC20 phosphorylation was quantified by densitometric scanning using a Scanalytics Masterscan interactive densitometer using Camscan/RFLPscan 1.01 software (CSPI, Inc., Billerica, MA, USA). We observed a linear relationship between the chemiluminescence signal (optical density × area of the band) and sample loading over the range 0–25 μl. Loading levels of 10 or 15 μl were therefore routinely used.
Western blotting
Sheets of tissue were blotted on filter paper, weighed and homogenized using a Brinkmann Polytron in 15 volumes of homogenization buffer (composition: 50 mM tris(hydroxymethyl)aminomethane-HCl (Tris-HCl), pH 7.5, 400 mM NaCl, 2 mM EGTA, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM DTT, 10 μM phenylmethyl sulphonyl fluoride (PMSF), 10 μg ml−1 leupeptin, 1 μg ml−1 pepstatin and 1 mM benzamidine). The homogenate was mixed with an equal volume of 2 × sodium dodecyl sulphate (SDS) sample buffer (Laemmli, 1970) and boiled. Proteins were separated by SDS-polyacrylamide gel electrophoresis (7.5-20 % acrylamide gradient) and blotted onto nitrocellulose (0.2 μm) in 25 mM Tris, 192 mM glycine, 20 % (v/v) methanol, 0.1 % (w/v) SDS, pH 8.3 at 40 V and 4°C. Membranes were blocked for 1 h with 5 % (w/v) dry milk in TBS-Tween (20 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 0.05 % (v/v) Tween 20). After three 10 min washes in TBS-Tween, a primary antibody raised against the RhoA-binding domain of ROKβ-was used at 1:200 dilution for detection of ROK. For RhoA detection, monoclonal antibody 26C4 (2 μg ml−1; Santa Cruz Biotechnology Inc.) was used. MLCK was detected with a polyclonal antibody raised against chicken gizzard MLCK (0.25 μg ml−1; Paul et al. 1995) and MYPT was detected with a monoclonal antibody raised against a 58 kDa fragment of chicken gizzard MYPT (1:2000 dilution; Okubo et al. 1994). Horseradish peroxidase-conjugated secondary antibodies, anti-rabbit and anti-mouse IgGs (both from Boehringer-Mannheim), were used at 1:5000 dilution. Membranes were incubated for 1 h each with primary and secondary antibody diluted in TBS-Tween with 1 % dry milk. Incubations were followed by three 10 min washes in TBS-Tween. The blots were developed with the Supersignal CL-HRP substrate system (Pierce Chemical Co.) after a final 10 min wash in TBS.
[35S]ATPγS experiments
Muscle strips were prepared as for LC20 phosphorylation experiments and permeabilized as described above. After washing (2 × 5 min) in pCa 9 standard solution and then in pCa 9 rigor solution (2 × 5 min) to remove residual ATP, strips were treated with 10 μM [35S]ATPγS (1 mCi ml−1) in pCa 9 rigor solution with additions as indicated in the figures. At the times indicated, groups of three muscle strips were snap-frozen in a dry ice-acetone slurry containing 10 % (w/v) TCA and 20 mM DTT. Strips were removed from the holders, frozen briefly again and washed (8 × 0.5 min) in acetone plus DTT. After lyophilization overnight, proteins were extracted in Eppendorf tubes with 200 μl of 2× SDS gel sample buffer for 2 h and then boiled. Proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. The wet gel enclosed in a sealed plastic bag was exposed in a Phosphorimager (Molecular Dynamics Storm 850) for 4–8 h for quantification of radioactivity. Radioactivity in the 130 kDa band was corrected for background and slight variations in protein loading levels, the latter obtained by scanning the stained gel (calponin or filamin bands) using a Pharmacia Biotech Image Master desktop scanning system (Uppsala, Sweden). Gels were also dried in a gel drier (model 583, BioRad) and exposed to X-ray film (Biomax MR, Kodak) for up to 14 days. The radiolabelled 130 kDa band was quantified by scanning the autoradiogram using the Image Master desktop scanning system.
Protein purification
ROK was purified using a modification of the procedures used by Leung et al. (1995) and Matsui et al. (1996). Bovine brains were transported on ice from a local abattoir. The tissue was cleaned of adhering blood vessels and white matter in the brainstem and hemispheres with a scalpel. After weighing, the tissue was homogenized in a Waring blender with three volumes of homogenization buffer (HB, composition: 25 mM Tris-HCl, pH 7.5; 1 mM DTT, 5 mM EGTA, 10 mM MgCl2, 10 μM PMSF, 1 μg ml−1 pepstatin, 1 μg ml−1 leupeptin and 10 % (w/v) sucrose). The homogenate was centrifuged at 20000g for 30 min. The pellet was resuspended in two volumes of HB as before and an equal volume of HB with 4 M NaCl was added with continuous overhead stirring. After stirring for 1 h, the suspension was centrifuged 2 × 1 h as before and the resulting pellets were discarded. The final supernatant was dialysed overnight against 3 × 8 l of buffer A (composition: 20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT).
Ammonium sulphate was slowly added to the dialysate (over 30 min) with overhead stirring to 40 % saturation (w/v) followed by centrifugation as before for 1–2 h. Supernatants were carefully discarded, the pellets were resuspended in adhering liquid plus ∼10 ml of buffer A and dialysed against 3 × 5 l of buffer A. After centrifugation as above, the supernatant was loaded on a Q-Sepharose column (Pharmacia Biotech, Sweden) previously equilibrated with buffer A at a flow rate of 120 ml h−1. The column was washed for 1 h with buffer A and bound proteins were eluted with a 500 ml 0–0.8 M NaCl gradient in the same buffer, 3 ml fractions were collected. ROK was detected in selected fractions by Western blotting after separation of proteins on 7.5-20 % SDS-PAGE gels. The primary antibody used was monoclonal antibody 1A1 raised against ROKα (1 μg ml−1) and the secondary antibody goat anti-mouse IgG (Boehringer Mannheim, 1:5000 dilution). Fractions containing ROKα were pooled and dialysed against 3 × 2 l buffer B (composition: 25 mM 2-(N-morpholino)ethanesulphonic acid (Mes)-NaOH, pH 6.0, 0.5 mM MgCl2, 0.05 % (w/v) Triton X-100, 0.1 M NaCl).
The ROK pool was subsequently loaded on a glutathione S-transferase (GST)-RhoA-GTPγS or a GST-RhoAV14-GTPγS affinity column previously equilibrated with buffer B plus 50 μM GTP at 15 ml h−1. The column was washed for 1–2 h with buffer B plus 50 μM GTP. Bound ROKα was eluted with 25 mM Tris, pH 8, 0.5 mM MgCl2, 0.05 % (w/v) Triton X-100, 0.1 mM EDTA and 0.1 M NaCl. ROK-containing fractions, identified by enzymatic assays and Western blotting, were frozen.
RhoA-GST and RhoAV14-GST were expressed in E. coli (strain pLysS, vector pGEX 2T) and purified from the bacterial cytosol according to Glover & Hames (1995) protocols 7, 8 and 10. RhoA-GST (7–10 mg) was incubated for 1 h at 30°C in 20 mM Tris-HCl, pH 7.5, 10 mM EDTA, 1 mM DTT, 5 mM MgCl2, 0.03 % (w/v) 3-(3-cholamido-propyl)dimethylammonio-1-propane-sulphonate (CHAPS), 1 mM 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 50 μM GTPγS to load RhoA with GTPγS. Glutathione-Sepharose (10 ml; Pharmacia Biotech), previously equilibrated with buffer A and dried, was then added. The mix was tumbled for 1 h at 4°C before packing and equilibration of the affinity column.
Chicken gizzard MLCK (Ngai et al. 1984), rat brain PKC (a mixture of Ca2+-dependent α, β and γ isoforms) (Allen et al. 1994) and bovine brain calmodulin (Walsh et al. 1984) were purified as described previously. Chicken gizzard LC20 was purified using a modification of the procedure of Hathaway & Haeberle (1983). Chicken gizzards were obtained from a local poultry packing plant and transported to the laboratory on ice. For PKC purification male Sprague-Dawley rats (300–500 g) were killed by inhalation of a lethal dose of halothane and decapitated, according to a research protocol consistent with the standards of the Canadian Council on Animal Care and approved by the local Animal Care Committee of the Medical Research Council of Canada.
Enzyme assays
ROK activity was assayed using a synthetic peptide (MYPT peptide, 0.2 mg ml−1) derived from the sequence surrounding the phosphorylatable threonine 654 (RQSRRSTQGVTLTD) of the 130 kDa myosin targeting subunit of chicken gizzard MLCP (Ichikawa et al. 1996) as substrate under the following conditions: 50 mM Tris-HCl, pH 7.5, 2 mM EDTA, 7 mM MgCl2, 1 mM DTT, 0.15 % (w/v) CHAPS and 0.35 μM ROK.
MLCK activity was assayed under the following conditions: 25 mM Tris-HCl, pH 7.5, 60 mM KCl, 4 mM MgCl2, 0.1 mM CaCl2, 1 mM DTT, 0.1 % (w/v) Tween 80, 10 μM LC20, 0.5 nM MLCK and 0.6 μM calmodulin.
PKC activity was assayed under the following conditions: 20 mM Hepes, pH 7.5, 10 mM MgCl2, 10 mM DTT, 0.15 mM CaCl2, 1 mg ml−1 histone III-S, mixed micelles (composed of 0.3 mg ml−1 phosphatidylserine and 62 μg ml−1 1,2-diolein in Triton X-100, 0.03 % (w/v)), and 0.6 nM PKC. All assays were performed in an assay volume of 25 μl and reactions were started by addition of [γ32-P]ATP (200–500 c.p.m. pmol−1) to 0.25 mM at 30°C. Reactions were stopped by spotting 22.5 μl of assay mix on Whatman P81 filter paper squares (1 cm × 1 cm) after 5 or 10 min (MLCK and PKC assays) or 30 min (ROK assay). Filter papers were then washed 3 times in phosphoric acid (0.5 %, v/v) and once in acetone followed by drying and Cerenkov counting.
Chemicals
HA1077, 1-(5-isoquinolinesulphonyl)-2-methylpiperazine (H-7), 1-(5-chloronaphthalene-1-sulphonyl-1H-hexahydro-1,4-diazepine (ML-9) and microcystin-LR were purchased from Calbiochem. 3-(2-(4-(Bis(4-fluorophenyl)-methylene)-1-piperidinyl)ethyl)-2,3-dihydro-2-thioxo-4[1H]-quinazolinone (R59022) was purchased from Sigma. Y-27632 (R-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide) was generously provided by Yoshitomi Pharmaceutical Industries, Ltd (Osaka, Japan). MYPT peptide was synthesized by Macromolecular Resources (Colorado State University). [γ-32P]ATP (>5000 Ci mmol−1) and [35S]ATPγS (>1000 Ci mmol−1) were purchased from Pharmacia Biotech. Triton X-100 (peroxide free) was purchased from Boehringer-Mannheim. L-α-Phosphatidylserine and 1,2-diolein were purchased from Serdary Research Laboratories (London, Canada). DMPC was from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Tween 20 (polyoxyethylene sorbitan mono-oleate) was purchased from BioRad. Leupeptin, pepstatin A and DTT were from ICN Biomedicals, Inc. (Aurora, OH, USA). All other chemicals were of analytical grade or better and were purchased from Canlab (Edmonton, AB, Canada) or Sigma. Antibodies to ROKα and ROKβ were generously provided by Drs Thomas Leung and Louis Lim, The National University of Singapore. Anti-ROKα is a mouse monoclonal antibody (1A1) raised against the kinase domain (residues 112–392; Leung et al. 1995) and anti-ROKβ is a polyclonal antiserum raised in rabbits against a region (residues 670–1027) containing the Rho-binding domain (Leung et al. 1996). These antibodies recognize the same population of proteins in various rat tissues and therefore do not distinguish between ROKα and β (Leung et al. 1996).
Statistics
Student's t test, paired or unpaired as appropriate, was used for single statistical comparisons. For multiple comparisons, one-way analysis of variance followed by Dunn's test (Fig. 8b) was used. P < 0.05 was considered significant. Summarized data are presented as means ±s.e.m.
Figure 8. Effect of HA1077 on carbachol-induced LC20 phosphorylation in intact guinea-pig ileum.
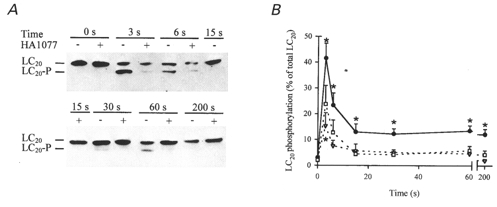
Intact guinea-pig ileum longitudinal smooth muscle strips were treated with carbachol (100 μM) in the absence and presence of HA1077 (6 or 30 μM) for the indicated times at which the tissue was snap-frozen and LC20 phosphorylation quantified as described in Methods. A, representative Western blots of LC20 after separation of the phosphorylated from the unphosphorylated species by urea-glycerol gel electrophoresis. B, quantitative data pooled from several experiments (n = 6–16). Control (•), 6 μM HA1077 (▿), 30 μM HA1077 (□). *Significant differences from LC20 phosphorylation levels at time zero.
RESULTS
Expression of RhoA and ROK in guinea-pig ileum
Figure 1 demonstrates by Western blotting with antibodies to ROKβ (lane 1) and RhoA (lane 2) that both ROK and RhoA are expressed in guinea-pig ileum longitudinal smooth muscle. The calculated molecular weights were as expected: ∼160 kDa for ROK and 22 kDa for RhoA. Figure 1 (lane 3) shows a Coomassie Blue-stained gel of the tissue homogenate used for Western blotting.
Figure 1. Identification of ROK and RhoA in guinea-pig ileum longitudinal smooth muscle.
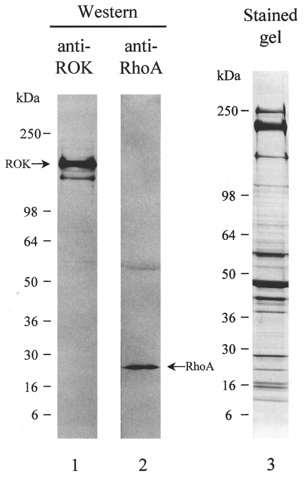
Tissue homogenates were subjected to SDS-PAGE and either stained with Coomassie Brilliant Blue (lane 3) or immunoblotted with anti-ROKβ (lane 1) or anti-RhoA (lane 2). The equivalent of 0.625 mg wet weight of tissue was applied to lane 1, 2.5 mg wet weight of tissue to lane 2 and 2.5 mg wet weight of tissue to lane 3. Results are representative of three independent experiments.
Effects of kinase inhibitors on Ca2+ sensitization of force in guinea-pig ileum
Addition of GTPγS (100 μM) to solution bathing β-escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips at a sub-threshold [Ca2+] (pCa 6.5) induced a contraction that peaked and then declined to a steady level corresponding to 25.9 ± 2.0 % (n = 20) of the preceding reference contraction in pCa 4.5 solution (Fig. 2a), i.e. GTPγS induced Ca2+ sensitization. Subsequent addition of the kinase inhibitors Y-27632, HA1077 and H-7 led to concentration-dependent relaxation to < 5 % residual force. Force was well maintained in the presence of vehicle alone over the same number of solution changes for the same duration (-0.7 ± 13.0 %, n = 5). The calculated IC50 values for inhibition of Ca2+ sensitization (1.7 ± 0.4 μM for Y-27632, 2.7 ± 0.7 μM for HA1077 and 3.1 ± 0.4 μM for H-7) correlated well (r2 = 0.99; Fig. 2b) with the reported Ki values for inhibition of ROK in vitro (Uehata et al. 1997).
Figure 2. Effects of kinase inhibitors on Ca2+ sensitization of force induced in permeabilized guinea-pig ileum by GTPγS.
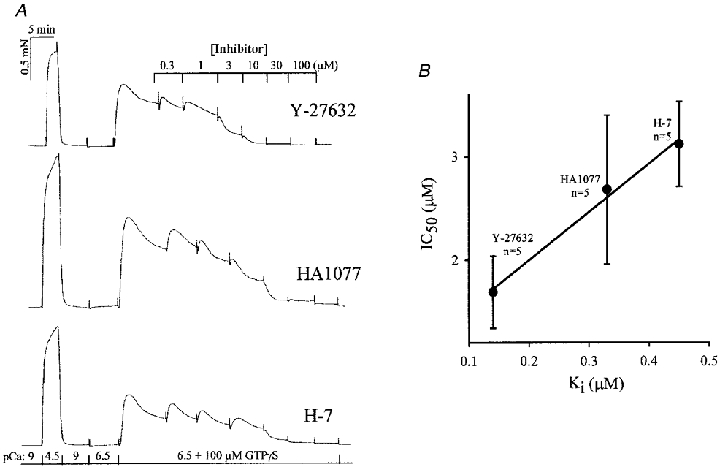
A, guinea-pig ileum longitudinal smooth muscle permeabilized with β-escin was contracted by increasing [Ca2+] from pCa 9 to 4.5 and relaxed by returning to pCa 9. Transfer to pCa 6.5 solution failed to elicit a significant contraction but subsequent transfer to pCa 6.5 + GTPγS (100 μM) evoked Ca2+ sensitization of force. The effects of increasing concentrations of three kinase inhibitors (Y-27632, HA1077 and H-7) on Ca2+ sensitization were recorded. B, correlation between IC50 values for inhibition of Ca2+ sensitization and the known Ki values for inhibition of ROK (n = 5).
HA1077 was used to inhibit ROK in most subsequent experiments due to its ready commercial availability. Figure 3 confirms its selectivity for inhibition of ROK over MLCK and PKC with IC50 values of 10.7 ± 2.0 μM (ROK), 95.2 ± 4.7 μM (MLCK) and 425 ± 41 μM (PKC). The PKC inhibitor chelerythrine (≤ 100 μM) and the tyrosine kinase inhibitor genistein (≤ 100 μM) had no significant effect on ROK activity (results not shown).
Figure 3. Sensitivity of ROK, MLCK and PKC to the kinase inhibitor HA1077.
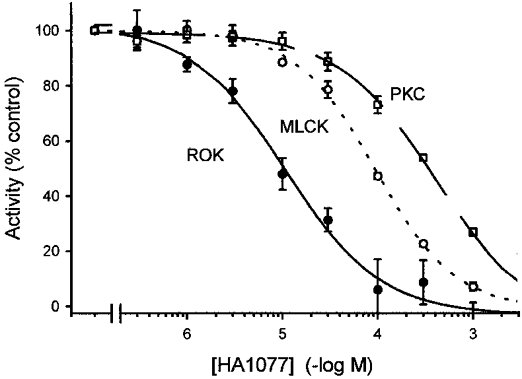
The effects of increasing concentrations of HA1077 on ROK (•), MLCK (○) and PKC activities (□) were determined as described in Methods. Activity in the presence of vehicle was expressed as 100 %.
Many of the subsequent experiments designed to achieve specific inhibition of ROK activity in intact and permeabilized smooth muscle were carried out at 30 μM HA1077. From the data in Fig. 3 it is apparent that, under the in vitro conditions used in this experiment, MLCK activity was inhibited by ∼20 % at this concentration of HA1077. It should be noted, however, that the concentration of MLCK in this experiment was low, 0.05 μg ml−1 or 0.5 nM, which was necessary to ensure linear phosphorylation time courses. The tissue concentration of MLCK in guinea-pig ileum longitudinal smooth muscle was calculated to be 1.8 ± 0.1 μM (n = 3) assuming 50 % cell water (Fig. 4a) so that significantly higher concentrations of HA1077 would be required to inhibit MLCK in situ. This estimate of the tissue concentration of MLCK is similar to values previously published for other smooth muscle tissues (2–4 μM, Walsh et al. 1984; Tansey et al. 1994) and is significantly higher than the tissue concentration of ROK (estimated to be 0.37 μM in chicken gizzard from the yield of purified enzyme reported by Feng et al. 1999). The ROK concentration used in Fig. 3 (0.35 μM) was close to this estimated tissue concentration. HA1077 inhibits ROK by competing with ATP. Figure 4B shows that increasing the ATP concentration in the in vitro MLCK assay towards the physiological concentration shifts the concentration- response curve to the right so that at 1 mM ATP 30 μM HA1077 had no significant inhibitory effect. The IC50 values were calculated to be 105.8 ± 4.7 μM HA1077 at 0.25 mM ATP and 273.5 ± 31.9 μM HA1077 at 1 mM ATP. Taken together, these results indicate that 30 μM HA1077 is unlikely to have any inhibitory effect on MLCK in situ. Confirmatory evidence will be presented later (Table 3).
Figure 4. Quantification of MLCK levels in guinea-pig ileum and the effect of [ATP] on inhibition of MLCK by HA1077.
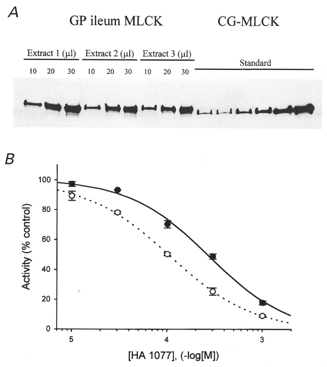
A, guinea-pig ileum longitudinal smooth muscle proteins were extracted as described in Methods, subjected to SDS-PAGE and immunoblotted with anti-MLCK. Three loading levels of three independent extracts and different known amounts of purified chicken gizzard MLCK (0.05, 0.1, 0.15, 0.2, 0.4 and 0.8 μg) were applied to the gel. MLCK bands were quantified by densitometric scanning. A standard curve relating the intensity of the chicken gizzard MLCK bands (optical density × area of the band) to micrograms of MLCK loaded was linear with an r2 value of 0.98. B, the effect of increasing concentrations of HA1077 on the activity of purified MLCK was assayed as described in Methods at 0.25 (○) and 1 mM ATP (•).
Table 3.
Effects of Ca2+, GTPγS (100 μm) and HA1077 on the rate of microcystin-LR-induced contraction of permeabilized guinea-pig ileum
| Conditions | t1/2 (s) | Steady-state force (mN) | n |
|---|---|---|---|
| pCa 9 | 326.7 ± 55.7 | 1.31 ± 0.20 | 8 |
| pCa 9+GTPγS | 305.2 ± 40.5 | 1.71 ± 0.21 | 8 |
| pCa 6.5 | 46.0 ± 2.6 | 1.69 + 0.25 | 7 |
| pCa 6.5+GTPγS | 47.9 ± 2.4 | 1.51 + 0.19 | 7 |
| pCa 6.5+GTPγS+6 μm HA1077 | 47.0 ± 4.2 | 1.72 + 0.11 | 7 |
| pCa 6.5+GTPγS+30 μm HA1077 | 46.1 ± 4.2 | 1.68 + 0.13 | 6 |
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were induced to contract by addition of microcystin-LR (10 μm) under the conditions indicated. The time to half-maximal contraction (t½) and amplitude of steady-state force were determined. Values are given as means ± s.e.m.
Using a protocol similar to that in Fig. 2, GTP (50 μM) and carbachol (100 μM) at pCa 6.5 increased force to a steady level of 25.7 ± 3.1 % (n = 6) of the reference contraction at pCa 4.5 (Fig. 5a). HA1077 caused a concentration-dependent relaxation to near-basal force, with maximal effect at ∼30 μM (n = 6). Contraction induced at pCa 5.75 amounted to 96.0 ± 4.0 % (n = 8) of the reference contraction at pCa 4.5 and was not affected by HA1077 at concentrations as high as 300 μM (n = 8; Fig. 5b). These results implicate ROK activation in agonist-induced Ca2+ sensitization.
Figure 5. Effects of HA1077 on Ca2+ sensitization of force evoked by agonist plus GTP and Ca2+-induced contraction.
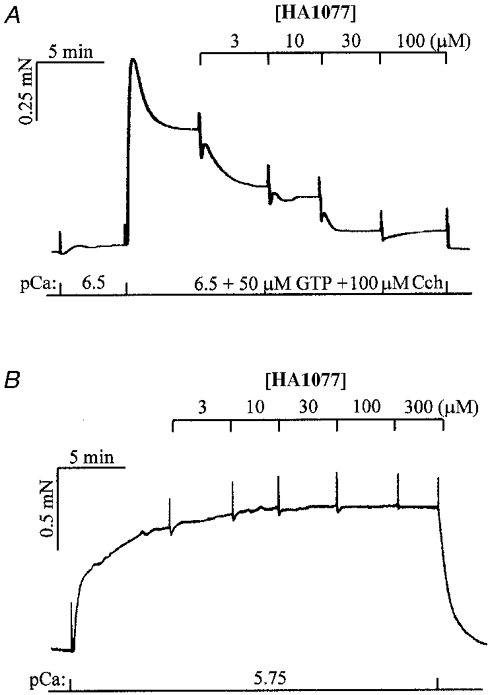
A, force in β-escin-permeabilized guinea-pig ileum longitudinal smooth muscle was activated at a sub-threshold [Ca2+] (pCa 6.5) by addition of 100 μM carbachol (Cch) and 50 μM GTP. Cumulative addition of HA1077 inhibited this Ca2+ sensitization. In control strips (n = 6), Cch + GTP-induced steady-state force at pCa 6.5 was maintained for at least 40 min. Ca2+ sensitization induced by Cch + GTP was inhibited by 100 μM atropine, verifying that the Cch effect was mediated by muscarinic receptors. B, force was maximally activated at pCa 5.75 and was unaffected by cumulative addition of HA1077 up to 300 μM.
Lack of Ca2+ sensitization in guinea-pig ileum in response to PKC activation
PKC activation has been implicated in Ca2+ sensitization in a variety of smooth muscle tissues (Masuo et al. 1994; Sato et al. 1994; Gailly et al. 1997; Buus et al. 1998; Li et al. 1998). As shown in Table 1, however, phorbol 12,13-dibutyrate (1 or 10 μM) failed to induce Ca2+ sensitization in β-escin-permeabilized guinea-pig ileum longitudinal smooth muscle. On the other hand, the same stock solution of phorbol ester significantly increased force at pCa 6.25 in β-escin-permeabilized guinea-pig taenia coli at a final concentration of 1 μM. Furthermore, treatment of permeabilized guinea-pig ileum longitudinal smooth muscle at pCa 6.25 with the diacylglycerol kinase inhibitor R59022 did not increase carbachol-induced force, as would be expected if PKC activation evoked Ca2+ sensitization, but actually significantly reduced carbachol-induced force (Table 2). The lack of detectable PKC-mediated Ca2+ sensitization in guinea-pig ileum longitudinal smooth muscle makes this tissue particularly well suited for investigation of the functional role of ROK.
Table 1.
Phorbol ester induces Ca2+ sensitization of force in permeabilized guinea-pig taenia coli but not ileum
| Steady-state force | ||
|---|---|---|
| [PDBu] (μM) | Taenia coli (% maximum) | Ileum (% maximum) |
| 0 | 31.6 ± 4.4 (n = 10) | 16.0 ± 2.5 (n = 12) |
| 1 | 78.0 ± 5.5* (n = 10) | 13.1 ± 4.4 (n = 4) |
| 10 | — | 16.8 ± 3.0 (n = 8) |
Steady-state force was measured in β-escin-permeabilized guinea-pig taenia coli or ileum at pCa 6.25 in the absence or presence of phorbol 12,13-dibutyrate (PDBu, 1 or 10 μm). Values were normalized to the reference contraction at pCa 4.5 in each experiment and are given as means ± s.e.m.
Significantly different from the corresponding response in the absence of PDBu (P < 0.001; Student's t test).
Table 2.
Inhibition of diacylglycerol kinase fails to increase carbachol-induced force in permeabilized guinea-pig ileum
| Steady-state force | |||
|---|---|---|---|
| Carbachol] (μm) | Control (% maximum) | R59022 (% maximum) | P |
| 1 | 17.7 ± 1.7 | 7.7 ± 2.2* | 0.005 |
| 3 | 27.1 ± 2.6 | 12.3 ± 2.7* | 0.004 |
| 10 | 36.3 ± 4.3 | 21.7 ± 3.9* | 0.03 |
Steady-state force was measured in β-escinpermeabilized guinea-pig ileum longitudinal smooth muscle in response to the indicated concentrations of carbachol at pCa 6.25 in the absence and presence of the diacylglycerol kinase inhibitor R59022 (10 μm). Values were normalized to the reference contraction at pCa 4.5 in each experiment and are given as means ± s.e.m. (n = 6).
Significantly different from the corresponding response in the absence of R59022 with the indicated P values.
Effects of HA1077 on LC20 phosphorylation in permeabilized guinea-pig ileum
To determine if ROK inhibition by HA1077 was associated with changes in the level of LC20 phosphorylation, permeabilized muscle strips were frozen 5 min after exposure to the conditions shown in Fig. 6. LC20 phosphorylation levels were very low in relaxed muscle strips at pCa 9 and did not increase significantly when the [Ca2+] was increased to pCa 6.5, consistent with the lack of a contractile response at this [Ca2+] (Figs 2 and 5). LC20 phosphorylation was significantly increased by GTPγS at pCa 6.5 and 5.75 and this was prevented by pretreatment with HA1077 (30 μM). On the other hand, the increase in LC20 phosphorylation induced by increasing [Ca2+] to pCa 5.75 without GTPγS was unaffected by 30 μM HA1077.
Figure 6. Effect of HA1077 (HA) on the GTPγS-induced increase in LC20 phosphorylation in permeabilized guinea-pig ileum.
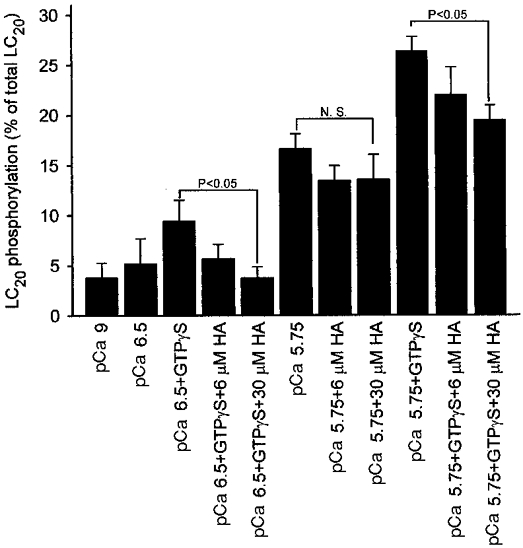
β-Escin-permeabilized guinea-pig ileum smooth muscle strips were exposed to the indicated conditions for 5 min, following which the strips were snap-frozen and LC20 phosphorylation levels quantified as described in Methods. Columns and bars represent the means and s.e.m.s. Columns 1–5, n = 5; column 6, n = 11; columns 7–11, n = 6. N.S., not significant.
Effects of HA1077 on force and [Ca2+]i in intact guinea-pig ileum muscle strips stimulated with carbachol
Stimulation of intact fura-2 AM-loaded guinea-pig ileum longitudinal smooth muscle strips with carbachol (100 μM) led to a transient increase in [Ca2+]i which settled at a sustained level significantly above resting [Ca2+]i (Fig. 7a). Force peaked rapidly, relaxed partially, then increased again before relaxing very slowly (Fig. 7b). Pre-treatment with HA1077 (30 μM) had no effect on the [Ca2+]i response to carbachol (Fig. 7C) but completely abolished the tonic phase of the contractile response (Fig. 7D). The phasic component of the contractile response to carbachol was slightly reduced by HA1077 (compare Fig. 7b and D).
Figure 7. Effects of HA1077 on carbachol-induced changes in [Ca2+]i and force in intact guinea-pig ileum.
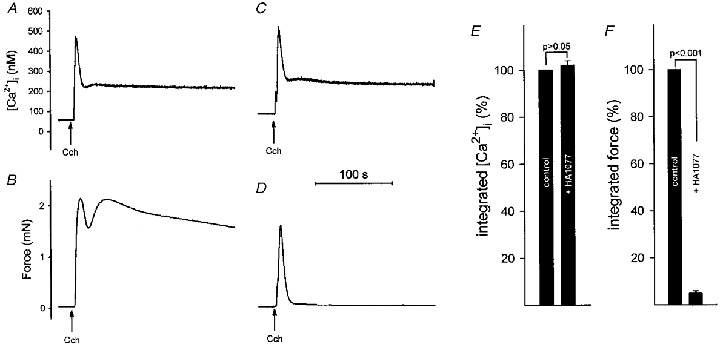
Intact guinea-pig ileum longitudinal smooth muscle strips were loaded with fura-2 AM. [Ca2+]i (A and C) and force (B and D) were recorded following stimulation with carbachol (100 μM) in the absence (A and B) and presence (C and D) of HA1077 (30 μM). HA1077 was present 4 min prior to, and during, the carbachol-induced contraction. Integrated and normalized [Ca2+]i (E) and integrated and normalized force (F) were recorded in controls and after exposure to HA1077 (n = 7).
Data from seven [Ca2+]i-force experiments are summarized in Fig. 7E and F. Whereas integrated [Ca2+]i was not affected by 30 μM HA1077, integrated force was markedly reduced.
Effect of HA1077 on LC20 phosphorylation in intact guinea-pig ileum
Intact guinea-pig ileum longitudinal smooth muscle strips were frozen prior to stimulation (0 s) and at 3, 6, 15, 30, 60 and 200 s after stimulation with carbachol (100 μM) in the absence or presence of HA1077 (6 or 30 μM) and LC20 phosphorylation levels were quantified. A typical experimental result is shown in Fig. 8A. The level of phosphorylation was close to zero prior to stimulation either in the absence or presence of HA1077. In the absence of HA1077, LC20 phosphorylation increased rapidly to a peak at 3 s and then declined to almost zero at 15 s followed by a second increase between 30 and 60 s. By 200 s, phosphorylation had fallen again to almost zero. This biphasic behaviour was observed in 10 of 13 experiments, but the times at which the first reduction and the second peak of LC20 phosphorylation took place were variable. The average data presented in Fig. 8b (n = 6–16), therefore, show only a rapid increase in LC20 phosphorylation that peaked at 3 s and then declined to a sustained level that was significantly greater than the resting level (P < 0.05). In the presence of 6 or 30 μM HA1077, LC20 phosphorylation also increased rapidly in response to carbachol but returned quickly to resting levels. HA1077 reduced the transient peak of LC20 phosphorylation and abolished the second peak (sustained component) of LC20 phosphorylation (Fig. 8a and B).
ROK induces LC20 phosphoryation via inhibition of MLCP
Previous analyses have indicated that the mechanism of increased LC20 phosphorylation induced by GTPγS or agonists plus GTP involves inhibition of MLCP activity rather than increased MLCK activity. To determine if the ROK-mediated increase in LC20 phosphorylation in guinea-pig ileum is due to MLCP inhibition, MLCK activation or direct phosphorylation of myosin by ROK, we carried out the following experiments. Firstly, to determine if ROK affects MLCK activity, we examined the effects of GTPγS in the absence and presence of HA1077 on the rates of contraction induced by the phosphatase inhibitor microcystin-LR (Table 3). Increasing [Ca2+] from pCa 9 to pCa 6.5 markedly reduced the half-time of microcystin-LR-induced contraction (t½) from >300 s to <50 s (P < 0.001), but the amplitude of steady-state force was the same in both conditions. GTPγS (100 μM) had no significant effect on t½ or the amplitude of steady-state force at pCa 9 or 6.5. Furthermore, HA1077 (6 or 30 μM) had no effect on t½ or steady-state force at pCa 6.5 in the presence of GTPγS. We conclude, therefore, that ROK has no effect on MLCK. These results also suggest that ROK does not phosphorylate myosin directly in situ.
To determine whether ROK activation leads to inhibition of MLCP, we examined the effect of HA1077 on the rate of relaxation under conditions of zero MLCK activity. Contraction of permeabilized guinea-pig ileum longitudinal smooth muscle was elicited at pCa 4.5 in the presence of 100 μM GTPγS. We then used three sets of conditions to inhibit MLCK activity: pCa 9, pCa 9 without ATP or an ATP regenerating system (rigor conditions) and pCa 9 without ATP in the presence of the MLCK inhibitor ML-9 (Table 4). Under all three conditions, the rate of relaxation was significantly enhanced by HA1077 (Table 4), suggesting that ROK activation leads to inhibition of MLCP. To test this hypothesis more directly, we measured LC20 dephosphorylation at various times during relaxation (Fig. 9). Strips were again contracted in pCa 4 solution with GTPγS for 5 min and then either maintained in the same solution for 5–10 min or transferred to solution containing HA1077 for 5–10 min. Strips were frozen immediately (0 s) or transferred to pCa 9 rigor solution with GTPγS in the absence or presence of HA1077 and frozen at the indicated times for quantification of LC20 phosphorylation. Inhibition of ROK by HA1077 clearly increases the rate of myosin dephosphorylation (the half-time of dephosphorylation was 50 ± 11 s (n = 6) in the absence of HA1077 and 15 ± 4 s (n = 6) in the presence of HA1077; P = 0.02), indicating that ROK activation leads to inhibition of MLCP.
Table 4.
Effect of HA1077 on the rate of relaxation of permeabilized guinea-pig ileum
| t½ | |||
|---|---|---|---|
| Solution | Control (s) | HA1077 (s) | P |
| pCa 9 | 34.6 ± 1.7 | 22.3 ± 1.4* | 0.0016 |
| pCa 9 rigor | 32.7 ± 0.45 | 21.0 ± 1.9* | 0.0008 |
| pCa 9 rigor+ 300 μm ML-9 | 50.4 ± 3.9 | 36.6 ± 2.2* | 0.013 |
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were maximally contracted at pCa 4.5 in the presence of GTPγS (100 μm). HA1077 (30 μm) or vehicle (control) was added 5 min prior to relaxation in three different solutions: pCa 9 containing ATP and an ATP regenerating system, pCa 9 without ATP or an ATP regenerating system (rigor conditions) or pCa 9 under rigor conditions in the presence of ML-9 (300 μm). All relaxing solutions contained GTPγ (100 μm). Values are given as means ± s.e.m. (n = 5).
Significantly different from the corresponding response in the absence of HA1077 with the indicated P values.
Figure 9. Effect of HA1077 on the rate of LC20 dephosphorylation in permeabilized guinea-pig ileum.
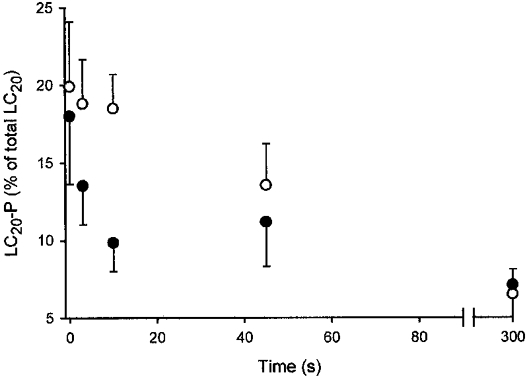
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were maximally contracted at pCa 4.5 in the presence of GTPγS (100 μM). HA1077 (30 μM, •) or vehicle (control, ○) was added 5–10 min prior to relaxation which was initiated at time zero by transfer to rigor solution at pCa 9 containing GTPγS (100 μM). Tissues were snap-frozen at the indicated times for quantification of LC20 phosphorylation levels (LC20-P) as described in Methods.
To test the hypothesis that the mechanism of inhibition of MLCP activity by ROK involves phosphorylation of MYPT, muscle strips were incubated with [35S]ATPγS in pCa 9 rigor solution containing 100 μM ML-9 and thiophosphorylation of MYPT visualized by autoradiography as described by Trinkle-Mulcahy et al. (1995). We observed time-dependent incorporation of 35S predominantly into two proteins of 42 and 130 kDa (Fig. 10a and B). In Western blotting experiments, the 130 kDa protein comigrated with MYPT (results not shown). Thiophosphorylation of the 130 kDa protein was associated with enhanced Ca2+ sensitivity and slowing of relaxation (increased t½) as shown in Fig. 10C and D, respectively. Collectively, these data support the hypothesis that phosphorylation of MYPT correlates with MLCP inhibition and Ca2+ sensitization of contraction.
Figure 10. Thiophosphorylation of a 130 kDa protein correlates with Ca2+ sensitization of contraction.
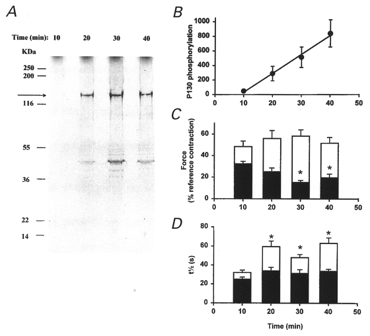
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated with 100 μM ML-9 in pCa 9 rigor solution and 10 μM [35S]ATPγS was added at time zero. Muscle strips were snap-frozen at the indicated times and proteins extracted and separated by SDS-PAGE as described in Methods. The gels were stained with Coomassie Blue and thiophosphorylated proteins detected by autoradiography. A, a representative autoradiogram. The 130 kDa thiophosphorylated protein (identified as MYPT by Western blotting) is indicated by the arrow. The positions of molecular weight markers are indicated to the left. Note the absence of LC20 thiophosphorylation. B, cumulative data (n = 3) showing the time course of MYPT thiophosphorylation. Values were normalized to the Coomassie Blue-stained calponin band to correct for variations in protein loading levels. C, to evaluate the mechanical effect of MYPT thiophosphorylation, permeabilized muscle strips were incubated in the absence (▪) or presence of ATPγS (□) as described above. Tissues were then washed in pCa 9 solution (3 × 5 min) and contracted in pCa 6.0 solution. *Significant difference (P < 0.05) from 10 min controls (n >= 10). D, after contraction in response to immersion in pCa 4.5 solution, relaxation was recorded at high chart speed to obtain t½ values for relaxation. *Significant difference from the 10 min plus ATPγS value. In C and D, values recorded in the presence of ATPγS differ significantly from controls at all times.
The effect of GTPγS on MYPT thiophosphorylation was then examined by coincubation of muscle strips with [35S]ATPγS and GTPγS in pCa 9 rigor solution. Results of a typical experiment are shown in Fig. 11a, which demonstrates increased thiophosphorylation of MYPT in the presence of GTPγS. The Coomassie Blue-stained filamin band was used to correct for variations in protein loading levels (Fig. 11b). Cumulative data (Fig. 11C) indicate significant increases in MYPT thiophosphorylation levels in the presence of GTPγS at 5 and 10 min. It is also noteworthy that no thiophosphorylation of LC20 was detected (Fig. 11a). Thiophosphorylation for 40 min (under conditions defined in Fig. 10) did not lead to attenuation of the subsequent sensitization of force by GTPγS at pCa 6.25 (Fig. 11D), suggesting that MYPT phosphorylation had not reached saturation by this time.
Figure 11. GTPγS enhances MYPT thiophosphorylation.
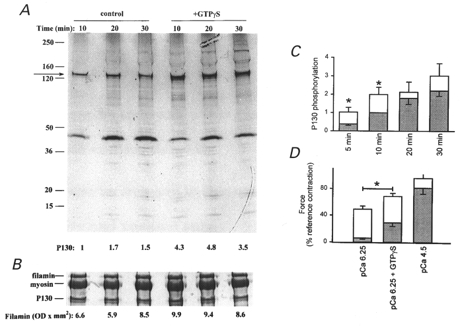
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated in pCa 9 rigor solution containing 10 μM [35S]ATPγS in the absence or presence of 1 μM GTPγS. Muscle strips were processed as described in the legend to Fig. 10. A, a representative autoradiogram. Numbers below each lane indicate the radioactivity in the 130 kDa band normalized to the 10 min control value. B, a portion of the Coomassie Blue-stained gel to show the filamin band that was used to correct for variations in protein loading levels. Numbers below the gel lanes denote the optical density × area of the filamin band in each lane. C, cumulative data (n >= 5) showing the time course of MYPT thiophosphorylation in the absence (shaded bars) and presence (open bars) of GTPγS. Values are corrected for background and protein loading and are normalized to the control (no GTPγS) value at 10 min. D, permeabilized muscle strips were incubated in pCa 9 rigor solution containing 100 μM ML-9 in the absence (shaded bars; n = 4) or presence (open bars; n = 4) of 10 μM ATPγS. After washing, muscle strips were contracted in pCa 6.25 solution. At the ensuing plateau, 100 μM GTPγS was added. Finally, muscle strips were contracted in pCa 4.5 solution.
To determine the mechanical effect of enhanced MYPT phosphorylation it was necessary to determine the reversibility of Ca2+ sensitization of force by GTPγS. A 10 min incubation with 100 μM GTPγS in pCa 9 rigor solution without ATPγS caused subsequent Ca2+ sensitization after more than 15 washes (5 min each) in fresh solution with no significant attenuation of the effect (results not shown). The effect of GTPγS is therefore irreversible within the time frame of the experiment, due perhaps to very slow dissociation from RhoA. Ca2+ sensitization by carbachol plus GTP, on the other hand, was rapidly reversible, requiring fewer than three 5 min washes (results not shown; see also Otto et al. 1996), and co-incubation of ATPγS (10 μM) with carbachol (100 μM) and GTP (10 μM) for 10 min, while not leading to increased basal force in the subsequent washes in pCa 9 solution (with MgATP), approximately doubled the subsequent response to pCa 6.25, as shown in Fig. 12. This effect was completely reversed by co-incubation with Y-27632 (30 μM) and attenuated by HA1077 (30 μM). The elevated response to pCa 6.25 after ATPγS pre-treatment (Figs 10C and 11D) was not affected by either Y-27632 or HA1077, suggesting that the kinase responsible for basal MYPT phosphorylation is probably distinct from ROK.
Figure 12. Addition of carbachol and GTP during ATPγS pre-incubation enhances the subsequent response to Ca2+ which is blocked by ROK inhibition.
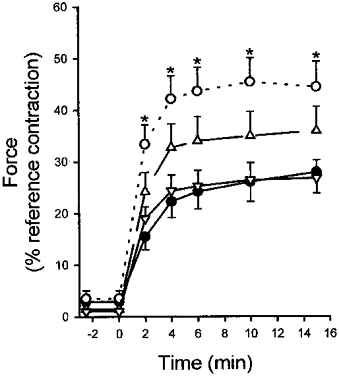
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were pre-incubated in pCa 9 rigor solution containing 10 μM ATPγS with the following additions: none (•); 100 μM carbachol and 10 μM GTP (○); 100 μM carbachol, 10 μM GTP and 30 μM Y-27632 (▿); or 100 μM carbachol, 10 μM GTP and 30 μM HA1077 (▵). Muscle strips were then washed in pCa 9 solution containing MgATP to ensure washout of acute sensitization by carbachol plus GTP. Force development upon re-addition of MgATP was not significant in either condition but differed during the subsequent pCa 6.25-induced contraction (initiated at time zero). *Significant difference compared with control (ATPγS alone).
Finally, the effect of ROK inhibition on GTPγS-induced MYPT thiophosphorylation was examined. As shown by the representative autoradiogram (Fig. 13a) and the cumulative data (Fig. 13B), the GTPγS-induced increase in MYPT thiophosphorylation was inhibited by HA1077.
Figure 13. Inhibition of the GTPγS-induced increase in MYPT thiophosphorylation by ROK inhibition.
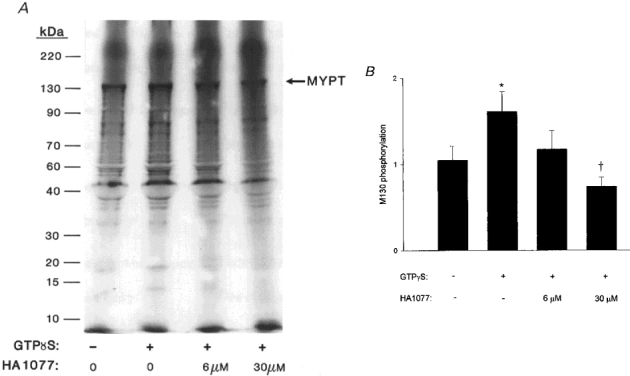
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated in pCa 9 rigor solution. At time zero, 10 μM [35S]ATPγS was added without or with 1 μM GTPγS in the absence or presence of HA1077 (6 or 30 μM) as indicated. Muscle strips were processed after 10 min as described in the legend to Fig. 10. A, a representative autoradiogram. B, cumulative data (n = 8) showing the GTPγS-induced increase in MYPT thiophosphorylation and its prevention by HA1077. *Significant difference from control. †Significant difference from value measured in the presence of GTPγS alone. More thiophosphorylated bands are visible in A than in Fig. 11A because the gel was exposed to the X-ray film for a longer period of time.
DISCUSSION
The results presented in this paper indicate that ROK activation is the predominant, if not the only, mechanism causing Ca2+ sensitization in guinea-pig ileum longitudinal smooth muscle. The high degree of selectivity of Y-27632 for ROK (Uehata et al. 1997) and the observed correlation between inhibition of Ca2+ sensitization and inhibition of isolated ROK by three inhibitors (Y-27632, HA1077 and H-7), suggests that the relevant inhibited kinase is indeed ROK.
HA1077, used in the majority of the present experiments, shows good selectivity for ROK over PKC and MLCK (Uehata et al. 1997; this study). However, it also inhibits cAMP- and cGMP-dependent protein kinases with Ki values ∼5-fold greater than for that ROK (Asano et al. 1989). This possible complication is irrelevant in the context of this work since inhibition of the cyclic nucleotide-dependent kinases would cause contraction rather than relaxation (cf. Lee et al. 1997, and references therein). Furthermore, we failed to observe relaxation of β-escin-permeabilized ileum by a combination of the adenylyl cyclase activator forskolin (10 μM) and the phosphodiesterase inhibitors rollipram (30 μM) and OPC3911 (3 μM) (results not shown).
The lack of effect of PDBu in guinea-pig ileum, which may relate to a low level of expression of a particular PKC isoform or the endogenous cytosolic protein inhibitor of myosin light chain phosphatase, CPI-17 (Li et al. 1998), suggests that conventional and/or novel PKC isoforms play a minor role (if any) in Ca2+-independent modulation of contractility in the ileum. This is in contrast to rat mesenteric small arteries in which PKC inhibition by calphostin C caused complete attenuation of Ca2+ sensitization (Buus et al. 1998) and rabbit femoral artery and portal vein in which a pseudosubstrate peptide inhibitor based on PKCα reduced Ca2+ sensitization and myosin phosphorylation (Gailly et al. 1997).
In permeabilized guinea-pig ileum, addition of GTPγS increased LC20 phosphorylation and this was reversed by ROK inhibition. At the lower [Ca2+] (pCa 6.5), changes in LC20 phosphorylation by GTPγS and HA1077 were relatively small (significantly different only after normalization to pCa 6.5 in each experiment) compared with the higher [Ca2+] (pCa 5.75) but may nevertheless be important if the LC20 phosphorylation-force relationship is very steep. This has indeed been demonstrated to be the case in several smooth muscles. For instance, Pfitzer & Arner (1998) showed that force changed from <10 to >90 % for an ∼8 % change in LC20 phosphorylation in the guinea-pig ileum. The present data thus support the hypothesis that the change in force by ROK inhibition is mediated by changes in LC20 phosphorylation in the permeabilized tissue.
To assess the functional effects of ROK inhibition under more physiological circumstances, intact strips were used. In strips loaded with fura-2 AM, contraction in response to carbachol was reduced by ROK inhibition to a transient peak coinciding with peak [Ca2+]i. [Ca2+]i on the other hand was unchanged. The effect of ROK inhibition by HA1077 in guinea-pig ileum is almost identical to the effect of treatment with toxin B from Clostridium difficile which glucosylates the Ras-related low molecular mass GTPases of the Rho subfamily thereby inactivating them. This treatment caused complete reduction of sustained contraction without associated changes in [Ca2+]i (Lucius et al. 1998). ROK may thus be the sole RhoA effector for Ca2+ sensitization in this tissue.
Even though ROK inhibition abolished the tonic phase of contraction this does not imply that the rise in [Ca2+]i and the consequent increase in MLCK activity is not important during this phase. It has been demonstrated frequently that extracellular Ca2+ is necessary for maintained contraction and that inhibition of MLCK reduces or abolishes maintained contraction. An interesting comparison can be made between our results in intact and permeabilized muscles. After a transient peak following stimulation of intact muscle with carbachol, [Ca2+]i settled at a steady level of 202 ± 33 nM which corresponds to a pCa of 6.69 and this was associated with a sizeable contraction that was, however, prevented by ROK inhibition. In the permeabilized tissue, stimulation with pCa 6.5 in the absence of GTPγS, i.e. a slightly higher [Ca2+], while drastically increasing the rate of contraction in response to the phosphatase inhibitor microcystin-LR, hardly elevated steady-state force relative to that achieved at pCa 9. Although a time-dependent change in the dependence of contraction on LC20 phosphorylation (latch) is likely to be important for this difference, our results also imply that physiological elevations in [Ca2+]i and consequent MLCK activation are not sufficient to cause a full force response unless GTPase-dependent pathways leading to MLCP inhibition are simultaneously activated. It therefore seems likely that maintained contraction in response to agonist stimulation depends on both MLCK and ROK activities.
LC20 phosphorylation in response to carbachol in the intact tissue was elevated only at 3 s in the presence of HA1077, whereas carbachol-induced LC20 phosphorylation in the absence of HA1077 was elevated for at least 200 s. The design of these experiments, with controls run versus each HA1077 concentration (6 and 30 μM) individually, precludes direct comparison of the effects of the two concentrations but allows us to conclude that selective ROK inhibition in the intact tissue is associated with an altered phosphorylation-time profile. Interestingly, Lucius et al. (1998) did not detect an increase in LC20 phosphorylation following toxin B treatment, even though the phasic component of the contractile response to carbachol plus GTP was retained. They concluded therefore that the carbachol-induced force transient occurred independently of myosin phosphorylation. Our results with HA1077 show that ROK inhibition reduces, but does not eliminate, the phasic increase in LC20 phosphorylation in response to carbachol treatment. Whether or not this lower level of LC20 phosphorylation accounts for the phasic contractile response is uncertain.
Our analysis of the dynamics of contraction and myosin phosphorylation indicates that inhibition of ROK affects the rate of dephosphorylation. The effects of agonist or GTPγS were similarly found to be associated with reduced MLCP activity (Kitazawa et al. 1991b) with no effect on MLCK activity. The use of microcystin and/or ATPγS in determination of the effect of GTPγS on phosphorylation rates in situ might cause activation of ROK-catalysed myosin phosphorylation by hyperphosphorylation of upstream signalling components. This would be detected by direct ROK inhibition and does not seem to be the case since HA1077 failed to affect rates of contraction and thus presumably the rate of phosphorylation in the present study. Although rates of phosphorylation were not measured directly, these contractions were much too slow to be limited by crossbridge turnover (∼1/s) and, if rates of phosphorylation were increased by GTPγS (contrary to several reports in the literature), an explanation would need to be invoked for the increased lag phase between phosphorylation and force since t½ values for contraction were unchanged.
There are additional observations which suggest that ROK acts primarily through inhibition of MLCP activity and not through direct phosphorylation of myosin. Firstly, activation of ROK by GTPγS did not induce Ca2+ sensitization of force at pCa 9 (results not shown) and did not thiophosphorylate LC20 whereas it did thiophosphorylate MYPT (Figs 11 and 13). Secondly, co-incubation of ATPγS with carbachol plus GTP at pCa 9 did not lead to significant force development upon re-addition of MgATP (as would have been expected if myosin had been phosphorylated) but only enhanced the subsequent response to pCa 6.25 (Fig. 12). Studies with purified ROK, on the other hand, have demonstrated that intact myosin and isolated LC20 are both good ROK substrates in vitro (Amano et al. 1996) and addition of a constitutively active fragment of ROK caused contraction via direct phosphorylation of myosin at serine-19, the MLCK site (Kureishi et al. 1997).
It is interesting to note that the effect of ROK inhibition did not appear to be attenuated by the lack of ATP in the solutions (Table 3). This may be due either to a slow turnover of phosphate on MYPT or to the presence of residual ATP in the tissue. The latter possibility is more likely since no qualified effort, such as apyrase treatment, was made to remove high energy phosphates and human ROK has been shown to have an unusually low Km for ATP (0.1 μM; Uehata et al. 1997), although Feng et al. (1999) determined a higher value (30.8 μM) for the chicken gizzard enzyme.
It is also clear from our results that there is no absolute Ca2+ requirement for GTPγS-induced MYPT phosphorylation. The Ca2+ dependence of sensitization of force and myosin phosphorylation thus appears to be downstream of RhoA/ROK activation and MYPT phosphorylation and is probably due to MLCK as discussed above.
Results from physiological studies such as this, and the studies of Kitazawa et al. (1991b), Kubota et al. (1992) and Noda et al. (1995), are thus not reconcilable with biochemical investigations with respect to direct phosphorylation of myosin. The cumulated physiological evidence argues that direct ROK-catalysed phosphorylation of myosin in the contractile apparatus is not of sufficient magnitude to cause contraction, at least in the absence of Ca2+. It could be speculated that ROK is spatially restricted, with limited access to myosin, but this would require a mobile messenger such as RhoA to translocate to the contractile apparatus following activation at the membrane. ROK isoform variation could perhaps also explain the lack of evidence for direct myosin phosphorylation and could contribute to variability of smooth muscle responses to neurotransmitters, autacoids and drugs in vivo. Whatever the case, the present study indicates that ROK plays an important role in myosin phosphorylation and contraction in intestinal smooth muscle and that this occurs through modulation of MLCP activity.
Acknowledgments
This study was supported by grants to M.P.W. (MT-13101) from the Medical Research Council of Canada, to P.H. (14x-28) from the Swedish Medical Research Council and to D.J.H. (HL-23615) from the National Institutes of Health. K.S. is supported by Fellowships from the Alberta Heritage Foundation for Medical Research, the Heart and Stroke Foundation of Canada and the Swedish Medical Research Council. M.P.W. is a Medical Scientist of the Alberta Heritage Foundation for Medical Research. The authors are grateful to Drs Thomas Leung and Louis Lim for generously providing antibodies to ROK, to Erik Persson for assistance with the pre-thiophosphorylation experiments, to Cindy Sutherland for protein purification, and to Lenore Youngberg for expert secretarial assistance.
References
- Akopov SE, Zhang L, Pearce WJ. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: effects of artery size and age. American Journal of Physiology. 1998;275:H930–939. doi: 10.1152/ajpheart.1998.275.3.H930. [DOI] [PubMed] [Google Scholar]
- Allen BG, Andrea JE, Walsh MP. Identification and characterization of protein kinase C ζ-immunoreactive proteins. Journal of Biological Chemistry. 1994;269:29288–29298. [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Asano T, Suzuki T, Tsuchiya M, Satoh S, Ikegaki I, Shibuya M, Suzuki Y, Hidaka H. Vasodilator actions of HA1077 in vitro and in vivo putatively mediated by the inhibition of protein kinase. British Journal of Pharmacology. 1989;98:1091–1100. doi: 10.1111/j.1476-5381.1989.tb12652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus CL, Aalkjaer C, Nilsson H, Juul B, Møller JV, Mulvany MJ. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. The Journal of Physiology. 1998;510:577–590. doi: 10.1111/j.1469-7793.1998.577bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. Journal of Biological Chemistry. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Letters. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Molecular Biology of the Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Gong MC, Somlyo AV, Somlyo AP. Possible role of atypical protein kinase C activated by arachidonic acid in Ca2+ sensitization of rabbit smooth muscle. The Journal of Physiology. 1997;500:95–109. doi: 10.1113/jphysiol.1997.sp022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DM, Hames BD. In: DNA Cloning 2: A Practical Approach. Expression Systems. 2. Glover DM, Hames BD, editors. Oxford: IRL Press; 1995. pp. 41–47. [Google Scholar]
- Gomez M, Swärd K. Long-term regulation of contractility and calcium current in smooth muscle. American Journal of Physiology. 1997;273:C1714–1720. doi: 10.1152/ajpcell.1997.273.5.C1714. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fuglsang A, Alessi D, Kobayashi S, Cohen P, Somlyo AV, Somlyo AP. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. Journal of Biological Chemistry. 1992;267:21492–21498. [PubMed] [Google Scholar]
- Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. Journal of Biological Chemistry. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins – ras-family or trimeric proteins or both – in Ca2+ sensitization of smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdödi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. Journal of Muscle Research and Cell Motility. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Hathaway DR, Haeberle JR. Selective purification of the 20,000-Da light chains of smooth muscle myosin. Analytical Biochemistry. 1983;135:37–43. doi: 10.1016/0003-2697(83)90726-1. [DOI] [PubMed] [Google Scholar]
- Himpens B, Matthijs G, Somlyo AV, Butler TM, Somlyo AP. Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. Journal of General Physiology. 1988;92:713–729. doi: 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. Journal of Biological Chemistry. 1992;267:8719–8722. [PubMed] [Google Scholar]
- Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. Journal of Biological Chemistry. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO Journal. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. Journal of Biological Chemistry. 1991a;266:1708–1715. [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1991b;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Nomura M, Kamm KE, Mumby MC, Stull JT. GTPγS-dependent regulation of smooth muscle contractile elements. American Journal of Physiology. 1992;262:C405–410. doi: 10.1152/ajpcell.1992.262.2.C405. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. Journal of Biological Chemistry. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. Journal of Biological Chemistry. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Molecular and Cellular Biology. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. Journal of Biological Chemistry. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. The Journal of Physiology. 1998;508:871–881. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Nilsson B-O, Hellstrand P. Inhibition of calcium entry preserves contractility of arterial smooth muscle in culture. Journal of Vascular Research. 1997;34:103–108. doi: 10.1159/000159207. [DOI] [PubMed] [Google Scholar]
- Lucius C, Arner A, Steusloff A, Troschka M, Hofmann F, Aktories K, Pfitzer G. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. The Journal of Physiology. 1998;506:83–93. doi: 10.1111/j.1469-7793.1998.083bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca2+-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. Journal of General Physiology. 1994;104:265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO Journal. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mita M, Walsh MP. α1-Adrenoceptor-mediated phosphorylation of myosin in rat tail arterial smooth muscle. Biochemical Journal. 1997;327:669–674. doi: 10.1042/bj3270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai PK, Carruthers CA, Walsh MP. Isolation of the native form of chicken gizzard myosin light chain kinase. Biochemical Journal. 1984;218:863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Kolber M, Van Breemen C. Norepinephrine and GTPγS increase myofilament Ca2+ sensitivity in α-toxin permeabilized arterial smooth muscle. Biochemical and Biophysical Research Communications. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K, Takuwa Y. Involvement of rho in GTPγS-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Letters. 1995;367:246–250. doi: 10.1016/0014-5793(95)00573-r. [DOI] [PubMed] [Google Scholar]
- Okubo S, Ito M, Takashiba Y, Ichikawa K, Miyahara M, Shimizu H, Konishi T, Shima H, Nagao M, Hartshorne DJ, Nakano T. A regulatory subunit of smooth muscle myosin bound phosphatase. Biochemical and Biophysical Research Communications. 1994;200:429–434. doi: 10.1006/bbrc.1994.1467. [DOI] [PubMed] [Google Scholar]
- Otto B, Steusloff A, Just I, Aktories K, Pfitzer G. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. The Journal of Physiology. 1996;496:317–329. doi: 10.1113/jphysiol.1996.sp021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ER, Ngai PK, Walsh MP, Gröschel-Stewart U. Embryonic chicken gizzard: expression of the smooth muscle regulatory proteins caldesmon and myosin light chain kinase. Cell Tissue Research. 1995;279:331–337. doi: 10.1007/BF00318489. [DOI] [PubMed] [Google Scholar]
- Pfitzer G, Arner A. Involvement of small GTPases in the regulation of smooth muscle contraction. Acta Physiologica Scandinavica. 1998;164:449–456. doi: 10.1111/j.1365-201x.1998.tb10698.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Leposavic R, Publicover NG, Sanders KM, Gerthoffer WT. Sensitization of the contractile system of canine colonic smooth muscle by agonists and phorbol ester. The Journal of Physiology. 1994;481:677–688. doi: 10.1113/jphysiol.1994.sp020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. Journal of Biological Chemistry. 1994;269:9912–9920. [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Walsh MP, Hinkins S, Dabrowska R, Hartshorne DJ. Smooth muscle myosin light chain kinase. Methods in Enzymology. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Walsh MP, Valentine KA, Ngai PK, Carruthers CA, Hollenberg MD. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochemical Journal. 1984;224:117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LP, van Lierop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. The Journal of Physiology. 1999;516:805–824. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


