Abstract
The metabolic activity of the brain has not been evaluated during physical exercise. In six volunteers substrate uptake by the brain was determined during graded exercise and recovery from maximal exercise by measuring the arterial-internal jugular venous concentration differences(a–v differences).
The a–v difference for lactate increased from 0.02 ± 0.08 mmol l−1 at rest to 0.39 ± 0.13 mmol l−1 during exercise and remained positive during 30 min of recovery (P < 0.05). The a–v difference for glucose (0.55 ± 0.06 mmol l−1 at rest) did not change significantly during exercise, but during the initial 5 min of recovery it increased to 0.83 ± 0.10 mmol l−1 (P < 0.05). The O2 a–v difference at rest of 3.11 ± 0.30 mmol l−1 remained stable during exercise, then increased during the initial 5 min of recovery (3.77 ± 0.52 mmol l−1) and remained high during the subsequent 30 min recovery period (3.62 ± 0.64 mmol l−1; P < 0.05). Thus the O2/glucose uptake ratio did not change during exercise (pre-exercise 5.95 ± 0.68; post-exercise 6.02 ± 1.39) but decreased to 4.93 ± 0.99 during the initial 5 min of recovery (P < 0.05). When lactate uptake was included, the resting O2/carbohydrate uptake ratio of 5.84 ± 0.73 was reduced to 4.42 ± 0.25 during exercise and decreased further during the recovery phase (to 3.79 ± 0.30; P < 0.05).
In contrast, in the resting and immobilised rat, lactate infusion to a level similar to that obtained during maximal exercise in humans did not affect the a–v difference for lactate.
The large carbohydrate uptake by the brain during recovery from maximal exercise suggests that brain glycogen metabolism is important in the transition from rest to exercise, since this would explain the significant post-exercise decrease in the O2/carbohydrate uptake ratio.
Controversy exists as to whether the metabolic activity of the brain as a whole increases during physical exercise. For example, there appears to be no change in brain O2 uptake during cycling (Zobl et al. 1965; Madsen et al. 1993), whereas during vigorous exercise on the treadmill an increase in brain O2 uptake and a tendency for glucose uptake to increase has been reported (Scheinberg et al. 1954). In the case of mental stress, there is no appreciable change in brain O2 uptake but there is an increase in cerebral glucose uptake (Madsen et al. 1995a). Thus, the rate of glucose uptake is enhanced compared to that of O2. Interestingly, the ‘uncoupling’ between the O2 and glucose uptake rates is sustained even after the cessation of brain activation (Madsen et al. 1995a,b) and may be associated with a decrease in the glycogen level in the brain (Madsen et al. 1995a).
During maximal exercise, blood lactate increases to as much as 30 mmol l−1 (Nielsen, 1999) and the brain is known to take up lactate (Ahlborg & Wahren, 1972). Lactate transport across mammalian plasma membranes is mainly carrier mediated (Poole et al. 1993) and a monocarboxylate transporter is found in rat brain endothelium cells (Gerhart et al. 1997). Brain tissue, including neurons (Dringen et al. 1993) and astrocytes (Tildon et al. 1993), possesses the capacity to take up and utilise lactate as an energy source. In fact, lactate rather than glucose may be the primary energy source during neuronal activation (Larrabee, 1996) when lactate is supplied by the glial cells to the neurons (Poitry-Yamate et al. 1995). A positive arterial-jugular venous concentration difference (a–v difference) for lactate has been demonstrated in the dog (Nemoto et al. 1974; Avogaro et al. 1990) and in humans during cardiopulmonary resuscitation (Rivers et al. 1991). Taken together, these reports led us to hypothesise that the metabolic rate for the brain is increased during exercise and also in the recovery phase when lactate, in addition to glucose and O2, is taken into consideration as a metabolic substrate. Therefore, we investigated the a–v differences for glucose, lactate and O2 at rest, during exercise and in the immediate recovery period. Since arterial CO2 tension increases during moderate exercise and decreases during maximal exercise (Jørgensen et al. 1992), we applied an index of the metabolic rate of the brain that would be independent of flow, i.e. we calculated the O2/carbohydrate uptake ratio. In a separate experiment in the rat, we investigated whether an increase in blood lactate per se would influence brain uptake of lactate.
METHODS
These experiments were approved by the Ethics Committee of Copenhagen (KF 01–369/97) and conformed with the standards set by the Declaration of Helsinki. The informed consent of the participants was obtained. Six healthy volunteers were studied (age 25 ± 2 years (mean ±s.d.), body weight 74 ± 5 kg, height 184 ± 3 cm). The subjects exercised in a semi-supine position on a modified Krogh cycle ergometer at 60 r.p.m. which elicits a maximal O2 uptake of ∼90 % of the value established during normal cycling (Galbo et al. 1987). The initial work rate was 30 W and this was increased every minute until exhaustion (280–320 W), resulting in a maximal O2 uptake of 3.8 ± 0.2 l min−1 and a ventilation rate of 136 ± 5 l min−1 (MedGraphics 2001, Medical Graphics). Cannulae were introduced percutaneously into the brachial artery of the non-dominant arm (19 G) and retrogradely into the internal jugular vein (14 G) and advanced to the bulb (Jacobsen & Enevoldsen, 1989). Blood samples were obtained anaerobically at rest, every minute during exercise and for the first 30 min of recovery. Blood gas variables (ABL 625, Radiometer) and lactate were determined (YSI2300, Yellow Springs Instruments).
The animal experiments were approved by the Animal Experimental Committee of the Danish Ministry of Justice. Six male Sprague-Dawley rats with a weight of 300–400 g were fasted for 22 h before surgery. The operations, lasting for less than 1 h, were carried out under halothane anaesthesia (4 % for induction; 1.25-1.5 % for maintenance) with 70 % N2O and 30 % O2. A catheter (19 G) filled with saline and heparin (30 U ml−1) was inserted in a femoral artery. Both femoral nerves were cut in order to immobilise the hind limbs. A local anaesthetic (0.15 ml 1 % lidocaine with 0.5 % noradrenaline) was instilled into the wounds, which were sutured and covered with an anaesthetic lotion (EMLA, Astra, Sweden). A similar catheter was inserted into the confluence of sinuses through a screw with a central bore hole placed just posterior to the lambdoid suture without penetrating the dura. The hind limbs and the pelvis were immobilised in a plaster of Paris cast. During recovery from anaesthesia, the rats were kept in a closed triangular shelter in order to minimise external stimulation and they were monitored by a video camera. Rectal temperature and arterial blood pressure remained constant. The animals remained undisturbed for a minimum of 3 h before the experiment. When the rats recovered from anaesthesia, they rarely moved their heads or forelimbs and they were not affected by blood sampling. Infusion of 2–4 ml of sodium lactate solution (0.9 g l−1) over 10 min secured the targeted plasma lactate concentration. Blood was analysed as described for the human experiments. After the experiment the rats were killed by i.v. infusion of barbiturate.
Data are means and s.d. For the human data, the exercise intensity (∼160 W) that resulted in an increase in plasma lactate was determined (the lactate ‘threshold’). Data were evaluated by a Friedman test encompassing average values at rest (two determinations), during exercise below and above the lactate threshold and during the first 5 min and the last 25 min of recovery. Significant deviations were evaluated with the Wilcoxon matched test by rank. A P value of < 0.05 was considered statistically significant.
RESULTS
The a–v difference for glucose did not change significantly during exercise (Table 1, Fig. 1). During the initial 5 min of recovery there was an increase to a peak of 0.83 ± 0.10 mmol l−1, followed by a gradual decline to slightly above resting values. The a–v difference for lactate increased from 0.02 ± 0.08 to 0.39 ± 0.13 mmol l−1 during moderate exercise and remained positive (0.24 ± 0.15 mmol l−1) over the 30 min recovery period. The a–v difference for O2 (3.11 ± 0.30 mmol l−1) did not increase significantly during exercise (3.50 ± 0.50 mmol l−1) but it did increase to 3.77 ± 0.52 mmol l−1 during the first 5 min of recovery, followed by a gradual decline to control levels over 30 min.
Table 1. Arterial and jugular venous variables at rest and in response to cycling for six humans.
| Rest | Exercise | Recovery | |||
|---|---|---|---|---|---|
| Below lactate threshold | Above lactate threshold | 0–5 min | 5–30 min | ||
| Pa,CO2(kPa) | 5.49 ± 0.31 | 5.57 ± 0.17 | 5.18 ± 0.22* | 4.70 ± 0.32* | 4.99 ± 0.37* |
| pHa | 7.41 ± 0.01 | 7.41 ± 0.01 | 7.37 ± 0.02* | 7.28 ± 0.04* | 7.33 ± 0.05* |
| pHv | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.31 ± 0.02* | 7.22 ± 0.05* | 7.28 ± 0.05* |
| [Hb]a (mmol l−1) | 8.61 ± 0.78 | 9.05 ± 0.55* | 9.51 ± 0.74* | 9.56 ± 0.73* | 8.94 ± 0.82* |
| [Hb]v(mmol l−1) | 8.85 ± 0.67 | 9.11 ± 0.66* | 9.63 ± 0.67* | 10.00 ± 0.52* | 9.08 ± 0.73* |
| Sa,O2 (%) | 97.2 ± 0.5 | 96.9 ± 0.3 | 96.4 ± 0.6 | 96.8 ± 0.6 | 96.3 ± 1.3 |
| Sv,O2 (%) | 59.3 ± 2.7 | 60.1 ± 1.6 | 58.5 ± 3.6 | 54.9 ± 4.1 | 54.6 ± 4.8 |
| Ca,O2 (mmol l−1) | 8.37 ± 0.76 | 8.77 ± 0.55* | 9.16 ± 0.73* | 9.25 ± 0.68* | 8.60 ± 0.71* |
| Cv,O2 (mmol l−1) | 5.25 ± 0.51 | 5.47 ± 0.47* | 5.63 ± 0.50* | 5.48 ± 0.37 | 4.94 ± 0.45 |
| [O2] a–v dif. (mmol l−1) | 3.11 ± 0.30 | 3.30 ± 0.14 | 3.50 ± 0.50 | 3.77 ± 0.52* | 3.62 ± 0.64* |
| ‘Corrected’, CO2 (mmol l−1) | — | 3.39 | 3.21 | 3.04 | 3.17 |
| [Glu]a (mmol l−1) | 5.37 ± 0.71 | 5.18 ± 0.45 | 4.89 ± 0.22 | 5.54 ± 0.47 | 5.06 ± 0.44 |
| [Glu]v (mmol l−1) | 4.82 ± 0.73 | 4.56 ± 0.46 | 4.25 ± 0.18 | 4.70 ± 0.44 | 4.44 ± 0.47 |
| Glu a–v dif. (mmol l−1) | 0.55 ± 0.06 | 0.62 ± 0.06 | 0.61 ± 0.13 | 0.83 ± 0.10* | 0.58 ± 0.09 |
| ‘Corrected’, CO2 (mmol l−1) | — | 0.63 | 0.56 | 0.67 | 0.50 |
| ‘Corrected’, O2 (mmol l−1) | — | 0.58 | 0.53 | 0.65 | 0.49 |
| [Lac]a (mmol l−1) | 0.90 ± 0.37 | 0.92 ± 0.26 | 3.85 ± 0.83* | 7.13 ± 2.19* | 4.53 ± 2.12* |
| [Lac]v (mmol l−1) | 0.88 ± 0.30 | 0.92 ± 0.26 | 3.40 ± 1.04* | 6.65 ± 1.98* | 4.02 ± 2.22* |
| Lac a–v dif. (mmol l−1) | 0.02 ± 0.08 | 0.01 ± 0.03 | 0.39 ± 0.13* | 0.48 ± 0.24* | 0.24 ± 0.15* |
| ‘Corrected’, CO2 (mmol l−1) | — | 0.01 | 0.36 | 0.39 | 0.21 |
| ‘Corrected’, O2 (mmol l−1) | — | 0.01 | 0.34 | 0.38 | 0.20 |
| (Glu + Lac/2) a–v dif. (mmol l−1) | 0.56 ± 0.09 | 0.62 ± 0.05 | 0.78 ± 0.13* | 1.07 ± 0.13* | 0.70 ± 0.11 |
| ‘Corrected’, CO2 (mmol l−1) | — | 0.63 | 0.73 | 0.87 | 0.61 |
| ‘Corrected’, O2 (mmol l−1) | — | 0.59 | 0.70 | 0.85 | 0.59 |
| O2/Glu a–v dif. | 5.95 ± 0.68 | 5.43 ± 0.39 | 6.02 ± 1.39 | 4.93 ± 0.99* | 6.48 ± 1.53 |
| O2/(Glu + Lac/2) a–v dif. | 5.84 ± 0.73 | 5.36 ± 0.33 | 4.42 ± 0.25* | 3.79 ± 0.30* | 5.32 ± 0.61 |
Values are means ± S.D. a, arterial value; v, venous value. dif., difference. PCO2, carbon dioxide tension; pH, pH of blood; Hb, haemoglobin; SO2, haemoglobin saturation; [O2], O2 content of blood; Glu, glucose; Lac, lactate. ‘Corrected’, a–v difference related to a 30% change in cerebral blood flow per kPa change in Pa,CO2 or a constant cerebral a–v dif. O2.
P < 0.05, compared to rest.
Figure 1. Arterial-internal jugular venous difference at rest, during exercise and in the immediate recovery period.
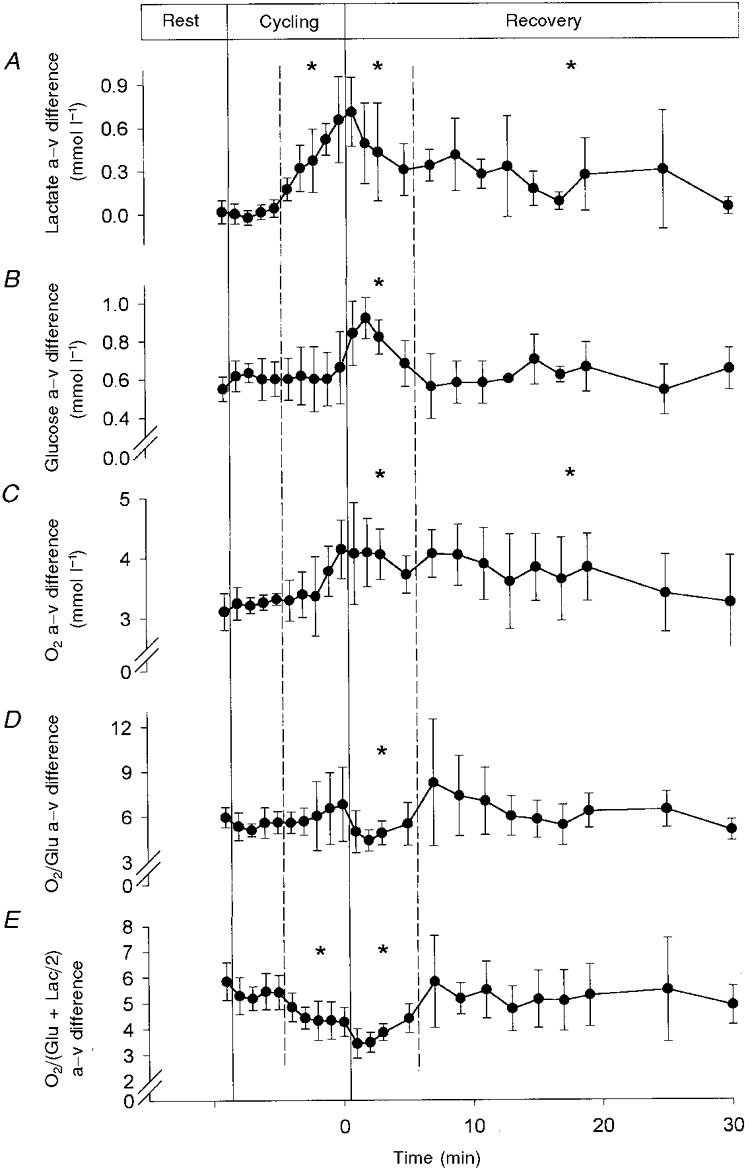
Data are means ±s.d. for six subjects. Arterial-jugular venous (a–v) differences for lactate (A), glucose (B) and O2 (C), and for molar O2/glucose (a–v difference O2/Glu; D) and O2/carbohydrate (a–v difference O2/(Glu + Lac/2); E) ratios. * P < 0.05, compared to rest.
These changes in the a–v difference did not change the O2/glucose uptake ratio during exercise (pre-exercise 5.95 ± 0.68, post-exercise 6.02 ± 1.39), but the ratio declined to 4.93 ± 0.99 for the first ∼5 min of recovery (Table 1, Fig. 1). When lactate was included in the ratio in order to account for total uptake of glucose equivalents, i.e. the O2/(glucose + lactate/2) uptake ratio, it declined from 5.84 ± 0.73 to 4.42 ± 0.25 during moderate exercise and further to 3.79 ± 0.30 during the recovery period.
Since the plasma lactate concentration per se could be important for the lactate uptake, we tested whether in the awake and resting rat an infusion of lactate would cause a positive a–v difference for lactate over the brain. This was not the case since the a–v difference did not change significantly from −0.11 ± 0.08 mmol l−1 at rest, with blood lactate ranging from 0.8 ± 0.3 to 10.5 mmol l−1.
DISCUSSION
There were two key results of this investigation. (1) The human brain takes up lactate during especially intense dynamic exercise and this uptake is maintained in the period of recovery from exercise. (2) During the initial few minutes after the termination of exercise brain glucose uptake, as indicated by the a–v difference for glucose was increased almost 2-fold compared to rest. These changes resulted in a significant decrease in the molar ratio of O2 to carbohydrate uptake, from a normal resting value close to 6, to 4.4 during exercise and to as low as 3.8 during the early recovery phase.
In the rat, brain activation results in a decrease in brain glycogen by 15 % (from ∼6.4 to 5.4 μmol g−1) and an increase in lactate uptake by the brain (Madsen et al. 1995a). Against that background the present results can be interpreted as follows. Brain activation enhances ATP turnover (at least in some parts of the brain) which is being generated initially from phosphocreatine breakdown and from glycolysis in a fashion analogous to the events that take place in skeletal muscle early during exercise. The fate of the lactate taken up by the brain is not clear. It has been suggested that lactate may serve as an energy fuel for the neurons, being oxidised to CO2 and water (Larrabee, 1996). The fact that the O2/carbohydrate uptake ratio decreased supports a role for lactate as well as glucose as fuel for oxidation, and suggests that all carbohydrate taken up by the brain may not be fully oxidised (Fox et al. 1988; Madsen et al. 1995a,b). Alternatively, increased flux in the glycolytic and tricarboxylic acid pathways could increase the concentration of intermediates, but if this were the case, the O2/carbohydrate uptake ratio would be expected to increase after exercise. In addition the brain lactate concentration may increase, but if lactate uptake by the brain was dominated by loading of its distribution volume, lactate would be expected to clear when the arterial concentration decreased during recovery.
It is not generally accepted that physical exercise causes neuronal activation. However, as determined by whole body positron emission tomography in humans after running, glucose uptake is higher in not only subcortical brain areas including the hypothalamus, but also in cortical regions including the primary sensorimotor cortex and the insular cortex when compared to that of non-exercising control subjects (Tashiro et al. 1998). The activation in the hypothalamus and the insular cortex may be related to endocrine and autonomic responses to exercise, respectively. It also appears that exercise-induced hyperventilation increases blood flow to the superolateral primary cortex (Fink et al. 1995).
When comparing the results from humans and rats, it seems that brain uptake of lactate depends on cerebral activation. A monocarboxylate transporter is distributed in the endothelial cells in the rat brain (Gerhart et al. 1997) and, in erythrocytes, a H+-monocarboxylate co-transporter accounts for 70–90 % of lactate transport across the physiological range of lactate concentrations (Gladden, 1996). In the physiological pH range, as in the present study, more than 99 % of lactate acid is dissociated into lactate anions and hydrogen ions, indicating that the diffusion of free acid is unlikely to provide an explanation for brain lactate uptake. Exchange of lactate for another anion such as Cl− or HCO3− probably plays only a minor role (Gladden, 1996), since hyperlactaemia did not evoke brain lactate uptake in rats in this study. Yet brain lactate uptake cannot always be explained by H+-monocarboxylate co-transporter in vivo. During hypoglycaemia in the anaesthetised dog a sodium lactate infusion induces brain lactate uptake (Nemoto et al. 1974; Avogaro et al. 1990), as does endogenous hyperlactaemia. Thus, it is probable that brain lactate uptake depends on an increase in cerebral metabolic demand and may be associated with a marked elevation in plasma hormone levels including catecholamines during intense exercise.
In addition to glucose and lactate, glutamate and other amino acids may serve as energy sources for the brain (Larrabee, 1995), and if this is so, substrate uptake would be larger than was noted in our study. In contrast to the evaluation of Madsen et al. (1993) based only on O2 uptake, our evaluation, which took both glucose and lactate into consideration, demonstrates that substrate uptake by the brain changes in proportion to work rate (Fig. 1). This was significant even when flow was normalised to arterial CO2 tension (Table 1). Thus, although the molar ratio between O2 and carbohydrate was higher both at rest and during exercise than when calculated as regional changes during visual stimulation (4.1 and 0.4, respectively; Fox et al. 1988), the a–v differences do confirm that exercise is associated with a decrease.
Arterial CO2 tension affects cerebral blood flow during exercise (Linkis et al. 1995), as it does at rest, and in turn affects the a–v difference. A CO2‘reactivity’ of 30 % kPa−1 was used when there was found to be no change in jugular venous flow or uptake of O2 during cycling (Madsen et al. 1993). After such ‘correction’, cerebral blood flow would be calculated to be reduced by 14 % during high intensity cycling and by 24 % immediately after exercise. When the correction was conducted as a constant a–v difference for O2 (Madsen et al. 1993), a similar trend was obtained (Table 1). Therefore, the increases in O2 and glucose should partly be regarded as a compensation for a reduced flow. However, the a–v difference for carbohydrates remained about 66 % higher during and immediately after exercise. Furthermore, both the middle cerebral artery blood velocity (Jørgensen et al. 1992, 1993; Helström et al. 1996) and flow in the internal carotid artery increase during exercise (Huang et al. 1992; Hellström et al. 1996). It is therefore likely that flow in the jugular vein, as determined by the Kety-Schmidt method, should increase (although by a lesser amount) during exercise, which means that the above calculation would be a conservative estimate of substrate uptake by the brain and the a–v difference ratio would be independent of absolute flow. Thus, changes in arterial CO2 tension will not influence the results qualitatively.
A reduced molar ratio of O2 to glucose uptake has also been obtained during a euglycaemic insulin clamp in patients with insulin-dependent diabetes mellitus, indicating an enhanced non-oxidative glucose metabolism (Grill et al. 1990). However, the fate of this extra glucose uptake is not known.
The increase in brain uptake of glucose and lactate during recovery after exercise suggests an important role for brain glycogen stores in the metabolic response of the brain to physiological activation, with subsequent repletion during the first minutes of recovery. We speculate that ‘central fatigue’, i.e. fatigue that has an apparent non-muscular origin (Secher, 1992), could relate to incomplete recovery of brain glycogen stores. Our results indicate that brain glucose is replenished upon cessation of activation and that lactate can substitute for glucose as a substrate in the human brain during especially intense physical exercise where blood lactate is at a high level.
Acknowledgments
This study was supported by The Danish National Research Foundation (Grant 504-4) and The Danish Medical Research Council (Grant 9502885). We thank Mette Secher and Yan Cai for expert technical assistance and Elizabeth Pemberton for revising the manuscript.
References
- Ahlborg RG, Wahren J. Brain substrate utilization during prolonged exercise. Scandinavian Journal of Clinical Laboratory Investigation. 1972;29:397–402. doi: 10.3109/00365517209080256. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Nosadini R, Doria A, Tremolada C, Baccaglini U, Ambrosio F, Merkel C, Nosadini A, Trevisan R, Fioretto P. Substrate availability other than glucose in the brain during euglycemia and insulin-induced hypoglycemia in dogs. Metabolism. 1990;39:46–50. doi: 10.1016/0026-0495(90)90146-4. [DOI] [PubMed] [Google Scholar]
- Dringen R, Wiesinger H, Hamprecht B. Uptake of L-lactate by cultured rat brain neurons. Neuroscience Letters. 1993;163:5–7. doi: 10.1016/0304-3940(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JDG, Innes JA, Wuyam B, Kobayashi I, Corfiels DR, Murphy K, Jones T, Frackowiak RSJ, Guz A. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. The Journal of Physiology. 1995;489:663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MM, Dence C. Nonoxidative glucose consumption during focal physiologic neural activation. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Galbo H, Kjær M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. The Journal of Physiology. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. American Journal of Physiology. 1997;273:E207–213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate transport and exchange during exercise. In: Rowell LB, Shephard JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 614–648. chap. 14. [Google Scholar]
- Grill V, Gutniak M, Björkman O, Lindoqvist M, Stone-Elander S, Seitz RJ, Blomqvist G, Reichard P, Widen L. Cerebral blood flow and substrate utilization in insulin-treated diabetic subjects. American Journal of Physiology. 1990;258:E813–820. doi: 10.1152/ajpendo.1990.258.5.E813. [DOI] [PubMed] [Google Scholar]
- Hellström G, Fischer-Colbrie W, Wahlgren NG, Jörgenstrand T. Carotid artery blood flow and middle cerebral artery blood velocity during physical exercise. Journal of Applied Physiology. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- Huang SY, Sun S, Droma T, Zhuang J, Tao JX, Mccullough RG, Mccullough RE, Micco JA, Reeves JT, Moore LG. Internal carotid flow velocity during exercise in Tibetan and Han residents of Lhasa (3.658 m) Journal of Applied Physiology. 1992;73:2638–2642. doi: 10.1152/jappl.1992.73.6.2638. [DOI] [PubMed] [Google Scholar]
- Jacobsen M, Enevoldsen E. Retrograde catherization of the right internal jugular vein for serial measurement of cerebral venous oxygen content. Journal of Cerebral Blood Flow and Metabolism. 1989;9:717–720. doi: 10.1038/jcbfm.1989.101. [DOI] [PubMed] [Google Scholar]
- Jørgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. Journal of Applied Physiology. 1992;72:1123–1132. doi: 10.1152/jappl.1992.72.3.1123. [DOI] [PubMed] [Google Scholar]
- Jørgensen LG, Perko G, Payne G, Secher NH. Effect of limb anesthesia on middle cerebral response to handgrip. American Journal of Physiology. 1993;264:H553–559. doi: 10.1152/ajpheart.1993.264.2.H553. [DOI] [PubMed] [Google Scholar]
- Larrabee MG. Lactate metabolism and its effects on glucose metabolism in an exercised neural tissue. Journal of Neurochemistry. 1995;64:1734–1741. doi: 10.1046/j.1471-4159.1995.64041734.x. [DOI] [PubMed] [Google Scholar]
- Larrabee MG. Partitioning of CO2 production between glucose and lactate in excised sympathetic ganglia, with implications for brain. Journal of Neurochemistry. 1996;67:1726–1734. doi: 10.1046/j.1471-4159.1996.67041726.x. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Ingvar DH, Skinhøj E. Brain function and blood flow. Scientific American. 1978;239:62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- Linkis P, Jørgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. Journal of Applied Physiology. 1995;78:12–16. doi: 10.1152/jappl.1995.78.1.12. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Denel GA. Metabolite flux to and from brain and levels of metabolites in brain of rats during rest, sensory stimulation, and recovery. Journal of Cerebral Blood Flow and Metabolism. 1995a;15(suppl 1):S77. [Google Scholar]
- Madsen PL, Hasselbalch SG, Hageman LP, Olsen KS, Bülow J, Holm S, Wildschiødtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. Journal of Cerebral Blood Flow and Metabolism. 1995b;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiødtz G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. Journal of Applied Physiology. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245. [DOI] [PubMed] [Google Scholar]
- Nemoto EM, Hoff JT, Severinghaus JW. Lactate uptake and metabolism by brain during hyperlactemia and hypoglycemia. Stroke. 1974;5:48–53. doi: 10.1161/01.str.5.1.48. [DOI] [PubMed] [Google Scholar]
- Nielsen HB. pH after competitive rowing: the lower physiological range? Acta Physiologica Scandinavica. 1999;165:113–114. doi: 10.1046/j.1365-201x.1999.00485.x. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. Journal of Neuroscience. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. American Journal of Physiology. 1993;264:C761–782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Rivers EP, Paradis NA, Martin GB, Goetting ME, Rosenberg JA, Smithline HA, Appleton TJ, Nowak RM. Cerebral lactate uptake during cardiopulmonary resuscitation in humans. Journal of Cerebral Blood Flow and Metabolism. 1991;11:479–484. doi: 10.1038/jcbfm.1991.91. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Blackburn LI, Rich M, Saslaw M. Effects of vigorous physical exercise on cerebral circulation and metabolism. American Journal of Medicine. 1954;16:549–554. doi: 10.1016/0002-9343(54)90371-x. [DOI] [PubMed] [Google Scholar]
- Secher NH. Central nervous influence on fatigue. In: Shephard RJ, Åstrand P-O, editors. Endurance in Sports. Oxford: Blackwell Scientific Publications; 1992. pp. 96–106. chap. 9. [Google Scholar]
- Tashiro M, Ito M, Fujimoto T, Fujiwara T, Sasaki H. Analysis of neuromuscular interaction by simultaneous measurement of cerebral and muscular energy consumption using whole body PET (abstract) In: Sargeant AJ, Siddons H, editors. From Community Health to Elite Sport. Proceedings of Third Annual Congress of the European College of Sports Science, Manchester, UK. Liverpool: Health Care Development; 1998. p. 183. [Google Scholar]
- Tildon JT, Mckenna MC, Stevenson J, Couto R. Transport of lactate by cultured rat brain astrocytes. Neurochemical Research. 1993;18:177–184. doi: 10.1007/BF01474682. [DOI] [PubMed] [Google Scholar]
- Zobl EG, Talmers FN, Christensen RC, Baer LJ. Effect of exercise on the cerebral circulation and metabolism. Journal of Applied Physiology. 1965;20:1289–1293. [Google Scholar]


