Abstract
The co-ordination between respiratory and postural functions of the diaphragm was investigated during repetitive upper limb movement. It was hypothesised that diaphragm activity would occur either tonically or phasically in association with the forces from each movement and that this activity would combine with phasic respiratory activity.
Movements of the upper limb and ribcage were measured while standing subjects performed repetitive upper limb movements ‘as fast as possible’. Electromyographic (EMG) recordings of the costal diaphragm were made using intramuscular electrodes in four subjects. Surface electrodes were placed over the deltoid and erector spinae muscles.
In contrast to standing at rest, diaphragm activity was present throughout expiration at 78 ± 17% (mean ± s.d.) of its peak inspiratory magnitude during repeated upper limb movement.
Bursts of deltoid and erector spinae EMG activity occurred at the limb movement frequency (≈2.9 Hz). Although the majority of diaphragm EMG power was at the respiratory frequency (≈0.4 Hz), a peak was also present at the movement frequency. This finding was corroborated by averaged EMG activity triggered from upper limb movement. In addition, diaphragm EMG activity was coherent with ribcage motion at the respiratory frequency and with upper limb movement at the movement frequency.
The diaphragm response was similar when movement was performed while sitting. In addition, when subjects moved with increasing frequency the peak upper limb acceleration correlated with diaphragm EMG amplitude. These findings support the argument that diaphragm contraction is related to trunk control.
The results indicate that activity of human phrenic motoneurones is organised such that it contributes to both posture and respiration during a task which repetitively challenges trunk posture.
Activation of the diaphragm occurs as a component of the postural response to single rapid upper limb movements in humans (Hodges et al. 1997). Postural responses of this type are thought to counteract the challenge to postural stability caused by reactive forces from the limb movement (e.g. Bouisset & Zattara, 1981; Friedli et al. 1984). In our previous study, we showed that electromyographic (EMG) activity of the diaphragm increased prior to the onset of activity of the muscle responsible for movement of the contralateral upper limb (i.e. feedforward) and that it occurred irrespective of the phase of respiration. This diaphragm EMG activity was associated with an increase in transdiaphragmatic pressure and an initial reduction in length of the costal diaphragm. Rapid upper limb movement of the type investigated previously has a brief duration (∼150-300 ms) and presents minimal disturbance to the respiratory function of the diaphragm. However, sustained tasks that interfere with the postural control of the trunk, as with repetitive movement of the upper limb (Zedka & Prochazka, 1997), present a challenge to the central nervous system (CNS) to co-ordinate respiratory and non-respiratory functions concurrently. Co-ordination of respiratory and non-respiratory functions of the diaphragm is also required during expulsive manoeuvres such as vomiting (e.g. Grelot & Miller, 1994), coughing (e.g. Leith et al. 1986), micturition, parturition and defaecation (e.g. Agostoni et al. 1960).
The diaphragm is thought to contribute to postural control of the trunk through elevation of the intra-abdominal pressure (Farfan, 1973; Grillner et al. 1978; Daggfeldt & Thorstensson, 1997). Thus, in response to repetitive upper limb movement the diaphragm may assist the mechanical support of the trunk by maintenance of intra-abdominal pressure at an elevated level through tonic (sustained) contraction or alternatively by phasic modulation of its activity in association with the reactive forces resulting from each movement. If respiration is to be maintained then either superimposed diaphragm activity must be modulated at the respiratory frequency or inspiration must be generated by other muscles.
The present study was designed to investigate these possibilities. The study aimed to evaluate, first, whether the diaphragm contracted throughout expiration during sustained repetitive limb movement. The second aim was to determine whether diaphragm activity was modulated phasically with the limb movement as well as respiratory activity. Finally, the contribution of the diaphragm to postural control was further investigated by evaluation of the influence of increasing peak acceleration of the upper limb on diaphragm activity. The results have previously been presented as an abstract (Hodges & Gandevia, 1999).
METHODS
Subjects
Successful studies were conducted on four male subjects of whom two were investigated on three separate occasions. The mean ±s.d. age, height and weight of the subjects were 34 ± 7 years, 1.79 ± 0.05 m and 81 ± 16 kg, respectively. Acceptable data could not be obtained from a fifth subject. Written informed consent was obtained and all procedures were approved by the institutional research ethics committee and conducted in accordance with the Declaration of Helsinki.
Electromyography
Recordings from the right costal diaphragm were made using bipolar fine-wire electrodes fabricated from multi-strand Teflon-coated stainless steel wire (7 strand, wire diameter 110 μm, A-M systems Inc., Everett, WA, USA). The two wires were gently twisted and the Teflon insulation was fused by heating. The cut ends of the wires were exposed (no additional insulation was removed to produce a receptive area of ∼300 μm2) and the tips (0.5-1 mm) were bent to form a hook. The electrodes were threaded into a hypodermic needle (0.70 mm × 50 mm) and then inserted into the diaphragm via the 7th or 8th intercostal space in the mid-clavicular line under the guidance of real-time ultrasound imaging using a 3 MHz vector array transducer (128XP/4, Acuson, Mountain View, CA, USA). Prior to needle insertion the diaphragm was visualised using the ultrasound transducer aligned parallel to the intercostal space to identify the approximate depth of the inner border of the diaphragm and to confirm that the selected site remained below the pleural reflection during deep inspiration (see De Troyer et al. 1997; Hodges et al. 1997). Approximately 0.5-1 ml lignocaine (lidocaine, 2% with adrenaline) was injected along the proposed path of insertion to the external surface of the diaphragm. The ultrasound reflection from the needle was visualised during the insertion and care was taken to maintain the bevel of the needle parallel to the diaphragm to increase the likelihood that the electrode tips would be located in the diaphragm. Prior to removal of the needle the electrode placement was confirmed by evaluation of the EMG signal. EMG activity recorded during inspiration, but not during forced expiration through pursed lips, confirmed the electrode's location in the diaphragm. EMG activity present during this task is indicative of activity from the internal intercostal muscle (Taylor, 1960). Following needle removal the location of the electrode was again confirmed from the EMG signal. Surface EMG electrodes (1 cm diameter, Ag-AgCl discs) were placed over deltoid (anterior and posterior portions) and erector spinae muscles adjacent to the L3 spinous process. A ground electrode was placed on the right shoulder.
Upper limb movement
Angular displacement of the upper limb about the shoulder in the sagittal plane was measured with a potentiometer attached to a light-weight bar strapped to the wrist with the axis of rotation aligned to the approximate centre of rotation of the glenohumeral joint. Motion of the upper limb was displayed on an oscilloscope along with markers to indicate the required range of motion to the subject. Subjects were instructed to maintain the elbow in full extension.
Respiratory phase
Expansion of the ribcage was monitored with an inductance plethysmograph placed around the chest at the level of the nipples. Preliminary studies identified a delay of less than 14 ms between the onset of ribcage motion measured with this device and the onset of inspiratory airflow at the mouth. The signal of ribcage motion measured with the inductance plethysmograph was affected to a small extent by movement of the upper limb (see Fig. 1). It was impossible to determine whether this was due to artefact or to real changes in volume of the thorax and no inference was drawn from this change in ribcage diameter associated with the upper limb movement.
Figure 1. Raw EMG and movement data from a typical subject before, during and after rapid upper limb movements.
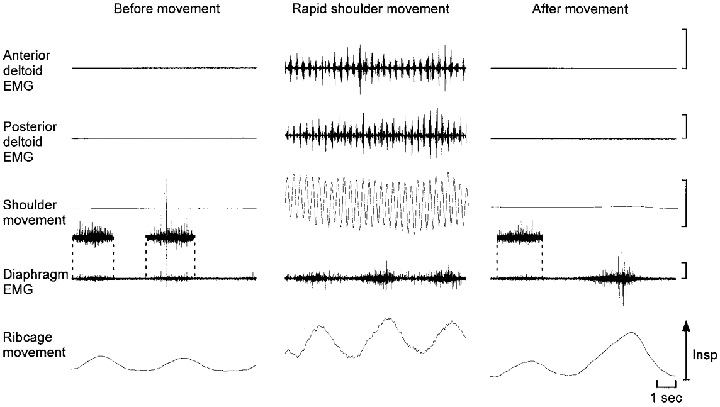
Subjects maintained quiet respiration prior to movement. In the post-movement period subjects were instructed to breath normally followed by a deep inspiration with deliberate expiration through pursed lips. EMG activity recorded from the diaphragm intramuscular electrodes during inspiration but not forced expiration (final panel) indicates that the electrode remained in the diaphragm. In the first and third panels the diaphragm EMG during inspiration is presented at three times the amplification. Note that the diaphragm EMG activity during repetitive movement of the upper limb as fast as possible is modulated with respiration and persists during expiration. Insp, inspiration. EMG calibration, 500 μV. Upper limb movement range, −15 to 15 deg.
Procedure
Subjects stood relaxed with the feet placed shoulder-width apart with the left arm attached to the potentiometer. Each trial commenced with three ‘quiet’ breaths (normal breathing, no limb movement) followed by a period of limb movement. Subjects performed unilateral upper limb motion in the sagittal plane between 15 deg flexion and 15 deg extension ‘as fast as possible’ for at least 10 s. This motion creates an anteroposterior and rotary perturbation to the trunk. After the limb movement three further quiet breaths were recorded followed by two large breaths with forced expiration through pursed lips to confirm that the needle remained in the diaphragm. Respiration was maintained throughout the upper limb movement. In separate trials the same procedure was performed except that the subjects were instructed to hold their breath at their end-expiratory level and to move their upper limb ‘as fast as possible’ for 10 s without breathing.
Additional experiments
Increasing frequency of movement
Upper limb movement was performed between 15 deg flexion and 15 deg extension starting at 1 Hz and increasing to maximal speed (approximately 3 Hz).
Increasing amplitude of movement
Upper limb movement performed at 1 Hz starting with movement between 5 deg flexion and 5 deg extension and the amplitude of movement increased to between 15 deg flexion and 15 deg extension.
Altered body position
Upper limb movement was performed in an identical manner to the main trials except that the subject was seated without back support but with the feet in contact with the floor.
Data analysis
Diaphragm EMG was recorded and differentially amplified with a gain of 10000–50000, bandpass filtered from 53 Hz to 3 kHz and sampled at 5 kHz. EMG data from the deltoid and erector spinae muscles were amplified 1000–50000 times, bandpass filtered from 53 Hz to 1 kHz and sampled at 2 kHz. Data were sampled with the use of Spike2 software (Cambridge Electronic Design, Cambridge, UK) and converted into a format suitable for signal processing with Matlab version 5.1 software (MathWorks, Natick, MA, USA), which was used for all analyses of EMG amplitude and frequency characteristics.
For comparison of the magnitude of diaphragm EMG between phases of the respiratory cycle, the root mean square (r.m.s.) EMG amplitude was calculated for 1 s epochs at the end of the inspiratory and expiratory phases. The r.m.s. value of baseline noise was subtracted from the r.m.s. EMG amplitude and all values were expressed as a percentage of the level recorded during quiet inspiration. A one-way ANOVA and Duncan's multiple-range test were used to compare EMG amplitude between respiratory phases. The relationship between upper limb acceleration and diaphragm or erector spinae EMG amplitude in the trials with upper limb movement at increasing frequency was evaluated by calculation of Pearson's correlation coefficient (r) between peak upper limb acceleration for consecutive repetitions of upper limb movement and the corresponding r.m.s. EMG for each muscle for a 100 ms epoch. For initial evaluation of the relationship between diaphragm EMG and upper limb movement the signals were averaged over multiple trials with alignment of the traces to the onset of upper limb flexion.
The relationship between the EMG and movement data was also analysed in the frequency domain. The power spectral density of the auto-correlations of the EMG and movement signals was calculated to identify the frequency of EMG bursts and the frequency of upper limb and ribcage motion. A requirement of parametric analysis in the frequency domain is that data are stationary (i.e. the signal must be random with consecutive segments non-significantly different from each other). To remove any non-stationarity from the data due to low-frequency drift, and to remove any movement artefact, the EMG data were high-pass filtered at 100 Hz (4th order zero-lag Butterworth filter) and then rectified and low-pass filtered at 30 Hz. The EMG, angular displacement of the upper limb and movement of the ribcage were then re-sampled at 1000 Hz so that all data could be directly compared with identical resolution for the Fourier transform analysis. Spectral analysis was performed using a Hanning window with no overlap. In the present study the high-frequency components within the multi-unit EMG were removed and only the low-frequency pattern of EMG bursts in association with the upper limb movement was evaluated. Analysis of the filtered and rectified EMG signal using a runs test on consecutive 1 s data intervals indicated that the data satisfied the conditions of stationarity (Bendat & Piersol, 1966). An additional factor that supports the validity of this analysis is that many of the findings were confirmed by analysis of data averaged from the onset of the movement (see Fig. 3). For comparison of the magnitude of the power spectral densities between muscles and subjects the data were normalised. The peaks in power spectra were expressed as a percentage of the maximal peak to indicate the relative distribution of the power at each frequency.
Figure 3. Power spectral densities and averaged data.
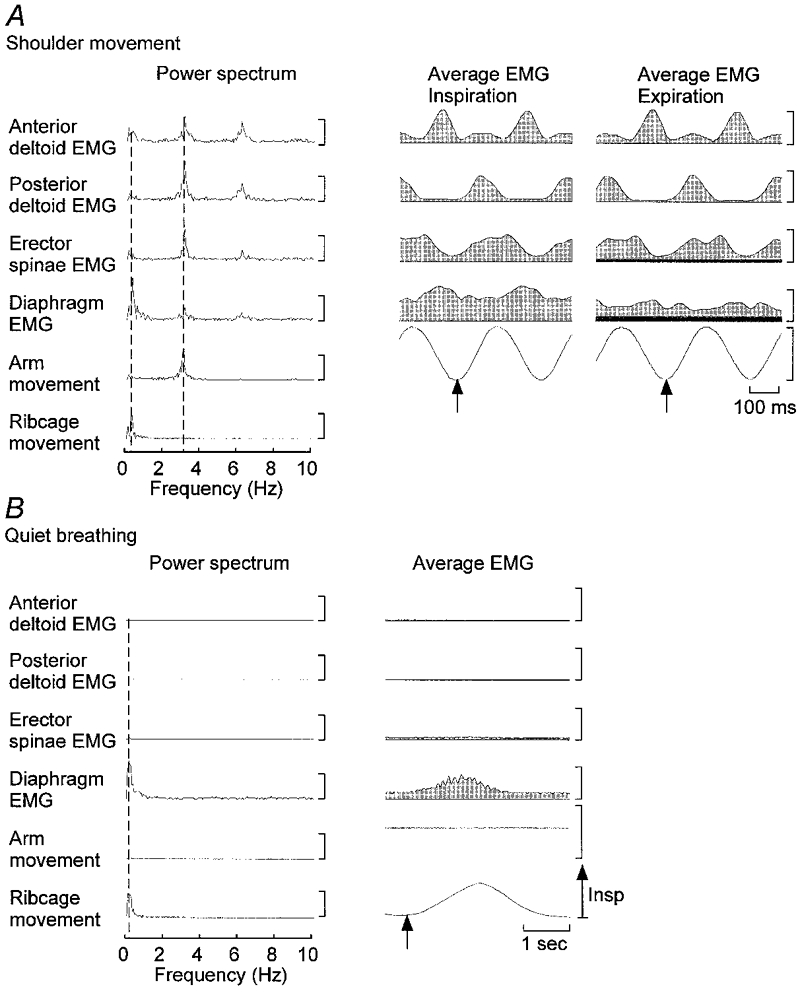
The power spectrum and averaged data are presented for a representative subject for breathing with upper limb movement (A) and breathing without movement (B). The power spectra in A and B were calculated for 20 s periods of movement with breathing and breathing without movement, respectively, for a different subject to that presented in Figs 1 and 2. The vertical dashed lines indicate the frequencies of respiration (left) and upper limb movement (right) in A and the frequency of respiration in B. In A note the peak in the upper limb movement signal at approximately 3 Hz and the corresponding peaks in all EMG signals at this frequency and the smaller peaks at twice the movement frequency. In addition note the peak in the ribcage and diaphragm signals at the respiratory frequency of approximately 0.4 Hz. In B peaks of the diaphragm and ribcage movement signals are present at the respiratory frequency. Averaged data were aligned to the onset of upper limb flexion in A and to the onset of inspiration in B, as denoted by the arrows. The background levels of diaphragm and erector spinae EMG activity are shown by dark grey region presented in the average data for the expiratory trials. Vertical calibrations for power spectra: anterior deltoid EMG, 2 μV2; posterior deltoid EMG, 4 μV2; erector spinae EMG, 2.5 μV2; diaphragm EMG, 12.5 μV2; upper limb movement, 12 deg2; ribcage signal, 2 V2. Vertical calibrations for the averaged EMG data: anterior deltoid, 10 μV; posterior deltoid, 20 μV; erector spinae, 5 μV; diaphragm, 20 μV. Upper limb movement range: −15 to 15 deg.
In addition, we evaluated relationship between signals (e.g. arm movement and diaphragm EMG) in terms of the similarity in frequency content between pairs of signals by calculation of the coherence. Coherence provides a formal measure of the correlation between the two signals in the frequency domain. A coherence of 1 indicates that the phase shift between the waveforms at a given frequency is constant and the amplitude of the signals at that frequency has a constant ratio (Bendat & Piersol, 1966). In general terms, coherence is a measure of the presence of a constant temporal (phase-locked) and spatial relationship between the phasic changes in two signals. The coherence was calculated from the cross-spectral density between two signals normalised by the power spectral density of each waveform. Throughout the text the coherence, frequency and amplitude data are presented as means ±s.d. across subjects (n = 4).
RESULTS
General observations
Figure 1 shows the diaphragm EMG recorded with intramuscular wires and the movement traces from a typical subject before, during and after a period of repeated movement of the upper limb as fast as possible between 15 deg flexion and 15 deg extension. In contrast to quiet respiration in relaxed standing, diaphragm EMG activity was present during the expiratory phase of the respiratory cycle when rapid upper limb movement was performed. During upper limb movement there was also clear modulation of diaphragm activity in phase with respiratory movement of the ribcage and clear modulation of deltoid and erector spinae EMG with movement of the upper limb. However, further analysis was required to determine whether there was movement-related modulation of diaphragm EMG activity (see below). The presence of diaphragm EMG activity during inspiration, but not during forced expiration through pursed lips, after the upper limb movement confirmed that the electrodes had remained in the diaphragm (see Methods). Acceptable recordings for up to several hours were obtained from approximately 75% of the electrodes inserted into the diaphragm. One or two electrode insertions were required for each subject. No side-effects other than mild pain and bruising occurred.
The level of diaphragm EMG activity during the last second of expiration when the subjects stood without moving their upper limb was less than 1% of that recorded during inspiration. When repetitive movement of the upper limb was performed this increased to 88.2 ± 24.3% (mean ±s.d.) of the diaphragm EMG magnitude during relaxed inspiration (P < 0.05) (Fig. 2C and D). In addition, the diaphragm EMG magnitude recorded during inspiration with repetitive upper limb movement increased to 153 ± 104% of the resting inspiratory magnitude (Fig. 2D).
Figure 2. Diaphragm EMG activity during movement with and without breathing.
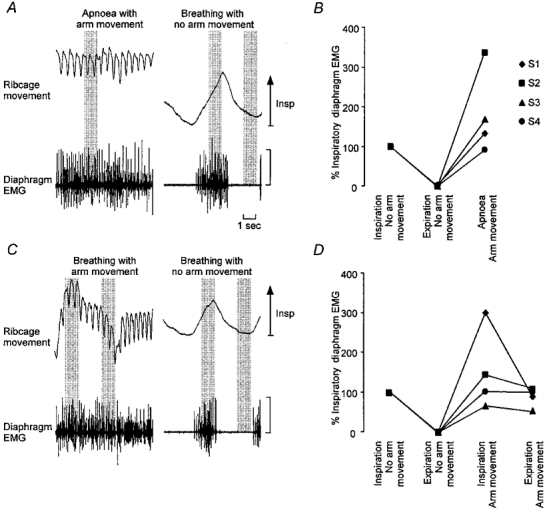
Raw diaphragm EMG from a typical subject during upper limb movement with apnoea and breathing is presented in A and C, respectively. Diaphragm r.m.s. EMG amplitudes for all subjects expressed as a percentage of the inspiratory r.m.s. EMG during upper limb movement with apnoea and breathing are presented in B and D, respectively. The 1 s segments selected for calculation of the r.m.s. EMG amplitude are indicated by the shaded vertical bars. Note the presence of diaphragm EMG activity during expiration with upper limb movement and when movement was performed without breathing. EMG calibration, 1 mV. Symbols indicate different subjects (S1–4).
Rapid repetitive movement of the upper limb during apnoea (i.e. breath holding at end-expiration prior to upper limb movement) also activated the diaphragm (Fig. 2a and B). This activity was 182 ± 108% of that during inspiration at rest (Fig. 2), although there was large variation between subjects.
Frequency analysis
The frequency of upper limb movement, identified as the frequency with peak power for the upper limb movement signal, was 2.87 ± 0.32 Hz across subjects (Fig. 3). The peak power for the anterior and posterior deltoid EMG was at 2.93 ± 0.37 Hz for both muscles. Smaller peaks were identified at twice the movement frequency in all subjects. These peaks partly resulted from small bursts of deltoid EMG at twice the movement frequency and partly because the EMG modulation was not precisely sinusoidal and therefore peaks occurred at the harmonic frequencies.
Due to motion of the ribcage with rapid upper limb movement, the frequency spectrum from the signal of ribcage movement had a peak at the movement frequency (2.81 ± 0.37 Hz) with an amplitude of 50.8 ± 37.6% of the maximum peak, which occurred at the respiratory frequency of 0.31 ± 0.15 Hz. While the majority of the power of the frequency spectrum for the diaphragm EMG occurred at the respiratory frequency (0.36 ± 0.09 Hz), a peak also occurred at the movement frequency (2.87 ± 0.30 Hz). The magnitude of this peak at the frequency of movement was 77.8 ± 44.3% of that at the respiratory frequency. This finding was consistent for all subjects. Similar to the deltoid EMG, smaller peaks were identified at twice the movement frequency in the diaphragm EMG signal. In trials in which no movement of the upper limb was performed all power in the diaphragm EMG signal occurred at the respiratory frequency (Fig. 3b). Conversely, in trials in which upper limb movement was performed without breathing, a peak was isolated to the movement frequency with no respiratory frequency peak. In all subjects the peak frequency in erector spinae occurred at the movement frequency (2.95 ± 0.45 Hz), but in one a small peak was present at the respiratory frequency (Fig. 3a).
With the selective multi-unit recordings from the costal diaphragm it was difficult to identify accurately the temporal characteristics of the burst of diaphragm EMG activity from single movements of the upper limb. Thus, trials were averaged by aligning them to the onset of upper limb flexion (Fig. 3). The averaged pattern of activity of each muscle was consistent with that identified from the power spectra (compare left and right panels in Fig. 3a and B). The averaged diaphragm EMG exposed either a single or double burst of activity that was in-phase with the burst of anterior deltoid activity. Diaphragm EMG between bursts was greater during inspiration than expiration. The presence of continuous diaphragm EMG during expiration indicates that the diaphragm was activated tonically in addition to the movement-related modulation in activity. All subjects showed a movement-related burst of diaphragm EMG with averaging, while some showed two bursts per cycle of upper limb movement (particularly during expiration).
As shown in Fig. 4 (left panel) for a typical subject and the group, the EMG signals for all muscles were highly coherent with the upper limb movement at the frequency of movement. Coherence values ranged from 0.56 ± 0.11 for the diaphragm to 0.80 ± 0.1 for the posterior deltoid. The greatest coherence between EMG signals occurred at the movement frequency (right panels) with, predictably, the highest coherence between the anterior and posterior deltoid (0.9 ± 0.04). Only the diaphragm EMG was consistently coherent with ribcage motion at the respiratory frequency (0.58 ± 0.21, Fig. 4 middle panels). However, in two subjects a peak coherence of 0.57 ± 0.01 (mean for two subjects) was identified between motion of the ribcage and erector spinae EMG signals at the respiratory frequency.
Figure 4. Coherence analysis.
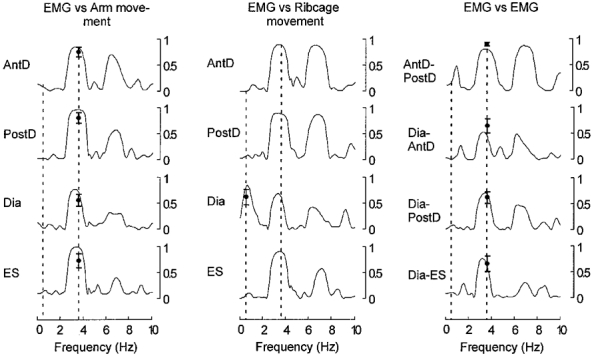
Coherence between signals are presented for a typical subject along with the group mean recorded at the movement frequency (vertical dashed line at ≈3.5 Hz) along with the group mean for the diaphragm- ribcage coherence at the respiratory frequency (vertical dashed line at ≈0.4 Hz). Coherence approached 1 for all muscles with movement and between muscles at the movement frequency. At the respiratory frequency diaphragm EMG is the only signal coherent with the ribcage motion at the respiratory frequency. Abbreviations: AntD, anterior deltoid; Dia, diaphragm; ES, erector spinae; PostD, posterior deltoid.
Factors affecting the activation of the diaphragm during repetitive upper limb movement
To investigate whether contraction of the diaphragm during limb movement was related to the control of forces associated with the movement, subjects moved the upper limb with gradually increasing acceleration. The level of diaphragm EMG activity was positively correlated with peak acceleration recorded during repetitive upper limb movements that began at approximately 1 Hz and increased in frequency until they were as rapid as possible (r = 0.69 to 0.88, P < 0.05) (Fig. 5). A similar relationship was identified for erector spinae (r = 0.84 to 0.95, P < 0.05). In additional trials (n = 4), the repetitive movement of the upper limb was performed at constant frequency with increasing amplitude. The relationship between peak upper limb acceleration and diaphragm or erector spinae EMG was similar to the trials with increasing frequency.
Figure 5. Relationship between trunk muscle EMG and acceleration of the upper limb.
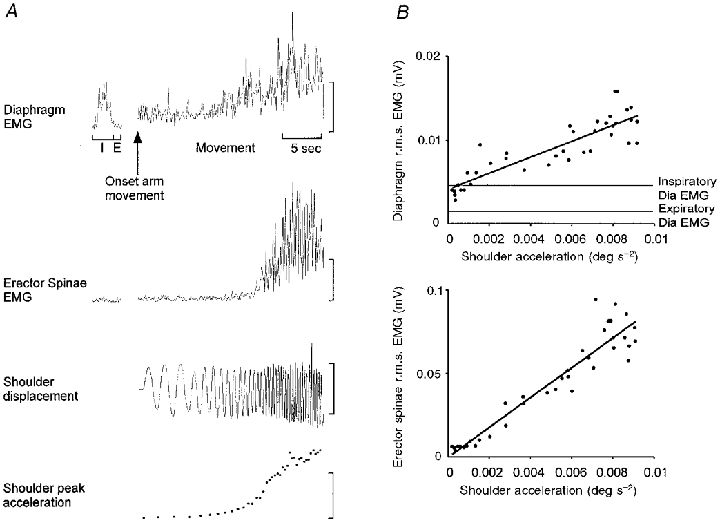
A, root mean squared EMG amplitude calculated for 100 ms epochs of diaphragm and erector spinae are presented for a trial in which the frequency of upper limb movement increased from approximately 1 Hz to movement performed as fast as possible. Root mean squared EMG activity during inspiration and expiration preceding movement is shown to the left. EMG amplitude increased as the frequency of voluntary movement increased. I, inspiration; E, expiration. Vertical calibrations: diaphragm EMG, 25 μV; erector spinae EMG, 1 mV; shoulder displacement range, 15 to −15 deg; shoulder peak acceleration, 0.006 deg s−2. B, correlation between peak upper limb acceleration and r.m.s. EMG of diaphragm and erector spinae. Mean inspiratory and expiratory r.m.s. EMG levels are shown for comparison. There is a linear relationship between peak upper limb acceleration and EMG amplitudes (P < 0.001).
To determine whether contraction of the diaphragm depended upon the posture of the subject, two subjects performed rapid upper limb movements as in the main study, but during sitting without back support. The pattern of diaphragm EMG was identical for both postures (Fig. 6). Diaphragm EMG occurred throughout the respiratory cycle. Spectral analysis indicated peaks of EMG that occurred in all muscles at the movement frequency, with an additional peak in the diaphragm EMG at the respiratory frequency (Fig. 6).
Figure 6. Diaphragm activity during rapid repetitive upper limb movement while sitting.
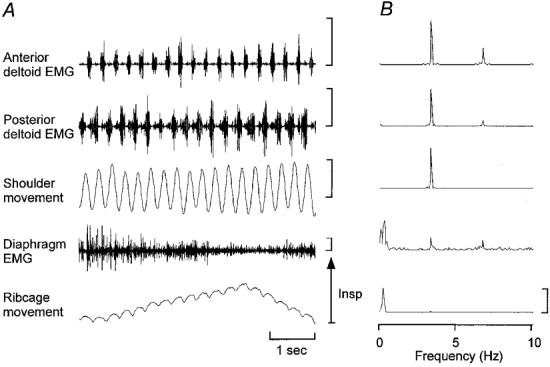
Representative raw EMG and movement signals for upper limb movement during sitting (A) and corresponding power spectral densities (B). Note that similar to the data presented in Fig. 3 there are peaks in the diaphragm EMG power spectrum at the frequencies of respiration and upper limb movement. EMG calibration, 500 μV. Upper limb movement range is −15 to 15 deg. Vertical calibrations for power spectra: anterior deltoid EMG, 1 μV2; posterior deltoid EMG, 1 μV2; upper limb movement, 6 deg2; diaphragm EMG, 15 μV2; ribcage signal, 1 V2.
DISCUSSION
The present results show that during continuous movement of a limb, activity of the diaphragm was sustained and modulated at both the respiratory and limb movement frequencies. The modulation of diaphragm EMG at two frequencies was identified in the frequency components of the EMG, and was corroborated by analysis of data from averaged trials. This activation of the diaphragm may assist mechanical stabilisation of the trunk in addition to the maintenance of ventilation.
Repetitive upper limb movement modified costal diaphragm activity in two ways that were unrelated to respiration. First, unlike respiration ‘at rest’ (Agostoni, 1964), the diaphragm contracted tonically throughout the respiratory cycle. If accompanied by abdominal muscle activity, this would elevate intra-abdominal pressure. This finding is consistent with the sustained elevation of transdiaphragmatic pressure during lifting (Hemborg et al. 1985) and tonic activation of the crural diaphragm throughout respiration during upper limb cycling in people with paraplegia (Sinderby et al. 1992). However, unlike previous reports of non-respiratory functions of the diaphragm, phasic modulation of diaphragm activity at the frequency of limb movement was superimposed on its respiratory and tonic activation. A model of several features of the diaphragm EMG is presented in Fig. 7A. Although other strategies to simplify the co-ordination between breathing and postural control have been described, such as glottis closure or expiration with lifting to maintain abdominal pressure (Hemborg et al. 1985), respiration did not appear to be compromised during the limb movement task.
Figure 7. Integration of respiratory and ‘postural’ inputs to phrenic motoneurones.
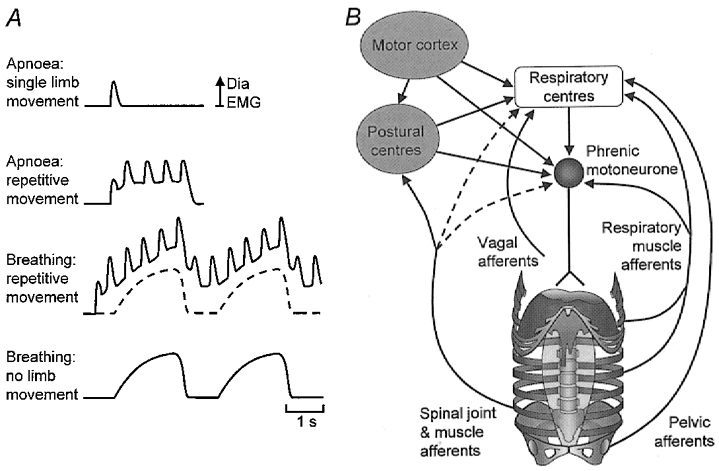
A, diaphragmatic representation of the four types of diaphragm EMG that have been identified (shown as rectified and low-pass filtered EMG). The upper trace shows a single burst of diaphragm EMG that occurs with single repetitions of upper limb flexion. In the second trace there is tonic activity in addition to superimposed phasic bursts of diaphragm EMG with repetitive upper limb movement. The third trace shows summation of inspiratory diaphragm EMG with the combined tonic and phasic modulation with repetitive upper limb movement. The lowest trace and the dashed line in the third trace show inspiratory diaphragm EMG. B, multiple inputs to the phrenic motoneurones arising from respiratory centres, (non-respiratory) higher centres and a variety of peripheral sources. Although the inspiratory drive arises predominantly from the ponto-medullary respiratory centres, ‘postural’ inputs may arise from higher centres, but during predictable repetitive movements some inputs may be reflex in origin and act at either spinal or supraspinal levels. Dashed lines indicate hypothesised inputs that have not been confirmed in the literature. (See Discussion for detail and references.)
In contrast to the changes in diaphragm activity, other trunk muscles responded simply with phasic modulation at the frequency of limb movement. Such activation has been recorded in trunk muscles during rapid and/or repetitive limb movements (Bouisset & Zattara, 1981; Friedli et al. 1984; Aruin & Latash, 1995; Hodges & Richardson, 1997; Zedka & Prochazka, 1997; Hodges et al. 1999). In a recent study of single repetitions of upper limb flexion, a burst of erector spinae EMG activity was initiated prior to limb movement and moved the trunk to prepare the spine for the reactive forces due to limb movement (Hodges et al. 1999).
Is the activity of the diaphragm related to the dynamic forces from movement of the limb? Due to the multi-segmented structure of the spinal column the reactive forces associated with upper limb movement may disrupt the relationship between adjacent spinal vertebrae (Panjabi et al. 1989), and the orientation of the spine (Hodges et al. 1999). The diaphragm may contribute to control of these factors either directly via increased intra-abdominal pressure (Bartelink, 1957; Grillner et al. 1978; Cresswell et al. 1994; Daggfeldt & Thorstensson, 1997) or by prevention of displacement of the abdominal contents (Farfan, 1973).
Raised intra-abdominal pressure may extend the lumbar spine and thus assist in the control of spinal orientation (Bartelink, 1957; Grillner et al. 1978; Daggfeldt & Thorstensson, 1997). Consistent with this proposal diaphragm activity increases in quadriplegic patients when positioned in trunk flexion (Sinderby et al. 1992) and trunk extensor activity decreases when abdominal pressure is increased by diaphragm contraction (Wedin et al. 1987). The rapid alternating limb movement in the present study would generate reactive forces in alternating directions. However, the phasic periods of increased diaphragm EMG in conjunction with limb movements were out-of-phase with those of the trunk extensor (erector spinae) and are, therefore, inconsistent with a contribution of the diaphragm to the control of trunk flexion. However, the phasic changes in diaphragm activity at the frequency of upper limb movement may be related to the rotational forces generated by the unilateral upper limb movement.
Translation and rotation between individual vertebrae are most effectively controlled by muscles with direct attachment to them (Bergmark, 1989). Although transversus abdominis and, to some extent, obliquus abdominis internus attach directly to the vertebrae via the thoracolumbar fascia, their ability to influence segmental motion is dependent on abdominal pressure. Maintenance of abdominal pressure by activity in diaphragm and abdominal muscles would minimise abdominal muscle shortening and maintain muscle force. Thus, tonic diaphragm activity in the present study may control segmental motion indirectly.
Given that diaphragmatic contraction can contribute to trunk control, the present results provide further confirmation of this function. First, the phasic changes in diaphragm EMG were phase- and amplitude-locked to the arm movement. Secondly, the amplitude of diaphragm EMG was linearly related to the peak acceleration of the limb and thus to the forces transmitted to the spine. Diaphragm activity was also not present with single movements of small distal segments of the upper limb (Hodges et al. 1997). Thirdly, the ‘postural’ activation of the diaphragm was unchanged when subjects performed an identical task when sitting with the trunk unsupported. The contribution of the diaphragm activity to trunk control is further strengthened by the previous findings that the response to a single upper limb movement began prior to the movement and therefore, could not result from afferent input due to the movement (Hodges et al. 1997).
How are the respiratory and ‘postural’ functions of the diaphragm organised? Our data suggest that diaphragm EMG has three components: increased tonic activity, phasic modulation with respiration and phasic modulation with movement (Fig. 7a). Two questions must be considered regarding the organisation of these components. First, where do the inputs arise from? Secondly, where are they integrated? There are at least four relevant inputs to phrenic motoneurones (Fig. 7b): descending drive from ponto-medullary respiratory centres, drives from non-respiratory supraspinal structures including the motor cortex, spinal interneuronal networks, and inputs from peripheral receptors. The respiratory component of diaphragm activity is derived largely from the respiratory centres (Monteau & Hilaire, 1991). In contrast there are several sources of postural input. For single repetitions of voluntary limb movement the perturbation to trunk stability is predictable and is controlled in a feedforward manner (Belen'kii et al. 1967; Bouisset & Zattara, 1981; Aruin & Latash, 1995; Hodges & Richardson, 1997; Hodges et al. 1999). This feedforward response recruits the diaphragm (Hodges et al. 1997) and is generated by supraspinal structures (including the cortex, basal ganglia and cerebellum) in parallel with the command for limb movement (Massion, 1992). Correspondingly, there are corticospinal projections to the human diaphragm (Gandevia & Rothwell, 1987) and cortical projections to the medullary respiratory centres (Bassal et al. 1981). The organisation of the postural responses associated with repetitive movement may rely on spinal reflexes, at least for the trunk extensor muscles (Zedka & Prochazka, 1997).
Several peripheral inputs modulate phrenic activity and thus may contribute to the postural response (Fig. 7b). Studies in humans (Banzett et al. 1981; Reid et al. 1985; Butler et al. 1995) and animals (Gill & Kuno, 1963; Corda et al. 1965; Duron & Caillol, 1973) have indicated that phrenic activity may be modulated by input from homonymous muscle afferents (muscle spindle and tendon organs). Other studies have found this input to be weak or absent, perhaps due to the small number of muscle spindles in the diaphragm (Sant'Ambrogio & Widdicombe, 1965). Modulation of diaphragm activity also occurs with input from the abdominal and lower intercostal muscles (Decima & von Euler, 1969; Remmers, 1973) and joint mechanoreceptors (De Troyer, 1997).
An additional input from the postural perturbation may arise in the vestibular apparatus. There is conflicting evidence from animal experiments with respect to the influence of vestibular input on phrenic activity (Massion, 1976; Rossiter et al. 1996). Regardless, this explanation for the changes in diaphragm activity is probably unlikely in our studies, as the stimulus to the vestibular apparatus would be small. Finally, other visceral (see Monteau & Hilaire, 1991) and pelvic (Miller, 1924; Yamamoto et al. 1961) inputs can reflexly activate the diaphragm and abdominal muscles.
Integration of the multiple postural and respiratory inputs to the phrenic motoneurone could theoretically occur at the level of the medullary respiratory centres (see Shannon, 1986), supramedullary sites or at a spinal level (Fig. 7b). Although spinal interneurones (Aminoff & Sears, 1971; Kirkwood et al. 1988) and motoneurones (Sears, 1964) integrate cortical and segmental inputs, this does not exclude involvement of supraspinal centres. Furthermore, it is not known whether the tonic and phasic movement-related drives identified in this study are organised at the same site in the CNS.
Acknowledgments
We thank Dr Richard Fitzpatrick for helpful discussion regarding signal processing and data analysis and Jane Butler for assistance with the data collection. Financial support was provided by the National Health and Medical Research Council of Australia.
References
- Agostoni E. Action of the respiratory muscles. In: Fenn WO, Rahn H, editors. Handbook of Physiology. Vol. 1. Washington: American Physiological Society; 1964. pp. 377–386. [Google Scholar]
- Agostoni E, Sant'ambrogio G, Delportillocarrasco H. Electromyography of the diaphragm in man and transdiaphragmatic pressure. Journal of Applied Physiology. 1960;15:1093–1097. doi: 10.1152/jappl.1960.15.6.1093. [DOI] [PubMed] [Google Scholar]
- Aminoff MJ, Sears TA. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. The Journal of Physiology. 1971;215:557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Experimental Brain Research. 1995;103:323–332. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Inbar GF, Brown R, Goldman M, Rossier A, Mead J. Diaphragm electrical activity during negative lower torso pressure in quadriplegic men. Journal of Applied Physiology. 1981;51:654–659. doi: 10.1152/jappl.1981.51.3.654. [DOI] [PubMed] [Google Scholar]
- Bartelink DL. The role of intra-abdominal pressure in relieving the pressure on the lumbar vertebral discs. Journal of Bone and Joint Surgery. 1957;39B:718–725. doi: 10.1302/0301-620X.39B4.718. [DOI] [PubMed] [Google Scholar]
- Bassal M, Bianchi AL, Dussardier M. Effets de la stimulation des structures nerveuses centrales sur l'activité des neurones respiratoires chez le chat. Journal de Physiologie. 1981;77:779–795. [PubMed] [Google Scholar]
- Belen'kii V, Gurfinkel VS, Palt'sev Y. Elements of control of voluntary movements. Biofizika. 1967;12:135–141. [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Measurement and Analysis of Random Data. New York: John Wiley and Sons; 1966. [Google Scholar]
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthopedica Scandinavica. 1989;60:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. A sequence of postural adjustments precedes voluntary movement. Neuroscience Letters. 1981;22:263–270. [Google Scholar]
- Butler JE, Mckenzie DK, Crawford MR, Gandevia SC. Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. The Journal of Physiology. 1995;487:273–281. doi: 10.1113/jphysiol.1995.sp020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M, van Euler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. The Journal of Physiology. 1965;178:161–177. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell AG, Oddsson L, Thorstensson A. The influence of sudden perturbations on trunk muscle activity and intra-abdominal pressure while standing. Experimental Brain Research. 1994;98:336–341. doi: 10.1007/BF00228421. [DOI] [PubMed] [Google Scholar]
- Daggfeldt K, Thorstensson A. The role of intra-abdominal pressure in spinal unloading. Journal of Biomechanics. 1997;30:1149–1155. doi: 10.1016/s0021-9290(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Decima EE, Von Euler C. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiologica Scandinavica. 1969;76:148–158. doi: 10.1111/j.1748-1716.1969.tb04459.x. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Role of joint receptors in modulation of inspiratory intercostal activity by rib motion in dogs. The Journal of Physiology. 1997;503:445–453. doi: 10.1111/j.1469-7793.1997.445bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, Mckenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. American Journal of Respiration and Critical Care Medicine. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- Duron B, Caillol MC. Investigation of afferent activity in the intact phrenic nerve with bipolar electrodes. Acta Neurobiologiae Experimentalis. 1973;33:428–432. [PubMed] [Google Scholar]
- Farfan HF. Mechanical Disorders of the Low Back. Philadelphia: Lea and Febiger; 1973. [Google Scholar]
- Friedli WG, Hallet M, Simon SR. Postural adjustments associated with rapid voluntary arm movements 1. Electromyographic data. Journal of Neurology, Neurosurgery and Psychiatry. 1984;47:611–622. doi: 10.1136/jnnp.47.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. The Journal of Physiology. 1987;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PK, Kuno M. Excitatory and inhibitory actions on phrenic motoneurones. The Journal of Physiology. 1963;168:274–289. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelot L, Miller AD. Vomiting – its ins and outs. News in Physiological Sciences. 1994;9:142. [Google Scholar]
- Grillner S, Nilsson J, Thorstensson A. Intraabdominal pressure changes during natural movements in man. Acta Physiologica Scandinavica. 1978;103:275–283. doi: 10.1111/j.1748-1716.1978.tb06215.x. [DOI] [PubMed] [Google Scholar]
- Hemborg B, Moritz U, Löwing H. Intra-abdominal pressure and trunk muscle activity during lifting. IV. The causal factors of the intra-abdominal pressure rise. Scandinavian Journal of Rehabilitation Medicine. 1985;17:25–38. [PubMed] [Google Scholar]
- Hodges PW, Butler JE, Mckenzie D, Gandevia SC. Contraction of the human diaphragm during postural adjustments. The Journal of Physiology. 1997;505:539–548. doi: 10.1111/j.1469-7793.1997.539bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Cresswell AG, Thorstensson A. Preparatory trunk motion precedes upper limb movement. Experimental Brain Research. 1999;124:69–79. doi: 10.1007/s002210050601. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Gandevia SC. Activation of the human diaphragm during a prolonged postural task. Proceedings of Australian Neuroscience Society. 1999;10:197. [Google Scholar]
- Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Experimental Brain Research. 1997;114:362–370. doi: 10.1007/pl00005644. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. The Journal of Physiology. 1988;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith DE, Butler JP, Sneddon SL, Brain JD. Cough. In: Mackelm PT, Mead J, editors. Handbook of Physiology. I. Washington: American Physiological Society; 1986. pp. 315–336. [Google Scholar]
- Massion J. Postural function of respiratory muscles. In: Duron B, editor. Respiratory Centres and Afferent Systems. Vol. 59. Paris: Colloques de l'INSERM; 1976. pp. 175–181. [Google Scholar]
- Massion J. Movement, posture and equilibrium: Interaction and coordination. Progress in Neurobiology. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Miller FR. Viscero-motor reflexes. II. American Journal of Physiology. 1924;71:84–89. [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Progress in Neurobiology. 1991;36:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Abumi K, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces. A biomechanical model. Spine. 1989;14:194–200. doi: 10.1097/00007632-198902000-00008. [DOI] [PubMed] [Google Scholar]
- Reid MB, Banzett RB, Feldman HA, Mead J. Reflex compensation of spontaneous breathing when immersion changes diaphragm length. Journal of Applied Physiology. 1985;58:1136–1142. doi: 10.1152/jappl.1985.58.4.1136. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Extra-segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. The Journal of Physiology. 1973;233:45–62. doi: 10.1113/jphysiol.1973.sp010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter CD, Hayden NL, Stocker SD, Yates BJ. Changes in outflow to respiratory pump muscles produced by natural vestibular stimulation. Journal of Neurophysiology. 1996;76:3274–3284. doi: 10.1152/jn.1996.76.5.3274. [DOI] [PubMed] [Google Scholar]
- Sant'ambrogio G, Widdicombe JG. Respiratory reflexes acting on the diaphragm and inspiratory intercostal muscle of the rabbit. The Journal of Physiology. 1965;180:766–779. doi: 10.1113/jphysiol.1965.sp007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. Some properties and reflex connexions of respiratory motoneurones of the cat's thoracic spinal cord. The Journal of Physiology. 1964;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R. Reflexes from respiratory muscles and costovertebral joints. In: Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing, sect. part 1. II. Baltimore: American Physiological Society, Waverly Press; 1986. pp. 431–447. exec. [Google Scholar]
- Sinderby C, Ingvarsson P, Sullivan L, Wickstrom I, Lindstrom L. The role of the diaphragm in trunk extension in tetraplegia. Paraplegia. 1992;30:389–395. doi: 10.1038/sc.1992.88. [DOI] [PubMed] [Google Scholar]
- Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. The Journal of Physiology. 1960;151:390–402. doi: 10.1113/jphysiol.1960.sp006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedin S, Leanderson R, Knutsson E. The effect of voluntary diaphragmatic activation on back lifting. Scandinavian Journal of Rehabilitation Medicine. 1987;20:129–132. [PubMed] [Google Scholar]
- Yamamoto S, Araki K, Kikuchi M. Abdominal muscle reflexes of pelvic nerve origin in cats. Experimental Neurology. 1961;4:345–357. doi: 10.1016/0014-4886(61)90061-9. [DOI] [PubMed] [Google Scholar]
- Zedka M, Prochazka A. Phasic activity in the human erector spinae during repetitive hand movements. The Journal of Physiology. 1997;504:727–734. doi: 10.1111/j.1469-7793.1997.727bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


