Abstract
Interneurones receiving excitatory input from group II muscle afferents of hindlimb nerves and located in the lower-lumbar (L6-L7) segments of the cat spinal cord were investigated using both extracellular and intracellular recording.
The interneurones were located mainly in the lateral parts of laminae IV-VII, dorsal and lateral to the main region in which interneurones with input from group I muscle afferents are located. Almost half the sample of interneurones (38 of 76) were characterized by an ipsilateral ascending projection within the lateral funiculus to the L4 level.
The most powerful group II excitation was produced by afferents of the quadriceps and deep peroneal muscle nerves (which discharged 70–80 % of extracellularly recorded neurones) while group II afferents of tibialis posterior, posterior biceps-semitendinosus and gastrocnemius soleus were also highly effective (discharging 45–55 % of extracellularly recorded neurones). A proportion of intracellularly recorded group II EPSPs were monosynaptic.
Seventy-five per cent of the extracellularly recorded interneurones were discharged by group II afferents of two or more muscle nerves and 43 % by afferents of three or more nerves.
Group I muscle afferents evoked small EPSPs in over one-quarter of the intracellularly recorded interneurones and virtually all were strongly excited by cutaneous afferents. Evidence of excitatory input from joint, interosseous and group III muscle afferents was also observed.
The properties of the interneurones are compared with those of others in the lumbosacral segments and the possibility that they may function as last-order premotor interneurones is discussed.
Apart from a weak monosynaptic projection to homonymous motoneurones, the reflex actions of muscle afferents in the group II range are mediated by spinal interneurones (for review see Jankowska, 1992). Several populations of interneurones with input from group II muscle afferents have now been investigated, but so far only one population of last-order pre-motor interneurones (i.e. interneurones connected directly to motoneurones) has been identified. These interneurones are located in midlumbar segments and have axons that project caudally to lower-lumbar levels where they produce excitation or inhibition of motoneurones (Edgley & Jankowska, 1987; Cavallari et al. 1987). In addition to their segmental inputs, midlumbar interneurones are also influenced by various descending motor pathways (Yates et al. 1989; Davies & Edgley, 1994) and by the central pattern generator circuits for stepping (Shefchyk et al. 1990). The midlumbar interneurones receive input predominantly from group II afferents of hip flexor, knee extensor and pretibial flexor muscles, a pattern of convergence which has led to the suggestion that they may be involved in triggering the transition from stance to swing during the step cycle (Edgley & Jankowska, 1987; Aggelopoulos et al. 1996). However, the midlumbar interneurones are relatively weakly excited by group II afferents of other hindlimb muscles such as knee flexors or ankle extensors, so the interneuronal circuits driven by signals from these muscles (Lundberg et al. 1987a) must be located in other segments of the lumbosacral enlargement.
Interneurones with powerful input from semitendinosus and gastrocnemius group II afferents have recently been discovered in the sacral segments but these appear not to make direct contact with motoneurones (Jankowska et al. 1993; Jankowska & Riddell, 1994; Maxwell et al. 1997). Although reflex circuits in the lower-lumbar segments have been extensively studied, most investigations have focused on interneurones in pathways from group I muscle afferents. These studies have included unidentified neurones located in the intermediate nucleus (e.g. Eccles et al. 1960; Hongo et al. 1966) and also functionally identified neurones interposed in pathways of reciprocal inhibition from group Ia muscle spindle afferents (Hultborn et al. 1971; Jankowska & Roberts, 1972b) or non-reciprocal inhibition from group Ib tendon organ afferents (e.g. Hongo et al. 1983; Harrison & Jankowska, 1985). Much less is known about interneurones with input from group II muscle afferents, although intracellular studies have revealed group II excitation in a small minority of interneurones with input dominated by group I muscle afferents (10 % of group Ib interneurones; Harrison & Jankowska, 1985) and there are also brief reports of interneurones powerfully excited by group II afferents but less, if at all, by group I afferents (Fukishima & Kato, 1975; Lundberg et al. 1987b; Harrison et al. 1994). In addition, recordings made from axons in the lateral funiculus at L6 indicate that the lower-lumbar segments contain a population of group II-activated interneurones with an ascending projection to the midlumbar segments (Harrison & Riddell, 1989).
The aim of the present study was to investigate the lower-lumbar segments for candidate pre-motor interneurones in group II reflex pathways. In view of the evidence that some group II-activated interneurones have an ascending projection to midlumbar levels, neurones with group II input were tested for a projection to the L4 segment. The search was concentrated on lateral aspects of the grey matter where group II muscle afferents of a range of hindlimb nerves have been found to evoke field potentials (see companion paper, Riddell & Hadian, 2000).
METHODS
Experiments were performed on 12 cats under deep general anaesthesia (chloralose; 70 mg kg−1i.v., maintained with further doses of 10 mg kg−1i.v. as required) in accordance with Home Office guidelines. The adequacy of the anaesthesia was verified throughout surgery by monitoring withdrawal reflexes, corneal reflexes, blood pressure and ECG. During recording, when the animals were paralysed with gallamine triethiodide (2–3 mg kg−1i.v., every 40 min) and artificially ventilated, the adequacy of anaesthesia was verified by monitoring the diameter of the pupils and continuously recording blood pressure. In addition, at least every 40 min, tests were made of the stability of blood pressure and heart rate when noxious stimuli were applied to the forepaws. At the end of the experiment, the animal was killed by administration of an overdose of sodium pentobarbital anaesthetic.
Other aspects of the preparation and much of the stimulation and recording procedures used are described in detail in the accompanying paper (Riddell & Hadian, 2000). Briefly, a range of hindlimb peripheral nerves were dissected and mounted on electrodes for electrical stimulation. These included: quadriceps (Q), posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius soleus (GS), plantaris (Pl), the caudal branch of sural (Sur), flexor digitorum longus (FDL, dissected free from the interosseous nerve, i.o.), tibialis posterior (TP), popliteus (Pop), deep peroneal (DP, i.e. tibialis anterior and extensor digitorum longus from which the mixed nerve branch to extensor digitorum brevis was removed), superficial peroneal (SP), saphenous (Saph) and the posterior nerve to the knee joint (J). The spinal cord was exposed by laminectomy from the fourth lumbar (L4) to sacral segments and at the level of the lowest thoracic (Th) segments.
Recordings were made from neurones in the L7 and S1 segments using glass microelectrodes filled with 2 M potassium citrate (1.5-2.5 μm tip diameter, 2–6 MΩ resistance) or, in three experiments, 2 % biocytin in 0.05 M Tris-HCl buffer containing 0.5 M potassium chloride. Neurones were searched for while tracking through the dorsal horn and the intermediate zone within regions in which monosynaptic field potentials evoked by group II afferents could be detected (Riddell & Hadian, 2000). A search was made for neurones discharged by electrical stimulation of muscle nerves at up to 5 times threshold (5T). The neurones were usually first recorded from extracellularly in order to establish the peripheral nerves by which they could be discharged and to test for an ascending projection. In order to identify interneurones with an ascending projection to the L4 level, stimuli (0.1 ms, up to 300 μA) were applied within the lateral funiculus at this level via a tungsten electrode. The electrode was inserted into the lateral funiculus, close to the border with the dorsal columns, to a depth of about 1.5-2.0 mm with its tip directed medially at an angle of 5 deg (Harrison & Jankowska, 1985). Where the latencies of responses to such stimuli were consistent with either an orthodromic or an antidromic origin (see Results), it was verified that the action potentials were conducted antidromically along an ascending axon by colliding them with orthodromic action potentials evoked synaptically by appropriately timed stimuli applied to a muscle nerve. Apparent collisions were examined to confirm that they occurred within the critical interval which was calculated after deducting 0.2 ms for the utilization time of the L4 stimulus. Neurones were also tested for an antidromic response to stimulation (0.2 ms current pulses of at least 500 μA and up to 1 mA applied transdurally) of the ipsilateral and contralateral lateral funiculi at Th13, irrespective of whether or not they were antidromically activated from L4. Neurones responding with antidromic impulses to such stimuli were excluded from the sample. An attempt was then made to penetrate the neurones, either in the same electrode track or in subsequent tracks 20–100 μm away. On successful penetration the absence of antidromic activation by thoracic stimuli and, if appropriate, antidromic responses to stimulation at L4 were confirmed.
Electrode tracks were reconstructed using marking electrodes left in situ at the end of the experiment as described in the accompanying paper (Riddell & Hadian, 2000). The locations of intracellularly recorded interneurones were then determined from information on the angle of the recording track in relation to the marking electrode and the depth of the recording along the track. In three experiments in which neurones were intracellularly labelled with biocytin, animals were perfused with a mixture of 1 % formaldehyde and 1 % glutaraldehyde in 0.1 M phosphate buffer introduced through the descending aorta. Serial sections of the spinal cord were cut on a vibratome and processed using an avidin-peroxidase reagent (Sigma). Mounted sections were examined and the labelled neurones partially reconstructed under a light microscope equipped with a camera lucida attachment.
RESULTS
Observations were made on a sample of 76 interneurones recorded from within the grey matter of the L6 and L7 segments, all of which were excited by group II afferents of one or more hindlimb muscle nerves. Fifty-five of the neurones were recorded from extracellularly, 28 were recorded from intracellularly and a small number (7) were recorded from both intra- and extracellularly. Seven neurones with group II input and an ascending projection beyond the lumbar enlargement (as determined by antidromic activation from Th13) were also encountered but are not considered further in this study.
Neurones projecting to L4
Interneurones excited by group II muscle afferents were tested for an ipsilateral ascending projection to the rostral L4 level by applying stimuli within the lateral funiculi. Nearly half of the sample of interneurones (37/76) were antidromically activated by such stimuli (thresholds of 10–300 μA, mean 170 μA) but since a search stimulus was usually applied at L4 while tracking for neurones, there may be a bias towards these interneurones in the sample.
Antidromic impulses evoked by stimulation at L4 were identified by their latency or by using the collision test. The minimal latency of an orthodromic response to such stimuli would involve (a) a delay for the electrical stimulus to generate an action potential (utilization time 0.2 ms; Jankowska & Roberts, 1972a), (b) time for axonal conduction between rostral L4 and the recording site (0.2-0.3 ms given a conduction distance of 20–30 mm and assuming a conduction velocity of 100 m s−1) and (c) a synaptic delay (0.3 ms). Impulses evoked at latencies of less than about 0.7 ms would therefore be too short to have been evoked orthodromically and can be considered antidromic (see Jankowska & Roberts, 1972a; Fern et al. 1988). Twelve of the 37 neurones were judged to be antidromically activated according to this criterion.
A further 25 interneurones responded to stimulation at L4 at latencies of 0.7 ms or more and for these neurones the nature of the response to the L4 stimulus was confirmed by establishing that collision between orthodromic and antidromic impulses occurred within the critical interval. Examples of recordings made from interneurones during tests for an ascending projection are shown in Fig 2C, Fig 3a and Fig 4D. In Fig. 2C, the top pair of records show an extracellularly recorded antidromic spike (arrow) evoked at a fixed short latency by stimulation at L4 and the middle pair of records illustrate the blocked antidromic spike subsequently recorded on intracellular penetration (see also Fig. 4D). Figure 3a shows an example of the collision test performed on an intracellularly recorded interneurone in which the spike-generating mechanism initially remained intact. Also shown in each of these figures is the lack of response to stimulation at the Th13 level.
Figure 2. Extracellular and intracellular recordings from an L4-projecting interneurone with excitatory input from group II muscle afferents.
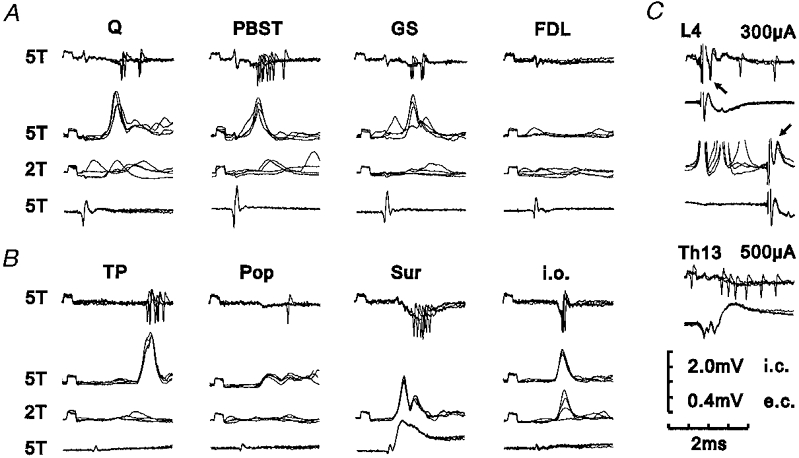
A and B, peripheral input from various muscle nerves and the Sur and i.o. nerves. Each panel of four records shows the effect of stimulation of the peripheral nerve indicated. The upper record of each panel is an extracellular recording of the discharges evoked by stimulation at 5T. The second record shows PSPs evoked by the same stimulation during intracellular recording, the third record shows the effect of reducing the stimulus strength to 2T and the lower record shows the afferent volley. In this and the following figures, negativity is downwards in extracellular and intracellular records from interneurones and upwards for records from the surface of the spinal cord. Note the lack of PSPs following stimulation of muscle nerves at 2T indicating that group II afferents are responsible for the PSPs and discharges evoked at 5T. C, tests of projection. Upper records of each pair are extracellular or intracellular recordings from the interneurone and lower records are from the surface of the spinal cord. The top and middle pairs of records show the antidromic response (indicated by arrows) of the interneurone to stimulation within the lateral funiculus at L4. The top pair of records show an extracellularly recorded antidromic spike and the middle pair of records show a blocked intracellularly recorded antidromic spike. The bottom pair of records show the absence of an antidromic response (extracellular recording) to stimulation at Th13. Each trace is composed of four superimposed sweeps. Calibration scale shows sensitivity for extracellular (e.c.) and intracellular (i.c.) recordings. Recording location, caudal L7.
Figure 3. Intracellular recordings from an L4-projecting interneurone with excitatory input from group I and group II muscle afferents.
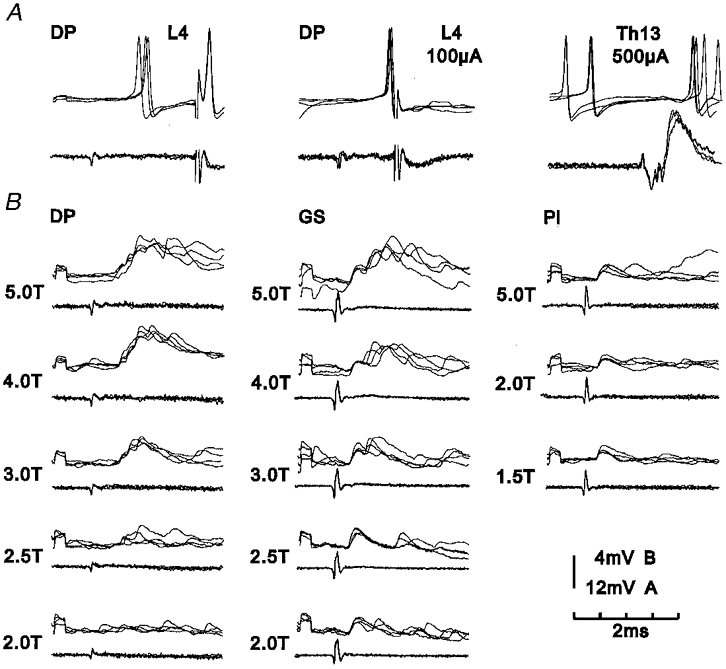
In both A and B, upper records of each pair are intracellular recordings from the interneurone and lower records are from the surface of the spinal cord (four superimposed sweeps). A, tests of projection. Left-hand records, orthodromic discharges evoked by group II afferents of the DP nerve, followed by an antidromic discharge in response to stimulation within the lateral funiculus at L4. Middle records, moving the timing of the L4 stimulus closer to that of the nerve stimulation leads to collision between orthodromic and antidromic impulses within the ascending axon and no antidromic response therefore occurs. Right-hand records, lack of antidromic response to stimulation at Th13. B, EPSPs evoked by stimuli applied to the DP, GS and Pl nerves at different stimulus strengths. Note that stimulation of DP evoked EPSPs attributable to group II afferents, stimulation of GS evoked EPSPs attributable to both group I and group II afferents and stimulation of Pl evoked EPSPs attributable to group I afferents alone. Recording location, rostral L7.
Figure 4. Intracellular recordings from an L4-projecting interneurone with excitatory input from group I and group II muscle afferents.
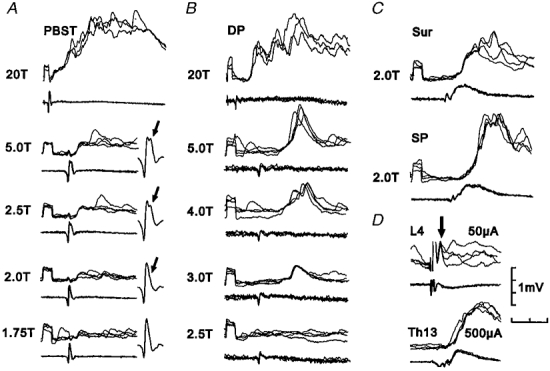
Upper records of each pair are intracellular recordings from the interneurone and lower records are from the surface of the spinal cord (four superimposed sweeps). A and B, EPSPs evoked by stimuli applied to the PBST and DP nerves at different stimulus strengths. Note stimulation of PBST evoked a small early EPSP which grew in parallel with the second (Ib) component of the group I volley (indicated by arrows in expanded records of volley). Stimulation of DP evoked EPSPs which grew in amplitude between 3T and 5T and are therefore attributable to group II afferents. Stimulation of both PBST and DP at 20T produced additional PSPs attributable to group III afferents. C, EPSPs produced by stimulation of cutaneous afferents. D, tests of projection. Top pair of records show a blocked antidromic spike (indicated by arrow) produced by stimulation within the lateral funiculus at L4. Bottom pair of records show lack of antidromic response to stimulation at Th13. Time calibration, 4 ms uppermost records of A and B, 2 ms all other records. Recording location, mid L7.
The conduction velocities of the ascending axons were measured between L4 and the recording site in the lower-lumbar segments. They were calculated after subtracting 0.2 ms for the latent period of activation (see Jankowska & Roberts, 1972a) and ranged from 23 to 74 m s−1 (mean 45 m s−1). These conduction velocities are similar to those of group II-activated axons recorded from in the lateral funiculus (mean 51 m s−1; Harrison & Riddell, 1989) and to those of L4 projecting interneurones mediating group I non-reciprocal inhibition (mean 48 m s−1; Brink et al. 1983; Fern et al. 1988).
Location of the interneurones
Rostro-caudal location
The rostro-caudal distribution of the sites where recordings were made from interneurones excited by group II muscle afferents is shown in Fig. 1. The histograms show the number of neurones recorded from in the rostral, middle and caudal thirds of the L6 and L7 segments. The histograms in Fig. 1a and B show, respectively, the rostro-caudal distribution of the intracellularly and extracellularly recorded samples of interneurones with an ascending projection to L4, while the histograms in Fig. 1D and E show the same information for the non-projecting sample of interneurones. It can be seen that the neurones investigated were located throughout the L6 and L7 segments though with some bias towards L7 and caudal L6. Tracking within the more rostral parts of the L6 segment was avoided in order to reduce the likelihood of recording from group II interneurones of the type that have been described in the midlumbar segments (Edgley & Jankowska, 1987). Similarly, tracking was not performed within 2 mm rostral of where the largest group II cord dorsum potentials were evoked by group II afferents of PBST in order to avoid recording from the population of group II interneurones which are common in the dorsal horn of the sacral segments (Jankowska & Riddell, 1994).
Figure 1. Location of the interneurones investigated.
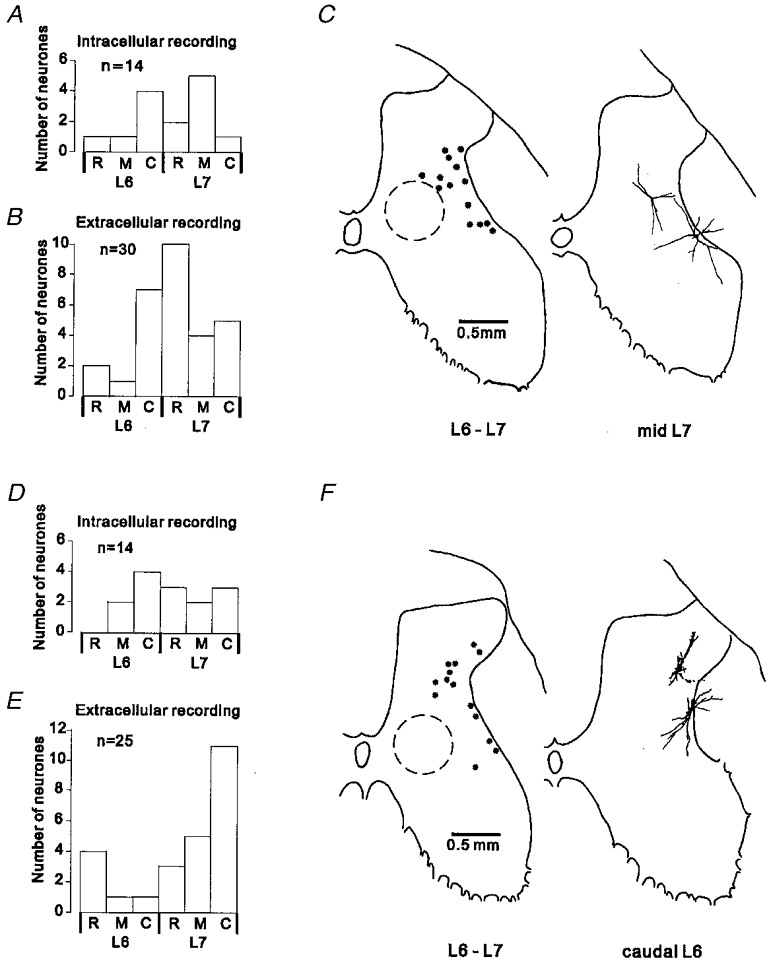
A and B, rostro-caudal distribution of the sample of interneurones with a projection to L4. The histograms show the number of interneurones recorded in the rostral (R), middle (M) and caudal (C) third of the L6-S1 segments. A, data for intracellularly recorded neurones. B, data for extracellularly recorded neurones (one neurone located in S1 not shown). C, locations of L4-projecting interneurones within the spinal grey matter. The outline on the left shows the locations of intracellularly recorded neurones, reconstructed using information on the recording angle and depth in relation to a marking electrode left in the recording track. Plots from different experiments were superimposed by alignment of the dorsal and lateral borders of the grey matter. The typical location of interneurones receiving excitatory input from group I muscle afferents is indicated by the circle drawn with a dashed line (data from Czarkowska et al. 1981; Jankowska et al. 1981). On the right-hand outline are shown partial reconstructions of two neurones intracellularly labelled with biocytin. D-F, segmental distribution and location within the grey matter of the sample of non-projecting interneurones. Same format as for A-C.
Location within the grey matter
An indication of the distribution of the recorded interneurones within the grey matter is provided in Fig. 1C and F. Representative outlines are shown of the grey matter in the transverse plane on which the locations of intracellularly recorded interneurones with (Fig. 1C) and without (Fig. 1F) an ascending projection to the L4 level are indicated (left-hand outlines). The right-hand outlines show partial reconstructions of neurones that were intracellularly labelled with biocytin.
The interneurones were distributed mainly in the ventro-lateral part of the dorsal horn and the lateral part of the intermediate zone; the majority were located in the lateral half or one-third of laminae V and VI of Rexed (1954), with a few lying more dorsally in laminae IV and a few more ventrally in lamina VII. The interneurones were therefore distributed in an area which lies dorsal and lateral to the main region in which interneurones with input from group I muscle afferents are located (indicated by the dashed circles in Fig. 1C and F; Czarkowska et al. 1981; Jankowska et al. 1981); an area similar to that in which field potentials evoked by group II muscle afferents can be recorded (Riddell & Hadian, 2000). There appears to be some tendency in the plots of Fig. 1C and F for the non-projecting interneurones to lie more dorsally than the L4-projecting neurones. However, the mean recording depths for the larger samples of extracellularly recorded interneurones with and without an ascending projection were not significantly different (Student's t test; mean depths 2.05 and 1.98 mm, respectively).
Excitatory input from muscle afferents
Afferent fibre origin
The sources of muscle afferent input to the interneurones were investigated by applying stimuli of graded intensity to muscle nerves. Stimuli of 2T are close to maximal for group I afferents of most muscle nerves while the majority of group II afferents can be recruited by increasing the stimulus intensity from 2T to 5T (Jack, 1978; Ellaway et al. 1982; Lundberg et al. 1987a). Group II muscle afferents can therefore be considered to contribute to the extracellularly recorded discharges of neurones when they are evoked by stimulation of muscle nerves at 5T, but not by stimulation of the same nerve at 2T, while group I afferents are likely to be primarily responsible for discharges evoked by stimuli of less than 2T.
All 55 of the interneurones investigated using extracellular recording were discharged by group II afferents of one or more muscle nerves, but none were discharged by stimulation at 2T. In the majority of cases where they were precisely determined (84 %, n = 86), stimulus strengths threshold for evoking discharges were greater than 2.5T (see Fig. 2).
In order to test the possibility that group I afferents might have weak actions, insufficient to evoke a discharge following single stimuli at 2T, pairs of stimuli (about 1.0-1.5 ms apart) were used in an attempt to produce temporal summation. The majority of the 33 neurones (similar numbers of L4-projecting and non-projecting neurones) tested in this way failed to respond even to double stimuli. A small number (7) were discharged by pairs of stimuli, but at latencies (> 2.5 ms with respect to the second shock) longer than would be expected for monosynaptic group I input. In addition, the thresholds for the responses were of the order of 1.8T which is already sufficient to activate the most excitable group II muscle afferents. It is therefore likely that the few examples of additional discharges seen using paired stimuli were due to activation of the most excitable group II afferents rather than temporal summation of sub-threshold actions from group I afferents.
The evidence obtained from extracellular recordings suggests that any input from group I muscle afferents to the interneurones is much weaker than that from group II afferents. This is supported by observations made during intracellular recordings from 28 interneurones. Excitatory postsynaptic potentials (EPSPs) evoked by group II muscle afferents appeared only when the stimulus intensity was raised to 1.8-2.5T (depending on the nerve) which was usually close to maximum for group I afferents as judged by the afferent volley. Increasing the stimulus intensity produced EPSPs of increasing amplitude until a maximum amplitude was reached at intensities of about 5T (see Fig. 3b, left and middle columns, and Fig. 4b).
For the majority of intracellularly recorded interneurones excited by group II muscle afferents, stimulation of a range of muscle nerves at intensities of up to 2T (i.e. intensities activating most if not all group I afferents) failed to evoke any PSPs. Figures 2 and 5 show examples of recordings from L4-projecting (Fig. 2) and non-projecting (Fig. 5) interneurones of this type. However, in about a quarter (8/28, 29 %) of the intracellularly recorded interneurones (4 L4-projecting and 4 non-projecting) there was evidence of convergence from both group I and group II afferents of muscle nerves. In some cases (6 nerves) both group I and group II EPSPs were evoked by the same nerve, as illustrated by the records in the middle column of Fig. 3B. In other examples (7 nerves) the group I EPSPs were evoked from nerves without any group II actions, as illustrated by the records in the right-hand column of Fig. 3b, and Fig. 4A. In these examples, the group I EPSPs were evoked at shorter latencies and at lower stimulus strengths than those attributed to group II afferents. Consistent with the lack of extracelluarly recorded discharges of neurones following stimulation of muscle nerves at 2T, group I EPSPs were always considerably smaller in amplitude (less than half) than the largest group II EPSPs evoked in the same neurone.
Figure 5. Intracellular recordings from a non-projecting interneurone with excitatory input from group II muscle afferents.
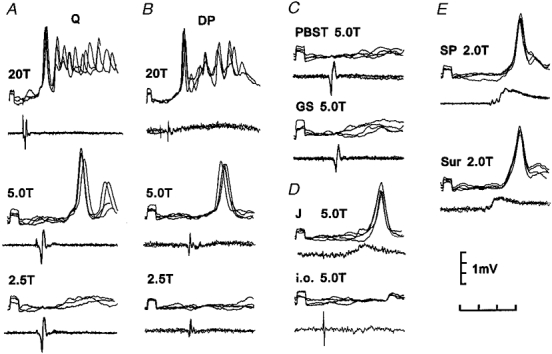
Upper traces of each pair are intracellular recordings from the interneurone and lower traces are recordings from the surface of the spinal cord (four superimposed sweeps). A-C, effects of electrical stimulation of muscle nerves at different stimulus strengths. D, EPSPs evoked by J and i.o. E, PSPs evoked by cutaneous afferents. Note that stimulation of both Q and DP at 20T produced additional PSPs attributable to group III afferents. Time calibration, 4 ms for upper records in A and B, 2 ms all other records. Recording location, caudal L6.
Stimuli just threshold for evoking group I actions, particularly those of PBST, were often of the order of 1.4T or more, which is higher than that required to excite the most excitable group I fibres. The possibility that group Ib afferents might be primarily responsible for the group I excitation was therefore investigated for four EPSPs evoked by group I afferents of PBST, where advantage could be taken of the separation usually visible in the group I afferent volley between fibres innervating muscle spindle primary endings (Ia) and fibres innervating Golgi tendon organs (Ib; cf. Bradley & Eccles, 1953). Graded stimulation of PBST was found to produce PSPs that grew in parallel with the second (Ib) component of the afferent volley as illustrated by the recordings in Fig. 4A. These observations suggest that group Ib afferents contribute to the group I excitation of this population of interneurones. However, the possibility remains that group Ia afferents also contribute to the group I EPSPs, particularly those evoked by nerves other than PBST.
The actions of muscle afferents of high electrical threshold were only occasionally investigated but raising the stimulus intensity from 5T to 20T, which is sufficient to activate a proportion of group III muscle afferents (Ellaway et al. 1982; Lundberg et al. 1987a), produced considerable additional excitation in all five of the intracellularly recorded interneurones investigated in this way. Such group III EPSPs could be evoked from nerves evoking group II EPSPs (as in the examples shown in Fig 4a and B and Fig 5a and B) or from nerves where stimuli of 5T were ineffective.
Nerve origin
The proportions of extracellularly and intracellularly recorded interneurones excited by input originating from different muscle and cutaneous nerves are shown in the histograms of Fig. 6. Since the pattern of peripheral input to L4-projecting and non-projecting interneurones was not obviously different, data for the two types of neurone have been pooled. Figure 6a shows that group II afferents of Q and DP evoked discharges in the largest proportion of interneurones (nearly 80 % and 70 %, respectively). Group II afferents of TP, PBST and GS (45–55 %) and Pop and FDL (about 30 %) were also an effective source of excitation while group II afferents of Pl and ABSM were the least effective of those tested.
Figure 6. Pattern of excitatory peripheral input to the sample of interneurones.
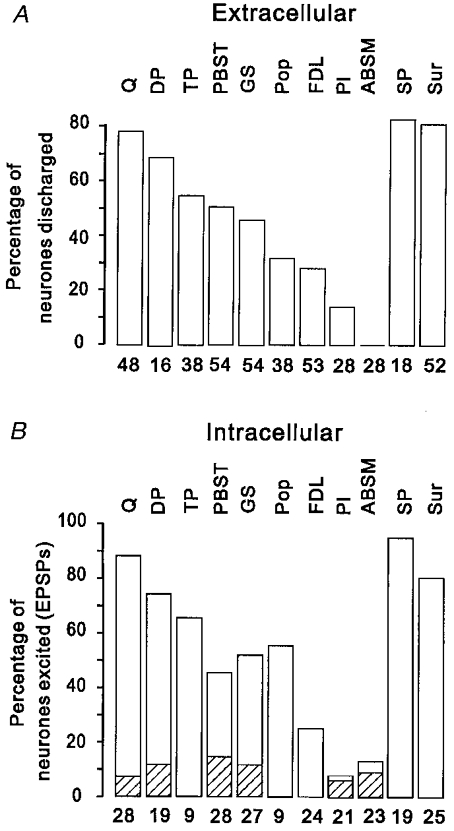
The histograms show the proportion of interneurones in which extracellularly recorded discharges (A) or intracellularly recorded EPSPs (B) were evoked by group II muscle afferents of the range of muscle nerves tested and by cutaneous afferents of the Sur and SP nerves. In B, the shaded portions of the histograms indicate the proportion of neurones in which EPSPs were evoked by group I muscle afferents of each of the muscle nerves tested. The nerves are indentified above the histograms and the number of neurones tested is shown beneath.
The frequency with which group II afferents of different muscle nerves evoked EPSPs in the intracellularly recorded interneurones is shown in Fig. 6B. As can be seen, the order of effectiveness of group II afferents of different nerves as a source of EPSPs was very similar to their effectiveness in evoking discharges. Furthermore, the proportion of neurones in which group II EPSPs were evoked by a given nerve was, with the exception of Pop, quite similar to the proportion of neurones in which the same nerve produced discharges. Taken together, this evidence suggests that transmission from group II afferents of most muscle nerves to the interneurones is highly secure, usually being sufficient to evoke a discharge of the neurone.
The hatched portions of the histograms in Fig. 6b indicate the proportion of neurones in which EPSPs were evoked by group I afferents of muscle nerves. As described above, these were seen much less frequently than group II EPSPs, even the most effective nerve (PBST) having group I actions on only 14 % of neurones. Furthermore, the effectiveness of different nerves as a source of group I input did not match their effectiveness as a source of group II input. For example, stimulation of Pl and ABSM, which produced the least effective group II actions, evoked group I EPSPs with the same frequency as stimulation of Q and DP, which produced the most effective group II actions. Group I afferents of FDL, TP and Pop never produced group I EPSPs even though they were an effective source of group II input.
There was considerable convergence onto single interneurones from group II afferents of different muscle nerves. Group II afferents of two or more muscle nerves discharged 75 % of the extracellularly recorded sample and evoked EPSPs in 90 % of the intracellularly recorded interneurones while group II afferents of three or more muscle nerves discharged 43 % of the extracellularly recorded sample and evoked EPSPs in 75 % of the intracellularly recorded interneurones (see Fig. 7a). There appeared to be a slightly lesser degree of convergence in the L4-projecting interneurones than in the non-projecting interneurones; group II afferents of three or more muscle nerves evoked discharges in 30 % and EPSPs in 85 % of L4-projecting interneurones but in non-projecting neurones the equivalent proportions were 56 % and 100 %.
Figure 7. Convergence from group II muscle afferents onto individual interneurones.
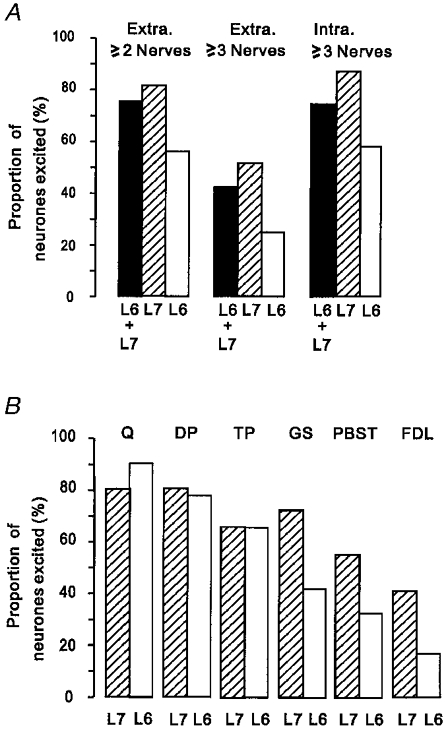
A, histograms showing the proportion of neurones discharged by group II afferents of two or more muscle nerves (left) and three or more muscle nerves (middle), and the proportion of neurones in which EPSPs were evoked by group II afferents of three or more muscle nerves (right). The filled bars represent neurones in both the L6 and L7 segments, the hatched bars neurones in the L7 segment and the open bars neurones in the L6 segment. Note the greater convergence in L7 than in L6. B, histograms showing a comparison of the proportions of interneurones in the L7 and L6 segments in which EPSPs were evoked by group II afferents of different nerves. The hatched bars represent neurones recorded in the L7 segment and the open bars represent neurones recorded in the L6 segment. Note that group II afferents of PBST, GS and FDL excited a larger proportion of neurones in the L7 segment than in L6, while afferents of Q, DP and TP were similarly effective in the two segments.
There was also a difference in the degree of convergence seen in neurones recorded in the L7 segment compared to those recorded in L6. Group II afferents of three or more muscle nerves discharged 51 % of neurones in L7 but only 25 % of those in L6 and evoked EPSPs in 88 % of neurones in L7 but in only 58 % of those in L6 (see Fig. 7a). Figure 7b shows that this is related to differences in the effectiveness of certain muscle nerves in the two segments. Group II afferents of Q, DP and TP were similarly effective in the L6 and L7 segments while group II afferents of GS, PBST and FDL produced EPSPs in a higher proportion of interneurones in the L7 segment than in L6. These observations are consistent with the relative frequency with which group II field potentials evoked by these nerves tend to be recorded in the L6 and L7 segments (see accompanying paper, Riddell & Hadian, 2000).
Synaptic linkage
The central delays (latencies) of EPSPs evoked by group II muscle afferents were measured in relation to the arrival of the group I afferent volley since volleys in group II muscle afferents could rarely be detected. The range of delays likely to be consistent with monosynaptic actions of the fastest group II afferents was estimated using methods described in detail elsewhere (Jankowska & Riddell, 1994). Briefly, the delay between the arrival of impulses in the fastest conducting group I and group II afferent fibres (conduction velocities taken as 110 and 70 m s−1, respectively) was calculated using measurements of conduction distance for each nerve. Where appropriate, the delay due to conduction in the dorsal columns was calculated, the level of entry of afferents of different nerves being determined by observing where the largest afferent volleys were evoked and conduction velocity in the dorsal columns being taken as half that in the periphery (Munson et al. 1980; Fern et al. 1988). The delay for conduction within the intraspinal terminals of group II afferents was taken to be 0.4-1.15 ms (see Fu & Schomburg, 1974) and a further 0.3 ms was allowed for a synaptic delay.
Central delays considered compatible with monosynaptic actions of the fastest conducting group II afferents were therefore 1.6-2.5 ms for GS, 1.3-2.2 ms for PBST, 1.3-2.3 ms for Q, 1.7-2.7 ms for DP and 1.6-2.4 ms for FDL, TP and Pop. The distribution of the latencies of discharges and EPSPs evoked by group II afferents of muscle nerves is shown in Fig. 7. The filled bars in the intracellular histograms indicate those EPSPs that are considered likely to have been evoked monosynaptically by the very fastest conducting group II afferents. The histograms show that for most of the nerves investigated, a proportion of the EPSPs evoked by group II afferents occurred at latencies which were compatible with a monosynaptic connection. The one exception was the action of group II afferents of popliteus (hatched bars) which evoked EPSPs only at latencies of 3.0 ms or more. Group II afferents of one or more muscle nerves produced EPSPs considered to be monosynaptic in 17 of the 28 (61 %) intracellularly recorded interneurones. It is possible that the actual proportion of monosynaptic connections from group II muscle afferents may be greater than that indicated in the histograms since most group II afferents conduct more slowly than the 70 m s−1 on which the estimates are based. The black bars under the histograms indicate the range of latencies of EPSPs that could be consistent with the monosynaptic actions of the full range of group II muscle afferents (i.e. with conduction velocities of 70 to 35 m s−1; see Matthews, 1972). However, it should be emphasized that at the longer end of these ranges, monosynaptic actions of slowly conducting group II afferents cannot be distinguished from possible di- or tri-synaptic actions of faster conducting group II afferents. The latencies of all 13 of the group I EPSPs seen were within the range (0.6-1.0 ms with respect to group I volleys) expected of the monosynaptic actions of group I muscle afferents (see for example Harrison & Jankowska, 1985).
Excitatory input from cutaneous afferents
In addition to muscle nerves, the actions of stimuli applied to one or more cutaneous nerves were investigated in virtually all of the sample of interneurones. Stimulation of cutaneous nerves, often at strengths close to nerve threshold, discharged 82 % of the extracellularly recorded sample and produced EPSPs in all but one of the intracellularly recorded neurones tested (n = 26); there was no difference between the L4-projecting and non-projecting neurones examined. Examples of extracellular recordings of discharges evoked by cutaneous afferents are shown in Fig. 2b while examples of intracellularly recorded EPSPs are shown in Fig 2b, Fig 4C and Fig 5E. The histograms of Fig. 6 show that cutaneous afferents of SP and Sur were almost equally effective in evoking discharges (Fig. 6a) or EPSPs (Fig. 6b) in the neurones and in fact, where the actions of Sur and SP were tested on the same neurones, both were usually effective.
The histograms of Fig. 6 also show that cutaneous afferents of both the SP and sural nerves evoked discharges (Fig. 6a) or EPSPs (Fig. 6b) in the sample of interneurones as frequently as group II afferents of the most effective muscle nerves. Furthermore, as illustrated by the examples of intracellular records (Fig 2b, Fig 4C and Fig 5E), EPSPs evoked by cutaneous afferents of both Sur and SP were often as large, if not larger, than those produced by the most effective group II muscle afferents. Cutaneous afferents therefore provide a powerful source of input to most of the group II interneurones.
Cutaneous afferents of the SP nerve enter mainly at L7, so their synaptic actions will involve a central delay due to intraspinal conduction (assumed to be similar to that for group II muscle afferents; Fu & Schomburg, 1974) and synaptic transmission. Afferents of the Sur nerve enter the cord mainly at S1, so impulses in these afferents must travel up to 10 mm in the dorsal columns to reach mid-L6 and will therefore be delayed by an additional 0.3 ms or so. Central delays considered compatible with the monosynaptic actions of cutaneous afferents are therefore 0.7-1.1 ms for SP and 1.0-1.4 ms for Sur. As can be seen from the histograms in Fig. 8, about half of the EPSPs evoked by Sur and SP had latencies that lie within these ranges so that at least a proportion of the cutaneous input to the interneurones is likely to be evoked monosynaptically. As with the actions of muscle afferents, this may be an underestimate if some of the longer latency EPSPs are evoked by slower conducting cutaneous afferents.
Figure 8. Latencies of discharges and EPSPs evoked in the sample of interneurones by afferents of various nerves.
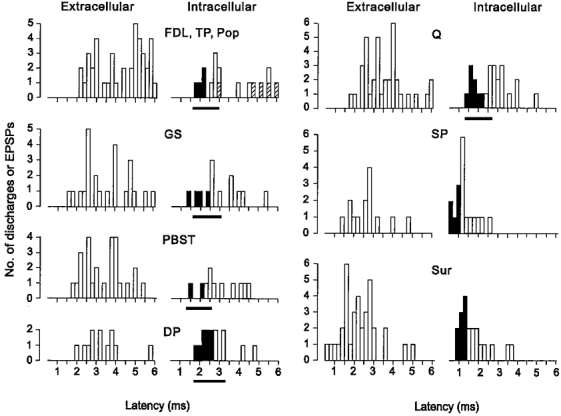
The histograms show the latencies of discharges (Extracellular) and EPSPs (Intracellular) measured from the onset (i.e. peak of the first positive deflection) of volleys in group I muscle afferents or cutaneous afferents. The latencies of discharges and EPSPs evoked from muscle nerves include only those evoked by group II afferents. The filled bars in the intracellular histograms indicate those EPSPs that are considered likely to have been evoked monosynaptically by the very fastest conducting group II afferents of muscle nerves or the fastest conducting cutaneous afferents (see text for further details). The latencies are grouped in 0.2 ms bins. The hatched areas in the histogram of the top left panel indicate EPSPs evoked by Pop group II afferents. The black bars under the histograms for muscle nerves indicate the range of latencies of EPSPs that could be consistent with the monosynaptic actions of the full range of group II muscle afferents (i.e with conduction velocities of 70–35 m s−1). Note, however, that at the longer end of these ranges monosynaptic actions of slowly conducting afferents cannot be distinguished from di- or tri-synaptic actions of faster conducting afferents. A few discharges and EPSPs which occurred at latencies between 6 and 8 ms have been omitted.
Excitatory input from joint and interosseous afferents
The effects of stimulation of the posterior nerve to the knee joint (J) and the interosseous nerve (i.o.) were investigated less systematically than the actions of muscle and cutaneous afferents. Nevertheless examples of excitatory actions of these afferents were seen during extracellular and intracellular recording. Figure 5D shows examples of intracellularly recorded potentials evoked by afferents of the J nerve and Fig. 2b shows an example of both extracellular discharges and EPSPs evoked by afferents of the i.o. The latencies of EPSPs evoked by J and i.o. afferents ranged from 1.3 to 3.2 ms (n = 9) and 2.3 to 5.9 ms (n = 6), respectively. The linkage of J and i.o. afferents is more difficult to estimate than that of other afferents (see for example Harrison & Jankowska, 1985) but at least some of the EPSPs are likely to represent monosynaptic actions of these afferents.
DISCUSSION
Most previous studies of interneurones in the lower-lumbar segments have concentrated on the medial region of laminae V-VI where neurones strongly excited by group I afferents but with little or no input from group II muscle afferents are located (e.g. Eccles et al. 1960; Hongo et al. 1966; Harrison & Jankowska, 1985). The present investigation shows that the regions located dorsal and lateral to this area contain interneurones which are strongly excited by group II muscle afferents but only weakly, if at all, by group I afferents.
Previous studies of lower-lumbar interneurones
Lundberg et al. (1987b) recorded intracellularly from a sample of 15 interneurones which were monosynaptically excited by group II muscle afferents (see also Harrison et al. 1994). Most of these were excited by group II afferents of several muscle nerves, including those providing input to the present population of neurones. In addition, evidence of convergent excitation from cutaneous, joint and group III muscle afferents was observed. Recordings have also previously been made from axons in the lateral funiculus belonging to interneurones activated by group II muscle afferents (Harrison & Riddell, 1989). These recordings were made after lesioning the dorsal columns (at the L5-L6 border) to interrupt primary afferent input to midlumbar interneurones and so the axons must have originated from neurones with cell bodies in the lower-lumbar or sacral segments. Since subsequent studies of group II interneurones in sacral segments have shown that these do not project to the L4 level (Jankowska & Riddell, 1994) the axons recorded by Harrison & Riddell (1989) almost certainly originated from neurones of the present population with somata in L6-L7.
The projection of lower-lumbar group II interneurones to L4 is a feature that they share with identified interneurones in pathways of group I non-reciprocal inhibition (Ib inhibitory interneurones) which are also located in the lower-lumbar segments (Hongo et al. 1983; Brink et al. 1983). However, the latter are located in a more medial region of the intermediate zone than the group II interneurones and are strongly excited by group I muscle afferents (both Ia and Ib). Furthermore, very few Ib interneurones (less than 10 %; Harrison & Jankowska, 1985) are excited by group II muscle afferents and although most are excited by cutaneous afferents, the linkage in this pathway is not monosynaptic as for many of the present group II interneurones. There are therefore several clear differences between group Ib inhibitory interneurones and group II interneurones with an ascending projection to the L4 level which indicate that the two types of neurone are activated by different sensory input and therefore contribute to different reflex pathways.
Comparison with group II interneurones in midlumbar and sacral segments
In the midlumbar segments group II muscle afferents project to two distinct regions of the grey matter making connections with functionally different populations of interneurones in the dorsal horn and intermediate zone (Edgley & Jankowska, 1987). In the sacral segments, group II afferents project only to interneurones in the dorsal horn (Jankowska & Riddell, 1994). Although, as described in the accompanying paper (Riddell & Hadian, 2000), group II afferents evoke field potentials in both the dorsal and intermediate grey matter of the lower-lumbar segments, the dorsal horn fields are much smaller than those in either the midlumbar or sacral segments and the separation into dorsal and intermediate fields is less distinct. It was not therefore possible to separate the lower-lumbar interneurones into different populations in relation to the type of group II field potentials evoked in proximity to the recording site. Furthermore, as a population, although the lower-lumbar interneurones were found to have properties in common with both the dorsal horn and intermediate zone group II interneurones previously described (e.g. in terms of convergence from cutaneous afferents and from group I muscle afferents, see below), these were not strictly related to their location in the grey matter.
As for group II interneurones located in both the dorsal horn and intermediate regions of other segments, the effects of stimuli of up to 5 times threshold applied to muscle nerves were dominated by the actions of group II muscle afferents. Group II inputs to the lower-lumbar interneurones were drawn from a wide range of muscle nerves. As in midlumbar interneurones (Edgley & Jankowska, 1987), group II afferents of Q and DP provided the most effective source of group II input, but group II afferents of several other nerves which have relatively weak effects on midlumbar interneurones (PBST, GS and to a lesser extent FDL) also provided appreciable excitatory input to the lower-lumbar interneurones. The excitation of lower-lumbar interneurones by group II afferents of Q is consistent with evidence obtained following selective interruption of transmission through either midlumbar or lower-lumbar interneurones, which shows that the reflex actions of these afferents are mediated by interneurones in both these regions (Cavallari & Pettersson, 1991). However, it remains to be determined whether the actions of Q in the midlumbar and lower-lumbar regions originate from the same or different heads of the muscle. Although a proportion of the group II EPSPs evoked by group II muscle afferents in lower-lumbar interneurones were monosynaptic, the longer latencies of some of the EPSPs and discharges evoked by group II afferents suggest that the interneurones also receive di- and oligo-synaptic input from group II afferents. If so, then part of this excitation, especially that produced by group II afferents of GS and PBST, might be mediated by group II interneurones in the dorsal horn of sacral segments (see Jankowska & Riddell, 1994). Some of these have axons that ascend for a short distance (a few millimetres) in the lateral funiculus and give rise to axon collaterals which arborize within the intermediate grey matter (Jankowska et al. 1993).
Lundberg and colleagues (Lundberg et al. 1987b) have noted that there is extensive convergence of group II actions from different muscle nerves of both flexors and extensors at the motoneuronal level. They sought to make functional sense of this by postulating that the convergence may arise from the actions of different subsets of interneurones with a much more limited convergence from group II afferents, thus allowing for the possibility of selection of particular patterns of group II reflex action. In support of this they presented evidence of limited convergence in a small sample of group II interneurones from the lower-lumbar segments. However, the present observations on a larger sample of interneurones suggest that the convergence of group II actions at the interneuronal level is already considerable; group II afferents of three or more muscle nerves discharged 43 % of the extracellularly recorded sample and evoked EPSPs in 75 % of the intracellularly recorded interneurones. This degree of convergence is similar to that seen for last-order pre-motor interneurones recorded in the intermediate region of the midlumbar segments (Edgley & Jankowska, 1987).
A high proportion of the group II interneurones in the dorsal horn of the midlumbar and sacral segments are excited by cutaneous afferents and some of these, especially in the sacral segments, are excited monosynaptically (Edgley & Jankowska, 1987; Jankowska & Riddell, 1994). In contrast, only around 25 % of the group II interneurones in the intermediate zone of the midlumbar segments are excited by cutaneous afferents and monosynaptic connections are infrequent (Edgley & Jankowska, 1987). In comparison, cutaneous afferents produced discharges in about 80 % of the extracellularly recorded interneurones of the present sample and produced EPSPs, many of which were monosynaptic, in all but one of those recorded intracellularly. The lower-lumbar interneurones, in common with the dorsal horn group II interneurones of other segments, are therefore powerfully excited by cutaneous afferents.
In addition to excitation from group II muscle afferents, last-order group II interneurones in the intermediate region of the midlumbar segments frequently receive convergent excitation from group I muscle afferents (62 % of neurones) which is sufficient to discharge 15 % of cells (Edgley & Jankowska, 1987). Some of the group II interneurones in the lower-lumbar segments were also excited by group I muscle afferents but this excitation was generally weak and insufficient to discharge the neurones even when attempts were made to produce temporal summation. This is in agreement with the generally low degree of spatial facilitation of transmission in group II reflex pathways that is produced by group I muscle afferents (Jankowska et al. 1996).
In each of the other populations of group II interneurones so far studied IPSPs were regularly evoked by stimulation of both muscle and cutaneous nerves. In contrast, IPSPs were only rarely observed in the lower-lumbar interneurones, a feature which has also been commented on by Lundberg et al. (1987b). This suggests that there is little inhibitory interaction between the lower-lumbar group II interneurones.
About half of the lower-lumbar group II interneurones of the present study were antidromically activated from the L4 level. While it is possible that a few interneurones projecting to L4 failed to be activated by the stimuli applied in the lateral funiculus, since most ascending axons were activated at strengths well below the maximum stimuli applied, it is unlikely that significant numbers of L4-projecting interneurones would have been missed. There therefore appear to be at least two functionally different populations of group II interneurones in the lower-lumbar segments and this raises the question of what their respective functions might be. One possibility is that the non-projecting interneurones are functionally analogous to the dorsal horn neurones of the midlumbar and sacral segments while the L4-projecting interneurones are functionally analogous to the intermediate zone interneurones. However, this seems unlikely to be the case since the two sets of interneurones were distributed within a broadly similar area and received a similar pattern of peripheral input. In particular, group I excitation, which characterizes intermediate zone but not dorsal horn interneurones, was seen in the same proportion of L4-projecting and non-projecting interneurones. The equivalence of the present population of interneurones to the dorsal horn and intermediate zone interneurones described in other segments therefore remains to be clarified; in particular, the question of whether, like group II interneurones in the intermediate zone of the midlumbar segments, some lower-lumbar interneurones make direct contact with motoneurones.
Are the lower-lumbar interneurones last-order pre-motor interneurones?
Lower-lumbar group II interneurones receive inputs from group II afferents of a wide range of nerves. Group II afferents of some of these nerves (PBST, GS, FDL) have relatively weak effects on group II last-order interneurones in midlumbar segments (Edgley & Jankowska, 1987) and yet are known to evoke postsynaptic potentials in motoneurones (e.g. Lundberg et al. 1987a). The only other population of neurones with appropriate group II inputs (in the sacral segments) appear not to project directly to motoneurones (Jankowska et al. 1993; Jankowska & Riddell, 1994). This evidence suggests that some of the present population of interneurones might be the last-order interneurones in reflex pathways from group II muscle afferents of these muscle nerves.
Since the lower-lumbar group II interneurones and the hindlimb motoneurones which are their potential targets lie within the same or nearby segments, the latencies of discharges of interneurones evoked by group II muscle afferents should be similar (within about 1.0 ms) to the latencies of group II PSPs in motoneurones for which they may be responsible. Comparison of the latency data for interneuronal discharges presented in Fig. 8 with the latencies of group II EPSPs and IPSPs of various nerve origin recorded in motoneurones (see Fig. 6 of Lundberg et al. 1987a), shows that the interneurones could theoretically be responsible for even the earliest EPSPs evoked in flexor motoneurones and that the range of latencies of interneuronal discharges span the ranges of latencies of both EPSPs and IPSPs in motoneurones.
The location of the group II lower-lumbar interneurones is also consistent with the possibility that some may be last-order interneurones projecting to motoneurones. The interneurones are distributed in those regions of the deep dorsal horn and the intermediate zone (laminae V-VII) where neurones are transneuronally labelled by wheat germ agglutinin-horseradish peroxidase retrogradely loaded into motoneurones of hindlimb muscles (Harrison et al. 1984; Jankowska, 1985).
There are two sets of preliminary observations relating specifically to the lower lumbar group II interneurones with an ascending projection to the L4 level which lend support to the idea that these interneurones are interposed in pathways to motoneurones and suggest, furthermore, that their actions may be inhibitory. Firstly, in experiments where the dorsal columns were sectioned at L6 to interrupt input from primary afferents to the midlumbar segments (see below), electrical stimulation of group II muscle afferents was found to produce IPSPs in midlumbar neurones (Hongo et al. 1983; Harrison et al. 1993). These IPSPs were attributed to the actions of lower-lumbar interneurones with an ascending projection passing through the lateral funiculus to the midlumbar level. Secondly, spike-triggered averaging of PSPs in populations of motoneurones recorded from the ventral roots has been used to examine the actions of a small number of group II-activated neurones which included some with a projection to L4 (type B interneurones of Rudomin et al. 1987). Discharge activity recorded from the interneurones was found to be correlated with inhibitory ventral root potentials, some of which occurred at latencies suggestive of a direct connection with motoneurones. Both sets of preliminary evidence therefore suggest that the group II-activated interneurones with a projection to L4 may be interposed in inhibitory pathways to motoneurones. Further investigations will be required to determine whether this is the case.
Acknowledgments
We wish to thank Anne Ward for excellent technical assistance. We gratefully acknowledge the support of the Wellcome Trust (grant ref. no. 043630/Z/91/Z/1.4U) and the McNaught Bequest. M.H. was supported on an Iranian Government studentship.
References
- Aggelopoulos NC, Bawa P, Edgley S. Activation of midlumbar neurones by afferents from anterior hindlimb muscles in the cat. The Journal of Physiology. 1996;497:795–802. doi: 10.1113/jphysiol.1996.sp021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K, Eccles JC. Analysis of the fast afferent impulses from thigh muscles. The Journal of Physiology. 1953;122:462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E, Harrison PJ, Jankowska E, Mccrea D, Skoog B. Post synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. The Journal of Physiology. 1983;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hindlimb muscles in the cat. The Journal of Physiology. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Pettersson LG. Synaptic effects in lumbar motoneurones evoked from group II muscle afferents via two different interneuronal pathways in the cat. Neuroscience Letters. 1991;129:225–228. doi: 10.1016/0304-3940(91)90467-8. [DOI] [PubMed] [Google Scholar]
- Czarkowska J, Jankowska E, Sybirska E. Common interneurones in reflex pathways from group Ia and Ib afferents of knee flexors and extensors in the cat. The Journal of Physiology. 1981;310:367–380. doi: 10.1113/jphysiol.1981.sp013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. The Journal of Physiology. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Types of neurones in and around the intermediate nucleus of the lumbosacral cord. The Journal of Physiology. 1960;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. The Journal of Physiology. 1987;389:675–690. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Murphy PR, Tripathi A. Closely coupled excitation of gamma motoneurones by group III muscle afferents with low mechanical threshold in the cat. The Journal of Physiology. 1982;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R, Harrison PJ, Riddell JS. The dorsal column projection of muscle afferent fibres from the cat hindlimb. The Journal of Physiology. 1988;401:97–113. doi: 10.1113/jphysiol.1988.sp017153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T-C, Schomburg ED. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiologica Scandinavica. 1974;91:314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Kato M. Spinal interneurones responding to group II muscle afferent fibres in the cat. Brain Research. 1975;90:307–312. doi: 10.1016/0006-8993(75)90311-x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Connolly G, Guzman-Villalba JM. Characteristics of input to the group II interneurones in the caudal lumbar segments of the cat spinal cord. The Journal of Physiology. 1994;480.P:46–47P. [Google Scholar]
- Harrison PJ, Connolly G, Jefford M. Inhibition of midlumbar interneurones by lumbosacral interneurones. The Journal of Physiology. 1993;473:16P. [Google Scholar]
- Harrison PJ, Hultborn H, Jankowska E, Katz R, Storai B, Zytnicki D. Labelling of interneurones by retrograde transsynaptic transport of horseradish peroxidase from motoneurones in rats and cats. Neuroscience Letters. 1984;45:15–19. doi: 10.1016/0304-3940(84)90322-7. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. The Journal of Physiology. 1985;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Riddell JS. Group II activated lumbosacral interneurones with an ascending projection to midlumbar segments of the cat spinal cord. The Journal of Physiology. 1989;408:561–570. doi: 10.1113/jphysiol.1989.sp017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Experimental Brain Research. 1966;1:338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. Inhibition of dorsal spinocerebellar tract by interneurones in upper- and lower-lumbar segments in the cat. The Journal of Physiology. 1983;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. The Journal of Physiology. 1971;215:613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology, presented to A. K. McIntyre. Cambridge University Press; 1978. pp. 155–176. [Google Scholar]
- Jankowska E. Further indications of enhancement of retrograde transneuronal transport of WGA-HRP by synaptic activity. Brain Research. 1985;341:403–408. doi: 10.1016/0006-8993(85)91084-4. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Johnannisson T, Lipski J. Common interneurones in reflex pathways from group Ia and Ib afferents of ankle extensors in the cat. The Journal of Physiology. 1981;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Perfilieva K, Riddell JS. How effective is integration of information from muscle afferents in spinal pathways. NeuroReport. 1996;7:2337–2340. doi: 10.1097/00001756-199610020-00012. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones in pathways from group II muscle afferents in sacral segments of the feline spinal cord. The Journal of Physiology. 1994;475:455–468. doi: 10.1113/jphysiol.1994.sp020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Szabo-Läckberg Z, Hammer I. Morphology of interneurones in pathways from group II muscle afferents in sacral segments of the cat spinal cord. Journal of Comparative Neurology. 1993;336:1–11. doi: 10.1002/cne.903370312. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Roberts W. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. The Journal of Physiology. 1972a;222:597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Roberts W. Synaptic actions of single interneurones mediating reciprocal Ia inhibition on motoneurones. The Journal of Physiology. 1972b;222:623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Experimental Brain Research. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Experimental Brain Research. 1987b;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold publishers Ltd; 1972. [Google Scholar]
- Maxwell DJ, Kerr R, Jankowska E, Riddell J. Synaptic connections in dorsal horn group II spinal interneurones: synapses formed with the interneurones and by their axon collaterals. Journal of Comparative Neurology. 1997;380:51–69. [PubMed] [Google Scholar]
- Munson JB, Fleshman JW, Sypert GW. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. Journal of Neurophysiology. 1980;44:713–738. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic atlas of the spinal cord in the cat. Journal of Comparative Neurology. 1954;100:297–380. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Hadian M. Field potentials generated by group II muscle afferents in the lower-lumbar segments of the feline spinal cord. The Journal of Physiology. 2000;522:97–108. doi: 10.1111/j.1469-7793.2000.0097m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Solodkin M, Jiménez I. Synaptic potentials of primary afferent fibres and motoneurones evoked by single intermediate nucleus interneurones in the cat spinal cord. Journal of Neurophysiology. 1987;57:1288–1313. doi: 10.1152/jn.1987.57.5.1288. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, Mccrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurones within the L4 spinal segment of the cat during brain stem evoked fictive locomotion. Experimental Brain Research. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Kasper EE, Wilson VJ. Effects of muscle and cutaneous hind-limb afferents on L4 neurones whose activity is modulated by neck rotation. Experimental Brain Research. 1989;77:48–56. doi: 10.1007/BF00250566. [DOI] [PubMed] [Google Scholar]


