Abstract
An autogenous vaccine was developed, using sonicated bacteria, with a strain of Streptococcus suis capsular type 1/2. The objectives of this study were to evaluate the antibody response following vaccination and to assess the changes in antibody levels in pigs from a herd showing clinical signs of S. suis capsular type 1/2 infection in 6- to 8-week-old pigs. An enzyme-linked immunosorbent assay using the vaccine antigen was standardized. Results from a preliminary study involving 2 control and 4 vaccinated 4-week-old pigs indicated that all vaccinated pigs produced antibodies against 2 proteins of 34 and 43 kDa, respectively, and, in 3 out of 4 vaccinated pigs, against the 117-kDa muramidase-released protein. For the serologic profile, groups of 30 pigs from the infected herd were blood sampled at 2, 4, 6, 8, and 10 weeks of age. The lowest antibody level was observed between weeks 6 and 8, presumably corresponding to a decrease in maternal immunity. A marked increase was seen at 10 weeks of age, shortly after the onset of clinical signs in the herd. For the vaccination field trial, newly weaned, one-week-old piglets were divided into 2 groups of 200 piglets each (control and vaccinated); blood samples were collected from 36 piglets in each group at 2-week intervals for 12 weeks. A significant increase (P 0.05) in antibody response was observed 4 weeks following vaccination and the level of antibodies stayed high until the end of the experiment. In the control group, the increase was only observed at 13 weeks of age, probably in response to a natural infection. The response to the vaccine varied considerably among pigs and was attributed, in part, to the levels of maternal antibodies at the time of vaccination. No outbreak of S. suis was observed in the control or vaccinated groups, so the protection conferred by the vaccine could not be evaluated.
Introduction
Streptococcus suis is the causative agent of a wide range of infections in pigs including meningitis, arthritis, pneumonia, septicemia, endocarditis, encephalitis, polyserositis, and abscesses (1). Thirty-five S. suis capsular types have been described (2,3,4,5), with capsular type 2 being the most prevalent in diseased animals, followed by capsular types 1/2 and 3 (6). Important economic losses are associated with S. suis, and since antibiotic therapy gives unsatisfactory results, research into vaccination has been stimulated.
Vaccines, formalin-killed or live attenuated, avirulent or virulent, given intramuscularly or intranasally, have been used either in piglets or in sows to attempt to control disease due to S. suis (7,8,9,10,11,12,13,14). However, inconsistent results have been obtained with vaccination. Possible explanations for the failure of the vaccines are degradation of protective antigens or loss of antigenicity of the strain due to the effects of heat or formalin (11), weak immunogenicity of the capsulated bacteria (15), or production of antibodies against antigens not associated with virulence factors (10). In addition, it is difficult to evaluate the possible interference of circulating antibodies with autogenous or commercial vaccines, since no reliable serological tools are available. Several S. suis capsular type 2 proteins have been suggested to induce protection in pigs (16,17). Among them are a 136-kDa protein known as the muramidase-released protein (MRP) (17) and a hemolysin (suilysin) (18), but these 2 proteins are not present in all virulent strains (19). The MRP has also been observed in capsular types 1, 1/2, and 14 (20). Some strains of S. suis produce MRP with lower (MRPs) or higher (MRP*) molecular weights (21). Virulence factors and antigens that induce protection for other important capsular types, such as capsular type 1/2, are unknown.
The objectives of this study were to evaluate the antibody response to an autogenous vaccine composed of a protein extract obtained from a field strain of S. suis capsular type 1/2 and to evaluate the serologic profile in a herd experiencing disease associated with this same strain.
Materials and methods
Bacterial strains
The strain of S. suis capsular type 1/2 used for the autogenous vaccine (strain #97-4114) originated from a pig that had died suddenly in a herd of 6- to 8-week-old pigs with clinical signs of S. suis infection. Culture conditions were as described previously (22). This strain, along with 5 other strains of S. suis type 1/2 isolated from pigs in the same herd and other herds belonging to the same owner, were compared by randomly amplified polymorphic DNA (RAPD) with primers OPB07, OPB10 and OPB17, as described by Chatellier et al (23). Presence of MRP in the strain used for the vaccine was tested by Western blot as previously described (19). Briefly, the supernatant was concentrated 100 times and mixed with an equal volume of solubilization buffer and separated in 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel. After electrophoresis, the supernatant material was transferred from the gel to nitrocellulose membranes in methanol-Tris-glycine buffer. After blocking with casein (2%, w/v), the membranes were incubated with monospecific anti-MRP antibodies (kindly supplied by Dr. U. Vecht, ID-DLO, Lelystad, The Netherlands). After washing with Tris-saline, peroxidase-labeled immunoglobulin G fraction of goat anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, Pennsylvania, USA) was added. Antibody binding was visualized following incubation with 0.06% 4-chloro-1-naphthol (Sigma Chemical Company, St. Louis, Missouri, USA) in cold methanol mixed with H2O2 in Tris-HCl. Molecular weights were calculated by comparison with standards of known molecular mass (high range) (Bio-Rad Laboratories, Mississauga, Ontario).
Autogenous vaccine
The strain #97-4114 of S. suis capsular type 1/2 was inoculated onto sheep blood agar, incubated for 24 h at 37°C, then inoculated into 10 mL of Todd-Hewitt broth (Difco Laboratories, Detroit, Michigan, USA) and incubated at 37°C for 18 h. Subsequently, bacteria were lysed by sonication, by 3 pulse cycles of 5 min each, at 80% duty cycle (Sonics & Materials, New Town, Connecticut, USA). After sonication, unlysed cells were removed by centrifugation at 3000 × g for 30 min. The supernatant was collected and dialyzed for 24 h in filtered water with a porous regenerated cellulose membrane with a molecular weight cut-off of 12 000 to 14 000 (Spectrum Medical Industries, Houston, Texas, USA). The solution was lyophilized and stored until use. The proteins present in the vaccine antigen solution were separated in 7.5% SDS-polyacrylamide gel and stained with Coomassie blue. The presence of MRP proteins in the antigen solution of the autogenous vaccine (concentrated 10 times) was verified by Western blot as described above.
Lyophilized antigen solution was rehydrated with sterile phosphate-buffered saline (PBS) (pH 7.4) and filtered with a disposable filter unit, first with 0.45 μm pore size and then with 0.22 μm pore size (Nalgene Company, Rochester, New York, USA). Protein concentration in the vaccine antigen solution was evaluated by a modified method of Lowry (24). Vaccines were prepared by the addition of adjuvant to obtain two different final protein concentrations, 1 mg/mL (1X) and 5 mg/mL (5X). Solutions were mixed for 1 h with an adjuvant (Rehydragel; Reheis Chemical Company, Berkeley Heights, New Jersey, USA) at 5% v/v, and then for another hour with another adjuvant (Emulsigen; MPV Laboratory, Ralston, Nebraska, USA) at 20% v/v. A placebo vaccine was prepared as described above, except that the antigen solution was replaced by PBS (pH 7.4).
Pilot study
A pilot study was undertaken in our research facilities to determine the appropriate dosage for the vaccine and to evaluate any adverse effects. Six 4-week-old specific pathogen-free (SPF) pigs were randomly divided into 3 groups. Two pigs were injected twice, IM, in the neck (both injections on the same side), at a 2-week interval, with 1 mL of 1X vaccine; 2 pigs were similarly injected with 5X vaccine and 2 pigs were injected with 1 mL of the placebo. The rectal temperature was recorded daily and the animals were observed for adverse effects during the week following the injections.
At the end of the experiment, the pigs were euthanized. Skin and muscle at the injection site and the 2 dorsal superficial cervical lymph nodes were collected from each animal. Lymph nodes and skin were examined macroscopically and histologically. Blood samples were collected before the first injection and subsequently at 2-week intervals until the pigs were 14 wk old. The experiment was conducted in accordance with the guidelines of the Canadian Council on Animal Care. An enzyme-linked immunosorbent assay (ELISA) test was carried out to evaluate the antibody levels against the S. suis type 1/2 strain (see section on serologic evaluation).
The pig sera were tested by Western blot; this was performed as described above, except that the vaccine antigen solution was concentrated 16 times, mixed with an equal volume of solubilization buffer, and separated in 7.5% SDS-polyacrylamide gel. The nitrocellulose membrane was incubated with 1/100 dilution of swine sera obtained before vaccination, 2 wk after each of the first dose and second doses of the vaccine. Immunogenic antigens were revealed using peroxidase-labeled immunoglobulin G (IgG) fraction of goat anti-swine IgG (Jackson Immunoresearch Laboratories).
Field trial
The vaccination trial was performed in a farrow-to-finish, high health status herd where clinical signs associated with infection with S. suis type 1/2 had been observed in piglets of 6 to 8 wk of age. The strain used in the autogenous vaccine originated from that herd. To determine the most appropriate time for vaccination, a serologic cross-sectional profile of sows and pigs of 2, 4, 6, 8, and 10 wk of age was established. Thirty pigs from each age group were sampled and an ELISA was used to demonstrate antibodies to the bacteria, as described below.
Newly weaned, 1-week-old pigs were divided into 2 groups of 200 pigs each and housed in 2 different rooms. Two weeks after weaning, the treatment group received 1 mL, IM, of the 1X vaccine and the control group was inoculated with the placebo solution. Each animal received a second injection 2 wk later. Pigs were transferred to a second nursery and grower-finisher facility, 3 and 10 wk after weaning, respectively. They were kept with the same penmates for the whole study. No feed additive or preventive treatment was allowed during the clinical trial. For each group, the number of pigs dead, the number of pigs treated, the reason for treatment, and the type of treatment were recorded daily. Each pig that died was necropsied and a sample of liver and spleen and a swab of the meninges were submitted for bacteriologic testing. The heart was opened and examined for the presence of endocarditis. For each group, all the piglets (n = 36) from the 2 pens located face-to-face in the middle of each room in the nursery were ear-tagged. Blood samples were collected from these pigs at weaning and subsequently at 2-week intervals until the pigs were 13 wk old.
Serologic evaluation
An indirect ELISA was developed for this study. The protein antigen used for the vaccine (as described in a previous section) was suspended in carbonate buffer (pH 9.6) at a final concentration of 10 μg/mL. Each well of flat-bottomed plates (PolySorb plates; Nunc-Immunoplates, Rochester, New York, USA) was coated with 100 μL of the antigen solution to give a final concentration of 1 μg/well. The plates were incubated overnight at 4°C, drained and washed 3 times with PBS containing 0.05% Tween 20 (PBS-T20; Sigma Chemical Company). Sera were tested at a dilution of 1/400, which was established as the best dilution that allows for differentiation between positive and negative sera (not shown), and added in 100-μL amounts to appropriate wells (in duplicate on the same plate). The negative and positive controls used were sera from a non-vaccinated pig and a vaccinated pig, respectively, and were added to each plate at the same dilutions as the field sera. These 2 pigs were 14 wk old at blood sampling, and originated from the pilot study, one from the control group and one from the 1X vaccine group (see results). All incubations were carried out at room temperature. After a 1-hour incubation of sera, plates were washed 3 times with PBS-T20 and then 100 μL of horseradish peroxidase- conjugated goat anti-swine IgG (Jackson Immunoreseach Laboratories) were added to each well for 1 h. Plates were washed and 0.4 mM 2,2′-azino-bis (3-ethyl-benzthiazoline-6-sulfonic acid) (ABTS) (Sigma Chemical Company) dissolved in 0.05 M citrate buffer (pH 4) with 0.5 M H2O2 was added to each well. After a 25-minute incubation period, absorbance was measured at 414 nm on a kinetic microplate reader (Molecular Devices, Menlo Park, California, USA). Results were reported as sample-to-positive (S/P) ratios, calculated as the optical density obtained for each serum divided by the optical density of the positive control on the same plate.
All sera from any one pig in the vaccination trial were tested on the same plate. For the serologic cross-sectional profile, sera from the 6 different age groups were represented in equal numbers on one plate. Sera were tested at least 3 different times. The average optical density obtained was only accepted when the coefficient of variation within and between plates was below 15%; otherwise, the serum was tested again. In addition, plates were only accepted when positive and negative control sera presented an optical density of 1.0 ± 0.15 and 0.2 ± 0.10, respectively.
Statistical analyses
For the study on serologic profile, the effect of age on S/P ratios was evaluated with a one-way analysis of variance. Tukey's post-hoc tests were used to examine differences between pairs of means. For the vaccination trial, a repeated measures general linear model with age as a within-subject factor was used. A priori contrasts were performed to determine differences between control and vaccinated groups at each sampling point (SAS v.6.12; SAS Institute, Cary, North Carolina, USA). Differences in the magnitude of the response to the vaccination were analyzed using a linear regression model (least-square means). Data were deemed to be normally distributed following visual and statistical evaluation. The level for statistical significance was set at 0.05 for all tests.
Results
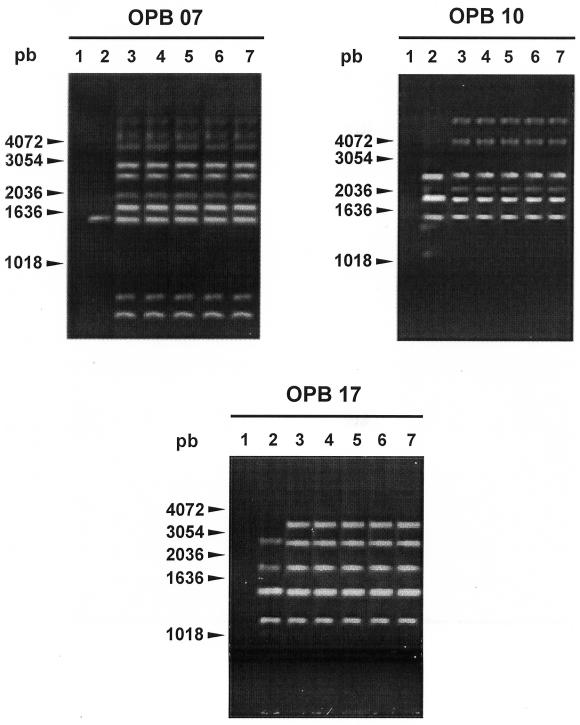
Of the 6 isolates recovered from diseased pigs, 5 were found to be genetically homogenous and one presented a different (but closely related) RAPD pattern. The arbitrarily selected strain for the autogenous vaccine (strain #97-4114) was genetically representative of other strains from the herds (Figure 1). The strain was MRPs+, as revealed by Western blot, and presented a protein of 117 kDa of molecular weight (not shown). The antigen solution of the autogenous vaccine contained different proteins of S. suis. Among them, the MRPs was found by Western blot (not shown).
Figure 1. Randomly amplified polymorphic DNA patterns generated with primers OPB17, OPB10 and OPB07 as described by Chatellier et al (20). Lane 1: negative control; lane 2: strain 97-4028; lane 3: strain 97-4114 (vaccine strain); lane 4: strain 96-5150; lane 5: 97-9993; lane 6: 96-5429; lane 7: 98-0425. All strains shown were Streptococcus suis capsular type 1/2 isolated from diseased pigs in herds belonging to the same owner.
In the pilot study, all pigs in the 2 vaccinated groups (1X and 5X) showed an increased ELISA S/P ratio 2 wk after the first injection and the S/P ratios stayed higher than at the beginning of the experiment until week 10. The mean S/P ratio was 0.5, 0.9, 1.1, 0.9, 0.9, and 0.9 for weeks 0, 2, 4, 6, 8, and 10, respectively. The administration of 5 times as much S. suis proteins extract did not result in higher antibody response in pigs. By comparison, the 2 pigs in the control group did not show any increase in ELISA S/P ratios following the injections until week 10. No adverse effect was observed following vaccination, even in pigs that received the 5X vaccine. No detectable lesions were observed from the skin and muscle at the injection site. At histological examination, no significant difference was noted between the lymph node near the vaccination site and the one on the other side and no granulomatous lesions were observed.
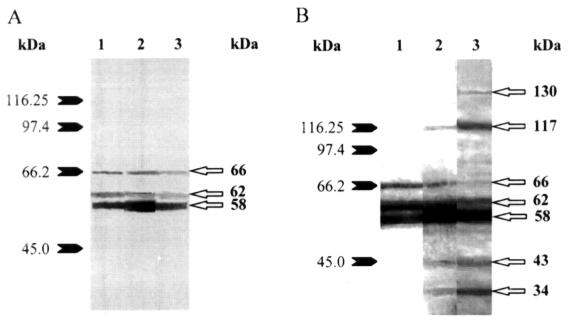
Figures 2 and 2B show Western blot results of sera from representative animals in the pilot study. All pigs in the control and vaccinated groups presented, before vaccination, antibodies against 3 different proteins of around 58, 62, and 66 kDa. In 5 pigs, antibodies against the latter 2 proteins decreased with age in both the vaccinated and control groups (Figures 2 and 2B), whereas antibodies against the 58 kDa protein increased in vaccinated animals. All vaccinated pigs presented post-vaccination antibodies against proteins of around 34 and 43 kDa. Responses to a protein of around 117 kDa (Figure 2B) were found in 3 vaccinated pigs, whereas those to proteins of around 78 and 101 kDa were observed in 2 and 3 pigs, respectively (data not shown). One pig showed an antibody response to a protein of around 130 kDa (Figure 2B).
Figure 2. Western blot analysis of proteins contained in the antigen solution of the autogenous vaccine for Streptococcus suis capsular type 1/2. Protein profiles were revealed using a serum from a control pig (Figure 2A) or from a vaccinated pig (Figure 2B). Lane 1: before first injection; lane 2: 2 wk after first injection (second injection); lane 3: 4 wk after first injection; Numbers on the left indicate molecular weight standards in kDa.
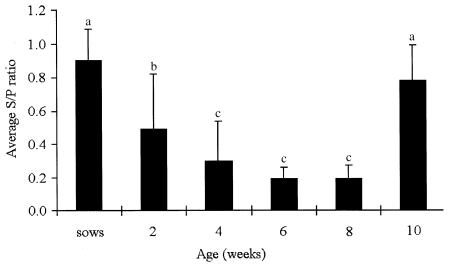
Results from the serological profile showed that sera collected from sows had an average ELISA S/P ratio of 0.90, with a range of 0.65 to 1.35 (Figure 3). At 2 wk of age, the piglets in this herd showed an average S/P ratio of 0.5, with a marked range of 0.02 to 1.17. The S/P ratios subsequently decreased significantly (P 0.0001), the lowest value occurred between 6 and 8 wk of age. A marked and significant increase (P 0.0001) in the average S/P ratio was observed in pigs of 10 wk of age, reaching a level similar to that obtained in the group of sows (P 0.2) (Figure 3).
Figure 3. Average ELISA S/P ratios and standard deviation for Streptococcus suis capsular type 1/2 obtained from unvaccinated sows and pigs of 2, 4, 6, 8, and 10 wk of age (30 animals per group). S/P ratio = optical density obtained by ELISA for each serum divided by the optical density of the positive control. Different superscripts are statistically significant at P 0.05.
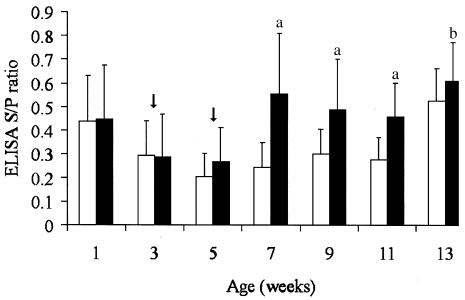
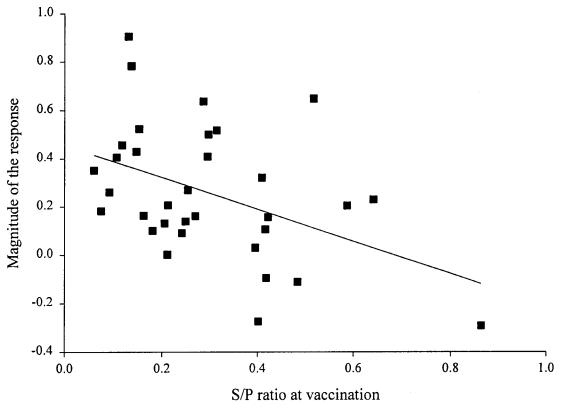
Four weeks following the first vaccination in the field trial, a significant increase in antibody response was observed in the vaccinated group (Figure 4). However, the S/P ratios varied considerably among the pigs in that group (average, 0.55; range, 0.13 to 1.16). The magnitude of the response to the vaccine varied according to the level of antibodies at vaccination (P 0.01, r2 = 0.2), being higher when maternal antibodies were low (Figure 5). The level of antibodies stayed high until the end of the experiment. In the control group, an increase in antibodies was only observed at 13 wk of age. No outbreak of S. suis was observed in these 2 groups of animals (control and vaccinated). No significant difference was observed between the 2 groups for mortality, number of treatments, and reasons for treatment. The causes of death were similar between the 2 groups and could not be associated with S. suis in any case.
Figure 4. Average ELISA S/P ratios and standard deviation for Streptococcus suis capsular type 1/2 obtained at weaning (7 to 10 d old) and at 2-week intervals. Control group (white band, n = 36) received a placebo and vaccinated group (grey band, n = 36) was injected with a Streptococcus suis autogenous vaccine 2 and 4 wk following weaning (arrows). Significant difference between the vaccinated and control group at P = 0.0001 (a) or P = 0.05 (b).
Figure 5. Magnitude of the response (S/P ratio) to a Streptococcus suis autogenous vaccine at 7 wk of age according to S/P ratio at vaccination time, 3 wk of age (n = 33) (y = 0.4555 – 0.6650x).
Discussion
Previous attempts to protect pigs against S. suis infection by vaccination have given equivocal results. Quessy et al (17) suggested that bacterial protein fractions could generate protection against S. suis capsular type 2 infections and proposed that the use of a combination of different proteins should be considered as a possibility for vaccination. In our study, the vaccine was prepared with a protein extract from a field strain of S. suis capsular type 1/2 and was shown to contain several proteins. Among them, a 117-kDa protein, which corresponds to a MRPs (21), was present.
In the pilot study, the antibody level following vaccination increased in vaccinated pigs as compared with control pigs and persisted during the vaccination trial for a minimum of 10 wk after vaccination. The importance of humoral immunity in the protection against S. suis infections has been demonstrated by Holt et al (25), who reported that protective response was serum-mediated and associated with the presence of IgM and IgG antibodies to surface components of the bacteria. The presence of antibodies, particularly IgG, to surface structures would increase the recognition of the bacteria by the immune system and stimulate phagocytosis (26). Since an infection model for serotypes other than type 2 has not been standardized, a protection assay was not attempted.
Results obtained in our study seem to indicate that although a relatively high antibody level against the cell wall proteins of S. suis was obtained, the reactive protein profile after vaccination was relatively heterogeneous. Prevaccination pig sera tested by Western blot demonstrated antibodies recognizing 3 proteins of around 58, 62, and 66 kDa. Antibodies against these proteins could be the result of a previous exposure to S. suis or other related bacteria. The decreased response to 2 of these proteins in most pigs and the increase in antibodies against the 58-kDa protein in some animals following either the injection of placebo or vaccine, suggest that the production of antibodies recognizing these proteins was independent of vaccination. It has been reported that several cross-reacting antigens, mainly proteins, are present among different serotypes of S. suis (15). All vaccinated pigs demonstrated an antibody response, following vaccination, to proteins present in the vaccine of approximately 34 and 43 kDa. Gottschalk et al (27) demonstrated that a 44-kDa cell wall protein could be a virulence factor as well as an important immunogen of S. suis capsular type 2. Quessy et al (17) demonstrated that vaccination of mice with proteins of 33 and 44 kDa resulted in protection against challenge with a homologous strain of capsular type 2. It is not known if proteins of these molecular weights of serotypes 2 and 1/2 are, in fact, related. In addition, antibodies against the MRPs protein of 117 kDa were obtained in 3 of 4 vaccinated animals. Quessy et al (17) demonstrated that antibodies against MRP were responsible for protection in mice against a homologous strain.
With modern swine production practices, such as medicated early weaning, many diseases can be controlled. However, the capacity to reduce or eliminate early colonizers, such as S. suis, is questionable with these techniques (28,29,30). Pijoan (30) proposed that to better control problems associated with this type of pathogen, new diagnostic techniques, such as serum profiling, should be used. In the herd studied, piglets were weaned at 7 to 10 d of age and clinical signs due to S. suis capsular type 1/2 were mostly observed in 6- to 8-week-old animals. The serologic profile revealed that antibody levels against the S. suis serotype 1/2 strain varied considerably among 2- and 4-week-old pigs. Differences in antibody levels among piglets in these age groups could be attributed to differences in maternal antibody levels and/or in the rate of absorption of maternal antibodies by the piglets. In our study, the ELISA S/P ratios of sows varied from 0.65 to 1.35. These results indicate that subpopulations (with high or low antibody levels) were present in our sample, which may be considered as a factor in the maintenance of bacterial transmission in herds with S. suis problems. Torremorell et al (31) suggested that most of the colonization of pigs by S. suis virulent strains occurs in animal after weaning, when maternal antibodies are at their lowest levels. The lowest antibody levels in this herd were observed in pigs at weeks 6 to 8, but the animals were probably highly infected at this time because the clinical signs of disease were observed. A marked increase in antibody response was noted at 10 wk of age; this probably corresponded to an active immunity after the breakout of clinical cases of S. suis infection in this herd. At that age, the average S/P ratio was similar to the one observed for sows in this herd.
Based on the kinetics of the antibody level obtained with the serum profiling, and considering the age at which clinical signs occurred in this herd, a vaccination of 3- and 5-week-old animals was chosen. While a good antibody response was observed in the vaccinated group, 2 doses and at least 4 wk after the first injection were required to obtain a significant increase. For the first time, interference between maternal antibodies and active production of antibodies against S. suis could be demonstrated. In fact, it was clear that animals with the lowest levels of antibodies against the strain of S. suis serotype 1/2 responded more effectively to vaccination. Although we could not prove that the antibodies at 1 wk of age were of maternal origin, the results are strongly indicative of this. Indeed, considering the age at which the first sample was taken (1 wk of age), and assuming that an infection had occurred at birth, it would be unlikely that this mounting active antibody response observed at 7 d would be decreasing 2 wk later.
No outbreak of S. suis was observed in these groups of animals (control and vaccinated), so the protection conferred by the vaccine could not be evaluated. Moreover, none of the mortalities in either the control or vaccinated group could be attributed to S. suis. For unknown reasons, the clinical signs of S. suis infection disappeared from the herd following the initiation of the vaccination trial.
In the present study, the development of an ELISA using a protein extract of S. suis as coating antigen presented an interest. It has been reported that serology for S. suis is more useful in vaccination studies or as a surveillance tool in high health status herds than as a diagnostic tool (32). The development of a strain-specific ELISA allowed the evaluation of the serologic profile of the animals, as has been shown recently for Actinobacillus suis infections (33). Knowledge of antibody kinetics is useful for implementation of a rational vaccination program. The strategy adopted should allow minimal interference between active and passive maternal immunity in piglets and maximal protection for pigs at the approximate time of onset of clinical signs. The experimental S. suis autogenous vaccine developed for capsular type 1/2 seemed to be effective for increasing the antibody level in animals. However, subsequent tests will be necessary to evaluate the efficacy of this vaccine for controlling the disease in field conditions.
Footnotes
Address correspondence and reprint requests to Dr. Sylvie D'Allaire, telephone: 450-773-8521 ext. 8473, fax: (450) 778-8120, e-mail: dallairs@medvet.umontreal.ca
Received June 19, 2001. Accepted November 27, 2001.
References
- 1.Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun 1997;21:381–407. [DOI] [PubMed]
- 2.Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol 1989;27:2633–2636. [DOI] [PMC free article] [PubMed]
- 3.Gottschalk M, Higgins R, Jacques M, Beaudoin M, Henrichsen J. Isolation and characterization of Streptococcus suis capsular types 9-22. J Vet Diagn Invest 1991;3:60–65. [DOI] [PubMed]
- 4.Gottschalk M, Higgins R, Jacques M, Beaudoin M, Henrichsen J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J Clin Microbiol 1991;29:2590–2594. [DOI] [PMC free article] [PubMed]
- 5.Higgins R, Gottschalk M, Boudreau M, Lebrun A, Henrichsen J. Description of six new capsular types (29–34) of Streptococcus suis. J Vet Diagn Invest 1995;7:405–406. [DOI] [PubMed]
- 6.Higgins R, Gottschalk M. Distribution of Streptococcus suis capsular types in 1998. Can Vet J 1999;40:277. [PMC free article] [PubMed]
- 7.Amass SF, Stevenson GW, Knox KE, Reed AJ. Efficacy of an autogenous vaccine for preventing streptococcosis in piglets. Vet Med 1999;94:480–484.
- 8.Amass SF, Stevenson GW, Vyverberg BD, Huxford TW, Knox KE, Grote LA. Administration of a homologous bacterin to sows prefarrowing provided partial protection against streptococcosis in their weaned piglets. Swine Health Prod 2000;8: 217–219.
- 9.Busque P, Higgins R, Caya F, Quessy S. Immunization of pigs against Streptococcus suis serotype 2 infection using a live avirulent strain. Can J Vet Res 1997;61:275–279. [PMC free article] [PubMed]
- 10.Holt ME, Enright MR, Alexander TJL. Immunisation of pigs with live cultures of Streptococcus suis type 2. Res Vet Sci 1988;45: 349–352. [PubMed]
- 11.Holt ME, Enright MR, Alexander TJL. Immunisation of pigs with killed cultures of Streptococcus suis type 2. Res Vet Sci 1990;48: 23–27. [PubMed]
- 12.Wisselink HJ, Vecht U, Stockhofe-Zurwieden N, Smith HE. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet Rec 2001;148:473–477. [DOI] [PubMed]
- 13.Torremorell M, Pijoan C, Dee S. Experimental exposure of young pigs using a pathogenic strain of Streptococcus suis serotype 2 and evaluation of this method for disease prevention. Can J Vet Res 1999;63:269–275. [PMC free article] [PubMed]
- 14.Oliveira S, Batista L, Torremorell M, Pijoan C. Experimental colonization of piglets and gilts with systemic strains of Haemophilus parasuis and Streptococcus suis to prevent disease. Can J Vet Res 2001;65:161–167. [PMC free article] [PubMed]
- 15.del Campo Sepulveda EM, Altman E, Kobisch M, D'Allaire S, Gottschalk M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet Microbiol 1996;52:113–125. [DOI] [PubMed]
- 16.Holt ME, Enright MR, Alexander TJL. Protective effect of sera raised against different fractions of Streptococcus suis type 2. J Comp Pathol 1990;103:85–94. [DOI] [PubMed]
- 17.Quessy S, Dubreuil JD, Caya M, Letourneau R, Higgins R. Comparison of pig, rabbit and mouse IgG response to Streptococcus suis serotype 2 proteins and active immunization of mice against the infection. Can J Vet Res 1994;58:220–223. [PMC free article] [PubMed]
- 18.Jacobs AAC, van den Berg AJG, Loeffen PLW. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec 1996;139:225–228. [DOI] [PubMed]
- 19.Gottschalk M, Lebrun A, Wisselink H, Dubreuil JD, Smith H, Vecht U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can J Vet Res 1998; 62:75–79. [PMC free article] [PubMed]
- 20.Luque I, Tarradas C, Astorga R, Perea A, Wisselink HJ, Vecht U. The presence of muramidase released protein and extracellular factor protein in various serotypes of Streptococcus suis isolated from diseased and healthy pigs in Spain. Res Vet Sci 1999;66:69–72. [DOI] [PubMed]
- 21.Vecht U, Wisselink HJ, Reek FH, Stockhofe-Zurwieden N, Smith HE. Diagnosis of several capsular serotypes of Streptococcus suis by phenotype and PCR and the relation with virulence for pigs. Proc Int Pig Vet Soc 1996;14:298.
- 22.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest 1990;2:249–252. [DOI] [PubMed]
- 23.Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol 1999;37:362–366. [DOI] [PMC free article] [PubMed]
- 24.Markwell MAK, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 1978;87:206–210. [DOI] [PubMed]
- 25.Holt ME, Enright MR, Alexander TJL. Studies of the protective effect of different fractions of sera from pigs immune to Streptococcus suis type 2 infection. J Comp Pathol 1989;100: 435–442. [DOI] [PubMed]
- 26.Wessman GE. Biology of the group E streptococci: a review. Vet Microbiol 1986;12:297–328. [DOI] [PubMed]
- 27.Gottschalk M, Higgins R, Jacques M, Dubreuil JD. Production and characterization of two Streptococcus suis capsular type 2 mutants. Vet Microbiol 1992;30:59–71. [DOI] [PubMed]
- 28.Clark LK, Hill MA, Kniffen TS, VanAlstine W, Stevenson G, Meyer KB, Wu CC, Scheidt AB, Knox K, Albregts S. An evaluation of the components of medicated early weaning. Swine Health Prod 1994;2 (3):5–11.
- 29.Amass SF, Clark LK, Wu CC. Source and timing of Streptococcus suis infection in neonatal pigs: Implications for early weaning procedures. Swine Health Prod 1995;3:189–193.
- 30.Pijoan C. Disease of high-health pigs: Some ideas on pathogenesis. Proc Leman Conf. St. Paul: University of Minnesota, 1995:16–17.
- 31.Torremorell M, Calsamiglia M, Pijoan C. Colonization of suckling pigs by Streptococcus suis with particular reference to pathogenic serotype 2 strains. Can J Vet Res 1998;62:21–26. [PMC free article] [PubMed]
- 32.Higgins R, Gottschalk M. Streptococcal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th edition. Ames: Iowa State University Press, 1999:563–578.
- 33.Lapointe L, D'Allaire S, Lacouture S, Gottschalk M. Serologic profile of a cohort of pigs and antibody response to an autogenous vaccine for Actinobacillus suis. Vet Res 2001;32:175–183. [DOI] [PubMed]