Abstract
Pretreatment of muscles with ionising radiation enhances tissue formation by transplanted myoblasts but little is known about the effects on muscle function. We implanted myoblasts from an expanded, male-donor-derived, culture (i28) into X-ray irradiated (16 Gy) or irradiated and damaged soleus muscles of female syngeneic mice (Balb/c). Three to 6 months later the isometric contractile properties of the muscles were studied in vitro, and donor nuclei were visualised in muscle sections with a Y chromosome-specific DNA probe.
Irradiated sham-injected muscles had smaller masses than untreated solei and produced less twitch and tetanic force (all by about 18 %). Injection of 106 myoblasts abolished these deficiencies and innervation appeared normal.
Cryodamage of irradiated solei produced muscle remnants with few (1–50) or no fibres. Additional myoblast implantation led to formation of large muscles (25 % above normal) containing numerous small-diameter fibres. Upon direct electrical stimulation, these muscles produced considerable twitch (53 % of normal) and tetanic forces (35 % of normal) but innervation was insufficient as indicated by weak nerve-evoked contractions and elevated ACh sensitivity.
In control experiments on irradiated muscles, reinnervation was found to be less complete after botulinum toxin paralysis than after nerve crush indicating that proliferative arrest of irradiated Schwann cells may account for the observed innervation deficits.
Irradiation appears to be an effective pretreatment for improving myoblast transplantation. The injected cells can even produce organised contractile tissue replacing whole muscle. However, impaired nerve regeneration limits the functional performance of the new muscle.
Transplantation of skeletal muscle precursor cells (myoblasts) has been performed in humans and animals to evaluate its potential as a therapy for hereditary muscle diseases (Partridge & Davies, 1995). Sufficient experimental data are now available to recognise problems specific to myoblast transplantations in addition to difficulties encountered with tissue transplantation such as initiation of immune responses or graft death (for reviews see Grounds, 1996; Tremblay & Guerette, 1997). One specific problem with myoblast transplantation is that the amount of donor-derived tissue appears to depend on the in vitro cultivation history of the implanted cells (Irintchev et al. 1997b). Non-cultivated muscle precursor cells of different species grafted as suspensions, muscle minces or slices produce abundant muscle tissue (Carlson, 1972; DiMario & Stockdale, 1995; Fan et al. 1996). However, cells from primary mouse cultures, similar to human or avian myoblasts propagated in vitro, generate little or no tissue in normal or dystrophic muscles (DiMario & Stockdale, 1995; Fan et al. 1996; Grounds, 1996; Tremblay & Guerette, 1997). Better results have been obtained with primary murine cultures expanded over several passages and highly enriched in myoblasts (Rando & Blau, 1994; Irintchev et al. 1997a). Finally, robust murine immortal lines form large amounts of contractile muscle but their use is limited by late neoplastic growth (Wernig et al. 1991; Wernig & Irintchev, 1995; Irintchev et al. 1998).
Apart from intrinsic or acquired properties of the cells, myogenicity in vivo depends on the host environment. Myoblasts from immortal lines produced larger progeny in cryodamaged than in intact or paralysed muscles, which led to a larger increase in contractile strength (Wernig et al. 1991). Intact muscles of normal mice appear to be a poor environment also for myoblasts from expanded primary cultures; only few donor cells survived for 1–4 months and muscle contractile properties did not improve (Irintchev et al. 1997a). However, when host muscles were damaged immediately before cell implantation, larger amounts of donor-derived tissue formed in direct correlation with the degree of damage inflicted (Irintchev et al. 1997a). Finally, myoblasts from the same cultures formed abundant and well-organised muscle tissue in the subcutaneous space (Irintchev et al. 1998). These findings indicate that new tissue formation is regulated by trophic and/or inhibitory factors in the host environment.
Modulation of the host environment by ionising pre-irradiation, which disables the proliferative potential of resident satellite cells, has been found to be an effective pretreatment for myoblast transplantation (Morgan et al. 1990, 1993; Huard et al. 1994; Kinoshita et al. 1994). However, only one study documents positive functional effects (Alameddine et al. 1994). In the present study, irradiation was applied before myoblast transplantation into undamaged or cryodamaged muscles resulting in enhanced – to varying degrees – formation of mature donor tissue in both models. However, it was also observed that after cryodamage the innervation of newly formed muscle was insufficient. Therefore, in order to elucidate the effects of irradiation on nerve regeneration additional experiments were performed i which muscle tissue and blood supply were left intact, but the innervation was blocked either by nerve crush or botulinum toxin. It has already been shown that X-ray irradiation hinders muscle reinnervation after toxin treatment but not after nerve crush (Gomez et al. 1982; Gomez & Love, 1984). Thus, if irradiation arrests Schwann cell proliferation, reinnervation after nerve crush should be less affected than toxin-induced sprouting, since in the former case axons grow into the old endoneurial tubes and no significant Schwann cell proliferation is needed.
The results have been published in part as an abstract (Wernig et al. 1999).
METHODS
Experimental groups of animals
Female Balb/c mice were purchased from Charles River Deutschland (Sultzfeld, Germany) and kept under standard laboratory conditions. All treatments were performed in accordance with the German law for protection of animals, anaesthetics being used where appropriate (see below). At the age of 3 months, all Balb/c animals used in transplantation experiments (n = 29) were subjected to local irradiation of the right hind limb. In one set of experiments, 1 day after radiation pretreatment the right soleus muscles were injected with myoblast suspension (n = 6) or, in the control group (n = 6), with vehicle solution (Hank's balanced salt solution, HBSS, Gibco). In a second set of experiments, irradiated soleus muscles were severely cryodamaged (see below) and cell (n = 8) or vehicle injections (n = 9) performed in the same session. In this case, without irradiation, the poor spontaneous recovery of the damaged muscles is significantly improved after cell implantation (Irintchev et al. 1997a). Additional mdx and Balb/c mice were used in control experiments on radiation efficacy and effects of irradiation on muscle reinnervation after nerve crush and botulinum toxin application (see below).
Pilot in vivo experiments to test radiation efficacy
Initially we considered the possibility that under our experimental conditions the effects of radiation may differ from previous results due to different radiation source, dose rate or type of muscle used (soleus versus tibialis anterior in previous experiments). We used an experimental design comparable to that of Morgan et al. (1990) and studied the expression of dystrophin in irradiated and non-irradiated soleus muscles of dystrophin-deficient mdx mice (n = 3, bred at our animal facility) implanted with normal C57Bl/10 myoblasts. One leg of each animal was irradiated (16 Gy, see below) and 2 days later both the left and right soleus muscles received one million myoblasts derived from hind limb muscles of a post-natal male C57Bl/10 mouse. This cell culture was established and propagated using fibroblast growth factor II as described previously (Irintchev et al. 1997a, b). In two of the animals ‘mild’ cryodamage was performed bilaterally before cell implantation (Irintchev et al. 1997a). Muscles were dissected 67–69 days after implantation, weighed and dystrophin detected with a monoclonal antibody in transverse muscle sections (see below). Higher muscle weights were found in the irradiated muscles in all three muscle pairs. This increase was obviously due to the incorporation of a much larger dystrophin-positive progeny of the implanted cells in the irradiated than in the non-irradiated muscles (Fig. 1). These observations are in accordance with previous results (Morgan et al. 1990, 1993).
Figure 1. Enhanced myogenesis by implanted myoblasts in irradiated muscle.
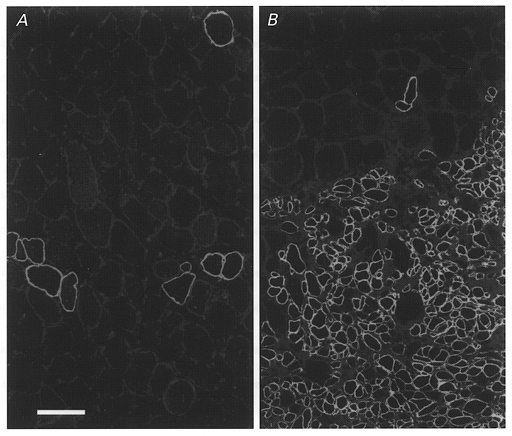
Dystrophin expression in representative transverse sections from the non-irradiated left soleus (A) and the irradiated right soleus (B) of a dystrophin-deficient mdx mouse 67 days after transplantation of myoblasts derived from a non-dystrophin deficient C57Bl/10 mouse. No muscle cryodamage had been imposed before implantation. A, a low number of large- and small-diameter muscle fibres in the non-irradiated muscle express dystrophin. B, dystrophin expression in numerous small-diameter fibres of donor origin is seen in the irradiated soleus muscle. Scale bar in A represents 50 μm and applies also to B.
Irradiation with X-rays
The animals were anaesthetised by intraperitoneal injections of 0.4 mg kg−1 fentanyl (Fentanyl-Janssen, Janssen, Neuss, Germany), 10 mg kg−1 droperidol (Dehydrobenzperidol, Janssen) and 5 mg kg−1 diazepam (Valium 10 Roche, Roche, Grenzach-Wyhlen, Germany) and fixed individually with adhesive tape onto small operation pads. A single dose of 16 Gy was delivered to the right lower hind limb of each animal using an RT 200 irradiation unit (200 kV, 0.5 mm copper filter, Müller, Hamburg, Germany). The focal skin distance and the dose rate were 30 cm and 2.3 Gy min−1, respectively. Local irradiation was ensured by a metal tube placed between the surface of the lower hind limb and the X-ray source.
Myoblast cultures
A primary myoblast culture (designated i28) was previously derived from hind limb muscles of a 7 day old male Balb/c mouse and used, after expansion over two to six passages, for myoblast transplantations (Irintchev et al. 1997a). For the present experiments, cells from this culture were grown on collagen type I-coated Petri dishes in Ham's nutrient mixture F-10 (Gibco BRL, Life Technologies, Eggenstein, Germany) supplemented with 20 % v/v fetal bovine serum (PAA Laboratories, Cölbe, Germany) and penicillin-streptomycin (100 U and 100 μg ml−1, respectively; Sigma, Deisenhofen, Germany). After three passages, the expanded primary cells, virtually all of which were myogenic (desmin positive), were trypsinised, centrifuged and resuspended in HBSS without Ca2+ and Mg2+. Following additional centrifugation, the cells were resuspended for implantation to a final concentration of 106 cells in 4 μl.
Cell implantations
One day after irradiation the animals were anaesthetised again and the right soleus muscle was surgically exposed along its length. The tip of a 26S gauge needle connected to a 10 μl Hamilton syringe was introduced into the muscle near the Achilles tendon and inserted further proximally within the muscle belly up to the proximal muscle aponeurosis. Four microlitres of cell suspension (106 myoblasts) or an equal volume of vehicle (HBSS) were injected while withdrawing the needle slowly. In two groups of animals, injections were performed without additional muscle treatment (6 cell-implanted and 6 vehicle-injected animals). In two other groups the soleus muscles were exposed to severe cryodamage just before cell implantation (n = 8) or vehicle injection (n = 9). The injury consisted of three repeated freezing-thawing cycles in situ. At each freezing cycle, the flat end (3 mm × 0.7 mm) of a copper rod precooled in liquid nitrogen was placed first on the proximal half of the muscle for 10 s and then immediately moved to the distal half were it was kept attached for additional 10 s. This repeated cryodamage, in contrast to single freezing of the mid-portion of soleus muscles, causes large functional and structural impairment after muscle regeneration (Irintchev et al. 1997a).
Following cell or vehicle injection the wounds were closed with 7–0 Ethilon polyamide threads (Ethicon, Norderstedt, Germany) and thereafter the animals were kept for several hours on a warm plate (37°C) to prevent hypothermia. All animals recovered well from the anaesthesia, did not need any special care and did not display apparent locomotory deficits in the post-operative period. Visible effects of the irradiation were delayed wound healing and loss of hair from the irradiated leg. In one irradiated animal the skin incision did not heal (see Results).
Nerve crush and botulinum toxin application
Muscle paralysis and axonal sprouting were induced in one hind limb by injection of 50 pg botulinum toxin type A (a gift from Professor Dreyer, Institute of Pharmacology, Giessen) dissolved in 5 μl physiological saline solution. Toxin application was performed unilaterally in 21 Balb/c mice aged 3–5 months under inhalation anaesthesia with halothane (2 % v/v Fluothane, ICI Pharma, Plankstadt, Germany) evaporated in a mixture of nitrous oxide (Hoechst, Frankfurt am Main, Germany) and oxygen (flow rates through the evaporator 0.8 and 0.4 l min−1, respectively). The tip of a 26S gauge needle connected to a 10 μl Hamilton syringe was introduced into the skin just distally from the fibula head and inserted about 15 mm distally alongside the soleus muscle. Toxin was applied while the needle was slowly withdrawn. One day before toxin application, 11 animals from this group were irradiated as described above. Nerve crush was performed in 31 female Balb/c mice at 3–5 months of age under inhalation anaesthesia (see above). The exposed soleus nerve in one limb was crushed once with fine forceps (Dumont 55 Inox, 0.05 mm × 0.02 mm tip, Fine Science Tools, Heidelberg, Germany) leaving the accompanying blood vessels intact. The operated limb of 14 animals was irradiated 1 day before nerve crush. Isometric force measurements were performed as described below at periods varying from 7 to 112 days after nerve crush or botulinum paralysis.
Isometric force measurements
The isometric contractile properties of myoblast-implanted and control soleus muscles were studied in vitro after post-operation recovery periods of 3–4 months (groups without cryodamage) or 3–6 months (groups with cryodamage) according to a previously described protocol (Badke et al. 1989; Irintchev et al. 1990; Wernig et al. 1990, 1995). The muscles were surgically removed with the intact supplying nerves under neuroleptanalgesia of the animals (see above) after which the mice were killed by cervical dislocation. The nerve-muscle preparations were mounted in a horizontal Lucite chamber (39 mm long × 15 mm wide × 8.5 mm deep) containing approximately 4 ml Tyrode solution (composition (mM): NaCl, 125; MgCl2, 1; CaCl2, 1.8; KCl, 5.4; NaHCO3, 24; and glucose, 10). A large volume, relative to the recording chamber, of this solution (1 l) was kept in a glass flask where it was aerated with a gas mixture of 95 % v/v O2 and 5 % v/v CO2 to maintain the pH at 7.2-7.3. The organ bath was continuously perfused with this buffered and warmed (to 25°C) physiological solution at a rate of 4 ml min−1. The distal (Achilles) tendon of the muscle was tied with silk thread to a force transducer; the proximal tendon was tied to a metal hook firmly attached to the chamber wall. The transducer consisted of a non-flexible plate made of synthetic material. One end of it, carrying a metal hook for muscle fixation, was immersed in the bath and the other one was attached to a slightly flexible metal stripe carrying two strain gauges (DMS LY41 1,5/350, Hottinger Baldwin Messtechnik, Darmstadt, Germany). The transducer system had a compliance of 1.2 mm N−1, unloaded resonant frequency of 330 Hz and a linear response between 0 and 300 mN. Change in muscle length reached about 1 % during maximal tetanic contractions.
For force measurements, muscles were adjusted to optimal length and stimulated directly with electrical pulses via silver electrodes in the bath (direct stimulation, 0.25 mm × 40 mm wire electrodes placed U-shaped at the remote sides of the bath, pulses of 0.5 ms duration, typically 20–25 V) or via nerve with a suction electrode (indirect stimulation, 0.1 ms duration, 3–6 V). The current and the polarisation voltage, measured in the bath filled with Tyrode solution, were 22 mA and 6 V, respectively, when 50 mA/20 V were delivered by the stimulator. The distortion of the rectangular pulses in the bath was small and closely resembled the distortions typical for silver-silver chloride electrode pairs. For both direct and indirect muscle stimulation, stimulus voltage was set to twice the value sufficient to evoke maximum twitch responses. Under such stimulation conditions, force evoked upon direct stimulation of intact and reinnervated muscles is not significantly reduced after application of d-tubocurarine indicating that ‘directly evoked’ contractions are indeed due to stimulation of muscle fibres (Badke et al. 1989). However, we cannot exclude that field stimulation may be subthreshold for very thin (atrophic, denervated) fibres which would result in some underestimation of force generation in partially innervated or denervated muscles. Previous experiments have shown that this potential problem cannot be solved by increasing stimulus intensity. An increase in voltage up to 5-fold above threshold values does not lead to an increase in force of normal or reinnervated muscles (Irintchev, 1992). An increase of pulse duration above 1 ms results in a force increase (up to 3-fold) but no plateau is reached for pulse durations between 1 and 200 ms.
The variables measured during force recordings included isometric twitch and tetanic (20, 50 and 100 Hz for 2 s) force, twitch time to peak (contraction time) and twitch half-relaxation time. For each stimulation frequency, muscles were stimulated first directly and then indirectly. Between two stimulations, muscles were allowed to recover for 3 min. The sequence of stimulations was: twitch, 20, 50, 100 Hz, twitch, 100 Hz. After this series, muscles were stimulated directly with 100 Hz for 2 s and acetylcholine (ACh) sensitivity was tested by rapid exchange of the normal perfusion solution with Tyrode solution containing 0.2 mM ACh perchlorate. The amplitude of the ACh-evoked contracture was expressed as a fraction of the amplitude of the preceding direct 100 Hz tetanus. Since perchlorate is known to alter muscle excitability at millimolar concentrations (Foulks et al. 1973; Dulhunty et al. 1992), we tested, in control experiments, the possibility that the evoked contractures were in part due to perchlorate effects unrelated to ACh receptors. Four contractures were induced with alternating 0.2 mM ACh perchlorate and 0.2 mM ACh chloride in six intact and four denervated (5 days after nerve crush) soleus muscles of adult Balb/c mice. To one-half of the muscles ACh perchlorate was first applied; the rest were initially treated with the chloride derivative. Values from individual measurements varied between 1.0 and 4.6 % of maximum tension for intact muscles and between 25 and 42 % for denervated muscles. For individual muscles, there was small variation in the repeated measurement values (not significant, one-way repeated measures analysis of variance) indicating that measurements with ACh perchlorate and ACh chloride produce similar results. Perchlorate effects were further studied in experiments in which sodium perchlorate at concentrations of 0, 0.2, 0.5, 1.0 and 2.0 mM was applied to two intact and two denervated (5 and 7 days) soleus muscles. Two known effects of perchlorate were observed in the normal, and to a lesser extent, in the denervated muscles: a dose-dependent increase of twitch force (direct stimulation) and a prolongation of the half-relaxation time. However, no contractures occurred upon exchange of the solutions even with 2 mM perchlorate, a concentration 10 times higher than the one used for estimating ACh sensitivity (0.2 mM). This shows that the contractures induced by ACh perchlorate are entirely due to ACh.
After the ACh sensitivity measurement, the muscle chamber was perfused with normal Tyrode solution for 15 min and then a final 100 Hz tetanus was evoked directly to estimate the degree of muscle deterioration during the contraction recording in vitro. The amplitude of this last (fourth) tetanus amounted to 96–103 % of the amplitude of the first 100 Hz tetanic contraction (mean 99 ± 2 %, 56 muscles). Performance upon nerve stimulation also remained stable over time. Thus, we conclude that the nerve-muscle preparations do not loose viability or suffer mechanical damage during the in vitro recordings. This is in agreement with previous observations in this laboratory (Badke et al. 1989) and with the well-preserved morphology of the same muscles observed in histological sections (except for irradiated and cell-implanted muscles which have been partially damaged during dissection; see Results).
Upon completion of the force recordings, the muscles were weighed, fixed at resting length on pieces of turkey liver and frozen in melting isopentane as described previously (Irintchev et al. 1997a). In addition, the twitch/tetanus ratio was calculated from the twitch and maximum tetanic force and specific force calculated as a ratio of maximum tetanic force to wet muscle weight. The latter parameter is usually corrected for actual muscle fibre length and known density of skeletal muscle to obtain a physiological estimate of force production per unit muscle cross-sectional area. This was not done since precise determination of representative muscle fibre length in regenerated, and even more for regenerated cell-implanted murine muscles may be difficult because of extensive abnormalities in the cytoarchitecture including short fibres which do not reach one or both aponeuroses of the soleus muscle (Ontell, 1986; Wernig et al. 1991).
Histology
Cryostat cross-sections 6 μm thick were cut from the mid-portion (end-plate region) of the muscles. Unfixed sections were stained with aqueous Toluidine Blue-borax solution (both at 1 % w/v) to reveal the general morphology. For immunocytochemistry, sections were fixed in pure acetone or methanol. Immunofluorescence identification of cell- and tissue-specific marker molecules was achieved as described previously (Irintchev et al. 1993, 1994, 1997a, c; Wernig & Irintchev, 1995). The following primary antibodies were used at optimal dilutions: anti-desmin (clone D33, Dako, Hamburg, Germany), anti-neural cell adhesion molecule (NCAM; clone H-28; Hirn et al. 1981), anti-dystrophin (clone DYS2, Novo Castra Laboratories, Newcastle upon Tyne, UK), anti-laminin (clone LAM-1, ICN, Meckenheim, Germany), two antibodies that recognise T-cell subsets anti-Ly2/CD8 (TIB 207, American Type Culture Collection, ATCC, Manassas, VA, USA) and anti-L3T4/CD4 (TIB 105, ATCC), and an antibody specific for macrophages, anti-MAC-1 (M1/70, Boehringer Mannheim, Mannheim, Germany). Fixed sections were overlaid with normal goat serum and then incubated overnight at 4°C with primary antibodies diluted in PBS containing 0.7 % w/v lambda-carrageenan (Sigma) and 0.02 % w/v sodium azide. After thorough washing in PBS, the first antibody was visualised with 5-((dichlorotriazin-2-yl)amino)-fluorescein (DTAF)-IgG raised against the appropriate species, or with biotinylated IgG and DTAF- or rhodamine-conjugated streptavidin. All secondary antibodies were affinity purified and purchased from Jackson ImmunoResearch Laboratories (Dianova, Hamburg, Germany).
In situ hybridisation with the Y chromosome-specific DNA probe Y1 (or 145SC5; Nishioka, 1988) was used to identify the donor cell nuclei of male origin present in the female hosts as previously described (Irintchev et al. 1997a). Labelling of the probe and detection of the hybridised DNA in paraformaldehyde-fixed tissue sections was performed with digoxigenin labelling and detection kit (non-radioactive, Boehringer Mannheim). Nuclei were stained with bis-benzimide (Hoechst No. 33258, Sigma).
Quantifications and statistical analysis
Total numbers of muscle fibre profiles and muscle fibre diameters were evaluated on videoprint reconstructions of complete muscle cross-sections (Toluidine Blue staining, final magnification × 610) as described previously (Irintchev et al. 1990; Wernig et al. 1990). Mean orthogonal diameters (mean of the longest axis and a short one passing through the middle of the longest at right angles) were measured using a digitizing tablet. Except for mechanically damaged fibre profiles, all fibres in a cross-section were evaluated.
Multiple comparisons of group mean values were performed with one-way analysis of variance and a subsequent Tukey's test using the SigmaStat 2.0 statistical software package (SPSS Europe, Erkrath, Germany). In some cases, upon indication for non-normal data distribution, a non-parametric statistical approach was used (Kruskal-Wallis one-way analysis of variance on ranks followed by Dunn's test for all pairwise comparisons). For all comparisons the accepted level of significance was 0.05 or less. Unless otherwise indicated, mean group values are given with standard deviations.
RESULTS
Cell implantation into irradiated muscles
Irradiated control muscles
Soleus muscles exposed to a single dose of 16 Gy and sham implanted (injection of salt solution) had smaller masses and lower tetanic force and twitch force (all by about 18 %) than the untreated contralateral muscles by 3–4 months after treatment (Table 1). Nerve-evoked muscle contractions were similar in amplitude and duration to those after direct electrical stimulation and ACh sensitivity was not significantly elevated, both indicating normal innervation (Table 1). Also, twitch contraction time, twitch/tetanus ratio (Table 1) and half-relaxation time (data not shown), all known to be increased in denervated or partially reinnervated muscles (Lewis, 1972; Drachman & Johnston, 1975; Badke et al. 1989; Irintchev et al. 1990), were not significantly different from control values. Force production per unit weight (specific force, Table 1) was normal. No signs of acute muscle fibre damage and regeneration, as well as of fibrosis or cellular infiltration, could be detected in sections stained with Toluidine Blue or for desmin, dystrophin, NCAM, MAC-1, CD4 or CD8. Split fibres and centralised myonuclei, persistent signs of previous muscle damage and regeneration in the mouse, were rare or absent. While total numbers of muscle fibres present in irradiated sham-implanted muscles were similar to those in untreated contralateral muscles (838 ± 34, n = 6 versus 827 ± 29, n = 5, not significant, Student's paired t test), fibre diameters were reduced (Fig. 2). In each of the six pairs of muscles studied, the mean fibre diameter of the irradiated muscle was smaller (P < 0.05, paired t test). The cross-sectional contractile area, calculated from number of fibres and mean fibre diameters, was, on average, 83 % of the area in the untreated muscles. Thus, force deficits (about 18 %) could be entirely attributed to smaller fibre diameters and cross-sectional area of contractile tissue. Accordingly, specific force calculated as a ratio of maximum force and the sum of muscle fibre cross-sectional areas was not different (218 ± 34 kN m−2 for irradiated muscles and 226 ± 33 kN m−2 for intact muscles, not significant, t test). The most likely reason for the small muscle size is diminished growth as the animals' body weights increased from 18 ± 0.6 g at the time of irradiation to 23 ± 1.5 g at the end of the observation period (see also Wakeford et al. 1991; Rosenblatt & Parry, 1993).
Table 1.
Isometric contractile properties and muscle weights of cell-or sham (vehicle)- injected soleus muscles after two different pretreatments
| Group no. | Maximum tetanic force Direct stim. (mN) | Maximum tetanic force Indirect stim. (mN) | Maximum twitch force Direct stim. (mN) | Maximum twitch force Indirect stim. (mN) | Twitch contraction time (ms) | Twitch/tetanus ratio | Wet muscle weight (mg) | Specific force (N g−1) | ACh (50 mg l−1) contracture (%) | No. of muscles |
|---|---|---|---|---|---|---|---|---|---|---|
| Irradiation without cryodamage | ||||||||||
| Sham-implanted muscles | ||||||||||
| 1 | 144 ± 16† | 144 ± 19† | 26 ± 2.0 | 26 ± 2.2 | 36 ± 3.4 | 0.18 ± 0.02 | 9.2 ± 0.9* | 16 ± 1.6 | 6.3 ± 1.9 | 6 |
| Cell-implanted muscles | ||||||||||
| 2 | 168 ± 18 | 166 ± 19 | 29 ± 5.6 | 28 ± 5.4 | 36 ± 3.3 | 0.17 ± 0.02 | 12 ± 1.6 | 14 ± 1.8 | 3.7 ± 1.5 | 4 |
| Untreated contralateral muscles | ||||||||||
| 3 + 4 | 176 ± 13 | 173 ± 14 | 31 ± 3.3 | 31 ± 3.3 | 39 ± 4.9 | 0.18 ± 0.02 | 11 ± 1.3 | 16 ± 1.7 | 4.4 ± 1.1 | 6 + 4 |
| Irradiation and cryodamage | ||||||||||
| Sham-implanted muscles | ||||||||||
| 5 | 7.3 ± 11* | 4.9 ± 11†b | 1.0 ± 1.6§ | 0.7 ± 1.5§b | 42 ± 4.0 | 0.14 ± 0.02 | 6.4 ± 1.8‡c | 1.3 ± 1.9* | 15 ± 0.3† | 9 |
| Cell-implanted muscles | ||||||||||
| 6 | 68 ± 28† | 20 ± 14† | 18 ± 7.2 | 5.3 ± 3.9 | 29 ± 5.8‡d | 0.27 ± 0.03* | 15 ± 4.5 | 4.6 ± 1.5† | 14 ± 3.6†a | 8 |
| Untreated contralateral muscles | ||||||||||
| 7 + 8 | 192 ± 25 | 189 ± 26 | 34 ± 4.9 | 33 ± 4.9 | 41 ± 3.3 | 0.18 ± 0.01 | 12 ± 1.5 | 16 ± 2.2 | 3.3 ± 1.3 | 5 + 6 |
Muscles were studied 3–6 months after (1) X-ray irradiation or (2) irradiation and cryodamage (repeated freezing/thawing) followed by cell implantation (‘cell-implanted muscles’ groups) or injection of salt solution (‘sham-implanted muscles’ groups); included are also data from the untreated (non-irradiated, non-injected) contralateral muscles of the same Balb/c mice. Direct stim. and Indirect stim., direct and indirect electrical stimulation of muscles as defined in the text. Contraction time was measured from direct twitch responses. Values are means ± S.D. The two groups of untreated contralateral muscles (one for non-implanted, one for cell implanted) in each set of experiments (irradiation without cryodamage, groups 3 + 4; and irradiation and cryodamage, groups 7 + 8) had very similar values and therefore, for brevity, overall means of the two groups are shown here; in the statistical analysis, however, these were treated as two independent groups. Statistical analysis (independently performed for groups 1–4 and 5–8): P < 0.05 compared with
all other groups
contralateral controls (one-way ANOVA–Tukey's test)
all other groups
contralateral controls (one-way ANOVA on ranks-Dunn's test). an = 2; bn = 7; cn = 8; dn = 4.
Figure 2. Muscle fibre diameters in untreated and in irradiated sham-implanted soleus muscles.
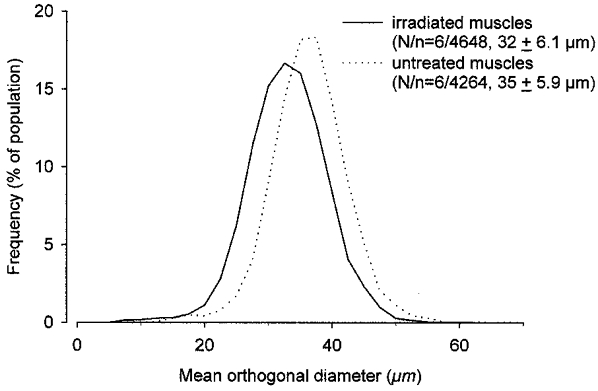
Relative frequency distributions of mean orthogonal diameters (see text for definition) of muscle fibres in the irradiated and sham-implanted right soleus muscles and in the untreated contralateral muscles of Balb/c mice studied 3–4 months after unilateral irradiation. Number of muscles studied (N) and measured muscle fibres (n), as well as group mean values ±s.d. are given in parentheses.
Irradiated cell-implanted muscles
Histocompatible primary myoblasts produce only few muscle fibres in non-irradiated muscles (Irintchev et al. 1997a) while many fibres are formed after irradiation. The difference can be seen on representative sections from myoblast-implanted dystrophic muscles of mdx mice (Fig. 1a and B). Accordingly, contractile strength and muscle mass in irradiated muscles increase (Table 1). On cross-sections, groups of small muscle fibre profiles, uncommon in control irradiated muscles, were present. The nuclei of these fibres, and occasionally nuclei in large-diameter muscle fibres, were of donor origin since they were labelled with the Y chromosome-specific cDNA probe (see below). No deficit in nerve-evoked force was present and sensitivity to bath-applied acetylcholine, contraction time and twitch/tetanus ratio were normal, indicating that all muscle fibres were innervated. In accordance with these physiological results was the finding that muscle fibres did not express NCAM outside the end-plate region (results not shown) which is the case in non-innervated fibres (see in Wernig et al. 1991, 1995; Irintchev et al. 1997a).
In 1 out of 6 animals, a tumour containing Y-positive nuclei formed in the irradiated limb and the implanted muscle was partially destroyed by the tumour masses which had grown mostly outside the soleus muscle. The tumour consisted of desmin- and NCAM-positive mononucleated cells and myotubes of varying calibre, and desmin- and NCAM-negative undifferentiated and/or non-muscle cells.
Cell implantation into irradiated and cryodamaged muscles
Severe cryodamage as performed here kills most cells along the length of the soleus muscle and regeneration occurs via repopulation with proliferating satellite cells from the undamaged muscle ends and from neighbouring muscles (Morgan et al. 1987; A. Irintchev & A. Wernig, unpublished observations). After regeneration, severely cryodamaged soleus muscles of Balb/c mice recover on average 33 % of the normal tetanic force (Irintchev et al. 1997a).
Irradiated and cryodamaged muscles
Three to 6 months after cryodamage of irradiated solei, tissue remnants were found which produced either no (n = 5) or low tetanic forces (3, 12, 19 and 32 mN, n = 4; see Table 1 and Fig. 3a). Muscles consisted mainly of loose fibrous and fatty tissues and wet weights were on average about half of normal (Table 1). Immunostaining for the muscle-specific proteins dystrophin or desmin revealed either complete absence of muscle fibres or presence of few fibres (1–50) with normal or, less frequently, small diameters (inset in Fig. 4). Most fibres with large diameters had peripherally located nuclei suggesting that they were normal soleus fibres which had escaped cryodamage. In a few large-diameter fibres central nucleation, a sign of previous muscle fibre repair, was present. This and the presence of some small fibres suggests that muscle regeneration had been markedly, but not absolutely, blocked by irradiation.
Figure 3. Donor myoblasts re-build irradiated/damaged host muscles.
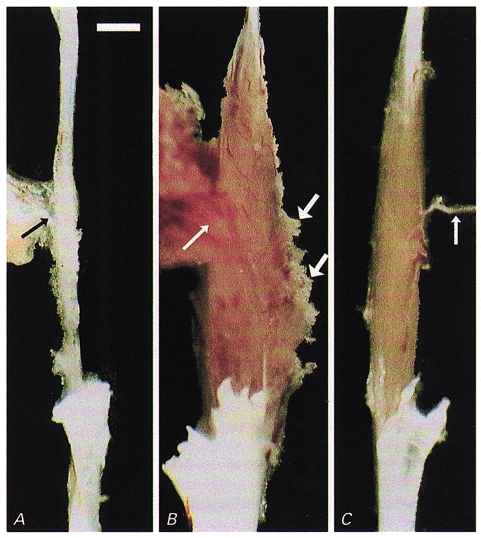
Macroscopic appearance of soleus muscles dissected with nerves (thin arrows) for force recordings. A, a fibrotic remnant (5 mg) of a Balb/c soleus muscle irradiated and cryodamaged 96 days previously which produced no force upon supramaximal stimulation and contained no muscle fibres. B, a large muscle (21 mg) had grown in the bed of an irradiated, damaged and cell-implanted muscle of another animal within 97 days after implantation. The muscle produced 45 mN maximum force upon electrical stimulation. Muscle tissue extruding from the muscle belly (thick arrows) had been damaged during dissection. C, for comparison, the untreated soleus contralateral to that in B is shown (10 mg, 177 mN). Scale bar in A represents 2 mm and applies also to B and C.
Figure 4. Histological appearance of a muscle repopulated by donor cells.
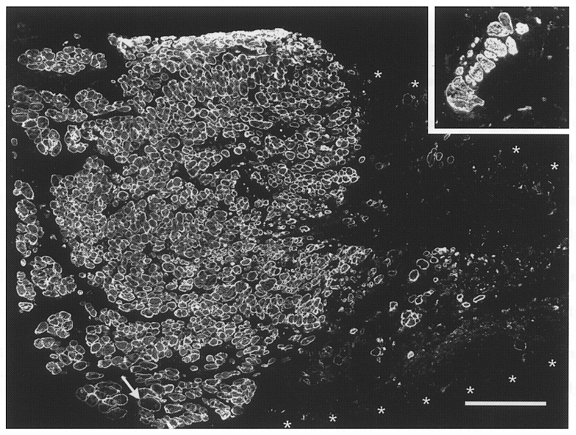
Immunofluorescent staining of desmin in a transverse section through the mid-belly of an irradiated/damaged and cell-implanted muscle studied 3 months after treatment. Contiguous and aligned small-diameter muscle fibres occupy about one-half of the muscle cross-sectional area (left half of the large panel). Fibres of large diameter (one at arrow) are very rare. The rest of the area (muscle border marked by asterisks) contains loose fibrous and fatty tissue (desmin negative) and very few desmin-positive muscle fibres. Inset, desmin-stained transverse section through the mid-portion of an irradiated/ damaged non-implanted muscle studied 4 months after treatment. Only a few muscle fibres, mostly of large calibre, are present in the soleus muscle remnant. Calibration bar represents 200 μm and applies also to the inset.
Cell-implanted muscles
Muscle masses comparable in size to or even larger than untreated muscles developed after implantation of 106 myoblasts into irradiated and cryodamaged solei (Table 1, Fig. 3B and C). Most muscle fibre profiles in these muscles were smaller than normal (Fig. 4), but their numbers were considerably higher than in intact muscles (1200–5200 on individual cross-sections, n = 9, versus 780–850, n = 7). In situ hybridisation with the Y chromosome-specific DNA probe revealed numerous labelled nuclei (Fig. 5). Due to the spot-like labelling of the nuclei, in a 6 μm thick cross-section of a soleus muscle from a normal male mouse only 63 % of the myonuclei were labelled; from 108 bis-benzimide-stained myonuclei in equally thick sections of an experimental muscle, 53 (49 %) were Y chromosome positive, indicating that most muscle fibres originated from the implanted donor cells. Non-repopulated areas of the host muscle were detectable on cross-sections of most muscles (Fig. 4, asterisks) but at other sites often donor-derived tissue extended beyond the apparent borders of the muscle. Under the microscope this ‘excessive’ muscle tissue appeared damaged indicating that connections to neighbouring muscles, disrupted during dissection, had existed (Fig. 3B, thick arrows). On average, only 59 % of the total cross-sectional area of the muscles (range 40–91 %, n = 9) was occupied by undamaged muscle tissue which accounts for the low specific forces developed in vitro (see below). As judged from the round or oval shapes of the fibre profiles in cross-sections (Figs 4 and 5), the muscle fibres were longitudinally aligned (except for a few misaligned fibres found in 2 out of 9 muscles studied).
Figure 5. Y chromosome-positive nuclear progeny of the implanted cells.
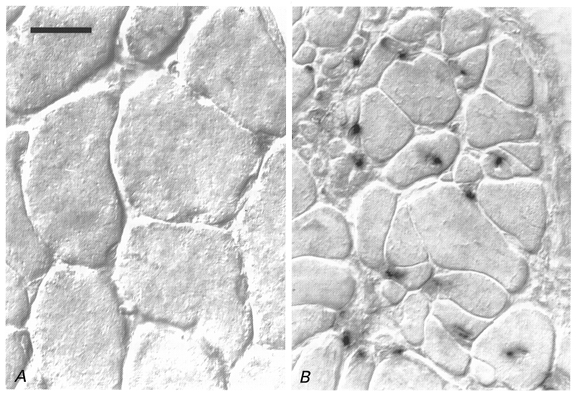
In situ hybridisation of a Y chromosome-specific cDNA probe on transverse sections of an irradiated/damaged muscle implanted with myoblasts derived from a male donor (B) and the contralateral intact soleus (A) of a female Balb/c mouse 4 months after treatment. No nuclear staining is present in or between muscle fibres of normal diameters in the intact muscle of the female animal (A). B, numerous donor-derived positive nuclei are incorporated into muscle fibres most of which have small diameters. Pictures were taken using differential interference contrast (Nomarsky) optics. Scale bar in A represents 20 μm and applies also to B.
Upon direct electrical stimulation, the new muscles developed tetanic forces of approximately 35 % of normal (Table 1). Force production per unit muscle weight was deficient (specific force in Table 1), obviously due to the presence of non-contractile as well as damaged contractile tissue (see above). The degree and/or maturity of innervation was apparently low since only 29 % of the maximum twitch or tetanic force could be elicited via nerve stimulation (Table 1). Also sensitivity to bath-applied ACh was more than 4 times higher than normal (Table 1) indicating the presence of numerous non-innervated muscle fibres with extrasynaptic ACh receptors. Insufficient innervation was also evident from expression of NCAM in numerous fibres with small diameters (not shown). In accordance with these observations was the finding of an increased twitch/tetanus ratio (Table 1). The significant shortening of the twitch contraction (Table 1) and half-relaxation time (not shown), however, was in contradiction with the other indices of incomplete reinnervation. The reason for this discrepancy is not clear.
Effects of X-ray irradiation on muscle reinnervation
Severe cryodamage not only destroys muscle fibres but also vascular and nerve supply, and irradiation might hinder regeneration of all of them. In order to understand the deficits in innervation described above, two types of denervation experiments were performed, in which muscle fibres and blood supply were left intact: crushing of the muscle nerve immediately before entry into the muscle and blocking transmission with botulinum toxin. After crushing, regenerating axons enter the denervated endoneural tubes which contain growth-promoting Schwann cells. In contrast, after botulinum toxin, axonal sprouts have to grow through the extracellular space to form new endplates on adjacent or even remote muscle fibres (see Schäfer & Wernig, 1998), a process which requires extensive growth of accompanying supportive Schwann cells. The results showed that both after nerve crush (Fig. 6A–C) and botulinum paralysis (Fig. 6D–F) recovery of force in irradiated muscles (•, continuous regression line) remained deficient as compared with non-irradiated muscles (^, dashed regression line) for 4 months. The changes with time observed in ACh sensitivity and the degree of innervation (ratio of indirectly versus directly evoked twitch force in per cent) indicated delayed and incomplete reinnervation, as compared with control non-irradiated muscles, in irradiated paralysed muscles (Fig. 6E and F) but not in irradiated muscles with nerve crush (Fig. 6B and C). The presence of significant differences could be verified when we analysed results from muscles studied at periods longer than 2 months after treatment (Table 2), time sufficient for stabilisation of muscle reinnervation without previous irradiation (Tonge, 1974a,b; Fig. 6). Force production upon nerve stimulation of irradiated and paralysed muscles was deficient and, accordingly, ACh sensitivity and twitch/tetanus ratio remained significantly elevated as compared with all other groups (Table 2). These results suggest that irradiation hinders reinnervation by arresting Schwann cell proliferation which may account for the poor degree of muscle innervation after cryodamage and irradiation (Table 1) where cryodamage-induced loss of Schwann cells does certainly occur.
Figure 6. Effects of X-ray irradiation on muscle reinnervation after nerve crush or paralysis with botulinum toxin.

After nerve crush (A–C) reinnervation appeared fast and was nearly complete in both non-irradiated (^) and irradiated muscles (•) as indicated by similarity of contractile force evoked by nerve and direct electrical stimulation (degree of innervation, C) and decreasing sensitivity to bath-applied acetylcholine (B, ACh-induced contracture amplitude as a percentage of maximum tetanic force). Reinnervation after botulinum paralysis (D–F) remained insufficient throughout the period of observation as seen from the low degree of innervation (F) and high sensitivity to bath-applied acetylcholine (E) in irradiated (•) versus non-irradiated muscles (^). Note the persisting force deficit in irradiated muscles as compared with non-irradiated solei (A and D) and the reduced maximum force in non-irradiated botulinum-treated muscles compared with nerve crush without irradiation (A and D). Each point represents one muscle. The plotted curves are non-linear regression lines for irradiated (continuous lines) and non-irradiated muscles (dashed lines) fitted with SigmaPlot 5.0 software (SPSS Europe, Erkrath, Germany).
Table 2.
Maximum tetanic force and innervation-related parameters of irradiated and non-irradiated muscles studied 61–112 days after nerve crush or botulinum paralysis
| Group | Maximum tetanic force (P0, mN) | ACh (0.2 mm) contracture (%P0) | Degree of innervation, twitch (%) | Twitch/tetanus ratio | No. of muscles |
|---|---|---|---|---|---|
| Nerve crush after irradiation | 102 ± 40* | 4.1 (2.7, 6.8) | 98 (90, 100) | 0.20 ± 0.02 | 5 |
| Nerve crush | 159 ± 29 | 2.3 (1.8, 3.0) | 100 (97, 100) | 0.19 ± 0.02 | 7 |
| Botulinum toxin after irradiation | 92 ± 22* | 30 (22, 34)§ | 61 (46, 65)§ | 0.37 ± 0.04† | 9 |
| Botulinum toxin | 136 ± 32 | 15 (12, 18) | 79 (75, 94) | 0.25 ± 0.02‡ | 8 |
Values are means ± s.d. or medians with 25th and 75th percentile in brackets. Degree of innervation, twitch: ratio of indirectly and directly evoked twitch contraction force in per cent. Statistically significant differences: P < 0.05 compared with:
the appropriate (denervated in a similar way) control group
all other groups
nerve crush group (one-way ANOVA-Tukey's test)
all other groups (one-way ANOVA on ranks – Dunn's test).
It is important to note that maximum tetanic force after irradiation and nerve crush remained below control levels despite apparently complete reinnervation (Fig. 6a, Table 2). This resembles the growth-related force deficit observed in irradiated intact muscle (see Table 1) and can be attributed to the inability of irradiated muscle satellite cells to provide sufficient numbers of myonuclei to muscle fibres recovering from atrophy.
DISCUSSION
The present experiments are part of a programme in which different pretreatments of muscles are performed to enhance the effects of myoblast implantation. The results show that pre-irradiation and cryodamage increase the efficacy of cell transplantation. While implantation of myogenic cells into untreated muscles had no functional effects (Wernig et al. 1991, 1995; Irintchev et al. 1997a), irradiated muscles increased in mass (+3 mg, i.e. 25 % of control weight) and produced more force (+24 mN, 17 %). In combination with severe cryodamage, by itself an effective pretreatment (+3 mg mass and +54 mN force over spontaneous regeneration, Irintchev et al. 1997a), irradiation was even more effective in promoting new muscle tissue formation (+9 mg, from Table 1). These comparisons raise three important issues. First, it is clear that donor-derived muscle masses, large enough to replace a host muscle, are obtained after irradiation and cryodamage. Second, combining cryodamage and irradiation had a potentiating effect which suggests different mechanisms of action. Third, even months after implantation the new muscle tissue was only partly innervated. Together with formation of muscle tissue outside the implanted soleus muscle, functional effects are thus less impressive than the production of donor-derived muscle tissue would suggest.
Recent evidence indicates that pre-irradiation of host muscles does not reduce cell death occurring in the early post-transplantation period but enhances the proliferation of the surviving myoblasts (Beauchamp et al. 1999). Why myoblast replication, and thus formation of donor tissue, are enhanced is not clear. It can be speculated that endogenous satellite cells have advantages over bulk-implanted myogenic cells in accomplishing muscle regeneration. Thus, when host satellite cells are inhibited by pre-irradiation, tissue repair will predominantly involve implanted cells. In favour of a cell competition hypothesis is also the finding that myoblasts injected into untreated muscle yield only few new muscle fibres, but form large amounts in the subcutaneous space which is devoid of satellite cells (Irintchev et al. 1998). In pre-irradiated (and cryodamaged) muscles, large amounts of donor-derived muscle tissue formed outside the implanted soleus muscle which indicates the presence of strong mitogenic signals. This is further supported by the formation of a tumour in one of the irradiated and implanted limbs. Tumours derived from implanted myoblasts developed in 6 out of 6 irradiated limbs in C57Bl/10 mice, but 0 out of 6 in non-irradiated limbs (A. Wernig, unpublished observations; see also unpublished observations by C. N. Pagel, communicated by Morgan et al. 1993). As shown in recent work (Beauchamp et al. 1999) irradiation appears to induce strong mitogenic signals which enhance myogenesis and may even cause tumour formation.
Previous work has shown that local irradiation may lead to impairment of Schwann cell proliferation and thus to inadequate support of axonal growth, end-plate formation and synaptic function while axonal growth capacity per se is not inhibited (Cavanagh, 1968; Gomez et al. 1982; Gomez & Love, 1984; Love, 1983). In the present investigation, reinnervation after nerve crush was hardly inhibited by irradiation indicating that the Schwann cells remaining in the denervated endoneurial tubes sufficiently support axonal growth and synapse formation. Even botulinum toxin-induced axonal sprouting was supported to some degree allowing new synapse formation and muscle reinnervation to 40–70 %. Similarly, new endplates form on donor-derived muscle fibres in irradiated, non-cryodamaged muscles. Only after nearly killing the muscle and the soleus nerve with its Schwann cells by repeated freezing-thawing, did the gap between the surviving nerve stump and the regenerating muscle become too large to be bridged by Schwann cells invading from the proximal nerve stem. Thus, only experimental conditions in which Schwann cells have to accompany growing axons into new territory over considerable distances or bridge too large gaps, lead to insufficient reinnervation after irradiation (see also Gomez et al. 1982; Scaravilli et al. 1986; Wernig & Herrera, 1986; Son et al. 1996).
It has previously been shown that at least a fraction of the satellite cells isolated from irradiated avian muscles retained their proliferative capacity in vitro (Mozdziak et al. 1996). In mdx mice even 30 Gy did not completely prevent regeneration (Quinlan et al. 1995). Also in the present investigation, some regeneration in damaged muscle occurred in spite of irradiation with 16 Gy and it appears that murine satellite cells are highly resistant to radiation as recently shown for avian muscle (Mozdziak et al. 1996). On the other hand, contractile muscle force and muscle mass of irradiated muscles did not recover within months after nerve crush in spite of complete reinnervation. This indicates that the presumed radiation-insensitive fraction of muscle precursor cells is not utilised for reversal of muscle atrophy. Whether these observations indicate the existence of different classes of satellite cells will have to be elucidated.
Acknowledgments
This work was supported by a Biomed 2 grant from the European Commission (BMH4-97-2767), a grant from the Deutsche Forschungsgemeinschaft (We 859/7) and the Vigoni programme. Stefanie Briel, Jennifer Heckroth and Karin Müller-Using are thanked for their excellent technical assistance. Martin Pfendtner was helpful with the in situ hybridisations. Professor Dr Hermann Rink at the Department of Experimental Radiology, University of Bonn, is acknowledged for performing the irradiations.
References
- Alameddine HS, Louboutin JP, Dehaupas M, Sebille A, Fardeau M. Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplantation. 1994;3:3–14. doi: 10.1177/096368979400300103. [DOI] [PubMed] [Google Scholar]
- Badke A, Irintchev A, Wernig A. Maturation of transmission in reinnervated mouse soleus muscle. Muscle and Nerve. 1989;12:580–586. doi: 10.1002/mus.880120709. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal discrete minority of precursors with stem cell-like properties as the myogenic source. Journal of Cell Biology. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BM. The regeneration of minced muscles. Monographs in Developmental Biology. 1972;4:1–128. [PubMed] [Google Scholar]
- Cavanagh JB. Effects of X-irradiation on the proliferation of cells in peripheral nerve during Wallerian degeneration in the rat. British Journal of Radiology. 1968;41:275–281. doi: 10.1259/0007-1285-41-484-275. [DOI] [PubMed] [Google Scholar]
- Dimario JX, Stockdale FE. Differences in the developmental fate of cultured and noncultured myoblasts when transplanted into embryonic limbs. Experimental Cell Research. 1995;216:431–442. doi: 10.1006/excr.1995.1054. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Johnston DM. Neurotrophic regulation of dynamic properties of skeletal muscle: effects of botulinum toxin and denervation. The Journal of Physiology. 1975;252:657–667. doi: 10.1113/jphysiol.1975.sp011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF, Zhu PH, Patterson MF, Ahern G. Actions of perchlorate ions on rat soleus muscle fibres. The Journal of Physiology. 1992;448:99–119. doi: 10.1113/jphysiol.1992.sp019031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Beilharz MW, Grounds MD. A potential alternative strategy for myoblast transfer therapy: The use of sliced muscle grafts. Cell Transplantation. 1996;5:421–429. doi: 10.1177/096368979600500309. [DOI] [PubMed] [Google Scholar]
- Foulks JG, Miller JA, Perry FA. Repolarization-induced reactivation of contracture tension in frog skeletal muscle. Canadian Journal of Physiology and Pharmacology. 1973;51:324–334. doi: 10.1139/y73-049. [DOI] [PubMed] [Google Scholar]
- Gomez S, Duchen LW, Hornsey S. Effects of X-irradiation on axonal sprouting induced by botulinum toxin. Neuroscience. 1982;7:1023–1036. doi: 10.1016/0306-4522(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Gomez S, Love S. Reinnervation of muscle in the mouse following nerve crush and X-irradiation. Quarterly Journal of Experimental Physiology. 1984;69:197–205. doi: 10.1113/expphysiol.1984.sp002780. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Commentary on the present state of knowledge for myoblast transfer therapy. Cell Transplantation. 1996;5:431–433. doi: 10.1177/096368979600500310. [DOI] [PubMed] [Google Scholar]
- Hirn M, Pierres M, Deagostini-Bazin H, Hirsch M, Goridis C. Monoclonal antibody against cell surface protein of neurons. Brain Research. 1981;214:433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Huard J, Verreault S, Roy R, Tremblay M, Tremblay JP. High efficiency of muscle regeneration after human myoblast clone transplantation in SCID mice. Journal of Clinical Investigation. 1994;93:586–599. doi: 10.1172/JCI117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A. University of Bonn; 1992. Reinnervation und Erholung des Muskels nach langzeitiger Denervation; p. 95. [Google Scholar]
- Irintchev A, Draguhn A, Wernig A. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience. 1990;39:231–243. doi: 10.1016/0306-4522(90)90236-w. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Langer M, Zweyer M, Theisen R, Wernig A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. The Journal of Physiology. 1997a;500:775–785. doi: 10.1113/jphysiol.1997.sp022057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Langer M, Zweyer M, Wernig A. Myoblast transplantation in the mouse: what cells do we use? Basic and Applied Myology. 1997b;7:161–166. [Google Scholar]
- Irintchev A, Rosenblatt JD, Cullen MJ, Zweyer M, Wernig A. Ectopic skeletal muscles derived from myoblasts implanted under the skin. Journal of Cell Science. 1998;111:3287–3297. doi: 10.1242/jcs.111.22.3287. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Salvini TF, Faissner A, Wernig A. Differential expression of tenascin after denervation, damage or paralysis of mouse soleus muscle. Journal of Neurocytology. 1993;22:955–965. doi: 10.1007/BF01218353. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated and regenerating mouse muscles. Developmental Dynamics. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zweyer M, Wernig A. Impaired functional and structural recovery after muscle injury in dystrophic mdx mice. Neuromuscular Disorders. 1997c;7:117–125. doi: 10.1016/s0960-8966(96)00422-1. [DOI] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Guerette B, Asselin I, Roy R, Tremblay JP. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle and Nerve. 1994;17:1407–1415. doi: 10.1002/mus.880171210. [DOI] [PubMed] [Google Scholar]
- Lewis DM. The effect of denervation on the mechanical and electrical responses of fast and slow mammalian twitch muscle. The Journal of Physiology. 1972;222:51–75. doi: 10.1113/jphysiol.1972.sp009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. An experimental study of peripheral nerve regeneration after X-irradiation. Brain. 1983;106:39–54. doi: 10.1093/brain/106.1.39. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Coulton GR, Partridge TA. Muscle precursor cells invade and repopulate freeze-killed muscles. Journal of Muscle Research and Cell Motility. 1987;8:386–396. doi: 10.1007/BF01578428. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. Journal of Cell Biology. 1990;111:2437–2450. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JE, Pagel CN, Sherratt T, Partridge TA. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. Journal of the Neurological Sciences. 1993;115:191–200. doi: 10.1016/0022-510x(93)90224-m. [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Schultz E, Cassens RG. The effect of in vivo and in vitro irradiation (25 Gy) on the subsequent in vitro growth of satellite cells. Cell and Tissue Research. 1996;283:203–208. doi: 10.1007/s004410050530. [DOI] [PubMed] [Google Scholar]
- Nishioka AY. Application of Y-chromosomal repetitive sequences to sexing mouse embryos. Teratology. 1988;38:181–185. doi: 10.1002/tera.1420380211. [DOI] [PubMed] [Google Scholar]
- Ontell M. Morphological aspects of muscle fiber regeneration. Federation Proceedings. 1986;45:1461–1465. [PubMed] [Google Scholar]
- Partridge TA, Davies KE. Myoblast-based gene therapies. British Medical Bulletin. 1995;51:123–137. doi: 10.1093/oxfordjournals.bmb.a072942. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle and Nerve. 1995;18:201–206. doi: 10.1002/mus.880180209. [DOI] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. Journal of Cell Biology. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflügers Archiv. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- Scaravilli F, Love S, Myers R. X-irradiation impairs regeneration of peripheral nerve across a gap. Journal of Neurocytology. 1986;15:439–449. doi: 10.1007/BF01611727. [DOI] [PubMed] [Google Scholar]
- Schäfer R, Wernig A. Polyclonal antibodies against NCAM reduce paralysis-induced sprouting. Journal of Neurocytology. 1998;27:615–624. doi: 10.1023/a:1006978429608. [DOI] [PubMed] [Google Scholar]
- Son YJ, Trachtenberg JT, Thompson WJ. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends in Neurosciences. 1996;19:280–285. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- Tonge DA. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. The Journal of Physiology. 1974a;241:127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge DA. Physiological characteristics of re-innervation of skeletal muscle in the mouse. The Journal of Physiology. 1974b;241:141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JP, Guerette B. Myoblast transplantation: a brief review of the problems and of some solutions. Basic and Applied Myology. 1997;7:221–230. [Google Scholar]
- Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle and Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Wernig A, Herrera AA. Sprouting and remodelling at the nerve-muscle junction. Progress in Neurobiology. 1986;27:251–291. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]
- Wernig A, Irintchev A. ‘Bystander’ damage of host muscle caused by implantation of MHC-compatible myogenic cells. Journal of the Neurological Sciences. 1995;130:190–196. doi: 10.1016/0022-510x(95)00034-y. [DOI] [PubMed] [Google Scholar]
- Wernig A, Irintchev A, Härtling A, Stephan G, Zimmermann K, Starzinski-Powitz A. Formation of new muscle fibres and tumours after injection of cultured myogenic cells. Journal of Neurocytology. 1991;20:982–997. doi: 10.1007/BF01187916. [DOI] [PubMed] [Google Scholar]
- Wernig A, Irintchev A, Lange G. Functional effects of myoblast implantation into histoincompatible mice with or without immunosuppression. The Journal of Physiology. 1995;484:493–503. doi: 10.1113/jphysiol.1995.sp020681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Irintchev A, Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. The Journal of Physiology. 1990;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Zweyer M, Irintchev A. Function of skeletal muscle formed after myoblast transplantation into irradiated mouse muscles. Pflügers Archiv. 1999;437:R145. doi: 10.1111/j.1469-7793.2000.t01-2-00333.x. abstract suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]


