Abstract
The input characteristics and distribution of climbing fibre field potentials evoked by electrical stimulation of various parts of the skin were investigated in the cerebellum of barbiturate anaesthetized rats. Climbing fibre responses were recorded in sagittally oriented microelectrode tracks across the mediolateral width of the anterior lobe.
Climbing fibres with similar response latencies and convergence patterns terminated in sagittal bands with widths of 0.5–1.5 mm. The principal organization of the anterior lobe with respect to input characteristics and locations of sagittal zones was similar to that in the cat and ferret. Hence, the sagittal bands in the rat were tentatively named the a, b, c1, c2 and d1 zones.
In contrast to the cat and ferret, the a zone of the rat was characterized by short latency ipsilateral climbing fibre input. Furthermore, it was divisible into a medial ‘a1’ zone with convergent, proximal input and a lateral ‘ax’ zone with somatotopically organized input. A forelimb area with similar location and input characteristics as the X zone of the cat was found, but it formed an integral part of the ax zone. A somatotopic organization of ipsilateral, short latency climbing fibre input was alsofound in the c1 zone.
Rostrally in the anterior lobe, climbing fibres activated at short latencies from the ipsilateral side of the body terminated in a somatotopically organized transverse band which extended from the midline to the lateral end of the anterior lobe.
The absence of the C3 and Y zones may be interpreted as a reflection of differences in the organization of the motor systems in the rat as compared with the cat. Skilled movements, which in the cat are controlled by the C1, C3 and Y zones via the anterior interposed nucleus, may in the rat be partly controlled by the ax zone via the rostrolateral part of the fastigial nucleus.
Based on anatomical studies of cerebellar myeloarchitecture and olivo-cortico-nuclear connectivity, Voogd (1964, 1969) divided the cerebellar cortices of the ferret and cat into seven longitudinal bands or sagittal zones, from medial to lateral, the A, B, C1, C2, C3, D1 and D2 zones. Similar techniques have since been used to demonstrate the existence of these zones in many other species (Voogd & Glickstein, 1998). Since each of the above zones innervate specific cerebellar and/or vestibular nuclei, which in turn innervate different ascending and descending systems (Voogd, 1964), the sagittal zones were put forward as the functional units of the cerebellum (see Ito, 1984). In a parallel line of investigations in the anterior lobe of the cat, Oscarsson showed that climbing fibre responses evoked on electrical stimulation of various limb nerves have distinct response latencies and convergence patterns in these longitudinal zones (Oscarsson, 1968, 1969; Larson et al. 1969a, b). Moreover, the response characteristics seem to be related to the motor function of the zone and input to the different zones is mediated by different spino-olivary pathways, i.e. pathways ascending through specific spinal funiculi and with synaptic relays in specific parts of the spinal cord and/or brainstem (Oscarsson, 1968, 1969, 1980; Larson et al. 1969a, b).
Using electrophysiological techniques, Ekerot & Larson (1979a) identified a previously unknown zone between the A and B zones in the vermis of the anterior lobe, the X zone. In a subsequent study, it was found that climbing fibres innervating the X zone branch and also supply the lateral C1 zone, which was hence renamed the Cx zone (Ekerot & Larson, 1982). Similarly, the lateral C3 zone shares branching climbing fibres with the Y zone of the anterior lobe (initially named the D2 zone), and the medial part of the C1 zone shares climbing fibre supply with the medial part of the C3 zone (Ekerot & Larson, 1982). Altogether, nine zones have now been identified in the cerebellar anterior lobe of the cat, the A, X, B, C1, Cx, C2, C3, D1 and Y zones.
Electrophysiological methodology has also been instrumental in demonstrating that the B and C3 zones are further divisible into microzones (Andersson & Oscarsson, 1978; Ekerot et al. 1991; see also Ekerot & Larson, 1979b). Microzones are narrow, longitudinal cortical strips defined by similar peripheral climbing fibre receptive fields and innervate specific parts of the efferent nucleus of the zone (Andersson & Oscarsson, 1978; Garwicz & Ekerot, 1994). It has been shown that the microzones of the C3 zone have specific nucleofugal output and hence appear to represent functional units (see Garwicz et al. 1998).
Several investigations suggest many overall similarities between the zonal organization of the rat cerebellum and that of the cerebella of the cat and ferret (Campbell & Armstrong, 1983; Azizi & Woodward, 1987; Buisseret-Delmas, 1988a, b; Buisseret-Delmas & Angaut, 1989, 1993; Atkins & Apps, 1997). However, the results from the above studies of the rat cerebellum are not entirely consistent and some parts of the anterior lobe that are difficult to reach from the dorsal surface of the cerebellum have not been investigated in detail. Physiological techniques have the advantage of outlining functional aspects of organization that are independent of macroanatomical boundaries, suggesting that such techniques can be used to clarify and outline details of the organization. For these reasons, we have used electrical stimulation of various parts of the skin to study the organization of climbing fibre input to the cerebellar anterior lobe of the rat.
Preliminary results have been presented (Jörntell et al. 1993; Ekerot et al. 1996).
METHODS
Preparation
Eleven adult Wistar rats weighing 200–450 g were initially anaesthetized with pentobarbital (60–80 mg kg−1; i.p., supplemented as necessary with 5–10 mg kg−1; i.v.). The level of anaesthesia was characterized by constricted pupils, complete muscle atonia before the administration of paralysing agents and stable blood pressure which did not change during the surgery, upon noxious pinch or electrical stimulation during the experiment. Cannulae were inserted into the trachea, the right jugular vein and the right femoral vein and artery. The animals were artificially ventilated and given a continuous infusion (buffered Ringer-acetate and glucose solution). The end-expiratory CO2 levels, arterial blood pressure and rectal temperature were continuously monitored and kept within physiological limits (4.0-4.5 %, 90–120 mmHg and 37.5-38.5°C, respectively). When necessary, metaraminol was added to the infusion to raise the blood pressure. All wound areas were infiltrated by lignocaine (lidocaine). The animals were paralysed with alcuronium chloride during recordings. The head of the animal was fixed in a frame by ear bars covered with lignocaine and by a nose ring. To increase the mechanical stability of the brain, cerebrospinal fluid was drained through a hole in the dura between the occipital bone and the first vertebra. A craniotomy was performed to expose cerebellar lobules V-VII on the left side and the overlying dura was cut. A pool of agar was built around the cerebellum and brain stem and filled with warm mineral oil to prevent the exposed parts from drying out.
Stimulation and recording
A glass-insulated tungsten microelectrode (exposed tip 30–50 μm) was inserted through the surface of lobule VI. The electrode was parasagittally oriented and tilted about 30 deg rostrally (Fig. 1a). The electrode was advanced by a motorized micromanipulator and the depth from the surface was indicated on the electronic unit controlling the manipulator. All electrode tracks were made in approximately the same transverse plane with mediolateral distances of 150–600 μm. Peripheral electrical stimulation evoked characteristic responses at the pial surfaces and in the successive molecular, Purkinje cell and granule cell layers (Eccles et al. 1967). Histological reconstruction of the electrode tracks in combination with notes on the sequential recording depths of these layers allowed sublobular localization of the recording points. Before data sampling, the electrode was adjusted to the depth within the molecular layer where the negative climbing fibre field potential reached its maximum (mossy fibre evoked activity was strongly depressed in the molecular layer due to the deep barbiturate anaesthesia, cf. Körlin & Larson, 1970). The onset latencies of these field potentials were defined as the time at which there was an obvious deviation from baseline noise. For each successive molecular layer encountered, stimulus-triggered recordings were saved on a computer disc (sweep length 51.2 ms, three sweeps, sampling time 100 μs, pretrigger time 10 ms).
Figure 1. Microelectrode trajectory and peripheral stimulation sites.

A, approximate insertion and orientation of the investigating microelectrode indicated in a sagittal section of the cerebellum. Shaded area corresponds to the granule cell layer. Roman numerals refer to the cerebellar lobules according to Larsell (1970). PF, primary fissure. B, approximate locations of the different stimulation sites (•). iFace, ipsilateral face; iF, ipsilateral forelimb; iFprox, ipsilateral forelimb, proximal part; cF, contralateral forelimb; iHprox, ipsilateral hindlimb, proximal part; iH, ipsilateral hindlimb; cH, contralateral hindlimb.
Pairs of percutaneous needle electrodes, insulated except at their tips, were used for electrical stimulation (100 μs shocks at 0.8 mA, 3 s interstimulus interval). When tested on the skin of one of the investigators, such stimulation was judged to activate mainly local fibres of Aβ type, although activation of smaller nerve trunks could also occur. There was no stepwise shortening of climbing fibre response latencies or increases in amplitudes when stimulus intensities were increased from threshold up to 2.0 mA. This indicates that no major nerve trunks were activated. In the experiments, up to eight different stimulation sites were used. The locations of these sites and their abbreviations as they appear in the text are shown in Fig. 1B. Since the contralateral femoral artery was cannulated to allow monitoring of the blood pressure, the distal part of the contralateral hindlimb could not be used for electrical stimulation. Instead, the skin overlying the gluteus muscles of this limb was cut and the needle pair was inserted blindly into these muscles in order to reach the sciatic nerve (cH in Fig. 1B).
Histology
After the experiment, the animal was killed with an overdose of barbiturate and perfused with a 10 % solution of formaldehyde in saline. After 8–14 h the cerebellum was removed. After at least 2 weeks storage in 10 % formaldehyde, the cerebellum was sagittally sectioned at 50 μm and stained with cresyl-violet. The trajectories of the different electrode tracks were then analysed. Sagittal sections were projected and redrawn to scale on a sheet of paper with the aid of a light projector, and the length of the Purkinje cell layer in individual sublobules was measured with a small distance measurement wheel.
The experiments were approved in advance by the local Swedish Ethics Committee.
RESULTS
Investigated areas of the cerebellar cortex and characteristics of the evoked climbing fibre activity
Recording microelectrodes were usually inserted in lobule VI and recordings were made in the molecular layers encountered along the electrode trajectory towards more rostral lobules (Fig. 1a). Table 1 summarizes the number of experiments from which recordings in different lobules were obtained. Local climbing fibre field potentials evoked from peripheral stimulation sites (Fig. 1B) were characterized by their maximal negativities being recorded in the deep third of the molecular layer close to the Purkinje cell layer and their reversal in the superficial part of the molecular layer. Furthermore, onset latencies of large evoked climbing fibre field potentials corresponded to the onset latencies of evoked complex spikes in the Purkinje cell layer.
Table 1.
Number of experiments covering specific lobules
| Vermis | Pars intermedia | iFace | Vermis | Pars intermedia | |||
|---|---|---|---|---|---|---|---|
| A | Lobule II | 5 | — | B | Lobule II | 2 | — |
| Lobule III | 6 | 5 | Lobule III | 2 | 2 | ||
| Lobule IV | 5 | — | Lobule IV | 1 | — | ||
| Lobule V | 6 | 7 | Lobule V | 2 | 3 | ||
| Lobule VI | 6 | 7 | Lobule VI | 2 | 3 |
The numbers of experiments in which specific lobules were investigated are given separately for vermis and pars intermedia. All data included here refer to investigations of the full mediolateral spans of vermis or pars intermedia in the plane of the electrode tracks. Note that the full mediolateral span of every lobule was not always covered. In pars intermedia, lobules II and III and lobules IV and V are separated by distinct fissures only in the most medial part and hence observations in these lobules are ascribed to lobules III and V, respectively. iF, cF, iH, iHprox, Tail and cH were used in all experiments described here (A) whereas iFprox was not included in two of them (both of which covered the entire anterior lobe) and iFace was included in three experiments (B).
Identification of sagittal zones: response latencies and convergence patterns of peripherally evoked climbing fibre activity
Figure 2 shows sample climbing fibre field potentials recorded at nine different locations between the medial vermis to the most lateral part of the anterior lobe (recording points 1–5 and 6–9 are from two different experiments). In the medial vermis (recording points 1–4), climbing fibre responses were evoked with short latencies from ipsilateral stimulation sites. In the lateral vermis (Fig. 2, panel 5), irregular responses with low amplitudes and long latencies were evoked from all four limbs. In medial pars intermedia (Fig. 2, panels 6 and 7), ipsilateral input with short response latencies was found again flanked on the lateral side by bilateral input with intermediate response latencies (Fig. 2, panel 8). In the most lateral part of the anterior lobe only occasional climbing fibre responses evoked at long latencies mainly from ipsilateral, distal stimulation sites were found (Fig. 2, panel 9).
Figure 2. Sample climbing fibre responses.

The tentative zonal identification of the recording points is indicated on top and the stimulation sites to the left; for abbreviations see Fig. 1B. Panel numbers refer to recording points indicated in Figs 5 and 6.
In all experiments, climbing fibre responses with the input characteristics described above were found in similar mediolateral sequences throughout most of the depths of the electrode tracks, indicating an organization resembling the sagittal zonation in the cat and ferret. Based on these similarities in input characteristics and locations, the five zones described above were tentatively named the a (medial vermis), b (lateral vermis), c1 (medial pars intermedia), c2 (lateral pars intermedia) and d1 (lateralmost pars intermedia) zones. Figure 3 summarizes the onset latencies of the climbing fibre responses in the five zones. For each stimulation site (see Fig. 1B), the frequency distribution of onset latencies fell into three relatively distinct groups (‘short’, ‘intermediate’ and ‘long’ response latencies). The frequency distributions within the different latency groups were similar in all experiments.
Figure 3. Climbing fibre response latencies in different sagittal zones.

Frequency histograms of climbing fibre response latencies for individual stimulation sites in different zones. Dotted vertical lines divide response latencies into three groups, short, intermediate and long. The range for the intermediate response latencies is indicated for each stimulation site. Only responses regularly exceeding 200 μV are included. Responses in the rostral short latency representation (see text) in the vermis and pars intermedia are included in the histograms of the a and c1 zones, respectively.
To show the general distribution of the zones, Fig. 4 illustrates the climbing fibre field potentials with short and intermediate response latencies in one experiment. The wide a zone was separated from the c1 zone by a relatively narrow b zone. The latter was usually not found rostral to lobule III. Lateral to the c1 zone, climbing fibres had intermediate response latencies (Fig. 4) and convergent input (Fig. 6) characteristic of the c2 zone. The d1 zone was located in the very small strip of the anterior lobe that remained lateral to the c2 zone, but in the experiment illustrated in Fig. 4 that strip could not be reached. In other experiments, we found irregular but relatively large responses with long latencies mainly from iH rostrally (see Fig. 2, panel 9 and Fig. 6) and from iF and iFace caudally (see Fig. 3) in this area (for abbreviations see Fig. 1B). In addition to revealing a zonal organization in the anterior lobe, the evoked climbing fibre responses also revealed an intrazonal topography within the a and c1 zones.
Figure 4. General distribution of climbing fibre responses.

Data from a sample experiment covering most of the anterior lobe. Amplitudes of climbing fibre field potentials recorded in the same lobule are plotted as a mountain range, the filling of which indicates the response latencies according to the groups defined in Fig. 3. Responses evoked from all eight stimulation sites (see Fig. 1) are superimposed. Recording points with no responses are indicated by horizontal lines. Track positions are indicated by arrows. Dashed lines indicate the distribution of sagittal zones. The position of the paravermal vein (PV) at the surface of lobule VI is also indicated. Med, medial; Lat, lateral.
Figure 6. Distribution of climbing fibre responses in pars intermedia.

Display as in Fig. 5.
Topographical organization of climbing fibre responses in the a and c1 zones
Figure 5 illustrates an experiment covering most of the hemivermis in addition to parts of the contralateral hemivermis and ipsilateral pars intermedia. The a zone is bounded by the short latency input from cH on the other side of the midline and by the long latency input to the b zone laterally. In a medial band within the a zone, climbing fibres received convergent input from the ipsilateral proximal stimulation sites iFprox, iFace, iHprox and Tail (see Fig. 2, panel 1, iFace not included in this experiment). Abbreviations defined in legend to Fig. 1B. Some degree of specificity was found in this band, most commonly exclusive input from iFace to lobule VI. Lateral to this band, there was a somatotopically organized zone with short latency input from the ipsilateral side of the body (see iF, iH and Tail in Fig. 2, panels 2–4). Input from iF was found in lobules V and VI, weak input from iFprox was found slightly rostral to this, input from iH was found further rostrally and input from Tail was found medial and rostral to the representation of iH.
Figure 5. Distribution of climbing fibre responses in the vermis.
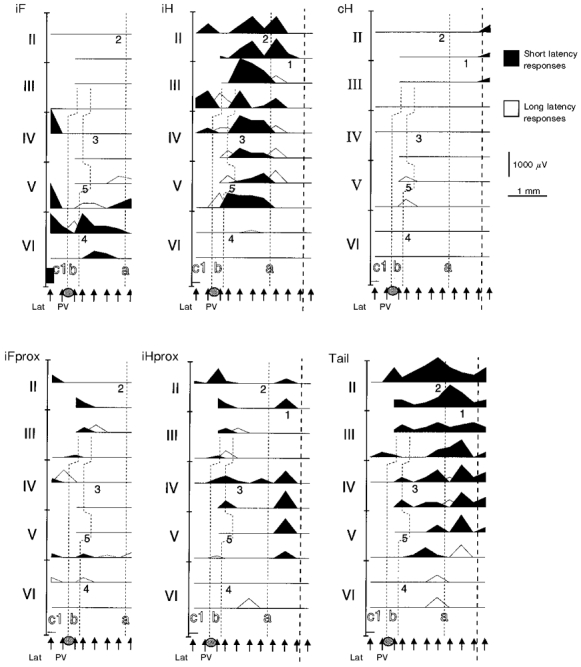
Similar display as in Fig. 4 but responses evoked from single stimulation sites are shown in separate panels. Numbers on plots correspond to the recording points illustrated in Fig. 2. Thick dashed line indicates midline.
In the c1 zone, large responses were evoked from iF caudally whereas iFprox evoked responses rostrally (Fig. 6). Medial to the iFprox input, short latency responses were evoked from the hindlimb (iH, iHprox).
Topographical organization of climbing fibre responses in the most rostral part of the anterior lobe
Figures 4–6 also show that ipsilateral climbing fibre input with short response latencies was dominating across the full mediolateral span of the anterior lobe in its most rostral parts. Furthermore, input from different stimulation sites was well separated topographically. In a sequence from the medial vermis to lateral pars intermedia, short latency input was evoked from Tail, iH-iHprox, iFprox and iFace. Conspicuously, the somatotopic organization in the most rostral part of the anterior lobe appeared to form a continuation of those in the lateral a zone and the c1 zone. Climbing fibre input characteristic of the b zone could not usually be found rostral to lobule III where the short latency responses spanned the mediolateral distance between the a and c1 zones. Similarly, local climbing fibre activity characteristic of the c2 zone extended rostrally only as far as lobule IV-V although non-local responses were sometimes seen further rostrally (see Fig. 2, panel 7 and Fig. 6).
Figure 7 summarizes the present findings by illustrating the zonal distribution across the cerebellar cortical surface. In the anterior lobe, large cortical areas are covered by the medial and lateral subdivisions of the a zone (here referred to as the ‘a1’ and ‘ax’ zones, see Discussion) whereas the c2 zone and in particular the d1 zone cover small cortical areas. This is mainly explained by the dramatic decrease in depth of the interfolial fissures laterally, which also results in the disappearance of macroanatomical boundaries between certain lobules and folia.
Figure 7. Climbing fibre sagittal zones and cortical areas.

A, in the rostral view of the cerebellum the approximate extents of the a1, ax, b, c1, c2 and d1 zones are indicated. Dashed line indicates midline. Thin, dotted vertical lines indicate the mediolateral position of three sagittal sections shown below to illustrate differences in the depth of fissures. Shaded areas in the sagittal sections indicate the granule cell layer. NI, nucleus interpositus. B, the zonal distribution and intrazonal somatotopic organization in the ax and c1 zones are indicated on an unfolded view of the Purkinje cell layer. The somatotopic organization of the a and c1 zones was reconstructed by first dividing the zonal widths in every lobule into thirds. For each stimulation site, data from all experiments were then pooled every such zonal third and the average amplitude of the short latency responses (minimal number of observations required was set to 3) was calculated and used for the reconstruction. Inset: recording points identified in the cerebella of individual animals superimposed on the unfolded cerebellum. Before superimposing recording points from different experiments, the mediolateral dimensions of the anterior lobe and the rostrocaudal dimensions of individual lobules were normalized. Recording points to the left of the cerebellar outline were from the contralateral side.
To be able to cover reasonable amounts of the cerebellar anterior lobe in each experiment, spacing between tracks generally had to be quite wide, relative to the widths of the individual zones. Estimates of precise zonal widths and of any possible changes in zonal widths along the rostrocaudal axis, which are known to occur in the cat and ferret (cf. Garwicz, 1997), could therefore not be made and Fig. 7 should only be viewed as a coarse outline in these respects.
DISCUSSION
The present investigation shows that sagittal zones can be distinguished in the cerebellar anterior lobe of the rat on the basis of response latencies and convergence patterns of climbing fibre activity evoked by peripheral electrical stimulation. The identified zones seem to correspond to the A, B, C1, C2 and D1 zones in the cat and ferret because of similarities in input patterns and anatomical locations. However, we found the a zone in the rat to be divided into two zones, ‘a1’ and ‘ax’ (see below). The ax and c1 zones were characterized by short latency climbing fibre responses with topographical distributions that suggested detailed somatotopic organization. Surprisingly, we also found ipsilateral, short latency climbing fibre input with a somatotopic organization that spanned the mediolateral width of the most rostral part of the anterior lobe. This transverse somatotopical organization has not been described in the cat, but the most rostral part of the cat cerebellar anterior lobe has not been investigated in detail with electrophysiological techniques.
Functional organization of climbing fibre input to the vermis
The short latencies of the climbing fibre responses in the medial vermis was a surprising find since the climbing fibre input to the A zone of the cat has been characterized as weak with irregular and long latency responses from mainly ipsilateral hindlimb nerves (Oscarsson & Sjölund, 1977). Anatomically, the A zone in the cat has been subdivided into two different longitudinal strips, A1 and A2, on the basis of differences in corticonuclear connections (Voogd, 1982). Lateral to the A zone in lobules V and VI, the X zone appears as an isolated island of short latency ipsilateral forelimb input located between two zones receiving long latency input, the A and B zones (Ekerot & Larson, 1979a, 1982). The a zone in the rat appeared to be organized in a medial part where climbing fibres received convergent input from proximal, ipsilateral stimulation sites and a larger lateral part where different distal, ipsilateral stimulation sites provided input to distinct subregions of the zone. The location of the forelimb area in this lateral zone corresponded to the location of the X zone in the cat but in the rat it formed an integral part of a somatotopic representation of the ipsilateral body. We therefore suggest that the lateral of the ‘a’ subzones be denoted the ‘ax’ zone, whereas the medial subzone be denoted the ‘a1’ zone. Note that at least part of our ax zone in lobules IV and V has corticonuclear connections with the interstitial cell group of the cerebellar nuclei (Buisseret-Delmas et al. 1993), which is the target of the corticonuclear projection of the X zone in the cat.
The gross topography of the ax zone, with iF, iFprox and iH represented separately from caudal to rostral parts of the anterior lobe, is similar to that of the C3 zone in the cat. A microzonal organization of the climbing fibre input to the C3 zone has been demonstrated using single unit recordings and natural stimulation of the skin (Ekerot et al. 1991). In the present study, climbing fibre field potentials were evoked by electrical stimulation of the skin, a technique lacking sufficient resolution to reveal a microzonal organization such as the one in the C3 zone (cf. Garwicz et al. 1996). However, given the similarities between the two zones, it is likely that the ax zone has a similarly detailed microzonal organisation as the cat C3 zone. In the cat, the A1 subdivision of the A zone innervates the rostromedial fastigial and medial vestibular nuclei whereas the lateral A2 subdivision innervates the rostrolateral fastigial nucleus (Voogd, 1982). In the rat, the cortical areas corresponding to our a1 and ax zones seem to have corticonuclear connections which are largely similar to those of the cat A1 and A2 zones, respectively (Buisseret-Delmas, 1988a). If the nucleofugal connections are similar in the rat as in the cat (see Voogd, 1964, 1982), the somatotopic representation in the ax zone would imply that the part of the reticular formation controlled by the rostrolateral fastigial nucleus exerts a finer and more specific motor control than the reticular formation is generally assumed to do. The convergent input from proximal parts of the body to the a1 zone is consistent with a role in posture and interlimb coordination.
The b zone in the present investigation was defined as a narrow band in the lateral vermis, which lacked responses characteristic of the ax and c1 zones and which had occasional long latency responses from stimulation sites on both sides of the body. By contrast, climbing fibre input to the B zone of the cat is characterized by relatively strong activation from large, bilateral receptive fields on stimulation of peripheral nerves (Oscarsson & Sjölund, 1977). Larger and more regular responses than in the present study were evoked from the sciatic nerve in the b zone of rats by Sjölund et al. (1981). A possible explanation for the difference is their use of sciatic nerve stimulation, which activates cutaneous as well as deep afferents. In the cat, deep input has been shown to effectively activate climbing fibres of the B zone (Garwicz et al. 1992). The lighter pentobarbital anaesthesia used by Sjölund et al. (1981) is another possible explanation.
Due to the largely negative definition criteria of the b zone in the present paper, its rostral extent (see Fig. 7) is somewhat uncertain, which makes the zonal organization in lobules II and III of the lateral vermis unclear. However, a strong ipsilateral input with short response latencies seemed to extend uninterruptedly between the ax and c1 zones, at least in lobule II and the rostral part of lobule III (Fig. 5), which is in contradiction with anatomical findings suggesting that the B zone has an uninterrupted course rostrally through lobules I-III (Buisseret-Delmas, 1988a; Voogd & Ruigrok, 1997).
Functional organization of climbing fibre input to pars intermedia
In pars intermedia of the rat cerebellar anterior lobe we could identify only three sagittal zones, the c1, c2 and d1 zones. The c1 zone in the rat was dominated by short latency input from the ipsilateral forelimb. The differential termination of climbing fibres activated from iF and iFprox-iH in, respectively, the caudal and rostral parts of the zone, is in coarse terms in agreement with the arrangement found in C3 zone of the cat (see Ekerot et al. 1991). Hence, as with the ax zone, it is suggested that the c1 zone may have a similar microzonal organization. A minor difference is that relatively large parts of the C1 and C3 zones in the cat receive input from the ipsilateral hindlimb (Oscarsson, 1969) whereas in the rat only a relatively small part in the rostromedial c1 zone received such input. The existence of a cx zone was not investigated in the present study since this would have required a study of the branching patterns of the climbing fibres innervating the c1 zone (Ekerot & Larson, 1982). Climbing fibre branching between areas that seem to correspond to the lateral parts of our c1 and ax zones has been demonstrated by anatomical tracing (Buisseret-Delmas et al. 1993, branching between their CX and X zones).
The climbing fibres of the c2 zone received input from all stimulation sites at intermediate or long response latencies with no apparent topographical organization. These input characteristics are similar to those of the C2 zone in the cat (see Garwicz et al. 1992). The c2 zone extended almost all the way out to the lateral end of the anterior lobe (Fig. 7). Still, a number of recording points were obtained in the cortical strip lateral to the c2 zone (Fig. 7B). This narrow cortical strip was denoted the d1 zone because of the long response latencies of the climbing fibres and their activation from distal, mainly ipsilateral stimulation sites, response properties which are similar to those of the D1 zone of the cat (Ekerot & Larson, 1979a; Garwicz et al. 1992).
Climbing fibre input to the most rostral part of the anterior lobe
Unexpectedly, we also found short latency climbing fibre input from the ipsilateral side of the body, which extended across the rostral pole of the anterior lobe. This transverse somatotopic representation was continuous with the somatotopic representations in the a and c1 zones, suggesting that the area as a whole is part of the same functional system. A similar arrangement exists in the cat where the C1 and C3 zones, as defined by olivo-cortical connections, fuse in lobules I and II of pars intermedia (Groenewegen et al. 1979).
Comparisons with the results from other investigations of cerebellar organization in the rat
Our results are generally in agreement with the results from three different anatomical tracing studies. Campbell & Armstrong (1983) traced climbing fibres after injections of tritiated leucine in the inferior olive and recognized zones that appear analogous to our a, c1, c2 and d1 zones. Azizi & Woodward (1987) retrogradely traced climbing fibres to the inferior olive after injections of horse radish peroxidase (HRP) or wheat germ agglutinin (WGA)-HRP into the cerebellar cortex and recognized zones that appear analogous to our a, b, c1 and c2 zones. A d1 zone was not recognized which was probably due to a lack of injections into the most lateral part of the anterior lobe. In a series of studies, Buisseret-Delmas (1988a, b) and Buisseret-Delmas & Angaut (1989, 1993) used anterograde and retrograde tracing techniques in combination and could delineate what appears to correspond to the a, b, c1 and c2 zones described in the present study. Although not treated as separate zones, they further recognized the medial and lateral subdivisions of the a zone, here denoted the a1 and ax zones. However, they also reported a C3 zone and further laterally in the anterior lobe zones that they named D0, D1 and D2 zones. Although a different strain of rats was used as compared with the present study, this seems an unlikely explanation for the discrepancies. Instead, it may be a matter of resolution as Buisseret-Delmas & Angaut apparently made only a few and relatively coarse tracer injections in the intermediate part of the anterior lobe (cf. Fig. 1 in Buisseret-Delmas, 1988b with inset in Fig. 7B in the present paper). In fact, there were only two injections in the anterior lobe that involved the postulated C3 zone, one of which involved also the C1 zone (which in the cat is known to have similar olivo-cortico-nuclear connections as the C3 zone, see Garwicz & Ekerot, 1994) and therefore is inconclusive and another one which covered a large part of the lateral anterior lobe from lobule II to lobule V (case C124, Buisseret-Delmas, 1988b). Although interpreted by the authors as a C3-D zone labelling, the present results suggest an alternative interpretation based on the presence of the transversely coherent cortical area in the most rostral part of the anterior lobe, which was not recognized in the anatomical studies. The lateral and caudal parts of the injection C124 might have involved our d1 zone whereas the rostromedial part might have involved the face part of our rostral somatotopic organization. This makes sense since C124 resulted in labelling in the lateral part of the anterior interposed nucleus, which in the cat is a known face-related area (Gibson et al. 1987). Regarding the D zones, our d1 zone was narrow and located immediately lateral to the c2 zone. Although the relatively weak input would not have allowed for a discrimination of several zones within this cortical area if they were present, because of the extreme narrowness it would have implied the presence in the cerebellar anterior lobe of physiological counterparts to the D0, D1 and D2 zones described by Buisseret-Delmas & Angaut (1989) seems unlikely.
Generality of zonal organization?
The functional organization of the cerebellar anterior lobe seems to be basically similar in all species investigated electrophysiologically, namely the cat (Oscarsson, 1980), the ferret (Garwicz, 1997) and now the rat. All three species have a, b, c1, c2 and d1 zones, which probably reflects similar overall motor control capabilities. An important difference is that the rat lacks the C3 and Y zones, which have detailed somatotopic organization and together with the C1 zone control skilled movements via the rubro- and corticospinal tracts (see Garwicz & Ekerot, 1994). Assuming that there is a general relationship between a detailed somatotopic organization and the control of skilled movements, the lack of the C3 and Y zones suggests that the control of skilled movements is distributed differently in the rat as compared with the cat. In the cat, the zones with detailed somatotopic organization are the C1, C3 and Y zones in pars intermedia whereas in the rat the somatotopically organized zones are the c1 zone in pars intermedia and the vermal ax zone, the latter of which appears to be missing in the cat. In addition, there is in the most rostral part of the rat cerebellar anterior lobe a transverse somatotopic representation, which may be part of the same system as the c1 and ax zones. It should be noted that rostral to lobules IV and V, the vermis of the cat and ferret have been investigated electrophysiologically only to a limited extent and it is quite possible that similar input as in the rat is to be found in these parts.
Acknowledgments
This work was supported by the Swedish Medical Research Council (project no 8291), the Medical Faculty of Lund University, the Royal Physiographic Society in Lund, the Magn. Bergvall Foundation, the Swedish Society for Medical Research, The Swedish Society for Medicine, Greta and Johan Kocks Stiftelser, Thorsten och Elsa Segerfalks Stiftelse, Crafoordska Stiftelsen.
References
- Andersson G, Oscarsson O. Climbing fiber microzones in cerebellar vermis and their projection to different groups of cells in the lateral vestibular nucleus. Experimental Brain Research. 1978;32:565–579. doi: 10.1007/BF00239553. [DOI] [PubMed] [Google Scholar]
- Atkins MJ, Apps R. Somatotopical organisation within the climbing fibre projection to the paramedian lobule and copula pyramidis of the rat cerebellum. Journal of Comparative Neurology. 1997;389:249–263. [PubMed] [Google Scholar]
- Azizi SA, Woodward DJ. Inferior olivary nuclear complex of the rat: morphology and comments on the principles of organization within the olivocerebellar system. Journal of Comparative Neurology. 1987;263:467–484. doi: 10.1002/cne.902630402. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C. Sagittal organization of the olivocerebellonuclear pathway in the rat. I. Connections with the nucleus fastigii and the nucleus vestibularis lateralis. Neuroscience Research. 1988a;5:475–493. doi: 10.1016/0168-0102(88)90038-7. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C. Sagittal organization of the olivocerebellonuclear pathway in the rat. II. Connections with the nucleus interpositus. Neuroscience Research. 1988b;5:494–512. doi: 10.1016/0168-0102(88)90039-9. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Angaut P. Sagittal organization of the olivocerebellonuclear pathway in the rat. III. Connections with the nucleus lateralis. Neuroscience Research. 1989;7:131–43. doi: 10.1016/0168-0102(89)90053-9. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Angaut P. The cerebellar olivo-corticonuclear connections in the rat. Progress in Neurobiology. 1993;40:63–87. doi: 10.1016/0301-0082(93)90048-w. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Yatim N, Buisseret P, Angaut P. The X zone and CX subzone of the cerebellum in the rat. Neuroscience Research. 1993;16:195–207. doi: 10.1016/0168-0102(93)90124-9. [DOI] [PubMed] [Google Scholar]
- Campbell NC, Armstrong DM. Topographical localization in the olivocerebellar projection in the rat: an autoradiographic study. Brain Research. 1983;275:235–249. doi: 10.1016/0006-8993(83)90985-x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. New York: Springer-Verlag; 1967. [Google Scholar]
- Ekerot C-F, Garwicz M, Schouenborg J. Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. The Journal of Physiology. 1991;441:257–274. doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C-F, Jörntell H, Garwicz M, Lou X-L. Electrophysiological identification of climbing fibre sagittal zones in the cerebellar anterior lobe of the rat. Society for Neuroscience Abstracts. 1996;22:640.3. [Google Scholar]
- Ekerot C-F, Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization of and termination in the anterior lobe. Experimental Brain Research. 1979a;36:201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Ekerot C-F, Larson B. The dorsal spino-olivocerebellar system in the cat. II. Somatotopical organization. Experimental Brain Research. 1979b;36:219–232. doi: 10.1007/BF00238906. [DOI] [PubMed] [Google Scholar]
- Ekerot C-F, Larson B. Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Experimental Brain Research. 1982;48:185–198. doi: 10.1007/BF00237214. [DOI] [PubMed] [Google Scholar]
- Garwicz M. Sagittal zonal organization of climbing fibre input to the cerebellar anterior lobe of the ferret. Experimental Brain Research. 1997;117:389–398. doi: 10.1007/s002210050233. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Apps R, Trott JR. Micro-organization of olivocerebellar and corticonuclear connections of the paravermal cerebellum in the cat. European Journal of Neuroscience. 1996;8:2726–2738. doi: 10.1111/j.1460-9568.1996.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Ekerot C-F. Topographical organization of the cerebellar cortical projection to nucleus interpositus anterior in the cat. The Journal of Physiology. 1994;474:245–260. doi: 10.1113/jphysiol.1994.sp020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwicz M, Ekerot C-F, Jörntell H. Organizational principles of cerebellar neuronal circuitry. News in Physiological Sciences. 1998;13:26–32. doi: 10.1152/physiologyonline.1998.13.1.26. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Ekerot C-F, Schouenborg J. Distribution of cutaneous nociceptive and tactile climbing fibre input to sagittal zones in cat cerebellar anterior lobe. European Journal of Neuroscience. 1992;4:289–295. doi: 10.1111/j.1460-9568.1992.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Robinson FR, Alam J, Houk JC. Somatotopic alignment between climbing fibre input and nuclear output of the cat intermediate cerebellum. Journal of Comparative Neurology. 1987;260:362–377. doi: 10.1002/cne.902600304. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J, Freedman SL. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. Journal of Comparative Neurology. 1979;183:551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Jörntell H, Ekerot C-F, Garwicz M. Functional organization of climbing fibre projection to the vermis in rat cerebellar anterior lobe. XXXII. Congress of the International Union of Physiological Sciences. 1993;322.15 [Google Scholar]
- Körlin D, Larson B. Differences in cerebellar potentials evoked by the group I and cutaneous components of the cuneocerebellar tract. In: Andersen P, Jansen JKS, editors. Excitatory Synaptic Mechanisms. Oslo: University Press; 1970. pp. 237–241. [Google Scholar]
- Larsell O. In: The Comparative Anatomy and Histology of the Cerebellum from Monotremes through Apes. Jansen J, editor. Minneapolis, MN, USA: The University of Minnesota Press; 1970. [Google Scholar]
- Larson B, Miller S, Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. The Journal of Physiology. 1969a;203:611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B, Miller S, Oscarsson O. A spinocerebellar path activated by the flexor reflex afferents from all four limbs. The Journal of Physiology. 1969b;203:641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the ventral spino-olivocerebellar path. The Journal of Physiology. 1968;196:453–478. doi: 10.1113/jphysiol.1968.sp008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. The Journal of Physiology. 1969;200:129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of olivary projection to cerebellar anterior lobe. In: Courville J, de Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven Press; 1980. pp. 279–289. [Google Scholar]
- Oscarsson O, Sjölund B. The ventral spino-olivocerebellar system in the cat. I. Identification of five paths and their termination in the cerebellar anterior lobe. Experimental Brain Research. 1977;28:469–486. doi: 10.1007/BF00236471. [DOI] [PubMed] [Google Scholar]
- Sjölund B, Björklund A, Wiklund L. The indolaminergic innervation of the inferior olive. 2. Relation to harmaline induced tremor. Brain Research. 1981;131:27–37. doi: 10.1016/0006-8993(77)90026-9. [DOI] [PubMed] [Google Scholar]
- Voogd J. Structure and Fibre Connexions. Van Gorcum, Assen; 1964. The Cerebellum of the Cat. Thesis. [Google Scholar]
- Voogd J. The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinás R, editor. Neurobiology of Cerebellar Evolution and Development. Chicago: American Medical Association; 1969. pp. 493–541. [Google Scholar]
- Voogd J. The olivocerebellar projection in the cat. In: Palay SL, Chan-Palay V, editors. The Cerebellum: New Vistas, Experimental Brain Research. suppl. 6. Berlin, Heidelberg, New York: Springer Verlag; 1982. pp. 134–161. [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends in Neurosciences. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Voogd J, Ruigrok TJH. Transverse and longitudinal patterns in the mammalian cerebellum. Progress in Brain Research. 1997;114:21–37. doi: 10.1016/s0079-6123(08)63356-7. [DOI] [PubMed] [Google Scholar]


