Abstract
The 5-HT3 receptor is a transmitter-gated ion channel of the Cys-loop superfamily. Uniquely, 5-HT3 receptor subunits (5-HT3A and 5-HT3B) possess a positively charged lysine residue within the putative channel lining M2 domain (4′ position). Using whole cell recording techniques, we examined the role of this residue in receptor function using wild-type (WT) and mutant 5-HT3A receptor subunits of murine origin transiently expressed in human embryonic kidney (HEK 293) cells.
WT 5-HT3A receptors mediated rapidly activating currents in response to 5-HT (10–90 % rise time, 103 ms; EC50, 2.34 μm; Hill coefficient, nH, 2.87). The currents rectified inwardly, reversed in sign at a potential of −9 mV and desensitized in the continuous presence of agonist (half-time of desensitization, t1/2, 2.13 s).
5-HT3A receptor subunits in which the 4′lysine was mutated to arginine, glutamine, serine or glycine formed functional receptors. 5-HT EC50 values were approximately 2-fold lower than for WT 5-HT3A receptors, but Hill coefficients, kinetics of current activation, rectification, and reversal potentials were unaltered.
Each of the mutants desensitized more slowly than the WT 5-HT3A receptor, with the arginine and glycine mutations exhibiting the greatest effect (5-fold reduction). The rank order of effect was arginine > glycine > serine > glutamine.
The single-channel conductance of the WT 5-HT3A receptor, as assessed by fluctuation analysis of macroscopic currents, was 390 fS. A similar value was obtained for the 4′lysine mutant receptors. Thus it appears unlikely that 4′lysine is exposed to the channel lumen.
Mutation of residues immediately adjacent to 4′lysine to glutamate or lysine resulted in lack of receptor expression or function. We conclude that 4′lysine does not form part of the channel lining, but may play an important role in 5-HT3 receptor desensitization.
The 5-HT3 receptor belongs to the Cys-loop superfamily of transmitter-gated channels that includes the nicotinic acetylcholine (nACh), glycine and GABAA receptors and the glutamate-gated anion channels (Maricq et al. 1991; Barnard, 1996). Neuronal 5-HT3 receptors mediate fast synaptic transmission, their activation resulting in rapid membrane depolarization due to the gating of an integral cation-selective channel (Derkach et al. 1989; Sugita et al. 1992; Roerig et al. 1997). In common with other members of the Cys-loop superfamily, the 5-HT3 receptor is a pentameric assembly of subunits (Boess et al. 1995). Two 5-HT3 receptor subunits, termed 5-HT3A (Maricq et al. 1991) and 5-HT3B (Davies et al. 1999) have been identified to date. Heterologously expressed receptors function with distinctive biophysical properties as either homo-oligomeric 5-HT3A or hetero-oligomeric 5-HT3A and 5-HT3B subunit complexes. Either subunit type is predicted to contain four hydrophobic transmembrane domains, M1-M4, based on hydropathy analysis and homology to the other members of the superfamily (Maricq et al. 1991; Davies et al. 1999). There is much evidence to support a role for the second membrane-spanning segment, M2, as the main channel-lining component for this family of receptor proteins at the level of the membrane bilayer (reviewed by Karlin & Akabas, 1995). Five M2 transmembrane domains are presumed to delineate the central pore in a pseudosymmetrical fashion (Unwin, 1993, 1995). Evidence from structural studies on the nACh receptor suggests that M2 lines the length of the channel as an α-helix with a kink towards the centre, a feature that may represent the gate of the channel (Unwin, 1993, 1995; cf. Karlin & Akabas, 1995).
The M2 domains of the cation-selective 5-HT3 and nACh receptors are bordered by negatively charged residues (see Fig. 1) which are referred to as the cytoplasmic (-4′), intermediate (-1′) and extracellular (20′) rings (Imoto et al. 1988; Konno et al. 1991; Imoto, 1993). Within the M2 domains, only polar and hydrophobic amino acids are usually found, consistent with the role of M2 as a membrane-spanning segment that allows ions to permeate. The existence of a positively charged lysine (4′K) residue within the pore-lining region is therefore an unexpected feature of both the 5-HT3A and 5-HT3B receptor subunits. The existence of this charged residue, towards the cytoplasmic side of M2, and a potential complementary negatively charged aspartate residue (D265), located in M1, was first noted by Maricq et al. (1991); these residues are conserved in the rat (Johnson & Heinemann, 1992; Isenberg et al. 1993), human (Belelli et al. 1995; Miyake et al. 1995) and guinea-pig (Lankiewicz et al. 1998) orthologues of the 5-HT3A subunit and in the human 5-HT3B subunit (Davies et al. 1999). Although the precise location of these residues is not known, their existence would be expected to be energetically unfavourable, reducing the likelihood that this region of the receptor could exist as an α-helix (Maricq et al. 1991). This destabilization of the structure of M2 may have profound effects upon the channel properties of the 5-HT3 receptor such as gating, rectification and conductance (Maricq et al. 1991).
Figure 1. Alignment of various transmitter-gated ion channel M2 regions.
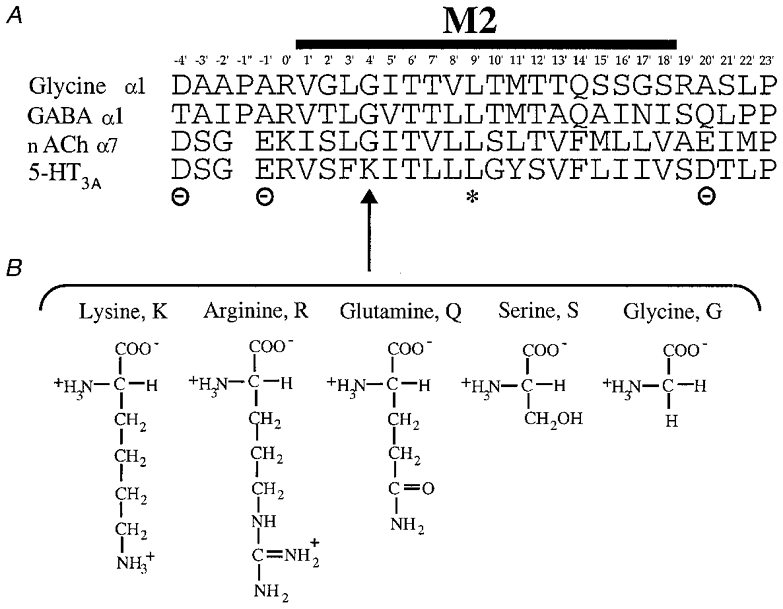
A, the amino acid sequence (single letter code) of the M2 region of the murine 5-HT3A receptor (Maricq et al. 1991) is shown aligned with the corresponding regions of the glycine (α1, Grenningloh et al. 1987) GABAA (α1, Schofield et al. 1987) and nACh (α7, Couturier et al. 1990) receptors. The asterisk indicates the position of the conserved leucine (9′L) residue involved in channel gating (Filatov & White, 1995; Labarca et al. 1995). The positions of the cytoplasmic (–4′), intermediate (–1′) and extracellular (20′) rings of charged residues bordering M2 in the cationic channels are also indicated. B, the amino acids substituted for 4′lysine (4′K, arrow) in this study: arginine (R), glutamine (Q), serine (S) and glycine (G) shown to illustrate the differences in their side chain charge and length.
In this study we have therefore investigated the role of the 4′K residue using homo-oligomeric receptors assembled from the ‘short’ splice variant of the mouse 5-HT3A receptor subunit (i.e. 5-HT3A(b); Hope et al. 1993). We used site-directed mutagenesis to replace 4′K with a series of amino acids with differing charge and/or side chain length: lysine was replaced by arginine (R), glutamine (Q), serine (S) or glycine (G) (see Fig. 1) and these mutants were expressed in HEK 293 cells and characterized using the whole cell recording configuration of the patch clamp technique. To support our hypothesis that 4′K faces away from the channel pore, we also mutated the adjacent 3′phenylalanine (3′F) and 5′isoleucine (5′I) residues, and examined their affect upon receptor function using radioligand binding and whole cell patch clamp techniques.
METHODS
Cell maintenance
HEK 293 cells (European Collection of Animal Cell Cultures, Porton Down, UK) stably expressing 5-HT3A receptors were developed using the eukaryotic expression vector pRc/CMV (InVitrogen, Abingdon, UK) containing the complete coding sequence for the 5-HT3A(b) subunit from N1E-115 neuroblastoma cells as previously described (Hargreaves et al. 1996). Cells were routinely grown until confluent (3–5 days) in a 1:1 mix of Dulbecco's modified Eagle's medium and F12 containing 10 % fetal calf serum and 500 μg ml−1 geneticin in 7 % CO2 and then passaged. Mutagenesis reactions were performed using the Kunkel method (Kunkel et al. 1985), and confirmed by DNA sequencing. Oligonucleotides used in the reactions were: K4′Q: GAGTGTGATCTGGAAGCTTACTCTCT-CAC; K4′G: GAGTGTGATCCCGAAGCTTACTCTCTCAC; K4′R: GAGTGTGATCCGGAAAGAGAC; K4′S: GAGTGTGATGCTGAAGCTTACTCTCTCAC; F3′K: CAGAAGGAGTGTAATTTTCTTGCTGACTCTCTCACC; F3′E: CAGAAGGAGTGTAATTTTTTCGCTGACTCTCTCACC; I5′K: CAGAAGGAGTGTCTTTTTAAAGCTGACTCTCTCACC; I5′E: CAGAAGGAGTGTTTCTTTAAAGCTGACTCTCTCACC; 4′K being at position 282 when using the complete sequence. For transient transfections, HEK 293 cells at 60–70 % confluency (∼48 h post- passage) were transfected with WT or mutant plasmid DNA by the calcium phosphate precipitation method (Chen & Okayama, 1988). For radioligand-binding studies cells were harvested 3 days after transfection. For electrophysiology experiments cells were grown on 35 mm plates (Falcon) and recordings performed on cells 1–4 days post-transfection.
Electrophysiological procedures
Whole cell currents were recorded at 17–23°C using either an EPC-9 converter headstage and amplifier (HEKA Elektronic, Darmstadt, Germany) controlled by Pulse software (HEKA version 7.85) or, for fluctuation analysis experiments, an EPC-7 converter headstage and amplifier (List Electronics L/m). Agonist-evoked currents were recorded at −60 mV unless otherwise stated. Culture dishes were continuously perfused (3–5 ml min−1) with an extracellular solution (E1) comprising (mM): 130 NaCl, 5.4 KCl, 2 MgCl2, 1.8 CaCl2, 30 glucose, 10 Hepes, titrated to pH 7.2 with NaOH. In experiments to investigate receptor desensitization a modified solution in which divalent cations were essentially absent (‘zero’ calcium solution; as E1, except (mM): 0.1 CaCl2, 0 MgCl2, 1.1 EGTA; ∼10 nM free [Ca2+]) was used. Patch pipettes were made from thin-walled borosilicate glass capillary tubing (Clark Electromedical GC120F-10) and filled with intracellular solution (mM: 140 CsCl, 1 MgCl2, 0.1 CaCl2, 1.1 EGTA (∼10 nM free [Ca2+]) 10 Hepes titrated to pH 7.2 with CsOH). Electrode resistances measured with these solutions were typically 2–5 MΩ. Liquid junction potentials arising at the tip of the patch pipette were calculated by the method of Barry & Lynch (1991), and potential measurements corrected post hoc.
In the majority of experiments drugs were applied via a U-tube device (Suzuki et al. 1990), allowing a complete change of agonist concentration at the cell membrane within 100 ms (Sepúlveda et al. 1991). Alternatively, in experiments where the single-channel conductance of the receptor was assessed by fluctuation analysis of whole cell currents, 5-HT (1 μm) was applied by diffusion from a coarse-tipped micropipette (approximately 10 μm diameter). The tip was positioned so as to achieve a relatively slowly developing response to the applied 5-HT. A minimum washout period of 3 min was left between agonist applications, unless otherwise stated, to allow complete recovery of the 5-HT3A receptor from desensitization.
Analysis of results
Agonist concentration-response data were fitted with the Hill equation using Kaleidagraph (Abelbeck Software):
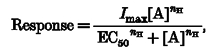 |
(1) |
where Imax is the maximal response, EC50 the concentration of agonist required for half-maximal effect, [A] the concentration of agonist, and nH the Hill coefficient.
Fluctuation analysis
Estimates of single-channel conductance of the 5-HT3A receptor were made by analysis of the random fluctuations in the 5-HT-evoked whole cell membrane currents. Signals were stored on DAT tape (DTR 1204, Biologic) for subsequent off-line analysis using the program SPAN 3.0 (Dempster, 1993). The signal was divided into a DC-coupled signal, low-pass filtered at a cut-off frequency of 1 kHz (Bessel characteristic, Flyde Electronics), and an AC-coupled signal (1–1000 Hz bandpass frequency; Bessel characteristic, Flyde Electronics) for the analysis of whole cell current noise. Continuous records of mean DC and current fluctuations in the presence and absence of 5-HT were replayed from DAT tape and digitized at a rate of 2 kHz and stored on a DELL XPSPS66s computer via a Digidata 1200 interface (Axon Instruments). Blocks of data were edited visually and those containing obvious artefacts were excluded from further analysis. The variance method (Cull-Candy et al. 1988; Lambert et al. 1989; Dempster, 1993) was used to estimate the current, i, flowing through a single channel using the equation (Dempster, 1993):
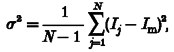 |
(2) |
where σ2 is the variance, Im the mean current, Ij the current sample j, and N the number of A/D samples in the block. The relationship between σ2 and Im is parabolic rising from zero to a maximum at P = 0.5 and falling to zero again as P approaches 1:
| (3) |
where n is the number of channels within the cell. When P is small (< 0.1) eqn (3) simplifies to the linear function:
| (4) |
Plots of σ2versus Im were obtained from blocks of data recorded during the entire exposure to microperfused 5-HT (1 μm). Single-channel current was estimated mostly from a linear function (eqn (4)) fitted to such data by least-squares regression analysis. Occasionally, a parabolic function resulted in a better fit (eqn (3)). Background variance, attributable to sources other than 5-HT-activated currents, was removed by subtracting the mean value of 16–32 blocks of data recorded prior to the application of the agonist. Single-channel chord conductances (γ) were calculated according to the equation:
| (5) |
where V is the holding potential, and Vrev, 5-HT is the reversal potential of the whole cell current through the receptor.
Radioligand binding
HEK 293 cells transfected to express WT or mutant 5-HT3A receptor subunits were washed twice with phosphate-buffered saline at room temperature; all subsequent steps were carried out at 1–4°C. Cells were scraped into 1 ml of Hepes buffer (10 mM, pH 7.4) containing the following proteinase inhibitors: 1 mM EDTA, 50 μg ml−1 soybean trypsin inhibitor, 50 μg ml−1 bacitracin and 0.1 mM phenylmethylsulphonyl fluoride (PMSF). Harvested cells were washed in Hepes, frozen and either used immediately or stored at −20°C. Protein concentration was estimated using the Bio-Rad Protein Assay with bovine serum albumin (BSA) as standard. Fifty micrograms of cell membranes were incubated in Hepes buffer containing 1 nM [3H]granisetron (81 Ci mmol−1, Dupont) in a final volume of 0.5 ml. One hundred nanomolar unlabelled granisetron or 1 μm (+)-tubocurarine was used to determine non-specific binding. Reactions were incubated for 1 h at 4°C, and were terminated by rapid vacuum filtration using a Brandel cell harvester onto Whatman GF/B filters presoaked for 3 h in 0.3 % (v/v) polyethyleneimine followed by two rapid washes with 4 ml ice-cold Hepes buffer. The filter discs were incubated for 4 h in 3 ml of scintillation fluid and radioactivity determined by scintillation counting.
All data are quoted as the means ±s.e.m. for n independent experiments. Where appropriate Student's t test for unpaired data was used and values of P < 0.05 were regarded as significant.
Drugs and reagents
All cell culture reagents were obtained from Gibco BRL (Paisley, UK), except fetal calf serum which was from Sigma (Poole, UK). 5-HT hydrochloride was from Research Biochemicals Inc. (St Albans, UK) and granisetron was a gift from SmithKline Beecham Pharmaceuticals (Welwyn, UK). All other reagents were obtained from Sigma (Poole, UK).
RESULTS
Electrophysiological characterization of the WT 5-HT3A receptor stably expressed in HEK 293 cells
Application of a supramaximal concentration (30 μm) of 5-HT to cells voltage clamped at a holding potential of −60 mV evoked rapidly activating inward currents (10–90 % rise time, 103 ± 9 ms; n = 9) in all cells tested (n = 90). Whole cell currents mediated by WT 5-HT3A receptors were completely and reversibly inhibited by 10 nM granisetron (n = 4), a selective 5-HT3 receptor antagonist (Fig. 2). Concentration-effect relationships for 5-HT revealed an EC50 of 2.34 ± 0.06 μm, a Hill coefficient of 2.87 ± 0.35 and a maximum current amplitude (Imax) of 656 ± 170 pA (n = 4; Fig. 2, Table 1). Current-voltage (I–V) relationships demonstrated modest inward rectification and a reversal potential of −9.0 ± 1.6 mV (n = 4; Fig. 3, Table 1).
Figure 2. Dose-response curves for WT and 4′K mutant 5-HT3A receptors.
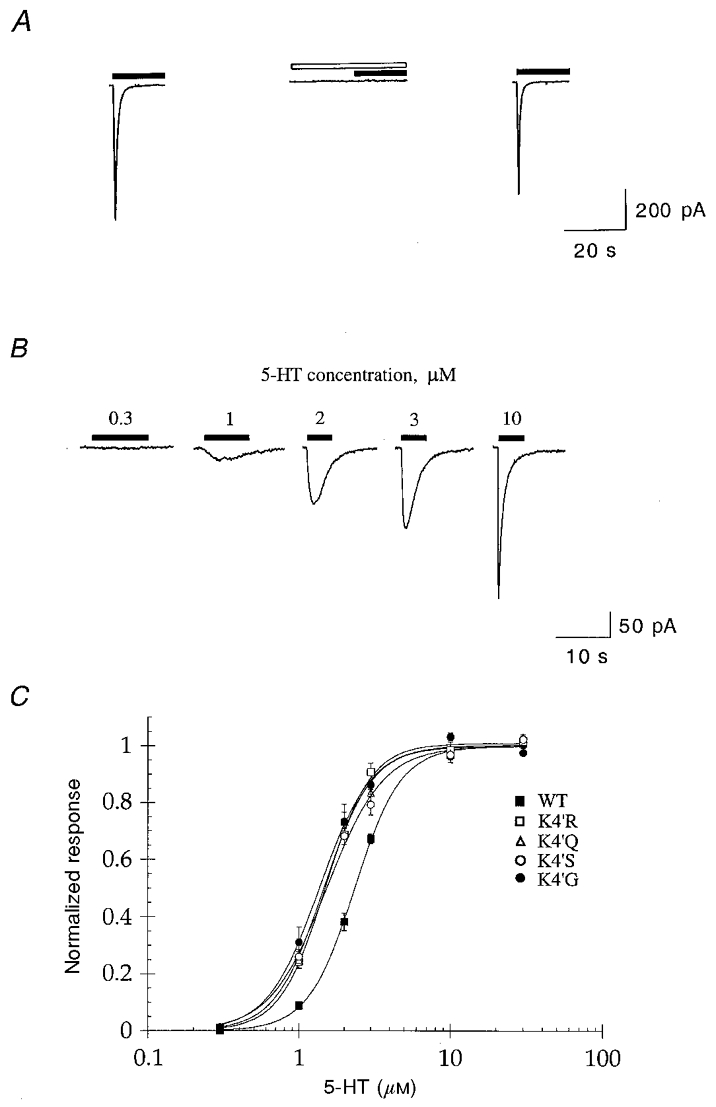
A, whole cell currents evoked by 10 μm 5-HT in transfected HEK 293 cells (filled bars) were completely and reversibly inhibited by 10 nM granisetron (open bar). Traces are from one experiment and are representative of four others. No responses were detected in untransfected cells (not shown). B, sample traces showing 5-HT-evoked (0.3-10 μm) whole cell currents in a HEK 293 cell expressing the WT 5-HT3A receptor. Traces are from one experiment and are representative of four others. C, 5-HT dose-response curves for WT, and 4′K mutant 5-HT3 receptors (n = 4). Data from each cell were normalized to the Imax value obtained from the Hill equation. The data obtained from these experiments are summarized in Table 1.
Table 1.
Electrophysiological data for WT and mutant 5-HT3 receptors
| M2 4′ amino acid | Imax (pA) | EC50 (μm) | nH | Vrev (mV) |
|---|---|---|---|---|
| K (WT) | 656 ± 170 | 2.34 ± 0.06 | 2.87 ± 0.35 | −9.0 ± 1.6 |
| R | 469 ± 89 | 1.50 ± 0.08* | 2.97 ± 0.18 | −6.8 ± 0.4 |
| Q | 775 ± 117 | 1.47 ± 0.06* | 2.77 ± 0.47 | −10.6 ± 0.9 |
| S | 979 ± 364 | 1.54 ± 0.04* | 2.39 ± 0.34 | −8.6 ± 2.3 |
| G | 545 ± 271 | 1.39 ± 0.10* | 2.73 ± 0.27 | −8.2 ± 1.9 |
Data are means ± s.e.m. (n = 4).
Significantly different from WT (Student's t test, P < 0.05). Amino acids: K, lysine; R, arginine; Q, glutamine; S, serine; G, glycine.
Figure 3. Current-voltage relationships for WT and 4′K mutant 5-HT3A receptors.
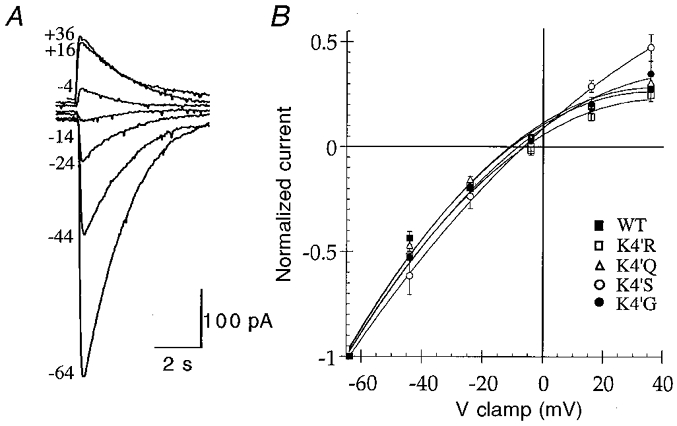
A, sample traces for current-voltage experiments conducted on the WT 5-HT3A receptor. 5-HT-evoked (10 μm, 2 s) whole cell responses were recorded at the holding potentials indicated to the left (mV). Traces are from one experiment and are representative of four others. B, I–V curves for WT and 4′K mutant 5-HT3A receptors were constructed by measuring the peak inward current evoked by 10 μm 5-HT, at the holding potentials (mV) shown (n = 4). The reversal potentials estimated from these data are given in Table 1.
Electrophysiological characterization of the 4′K mutant 5-HT3A receptors
All of the 4′K mutant receptors were functional and resembled the WT receptor in a number of aspects. The rates of activation of currents mediated by 4′K mutant receptors in response to a maximal concentration of agonist (30 μm 5-HT) were not significantly different from WT (Table 2) and the responses were completely and reversibly blocked by 10 nM granisetron (data not shown). I–V curves constructed for the mutant receptors showed similar rectification properties to the WT receptor and the reversal potentials determined were not significantly different from WT (Fig. 3, Table 1). However, the concentration-effect relationship for 5-HT differed slightly between the WT and mutant receptors. Each of the mutants exhibited a small (approximately 2-fold) increase in the potency of 5-HT compared to the WT receptor (Fig. 2; Table 1), but there were no significant differences in the Hill coefficients or the Imax values obtained (Table 1).
Table 2.
Activation and desensitization of WT and mutant 5-HT3 receptors
| M2 4′ amino acid | Activation (10–90% rise time, ms) | Desensitization (t1/2, s) |
|---|---|---|
| K (WT) | 103 ± 9 (11) | 5.0 ± 0.5 (12) |
| R | 106 ± 15 (7) | 26.6 ± 2.8 (11)* |
| Q | 115 ± 16 (6) | 8.2 ± 0.8 (11)* |
| S | 99 ± 10 (6) | 11.2 ± 1.2 (10)* |
| G | 119 ± 6 (5) | 20.3 ± 2.0 (11)* |
Data are means ± s.e.m. of (n) independent experiments.
Significantly different from WT (Student's t test, P < 0.05).
Desensitization of the WT and 4′K mutant 5-HT3A receptors
During the initial electrophysiological characterization of the 4′K 5-HT3A receptor mutants, it was noted that such receptors appeared to desensitize more slowly than the WT receptor. Since Ca2+ is postulated to be intimately involved in the mechanism of desensitization of the 5-HT3 receptor (Yakel et al. 1993) and perturbation of channel kinetics could lead to altered Ca2+ influx, experiments to compare the desensitization of the WT and mutant receptors were conducted in a modified solution in which the extracellular concentration of Ca2+ ([Ca2+]o) was buffered at ∼10 nM (‘zero’ Ca2+ solution, see Methods).
Whole cell currents mediated by WT and 4′K mutant 5-HT3A receptors desensitized completely in response to prolonged applications of agonist. The macroscopic current mediated by the WT 5-HT3A receptor in response to a maximal concentration of agonist (30 μm 5-HT) decayed to 50 % of peak amplitude (i.e. t1/2) in 2.13 ± 0.21 s (n = 10) when [Ca2+]o was set at 1.8 mM. In ‘zero’ Ca2+ solution, the rate of current decay was reduced considerably (t1/2 = 5.0 ± 0.5 s; n = 12; Fig. 4, Table 2). Similar experiments performed on the 4′K mutant 5-HT3A receptors indicated that each desensitized at a significantly slower rate compared to the WT receptor (Fig. 5, Table 2). The rank order of effect of the 4′ amino acid was R > G > S > Q > K (WT). The most profound effect, observed for the K4′R mutant, resulted in more than a 5-fold reduction in the rate of 5-HT3A receptor desensitization (Fig. 5, Table 2).
Figure 4. Effect of extracellular Ca2+ on desensitization of the 5-HT3A receptor.
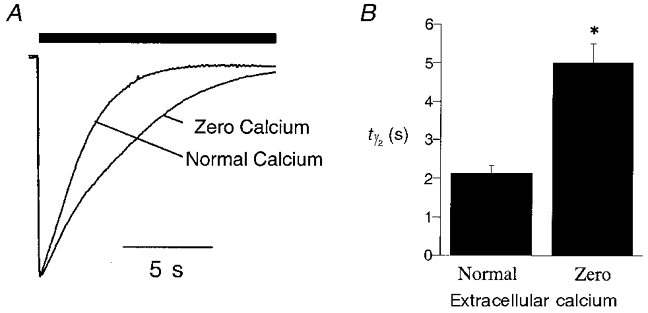
The rate of desensitization of WT receptor whole cell currents was studied in ‘normal’ (E1; 1.8 mM calcium) or ‘zero’ calcium (∼10 nM) extracellular solutions (see Methods). A, sample traces showing the effect of extracellular Ca2+ on the rate of desensitization of WT receptor whole cell currents in the continued presence of a maximal concentration of agonist (30 μm 5-HT, filled bar). B, graph illustrating the difference in the rate of desensitization of whole cell current recorded in ‘normal’ or ‘zero’ extracellular Ca2+. The data represent the time taken for the current to decay to half of its peak value in the continued presence of agonist (i.e. half-time of desensitization, t1/2; n = 10–12). *Statistically different from responses evoked in normal extracellular solution (P < 0.05).
Figure 5. 4′K mutants desensitize more slowly than the WT 5-HT3A receptor.
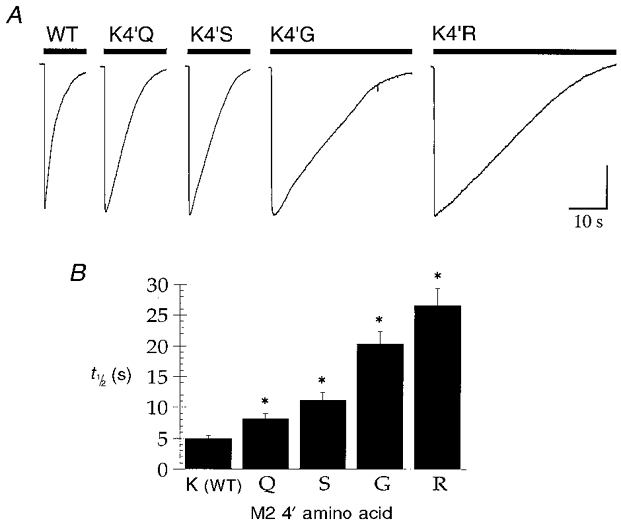
A, sample traces from experiments to estimate the rate of desensitization of WT and 4′K mutant receptors. Currents were evoked by application of a maximal concentration of 5-HT (30 μm, filled bar) to cells expressing WT or mutant receptors. Experiments were conducted in ‘zero’ Ca2+ extracellular solution (see Methods). The sample traces shown are from single experiments and are representative of 10–12 similar experiments. The vertical calibration bar corresponds to 200, 900, 550, 430 and 450 pA for WT, K4′Q, K4′S, K4′G and K4′R, respectively. B, graph summarizing the results of experiments to quantify the effect of 4′K substitutions upon 5-HT3A receptor desensitization. The data represent the time taken for the current to decay to half of its peak value in the continued presence of agonist (i.e. half-time of desensitization, t1/2; n = 10–12).
Single-channel conductance of the WT and 4′K mutant 5-HT3 receptors expressed in HEK 293 cells
Microperfusion of 1 μm 5-HT to HEK 293 cells expressing the WT 5-HT3A receptor resulted in slowly activating currents particularly amenable to fluctuation analysis. Figure 6a provides an example of such a response recorded as low-gain DC-coupled and high-gain AC-coupled traces. The latter allows the increase in current fluctuation due to the gating of 5-HT3 receptor channels to be clearly discerned. Fluctuation analysis of such responses usually yielded a linear relationship between the amplitude of the macroscopic current and current variance, the gradient of which was used to estimate the amplitude of single-channel events. From this value and Vrev,5-HT, the underlying chord conductance (γ) of the WT 5-HT3A receptor channel was estimated to be 390 ± 15 fS (n = 4) at a holding potential of −60 mV. Similar experiments were also performed on HEK 293 cells expressing the 4′K mutant 5-HT3A receptors: K4′Q, K4′R, K4′S and K4′G. Suitable whole cell currents were obtained by microperfusion of 1 μm 5-HT (Fig. 6). Fluctuation analysis of these responses yielded linear relationships between macroscopic current amplitude and current variance (Fig. 6), and the estimates of single-channel conductance obtained (n = 4) for each of the mutants were not significantly different from WT (Table 3). Some data were also re-analysed with the high-pass filtering reduced from 1 to 0.1 Hz. This increased bandwidth should, theoretically, have included events of up to 10 s duration. The estimates of single-channel conductance so obtained were very similar to those found with the higher cut-off frequency indicating that a significant loss of signal, as a result of a possible change in the open time of the mutant receptors, was unlikely.
Figure 6. Estimation of the single-channel conductance of the WT and 4′K mutant 5-HT3A receptors.
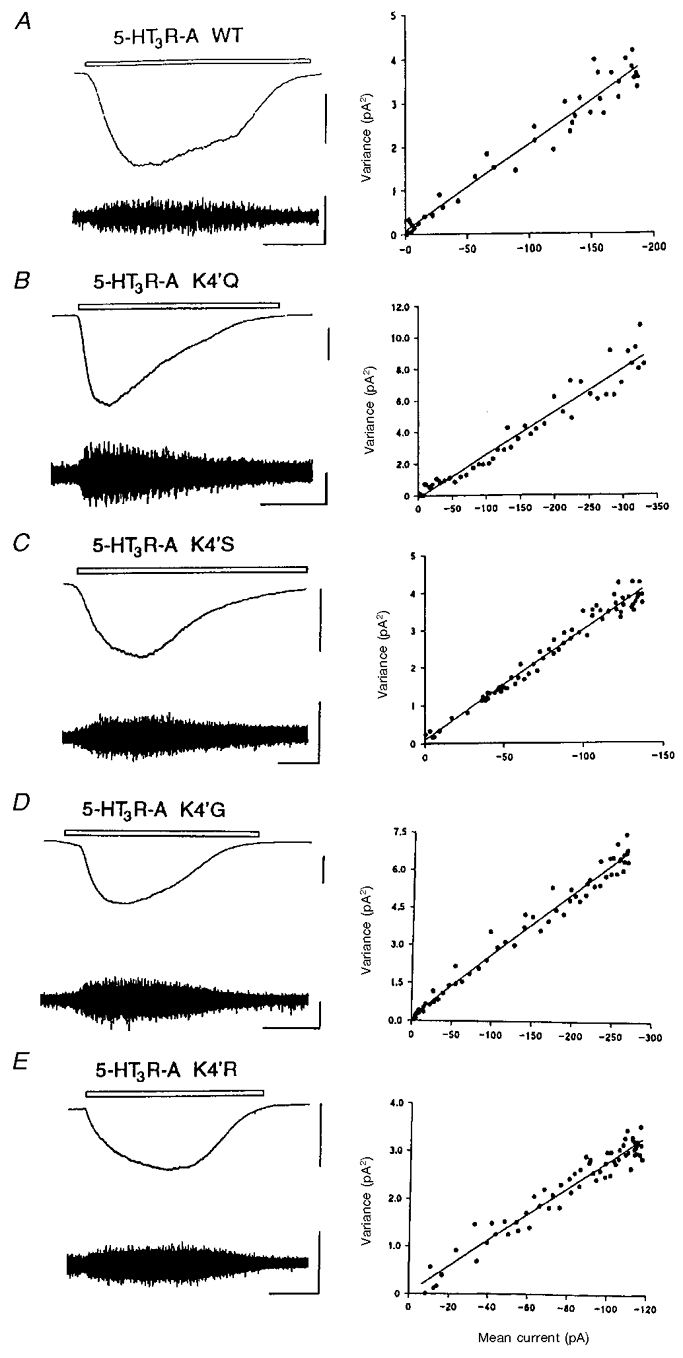
Examples from single cells of low-gain DC-coupled records (top left trace in each panel) and high-gain AC-coupled (1–1000 Hz bandwidth) records (bottom left trace in each panel) of inward currents evoked by application of 1 μm 5-HT applied by microperfusion for the period indicated (open bar) to HEK 293 cells expressing WT (A), K4′Q (B), K4′S (C), K4′G (D) and K4′R (E) 5-HT3 receptors. The vertical scale bars represent 100 and 5 pA for the DC- and AC-coupled traces, respectively. The horizontal calibration bar represents 20 s. The holding potential in all experiments was −60 mV. In each case the right panel represents the relationship between the variance of the AC-coupled current (pA2, vertical axes) and the DC amplitude (pA, horizontal axes) throughout the 5-HT-evoked response after background current variance in the absence of 5-HT has been subtracted. The slope of the line fitted to the data points, by linear regression analysis, provides an estimate of the underlying unitary conductance. In the examples shown above, the single-channel conductance (γ) was estimated to be 346, 473, 509, 428 and 484 fS for the WT, K4′Q, K4′S, K4′G and K4′R receptors, respectively.
Table 3.
Single-channel conductance of recombinant and native 5-HT3 receptors
| 5-HT3 receptor | Conductance (fS) | n |
|---|---|---|
| HEK 293 | ||
| WT | 390 ± 15 | 4 |
| K4′R | 478 ± 42 | 4 |
| K4′Q | 441 ± 35 | 4 |
| K4′S | 455 ± 28 | 4 |
| K4′G | 429 ± 32 | 4 |
| SCG neurones† | 3689 ± 357* | 5 |
Data shown are the means ± s.e.m. of n independent experiments.
Significantly different from HEK 293 data (Student's t test, P < 0.05).
M. J. Gunthorpe & J. A. Peters, unpublished observations.
Characterization of the 3′F and 5′I mutant 5-HT3A receptors
The functional data obtained from the 4′ mutants suggested that the residue at this position does not face into the channel. We therefore examined mutations at the 3′ and 5′ positions to provide some support for this hypothesis. Correct assembly of the 3′F and 5′I mutant 5-HT3A receptors was assessed using a binding assay with the high affinity radiolabelled antagonist [3H]granisetron to membranes prepared from transfected HEK 293 cells. Specific binding of [3H]granisetron, at a concentration (1 nM) approximating to the Kd for the WT 5-HT3A receptor, was not significantly different from zero for glutamate substituted F5′E (8 ± 6 fmol (mg protein)−1) and lysine substituted F5′K (15 ± 12 fmol (mg protein)−1) mutant receptor subunits (each n = 3). Similarly, specific binding was very low for the I3′E mutant 5-HT3A receptor (40 ± 7 fmol (mg protein)−1, n = 3), although reasonable levels were observed for the I3′K mutant receptor (590 ± 95 fmol (mg protein)−1, n = 3). However, the function of the I3′E and I3′K mutant receptors, which was assessed using whole cell recording performed on transiently transfected HEK 293 cells, revealed no response to a saturating concentration (30 μm) of 5-HT for > 20 cells for each cDNA transfected. Using identical procedures, responses were observed in 20–40 % of cells transfected with wild-type cDNA.
DISCUSSION
The present investigation has explored the influence of the positively charged M2 4′lysine residue upon the function of the recombinant 5-HT3A receptor. The data indicate this residue to be a determinant of the desensitization kinetics, but not the low conductance, of the receptor. Several properties of the receptor were not greatly affected when the 4′K residue was replaced by a series of amino acids that differ in side chain charge and length including arginine (R), glutamine (Q), serine (S) or glycine (G). In those instances, the expressed receptors were functional and displayed rates of activation and Hill coefficients similar to those of the WT 5-HT3A receptor, suggesting that the overall structure of the mutant proteins was not grossly altered.
4′ mutations and single-channel conductance
The single-channel conductance of the 5-HT3 receptor native to neurones and neuronal cell lines varies by approximately 60-fold between preparations (i.e. 0.3-19 pS; Lambert et al. 1989; Yang, 1990; Peters et al. 1993; Hussy et al. 1994; Jones & Surprenant, 1994; Zhong et al. 1999) and there is evidence to suggest the expression of receptors with distinct conductances within the same cell (Derkach et al. 1989; Yang et al. 1992; Hussy et al. 1994). Recent studies (Davies et al. 1999) describing a very large difference in the conductance of 5-HT3 receptors assembled from human 5-HT3A (< 1 pS; Brown et al. 1998), or human 5-HT3A and 5-HT3B subunits (∼17 pS; Davies et al. 1999), provide a potential explanation for such variation.
However, one unresolved issue is why the single-channel conductance of the homo-oligomeric 5-HT3A receptor is so small. The present work eliminates one possibility: the 4′lysine residue that is unique, within the Cys-loop receptor family, to 5-HT3 receptor subunit M2 sequences. The presence of positively charged residues within the pore region of cation-selective ion channels, as exemplified by the Q/R site of AMPA receptors assembled from GluR2 subunits, is known to markedly suppress single-channel conductance (Swanson et al. 1997). By analogy, if the 4′lysine were to reside upon the face of the M2 α-helix lining the channel lumen, the resulting ring of five positive charges would be expected to influence cation flux dramatically. The lack of effect of any of the four amino acid substitutions upon single-channel conductance argues strongly against this possibility. None of the mutant receptors had a single-channel conductance that was significantly different from that of 390 fS found for the WT 5-HT3A receptor (Fig. 6, Table 3), a value that compares well with previous estimates of 360 fS (Hussy et al. 1994) and 420 fS (Gill et al. 1995) obtained by fluctuation analysis. This negative finding is unlikely to reflect an inability to detect subtle changes in conductance by fluctuation analysis, because we and others have previously employed this technique to demonstrate the inwardly rectifying properties of the 5-HT3A receptor channel and the modulation of its conductance by extracellular divalent cations (Hussy et al. 1994; Brown et al. 1998). That the analysis technique is capable of detecting differences in the conductance of 5-HT3 receptor channels is additionally shown by the channel conductance of 3.7 pS obtained for the receptor endogenous to rat superior cervical ganglion (SCG) neurones (Table 3; M. J. Gunthorpe & J. A. Peters, unpublished observations). This value is similar to previous estimates of 2.6 pS and 3.4 pS obtained for rat (Yang et al. 1992) and 3.4 pS for mouse (Hussy et al. 1994) SCG neurones, respectively, using fluctuation analysis. Thus it appears that the presence of a charged residue at the 4′ position of the M2 domain does not contribute to the low single-channel conductance of the 5-HT3A receptor. Likewise, the exchange of uncharged alanine and polar serine residues located at the 4′ position of muscle-type nicotinic acetylcholine γ and ε subunits does not contribute to the difference in conductance observed for fetal (α12β1γδ) and adult (α12β1εδ) receptors (Herlitze et al. 1996).
The present observations add support to a spatial arrangement inferred from application of the substituted cysteine accessibility method to nicotinic and GABAA receptor subunits, whereby the 4′ residue faces into the protein interior (Akabas et al. 1994; Xu & Akabas, 1996). It is conceivable, as originally suggested by Maricq et al. (1991), that the 4′lysine forms a salt bridge with an aspartate residue (D265) located within the adjacent M1 sequence. The latter is also unique to the 5-HT3A and 5-HT3B subunit sequences, since other Cys-loop receptors express either serine, alanine or an aliphatic residue at this position. The lack of effect of any of the mutations at the 4′ position upon channel conductance might provide indirect evidence that salt bridge formation does in fact occur. Modelling of the 5-HT3A receptor ion channel suggests that even if the 4′lysine residue is located upon the face of the α-helix opposite to the lumen, it might still have a through space electrostatic effect sufficient to influence channel conductance (M. S. P. Sansom, personal communication). The absence of such an effect implies neutralization of the positive charge through salt bridge formation or, far more less likely in view of the side chain pKa of lysine (negative log of the acid dissociation constant, 10.4 in solution), deprotonation in the hydrophobic interior of the receptor protein.
If the above scenario is correct, the residues present at the 3′ (i.e. F) and 5′ (i.e. I) positions might reasonably be predicted to interact hydrophobically with the adjacent α-helix at either side, allowing the polar residues 2′threonine (2′T) and 6′serine (6′S) to line the pore. Thus, it is perhaps unsurprising that changing 3′F to either glutamate (E), or lysine, and 5′I to glutamate, resulted in either complete lack or very low levels of binding of the selective antagonist [3H]granisetron to membranes prepared from transfected cells. Interestingly, retention of binding was observed when the 5′I was replaced by lysine. This suggests that the structural changes introduced by the charged residues are better tolerated by the protein at this position, although only lysine, perhaps because it is a more flexible residue than glutamate, can presumably be accommodated. However the resulting receptors appeared to be non-functional, suggesting that either these lysine residues at least partly face the pore in the open channel conformation and prevent ion flow, or that in these mutant receptors the channel cannot actually open.
4′ mutations and agonist sensitivity
Irrespective of the substitution made, a small (approximately 2-fold) decrease in the EC50 for 5-HT was observed. Given the location of the residue, this effect is unlikely to result from direct influence upon the binding site for 5-HT. Instead, M2 mutants of the 5-HT3A and other transmitter-gated channels (e.g. Revah et al. 1991; Yakel et al. 1993) may produce an alteration in the stability of open versus closed states of the receptor (Filatov & White, 1995; Labarca et al. 1995). Such effects on receptor function have, in some instances, been correlated with the physico-chemical character of the amino acid substituted. For example, in a study of recombinant 5-HT3A receptors expressed in Xenopus oocytes, polar substitutions at 9′leucine (9′L) resulted in receptors with enhanced agonist sensitivity (EC50, 0.3-0.6 μm) compared to either WT (1.4 μm) or hydrophobic substitutions (1–1.3 μm; Yakel et al. 1993). A similar rank order of effect was found for equivalent substitutions in the nicotinic α7 nACh receptor (Revah et al. 1991). By contrast, all four 4′K substitutions examined in the present study, although involving amino acids with very different properties, produced a similar increase in the potency of 5-HT.
4′K mutations and desensitization
WT and 5-HT3A receptors mutated at the 4′K position all desensitized completely in the prolonged presence of a supramaximal concentration of agonist, but the rate of current decay was substantially slower for all four mutant subunits. It has been reported (Yakel et al. 1993) that the rate of desensitization of WT receptors is accelerated by the presence of divalent cations, particularly [Ca2+], in the extracellular solution (but see Boddeke et al. 1996). In the present study, the macroscopic current response in ‘zero’ calcium solution ([Ca2+]o∼10 nM) desensitized approximately 2-fold more slowly than currents recorded with [Ca2+]o set at 1.8 mM (Fig. 4). Therefore, experiments examining the kinetics of desensitization were conducted in the nominal absence of extracellular Ca2+ to eliminate a potentially confounding influence that could result from differences in Ca2+ influx between the mutated channels, although the latter possibility was not addressed specifically.
The rank order of effect of the 4′K substitutions on 5-HT3A receptor desensitization was arginine > glycine > serine > glutamine > lysine (WT); the arginine substitution resulting in more than a 5-fold slower rate of desensitization compared to WT (Fig. 5, Table 2). Thus, the quantitative effect of the 4′K mutations upon desensitization, unlike agonist potency (see above), is dependent upon the amino acid substituted. However, the effect of the 4′K mutations upon desensitization does not correlate simply with the properties of the side chain of the substituted amino acid. Replacement of the M2 4′lysine residue by the like-charged arginine residue, which has been described as a ‘safe’ substitution in site-directed mutagenesis (Bordo & Argos, 1991), in fact caused the greatest reduction in desensitization rate. In other classes of ion channel, the replacement, by lysine, of arginine residues participating in putative salt bridges that might stabilize protein structure has variable effects. For the cystic fibrosis conductance regulator (CTFR), such substitution within the sixth transmembrane domain (i.e. R347K) is well tolerated and is postulated to maintain the stability of the pore in a high conductance state (Cotten & Walsh, 1999). By contrast, at an inwardly rectifying potassium channel (IRK1), the mutation R148K located within the pore (or H5) region produces non-functional channels (Yang et al. 1997). Yang et al. hypothesize that this mutation disrupts a salt bridge that is exposed in the IRK1 channel. We suggest that such disruption of the putative salt bridge between the 4′ and the M1 D265 residues is less likely within the hydrophobic core of the 5-HT3A receptor protein (see also Cotten & Walsh, 1999). The lack of effect of the mutation upon channel conductance (via a through space electrostatic effect – see above) is consistent with this interpretation. Instead, the slowing of the rate of desensitization produced by the introduction of the arginine residue might be related to the reduced flexibility of this residue compared to lysine, which could result in local structural changes that are necessary to allow the formation of the salt bridge; further experiments are necessary to determine if such changes might affect the rate of the conformation change(s) necessary for entry into the desensitized state(s), which might provide an explanation for our data.
If the above interpretation is correct, how can the influence of the remaining amino acid substitutions upon desensitization rate be explained? In the absence of structural information, it might be speculated from the general principles of protein folding that mutations of the 4′ residue that leave the negatively charged D265 residue unpaired in the membrane will lead to a re-orientation within the protein that reduces the energy of this ion within a low dielectric constant (e.g. Perutz, 1979). The conformational freedom available to ‘solvate’ the D265 residue might correlate with the volume of the side chain of the amino acid substituting for the 4′lysine residue. Indeed, the increase in the t1/2 of desensitization does correlate inversely with side chain volume (i.e. Q > S > G). This scheme is, of course, purely conjectural. However, it is interesting to note that changes in the channel gating kinetics of the muscle nACh receptor produced by mutation of a valine residue within the TM3 domain can be related to the volume of the substituted residue (Wang et al. 1999).
Conclusions
In conclusion, the results presented here demonstrate that the lysine residue at the 4′ position in the M2 domain of 5-HT3A receptor has an important role in receptor function but is not the determinant of the low single-channel conductance of this receptor. Most of the biophysical properties of the receptor examined are unaffected by the substitution of lysine with amino acids of differing charge and/or side chain length, but each caused a reduction in the rate of desensitization. Combined with data from the 3′F and 5′I mutants, it would therefore appear that 4′K, and by analogy the 4′ location of other Cys-loop transmitter-gated channels, is not exposed to the channel lumen during the gating or the open state of the channel but it is probably involved in the conformational transition from the open to the desensitized state(s) of the receptor.
Acknowledgments
This work was supported by grants from the Wellcome Trust to S.C.R.L., J.A.P. and J.J.L. M.J.G was a MRC student and S.C.R.L. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science.
References
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α-subunit. Neuron. 1994;13:919–927. doi: 10.1016/0896-6273(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Barnard EA. The transmitter-gated channels: a range of receptor types and structures. Trends in Pharmacological Sciences. 1996;17:305–309. [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. Molecular Pharmacology. 1995;48:1054–1062. [PubMed] [Google Scholar]
- Boddeke HW, Meigel I, Boeijinga P, Arbuckle J, Docherty RJ. Modulation by calcineurin of 5-HT3 receptor function on NG108–15 neuroblastoma × glioma cells. British Journal of Pharmacology. 1996;118:1836–1840. doi: 10.1111/j.1476-5381.1996.tb15611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boess FG, Beroukhim R, Martin IL. Ultrastructure of the 5-hydroxytryptamine3 receptor. Journal of Neurochemistry. 1995;64:1401–1405. doi: 10.1046/j.1471-4159.1995.64031401.x. [DOI] [PubMed] [Google Scholar]
- Bordo D, Argos P. Suggestions for ‘safe’ residue substitutions in site-directed mutagenesis. Journal of Molecular Biology. 1991;217:721–729. doi: 10.1016/0022-2836(91)90528-e. [DOI] [PubMed] [Google Scholar]
- Brown AM, Hope AG, Lambert JJ, Peters JA. Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A) The Journal of Physiology. 1998;507:653–665. doi: 10.1111/j.1469-7793.1998.653bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Okayama H. Calcium phosphate-mediated gene-transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- Cotton JF, Walsh MJ. Cystic fibrosis-associated mutations at arginine 347 alter the pore architecture of CFTR. Journal of Biological Chemistry. 1999;274:5429–5435. doi: 10.1074/jbc.274.9.5429. [DOI] [PubMed] [Google Scholar]
- Couturier S, Erkman L, Valera S, Rungger D, Bertrand S, Boulter J, Ballivet M, Bertrand D. Alpha 5, alpha 3, and non-alpha 3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. Journal of Biological Chemistry. 1990;265:17560–17567. [PubMed] [Google Scholar]
- Cull-Candy SG, Howe JR, Ogden DC. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. The Journal of Physiology. 1988;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Dempster J. Computer Analysis of Electrophysiological Signals. London: Academic Press; 1993. [Google Scholar]
- Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Filatov GN, White MM. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Molecular Pharmacology. 1995;48:379–384. [PubMed] [Google Scholar]
- Gill CH, Peters JA, Lambert JJ. An electrophysiological investigation of the properties of a murine recombinant 5-HT3 receptor stably expressed in HEK 293 cells. British Journal of Pharmacology. 1995;114:1211–1221. doi: 10.1111/j.1476-5381.1995.tb13335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Hargreaves AC, Gunthorpe MJ, Taylor CW, Lummis SCR. Direct inhibition of 5-hydroxytryptamine3 receptors by antagonists of L-type Ca2+ channels. Molecular Pharmacology. 1996;50:1284–1294. [PubMed] [Google Scholar]
- Herlitze S, Villaroel A, Witzemann V, Koenen M, Sakmann B. Structural determinants of channel conductance in fetal and adult rat muscle acetylcholine receptors. The Journal of Physiology. 1996;492:775–787. doi: 10.1113/jphysiol.1996.sp021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope AG, Downie DL, Sutherland L, Lambert JJ, Peters JA, Burchell B. Cloning and functional expression of an apparent splice variant of the murine 5-HT3 receptor-A subunit. European Journal of Pharmacology. 1993;245:187–192. doi: 10.1016/0922-4106(93)90128-v. [DOI] [PubMed] [Google Scholar]
- Hussy N, Lukas W, Jones KA. Functional properties of a cloned 5-hydroxytryptamine ionotropic receptor subunit: comparison with native mouse receptors. The Journal of Physiology. 1994;481:311–323. doi: 10.1113/jphysiol.1994.sp020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K. Ion channels: molecular basis of ion selectivity. FEBS Letters. 1993;325:100–103. doi: 10.1016/0014-5793(93)81422-v. [DOI] [PubMed] [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Isenberg KE, Ukhun IA, Holstad SG, Jafri S, Uchida U, Zorumski CF, Yang J. Partial cDNA cloning and NGF regulation of a rat 5-HT3 receptor subunit. NeuroReport. 1993;5:121–124. doi: 10.1097/00001756-199311180-00006. [DOI] [PubMed] [Google Scholar]
- Johnson DS, Heinemann SF. Cloning and expression of the rat 5-HT3 receptor reveals species-specific sensitivity to curare antagonism. Society for Neuroscience Abstracts. 1992;18:249. [Google Scholar]
- Jones KA, Surprenant A. Single channel properties of the 5-HT3 subtype of serotonin receptor in primary cultures of rodent hippocampus. Neuroscience Letters. 1994;174:133–136. doi: 10.1016/0304-3940(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Konno T, Busch C, van Kitzing E, Imoto K, Wang F, Nakai J, Mishina M, Numa S, Sakmann B. Rings of anionic amino acids as structural determinants of ion selectivity in the acetylcholine receptor channel. Proceedings of the Royal Society. 1991;B 244:69–79. doi: 10.1098/rspb.1991.0053. [DOI] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proceedings of the National Academy of Sciences of the USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Peters JA, Hales TG, Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. British Journal of Pharmacology. 1989;97:27–40. doi: 10.1111/j.1476-5381.1989.tb11920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Peters JA, Hope AG. 5-HT3 receptors. In: North RA, editor. Ligand- and Voltage-Gated Ion Channels. Boca Raton: CRC Press Inc.; 1995. pp. 177–212. [Google Scholar]
- Lankiewicz S, Lobitz N, Wetzel CHR, Rupprecht R, Gisselmann G, Hatt H. Molecular cloning, functional expression, and pharmacological characterization of 5-hydroxytryptamine3 receptor cDNA and its splice variants from guinea pig. Molecular Pharmacology. 1998;53:202–212. doi: 10.1124/mol.53.2.202. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Molecular Pharmacology. 1995;48:407–416. [PubMed] [Google Scholar]
- Perutz MF. Electrostatic effects in proteins. Science. 1979;201:1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Peters JA, Malone HM, Lambert JJ. An electrophysiological investigation of the properties of 5-HT3 receptors of rabbit nodose ganglion neurones in culture. British Journal of Pharmacology. 1993;110:665–676. doi: 10.1111/j.1476-5381.1993.tb13863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi J-L, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J-P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Robertson B, Bevan S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. British Journal of Pharmacology. 1991;102:272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. Journal of Neuroscience. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Sepúlveda M-I, Lummis SCR, Martin IL. The agonist properties of m-chlorophenylbiguanide and 2-methyl-5-hydroxytryptamine on 5-HT3 receptors in N1E-115 neuroblastoma cells. British Journal of Pharmacology. 1991;104:536–540. doi: 10.1111/j.1476-5381.1991.tb12464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Shen KZ, North RA. 5-Hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992;8:199–203. doi: 10.1016/0896-6273(92)90121-s. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tachibana M, Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. The Journal of Physiology. 1990;421:645–662. doi: 10.1113/jphysiol.1990.sp017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. Journal of Neuroscience. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 Å resolution. Journal of Molecular Biology. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Wang H-L, Milone M, Ohno K, Shen X-M, Tsujino A, Batcchi AP, Tonali P, Brengman J, Engel AG, Sine SM. Acetylcholine receptor M3 domain: stereochemical and volume contributions to channel gating. Nature Neuroscience. 1999;2:226–233. doi: 10.1038/6326. [DOI] [PubMed] [Google Scholar]
- Xu M, Akabas MH. Identification of channel-lining residues in the M2 membrane-spanning segment of the GABAA receptor α1 subunit. Journal of General Physiology. 1996;107:195–205. doi: 10.1085/jgp.107.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL, Lagrutta A, Adelman JP, North RA. Single amino acid substitution affects desensitization of the 5-hydroxytryptamine type 3 receptor expressed in Xenopus oocytes. Proceedings of the National Academy of Sciences of the USA. 1993;90:5030–5033. doi: 10.1073/pnas.90.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. Journal of General Physiology. 1990;96:1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mathie A, Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. The Journal of Physiology. 1992;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yu M, Jan YN, Yang LY. Stabilization of ion channel selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proceedings of the National Academy of Sciences of the USA. 1997;94:1568–1572. doi: 10.1073/pnas.94.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Electrophysiological characterization of 5-HT receptors on rat petrosal neurons in dissociated cell culture. Brain Research. 1999;816:544–553. doi: 10.1016/s0006-8993(98)01232-3. [DOI] [PubMed] [Google Scholar]


