Abstract
The purpose of the present study was to investigate the acid-base status and the serum concentration of organic acids in puppies with naturally occurring canine parvoviral enteritis. Between July 1999 and July 2000, 25 client-owned puppies admitted to the St. Louis Animal Emergency Clinic South for treatment of enteritis caused by parvovirus infection were used in our study. Control blood samples were collected from 22 healthy puppies less than 9 months of age. Serum organic acid concentrations were quantitatively determined by HPLC. Puppies infected with parvovirus had significantly lower plasma concentrations of sodium, potassium, chloride, and bicarbonate than controls. Although serum L-lactate tended to increase in some puppies with canine parvoviral enteritis, our study demonstrated that most affected puppies developed only mild compensated metabolic acidosis. None of the affected puppies had an elevated serum D-lactate concentration at admission.
Introduction
Canine parvovirus (CPV) infection has been identified as a leading cause of enteritis in dogs since the late 1970s and has remained so despite the wide availability of effective vaccines (1,2). Although treatment of dogs with CPV enteritis is often successful, many dogs (up to 48%) (3) die of complications related to septicemia (4,5) or are euthanized because of anticipated financial burden (6). In a study conducted at the University of Missouri-Columbia, it was found that even when dogs that were euthanized were excluded from consideration, the mortality rate of dogs with CPV enteritis was unacceptably high (21%) (6).
Despite good epidemiological studies, a major problem remains: how to improve survival. An increased concentration of D-lactate, the stereoisomer of L-lactate, has recently been reported in acidemic diarrheic calves without signs of dehydration (7,8). It is believed that malabsorptive diarrhea could promote bacterial fermentation of undigested nutrients in the ileum and the large intestine, resulting in the production of D-lactate by bacteria and its subsequent absorption into the blood (9). In light of the fact that mammalian cells do not possess the enzyme D-lactate dehydrogenase, responsible for the production of the D-lactate, only limited amounts of D-lactate could be produced by mammalian cells through the glyoxalase pathway (10). Because of this, at least some D-lactate is excreted unmetabolized in the urine (10). Some D-lactate, after transformation into pyruvate, is oxidized or utilized for gluconeogenesis. Renal clearance is, nonetheless, much slower for D-lactate than for L-lactate (11).
The purpose of the present study was to investigate the acid-base status and the serum concentration of organic acids in dogs admitted with acute CPV enteritis. We hypothesized that puppies with naturally occurring CPV enteritis develop metabolic acidosis secondary to the production of D-lactate by the bacterial population within the lumen of the large intestine. Therefore, the finding of a novel mechanism for acidosis could have considerable impact on our thinking of the causes of morbidity in CPV enteritis.
Materials and methods
Between July 1999 and July 2000, client-owned dogs admitted to the St. Louis Animal Emergency Clinic South, St. Louis, Missouri, USA, for treatment of naturally occurring canine parvoviral enteritis were used in our study. Enrolment criteria included positive results for a fecal enzyme-linked immunosorbent assay (ELISA) antigen test (Synbiotics Parvo Witness Test; Synbiotics, San Diego, California, USA), clinical signs of vomiting, diarrhea prior to admission, and a signed client consent. Prior to any treatments, acid-base parameters (pH, bicarbonate concentration, partial pressure of carbon dioxide, and base deficit) of venous blood were determined within 15 min of blood collection (2 mL in lithium-heparin tubes) using a portable blood gas analyzer (i-Stat, Heska, Waukesha, Wisconsin, USA). Plasma sodium (Na+), potassium (K+), chloride (Cl−), glucose, and blood urea nitrogen (BUN) concentrations were also determined simultaneously with the portable blood gas analyzer. For the determination of organic acid concentrations, a second 3-mL blood sample was collected and placed in a tube without anticoagulant (Vacutainer; Becton-Dickinson, Rutherford, New Jersey, USA). On clotting, serum was separated by centrifugation and frozen at −20oC until analyzed.
Dogs with CPV enteritis were treated intravenously (IV) with fluids (2.5% dextrose in half-strength saline supplemented with potassium chloride) and ampicillin sodium (22 mg/kg body weight (BW)), 3 times a day. Prochlorperazine (0.3 mg/kg) was used intramuscularly (IM) as needed to control vomiting. Collection of blood was repeated 24 h after admission to assess the degree of acid levels after treatment. Control blood samples were collected as previously described from healthy dogs less than 9 mo of age. These control dogs were pets in the St. Louis area volunteered by their owners.
Anion gap was calculated as the difference between the sum of the serum concentrations of the measured cations (Na+ + K+) and the measured anions (Cl− + HCO3−). Serum acetate, pyruvate, DL-lactate, and β-hydroxybutyrate concentrations were determined, using the ion exclusion method (Waters-Millipore, Milford, Massachusetts, USA) for the separation of organic acids (12). The steriospecific analysis of serum lactate enantiomer concentrations was accomplished by high-performance liquid chromatography (HPLC) (13). The HPLC system consisted of a pump (Waters Model 626; Millipore), a tunable wavelength UV absorbance detector (Waters 2487; Millipore) and an autoinjector (Waters 717 plus; Millipore). Data collection, integration, and calibration were performed using a chromatography manager (Waters Millennium v.32; Millipore).
Statistical analysis
Data were analyzed with the statistical program (Systat v.8.0; SPSS, Chicago, Illinois, USA). The mean concentration of all organic acids was calculated for the controls and the puppies with naturally occurring canine parvoviral enteritis. The analysis of variance was used to compare blood gas components and measured concentrations of lactic acid, enantiomers, and other organic acids. The Tukey pairwise comparisons test was used when significant differences were detected for some of the parameters. Linear regression analysis was used to investigate the correlation between blood HCO3− and D-lactate concentration. All values were expressed as mean ± standard deviation (SD) and a P value of 0.05 was considered significant.
Results
Dogs
Between September and November 1999, control blood samples were collected from 22 client-owned healthy dogs. The control group consisted of 12 males and 10 females. The mean age of the controls was 21.5 ± 8.7 wk, with a range of 6 wk to 32 wk. Between July 1999 and July 2000, 25 client-owned dogs admitted for treatment of CPV enteritis were used in our study. The dogs with CPV enteritis consisted of 13 males and 12 females. The mean age of affected dogs was 19 ± 8.3 wk old, with a range of 4 wk to 42 wk. All affected dogs had a complete physical examination and received supportive treatment. Twenty-three of the 25 dogs recovered from treatment of CPV enteritis. Two dogs were euthanized for financial reasons. It was unknown in most dogs with CPV enteritis whether they had been vaccinated prior to admission.
Blood gas and serum concentration of organic acids
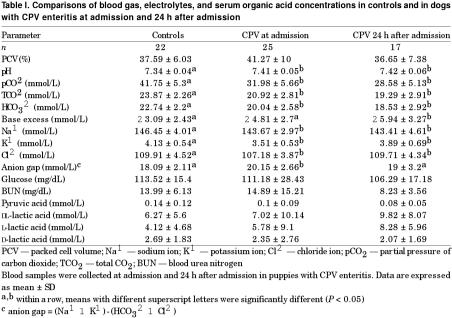
In comparison with the control group, blood gas analysis of puppies admitted with CPV enteritis revealed a significant decrease in HCO3− but a significant increase in blood pH (Table I). Significant decreases in the mean concentrations for plasma total CO2 (TCO2) and partial pressure of carbon dioxide (pCO2) were also found. Dogs with CPV enteritis had a significant higher anion gap at admission than the control group and a lower plasma concentration of Na+, K+, and Cl−. Overall, serum L-lactate tended to increase while D-lactate tended to decrease in puppies with CPV enteritis. In light of the high level of serum β-hydroxybutyric acid identified by HPLC in several puppies with CPV enteritis, confirmation of this compound was performed using a triple quadrupole mass spectrometer (Micromass Quatro IIE; Micromass, Altrincham, United Kingdom). Coeluting peaks were found with glycerin, a humectant of the centrifugal filter unit used to remove protein from samples. Therefore, our HPLC method was inaccurate for measuring β-hydroxybutyrate. Although we had been able of measuring serum acetate in different species with our HPLC method (12,14), no detectable level of serum acetate was found in our canine samples. For 22 healthy and 25 dogs with CPV enteritis, there was no significant correlation between blood HCO3− and D-lactate concentration.
Table I.
Discussion
This study represents the first attempt to document the possibility that most puppies with naturally occurring CPV enteritis admitted to the St. Louis Animal Emergency Clinic South develop metabolic acidosis. Venous blood pH is usually considered to be normal between 7.32 and 7.44 (15). Although blood pH was significantly higher in puppies admitted with CPV in comparison with the control group, the range was within normal limits. At admission, the dogs with CPV enteritis demonstrated mainly hyponatremia, hypokalemia, and hypochloremia. These findings were expected because diarrhea with villous atrophy usually results in a net loss of water, Na+, K+, and Cl−, as well as nutrients (16). Although the dogs with CPV demonstrated a decrease in HCO3− concentration, complete correction of metabolic acidosis was achieved in most subjects. Respiratory compensation could be an explanation (15). No significant increases were found, however, in the packed cell volume and BUN in the dogs with CPV enteritis; therefore, severe dehydration was not present. No IV fluids had been given prior to sample collection at the clinic or by referring veterinarians.
In a previous study conducted at the St. Louis Animal Emergency Clinic South, it was found that the mortality rate of dogs with parvovirus enteritis was unacceptably high (21%) (6). The survival rate of our puppies with CPV was not consistent with the previous study. This finding confirms that most of our puppies admitted with CPV were not severely dehydrated.
Rapid dehydration usually contributes to the metabolic acidosis in the pathophysiology of secretory diarrhea (16). This study showed that D-lactate did not increase in the serum of dogs with acute CPV enteritis. In comparison with a previous study in diarrheic calves (9), it is unlikely that the passage of D-lactate from the intestinal lumen to the blood was achieved in our puppies with the most severe metabolic acidosis. In diarrheic neonatal calves, gastrointestinal dysfunction with small intestinal malabsorption and maldigestion in the small intestine may result in the formation of D-lactate (17). The undigested lactose may aggravate diarrhea by providing additional substrate for the development of intestinal bacterial overgrowth or by osmotically drawing fluids into the lumen after fermentation of undigested lactose in the large intestine. A small number of dogs was used in this study, but it is unlikely that data from additional dogs would identify a contributory role for D-lactate to the onset of acidosis in CPV enteritis.
In dogs with CPV enteritis, the inflammatory response initiated by the viral disease and associated endotoxemia could play a more important role in the pathophysiology of the disease than the formation of D-lactate. Clinical signs of vomiting and diarrhea have usually been associated with viral destruction of rapidly dividing cells of the intestine and bone marrow (18). Mortality associated with CPV enteritis has appeared to result from bacteremia and endotoxemia (19). In addition, dogs with this disease have a high prevalence of clinical thrombosis or phlebitis and laboratory evidence of hypercoagulability without disseminated intravascular coagulopathy (20). In conclusion, metabolic acidosis is readily compensated or corrected in dogs with CPV enteritis and not exacerbated by D-lactate production.
Footnotes
Acknowledgments
The authors thank Pierre Trottier and Grant M. Laxdal for their technical assistance.
Address correspondence to Dr. Nappert. Reprints will not be available.
Dr. Nappert's current address is l'Hôpital vétérinaire Lachute, 431 rue Principale, Lachute, Quebec J8H 1Y4, fax: 450-562-1320, e-mail: nappertg@ultim.net.
Received May 15, 2001. Accepted September 28, 2001.
This work was supported by the Committee on Research, Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, University of Missouri-Columbia.
References
- 1.Parrish CR. Emergence, natural history, and variation of canine, mink and feline parvovirus. Adv Virus Res 1990;38:403–450. [DOI] [PMC free article] [PubMed]
- 2.Pollock RVH, Coyne MJ. Canine parvovirus. Vet Clin North Am Small Anim Pract 1993;23:555–568. [DOI] [PMC free article] [PubMed]
- 3.Dimmitt R. Clinical experience with cross-protective anti-endotoxin antiserum in dogs with parvoviral enteritis. Canine Pract 1991; 16(3):23–26.
- 4.Turk J, Miller M, Brown T, et al. Coliform septicemia and pulmonary disease associated with canine parvoviral enteritis: 88 cases (1987–1988). J Am Vet Med Assoc 1990;196:771–773. [PubMed]
- 5.Macintire DK, Smith-Carr S. Canine parvovirus. Part II. Clinical signs, diagnosis, and treatment. Compend Contin Educ Pract Vet 1997;19:291–302.
- 6.Mann FA, Boon GD, Wagner-Mann CC, et al. Ionized and total magnesium concentrations in blood from dogs with naturally acquired parvoviral enteritis. J Am Vet Med Assoc 1998;212: 1398–1401. [PubMed]
- 7.Grove-White DH, Aber G. Pathophysiology and treatment of metabolic acidosis in the diarrheic calf. 19th World Buiatrics Congr 1996:102–107.
- 8.Schelcher F, Marcillaud S, Braun JP, et al. Metabolic acidosis without dehydration and no or minimal diarrheoa in suckler calves is caused by hyper D-lactataemia. 20th World Buiatrics Congr 1998:371–374.
- 9.Omole OO, Nappert G, Naylor JM, et al. Both L- and D-lactate contribute to metabolic acidosis in diarrheic calves. J Nutr 2001;131:2128–2131. [DOI] [PMC free article] [PubMed]
- 10.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 1990;269:1–11. [DOI] [PMC free article] [PubMed]
- 11.Giesecke D, Stangassinger M. Lactic acid metabolism. In: Ruckebusch Y, Thivend P, eds. Digestive Physiology and Metabolism in Ruminants. Westport: Avi Publishing, 1980: 523–539.
- 12.Nappert G, Zello GA, Naylor JM. Examination of metabolism of viscera drained by the portal vein in neonatal calves, using short-term intravenous infusions of glutamine and other nutrients. Am J Vet Res 1999;60:437–445. [PubMed]
- 13.Omole OO, Brocks DR, Nappert G, et al. High-performance liquid chromatographic assay of (±)-lactic acid and its enantiomers in calf serum. J Chromatogr B 1999;727:23–29. [DOI] [PubMed]
- 14.Nappert G, Johnson PJ. Determination of the acid-base status in 50 horses admitted with colic between December 1998 and May 1999. Can Vet J 2001;42:703–707. [PMC free article] [PubMed]
- 15.Autran de Morais HS, DiBartola SP. Mixed acid-base disorders. Part II. Clinical disturbances. Compend Contin Educ Pract Vet 1994;16:477–488.
- 16.Argenzio RA. Pathophysiology of diarrhea. In: Anderson NV, ed. Veterinary Gastroenterology. 2nd ed. Philadelphia: Lea & Febiger, 1992:163–172.
- 17.Omole OO. Lactic acidosis in neonatal diarrheic calves [MSc thesis]. University of Saskatchewan, Saskatoon, Saskatchewan, 1999.
- 18.Smith-Carr S, Macintire DK, Swango LJ. Canine parvovirus. Part I. Pathogenesis and vaccination. Compend Continu Educ Pract Vet 1997;19:125–133.
- 19.Otto CM, Drobatz K, Soter C. Endotoxemia and tumor necrosis factor activity in dogs with naturally occurring parvoviral enteritis. J Vet Intern Med 1997;11:65–70. [DOI] [PubMed]
- 20.Otto CM, Rieser TM, Brooks MB, Russell MW. Evidence of hypercoagulability in dogs with parvoviral enteritis. J Am Vet Med Assoc 2000;217:1500–1504. [DOI] [PubMed]