Abstract
We here review mechanisms that can regulate the activity of myosin II, in smooth muscle and non-muscle cells, by modulating the Ca2+ sensitivity of myosin regulatory light chain (RLC) phosphorylation. The major mechanism of Ca2+ sensitization of smooth muscle contraction and non-muscle cell motility is through inhibition of the smooth muscle myosin phosphatase (MLCP) that dephosphorylates the RLC in smooth muscle and non-muscle. The active, GTP-bound form of the small GTPase RhoA activates a serine/threonine kinase, Rho-kinase, that phosphorylates the regulatory subunit of MLCP and inhibits phosphatase activity. G-protein-coupled release of arachidonic acid may also contribute to inhibition of MLCP acting, at least in part, through the Rho/Rho-kinase pathway. Protein kinase C(s) activated by phorbol esters and diacylglycerol can also inhibit MLCP by phosphorylating and thereby activating CPI-17, an inhibitor of its catalytic subunit; this mechanism is independent of the Rho/Rho-kinase pathway and plays only a minor, transient role in the G-protein-coupled mechanism of Ca2+ sensitization. Ca2+ sensitization by the Rho/Rho-kinase pathway contributes to the tonic phase of agonist-induced contraction in smooth muscle, and abnormally increased activation of myosin II by this mechanism is thought to play a role in diseases such as high blood pressure and cancer cell metastasis.
Myosin II, the major molecular motor of muscle and most non-muscle cells, is regulated not only by fluctuations in cytoplasmic calcium ([Ca2+]i), but also by other important signalling mechanisms. Thus, whereas excitation- contraction coupling in vertebrate striated muscles is under membrane potential control and contraction is initiated by binding of Ca2+ to a thin (actin) filament-associated protein, troponin, contractility of smooth muscle is regulated not only by such electromechanical coupling and [Ca2+]i, but also by membrane potential-independent, pharmacomechanical coupling (Somlyo & Somlyo, 1968; reviewed in Somlyo & Somlyo, 1994; Somlyo et al. 1999a). Furthermore, both smooth muscle and non-muscle myosin II are regulated by phosphorylation/dephosphorylation of the myosin regulatory light chain (RLC) by, respectively, Ca2+-calmodulin-regulated myosin light chain kinase (MLCK) and myosin phosphatase (MLCP, also known as SMPP-1M); actin activation of myosin II is the result of RLC phosphorylation on Ser 19 (Adelstein & Conti, 1975; reviewed in Hartshorne, 1987; Tan et al. 1992; Somlyo & Somlyo, 1994; Gallagher et al. 1997). Therefore, it was to be expected that the Ca2+-independent mechanisms that regulate smooth muscle myosin II will also regulate non-muscle myosin II and non-muscle motility (Somlyo & Somlyo, 1994). The small GTPase, RhoA, and its upstream activators and downstream effectors play a major role in these processes and are the subject of this brief review.
Increased RLC (Ser 19) phosphorylation of smooth and non-muscle myosin II can be effected not only by increasing [Ca2+]i and, thereby, the activity of MLCK, but also by inhibiting MLCP. This was the basis of the suggestion (Somlyo et al. 1989), verified experimentally (Kitazawa et al. 1991), that contractions induced at constant [Ca2+]i by certain agonists and by GTPγS were due to inhibition of a myosin phosphatase. The inhibitory signal for ‘Ca2+ sensitization’ is communicated by RhoA to a Rho-kinase that phosphorylates the M110-130 regulatory subunit and inhibits the catalytic activity of MLCP, resulting in increased RLC phosphorylation, contraction and cell motility (Fig. 1). Experimental evidence that led to these conclusions is summarized below.
Figure 1. Regulation of myosin II in smooth and non-muscle cells.
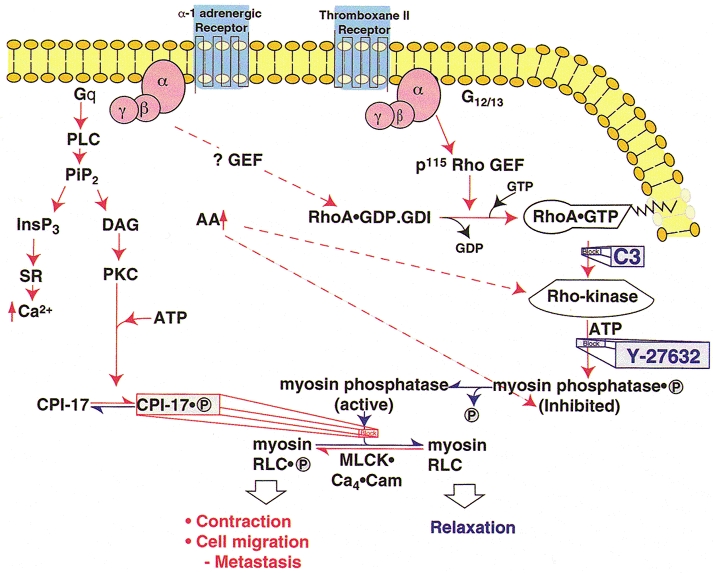
Pathways indicated in red activate myosin II, resulting in contraction, cell migration and cancer metastasis. Pathways that reduce myosin II activity are shown in blue. The major, Ca2+-independent pathway that increases myosin II activity is through activation of Rho-kinase by RhoA.GTP, phosphorylation of the regulatory subunit of myosin phosphatase (MLCP) by Rho-kinase and some other kinases (see text), resulting in inhibition of MLCP activity and increased myosin RLC phosphorylation. Increases in arachidonic acid, due to a variety of stimuli, can also activate Rho-kinase and, at least in vitro, also inhibit MLCP activity by dissociating the regulatory (M110-130) from the catalytic (PP-1C) subunit. The third pathway shown that enhances myosin II activity is through phosphorylation of CPI-17 by protein kinase C (PKC) leading to direct inhibition of PP-1C by the phosphorylated CPI-17 (CPI-17.P). Each of these mechanisms requires the presence of an active kinase that can phosphorylate Ser19 of RLC and increase myosin II activity by inhibiting MLCP even at constant [Ca2+]i (‘Ca2+ sensitization’). These mechanisms operate in parallel with and independently of the activation of MLCK by Ca2+ released from the sarcoplasmic reticulum/endoplasmic reticulum (SR/ER) by InsP3 or by Ca2+ influx (not shown). Abbreviations: PLC, phospholipase C; PiP2, phosphatidylinositol-bis-phosphate; InsP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C; GEF, guanine nucleotide exchange factor; MLCK, myosin light chain kinase; Cam, calmodulin; RLC, regulatory light chain; C3, Clostridium botulinum, exoenzyme that ADP-ribosylates Asn 41 of RhoA, inhibits RhoA activity and blocks its translocation to the membrane; Y-27632, selective inhibitor of Rho-kinase.
Although G-protein-coupled regulation of a myosin phosphatase was recognized over a decade ago (Somlyo et al. 1989), the regulated phosphatase (MLCP) that dephosphorylates the RLC of intact myosin was identified only recently. It consists of a 110–130 kDa regulatory (M110-130), an ∼37 kDa catalytic (PP-1C; variously described as β or δ) subunit, and a 20 kDa subunit of unknown function (Alessi et al. 1992; Shirazi et al. 1994; Shimizu et al. 1994; Haystead et al. 1995; reviewed in Hartshorne et al. 1998). It is also present in non-muscle (e.g. Nakai et al. 1997; Murányi et al. 1998; Suzuki et al. 1999; Essler et al. 1999; reviewed in Hartshorne et al. 1998), including human prostate cancer cells (Somlyo et al. 1999b). Selectivity of PP-1C for myosin is conferred by the M110-130 subunit that potentiates dephosphorylation of myosin by PP-1C, in vitro (Alessi et al. 1992; Shirazi et al. 1994; Shimizu et al. 1994; reviewed in Hartshorne et al. 1998) and in permeabilized smooth muscle (Gailly et al. 1996). Phosphorylation of M110-130 in its C-terminal half by Rho-kinase (Kimura et al. 1996) and some other kinase(s) on the same site (Feng et al. 2000) inhibits MLCP activity. Certain N-terminal fragments of M110-130 can cause Ca2+ sensitization, probably through competitive inhibition of the endogenous protein (Zhou et al. 1999).
Agonists acting on receptors coupled to Gq induce both Ca2+ sensitization and activation of phospholipase C (PLC)-mediated hydrolysis of phosphatidylinositol-bis-phosphate to inositol-1,4,5-trisphosphate (InsP3) and diacylglycerol to, respectively, cause Ca2+ release and activate protein kinase C (PKC); the concurrence of these effects implicated Gαq in both processes (Somlyo & Somlyo, 1994). However, Ca2+ release can be dissociated from Ca2+ sensitization (Kobayashi et al. 1991), and InsP3 does not Ca2+ sensitize smooth muscle. These results exclude PLC products as major Ca2+-sensitizing effectors of Gαq, consistent with the view that conventional and novel PKCs (for historical antecedents, see Andrea & Walsh, 1992; Somlyo et al. 1999a) play only a small and transient (Iizuka et al. 1999) or no role in G-protein-coupled Ca2+ sensitization (Jensen et al. 1996; Walker et al. 1998; Strassheim et al. 1999). Furthermore, some very potent Ca2+-sensitizing agonists, such as U-46619, cause little or no detectable release of intracellular Ca2+ (Bradley & Morgan, 1987; Himpens & Somlyo, 1988; Himpens et al. 1990), as would be expected of receptors coupled to Gαq/11. Subsequent studies of non-muscle cells revealed that RhoA can be activated by α subunits of other trimeric G-proteins, Gα12,13, via linked guanine nucleotide exchange factors (GEFs; Hart et al. 1998; Kozasa et al. 1998; Gohla et al. 1999). The receptors activated by U-46619 are coupled to G12,13 that, unlike Gαq/11, do not activate PLC, suggesting that the disproportionately high Ca2+-sensitizing (compared with Ca2+ releasing) effect of U-46619 (Himpens et al. 1990) is mediated by the Gα12,13 family. A Gα-GAP (GTPase-activating protein), p115 Rho-GEF, interacts with Gα13 and is probably the upstream ‘convector’ between this trimeric G-protein and RhoA (Kozasa et al. 1998; Hart et al. 1998). It now appears that several trimeric G-proteins, including Gαq, Gα12,13 and Gαi-2, can activate RhoA, depending on the receptors and cell types involved (Katoh et al. 1998; Croxton et al. 1998; Klages et al. 1999; Hirshman & Emala, 1999).
RhoA, a monomeric G-protein, is, like most GTPases, active when it contains bound GTP and inactive when the bound nucleotide is GDP. RhoA.GTP does not directly inhibit MLCP, as indicated by its lack of effect on extensively permeabilized (with Triton X-100) smooth muscle (Gong et al. 1996), and Ca2+ sensitization requires its translocation to a relatively intact plasma membrane (Gong et al. 1997; Fujihara et al. 1997; Taggart et al. 1999). In resting smooth muscle (Gong et al. 1997) as in other cells (Bourmeyster et al. 1992; Abo et al. 1994; Bokoch et al. 1994), the cytosolic, inactive forms of RhoA and Rac are complexed with RhoGDI (guanine nucleotide dissociation inhibitor). The hydrophobic geranyl-geranylated tail of RhoA inserts into a hydrophobic cavity of GDI and binding is reinforced by protein-protein interactions between the N-terminus of GDI and a highly conserved (among Rho-family proteins) epitope of RhoA (Fig. 2; Gosser et al. 1997; Longenecker et al. 1999 and references therein). Cytosolic RhoA.RhoGDI is activated by Rho-GEFs (guanine nucleotide exchange factors) that stimulate nucleotide exchange on RhoA (GTP replaces GDP), followed by dissociation of RhoA.GTP from the complex and translocation to the plasma membrane, while GDI is retained in the cytosol (Gong et al. 1997; Fujihara et al. 1997; Read et al. 2000; reviewed in Cherfils & Chardin, 1999).
Figure 2. Structural features of the complex between RhoA and RhoGDI.
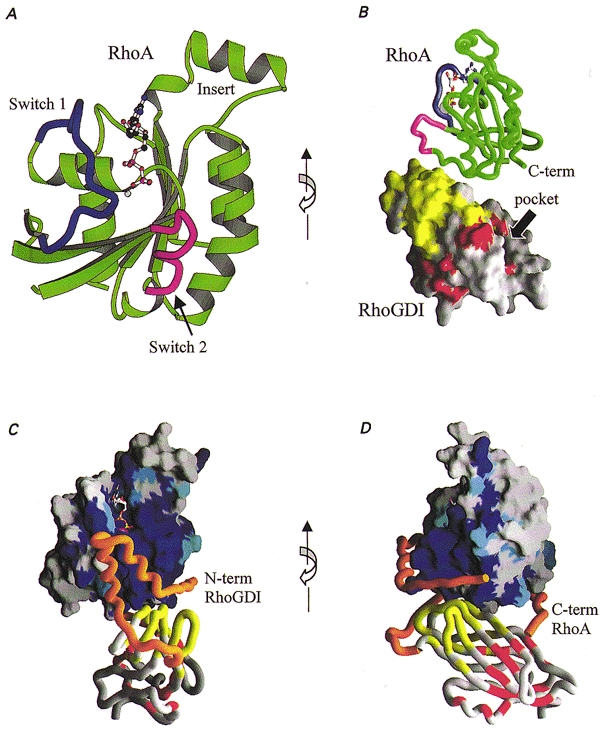
A, ribbon drawing of the crystal structure of RhoA highlighting the switch 1 (blue) and switch 2 (magenta) regions (Wei et al. 1997). B, crystal structure of the RhoA-RhoGDI complex (Longenecker et al. 1999) reveals the mutual disposition of the individual structures of RhoA and RhoGDI. The length of the complex is approximately 77 Å (7.7 nm). The surface of RhoGDI is coloured according to resonance shifts observed in solution by NMR (Gosser et al. 1997). RhoGDI residues affected by addition of Cdc42 (unprenylated) are coloured yellow, and those affected by addition of a prenylated peptide are red. C and D, models (orange) of the N-terminal domain of RhoGDI (residues 24–68) and of the C-terminal extension of RhoA (residues 181–190) display additional structural features of the complex. The conservation of amino acids among the Rho-family (rho, rac, Cdc42) is depicted on the surface of RhoA as a gradation of blue, where dark blue represents 100 % conservation. The large conserved surface is consistent with the ability of RhoGDI to bind each of the above Rho-family GTPases. A ribbon drawing of RhoGDI is coloured as in B. The views in B and D are rotated about the vertical axis relative to A and C.
Bacterial exoenzymes that ADP-ribosylate Asn 41 (C3, EDIN) or monoglucosylate Thr 37 (C. difficile toxin B) of RhoA (reviewed in Sehr et al. 1998) block its biological activity; ADP-ribosylation also prevents its translocation to the plasma membrane (Gong et al. 1997; Fujihara et al. 1997; Croxton et al. 1998). These agents inhibit Ca2+ sensitization of RLC phosphorylation and force in smooth muscle (Noda et al. 1995; Itagaki et al. 1995; Gong et al. 1996; Fujihara et al. 1997; Otto et al. 1996) and stress fibre formation in non-muscle cells (reviewed in Hall, 1994).
The Ca2+-sensitizing effector of RhoA.GTP is a serine/ threonine-kinase (we refer to it generically as ‘Rho-kinase’) that contains a Rho-binding domain, has two identified isoforms and is activated by Rho.GTP (Leung et al. 1995; Ishizaki et al. 1996; Matsui et al. 1996). Both α and β isoforms are present in smooth muscle (Yoshii et al. 1999), with ROKα (ROCK-II) predominant in gizzard (Feng et al. 1999). The detailed mechanism of Rho-kinase activation by RhoA.GTP is not known. However, the requirement for RhoA to translocate to the plasma membrane to cause Ca2+ sensitization and the lack of Ca2+ sensitization by a recombinant, non-prenylated (E. coli-expressed) RhoA.GTP that does not bind to the membrane (Gong et al. 1996), suggest that activation of Rho-kinase by RhoA.GTP, in analogy with the Ras-activated Raf-kinase, occurs upon recruitment of both proteins to the plasma membrane (Leung et al. 1995). Activated Rho-kinase phosphorylates the regulatory subunit of MLCP and inhibits myosin phosphatase activity (Kimura et al. 1996). MLCP can also be phosphorylated on the same inhibitory site (Feng et al. 2000) and inhibited by other, yet unidentified kinases (Trinkle-Mulcahy et al. 1995; Ichikawa et al. 1996; reviewed in Hartshorne et al. 1998).
In addition to RhoA.GTP, arachidonic acid, a Ca2+-sensitizing agent released by certain agonists (Gong et al. 1995; Gailly et al. 1997), can also activate Rho-kinase (Fu et al. 1998; Feng et al. 1999) and may contribute an ancillary pathway of RhoA-mediated Ca2+ sensitization. In vitro, arachidonic acid dissociates the regulatory from the catalytic subunit of MLCP (Gong et al. 1992) leading to a several-fold reduction in myosin-targeted phosphatase activity.
The Ca2+-sensitizing Rho/Rho-kinase pathway can be inhibited at several stages: at the receptor by antagonists to the activating agonist, by hydrolysis of bound GTP to GDP facilitated by GAPs, by complexation of free RhoA with GDI (for smooth muscle, see Longenecker et al. 1999), through deactivation/inhibition of Rho-kinase, and by site-specific dephosphorylation of M110-130. RhoA-induced Ca2+ sensitization can also be inhibited by Rnd1, a physiological RhoA antagonist that inhibits Ca2+ sensitization induced by carbachol, GTPγS, and recombinant RhoA (Loirand et al. 1999). Rnd1 is a prenylated Rho-related protein that is constitutively GTP bound and associated with the cell membrane; its expression is stimulated by progesterone or oestrogen. It is, therefore, important to realize that contractions initiated, maintained and terminated through the RhoA/Rho-kinase pathway are the result of a multiple-step process having complex kinetics.
Feedback inhibition of Rho/Rho-kinase-mediated Ca2+ sensitization may occur through inhibition of MLCK, because GTPγS and carbachol also increase MLCK phosphorylation of an inhibitory site (Tang et al. 1993). This may be due to MLCK's inhibitory site being dephosphorylated by MLCP or by another protein phosphatase that is inhibited by a G-protein-coupled mechanism, or to phosphorylation and inhibition of MLCK by PAK, a serine/threonine kinase activated by other Rho-subfamily GTPases, Cdc42 and Rac (Sanders et al. 1999). Val 12 Cdc42 and L61 Rac1 (constitutively active mutants) can inhibit Ca2+ sensitization of force induced by constitutively active Val 14 RhoA in permeabilized smooth muscle (M. Gong, P. Read, R. Nakamoto, A. V. Somlyo & A. P. Somlyo, unpublished observation). It remains to be determined whether these effects of Rac and Cdc42 are due to activation of PAK.
The Rho/Rho-kinase pathway plays an important physiological role in intact smooth muscle. The tonic phase of agonist-induced contractions, previously ascribed solely to maintained Ca2+ influx, is inhibited by a Rho-kinase inhibitor (Uehata et al. 1997; Fu et al. 1998), by a cell permeant chimera (DC3B) of C3 that selectively inactivates RhoA (Fujihara et al. 1997) and by the somewhat less selective toxin B, without any accompanying decrease in [Ca2+]i (Lucius et al. 1998). These results suggest that the slow (tonic) phase of contractions induced by carbachol in intestinal smooth muscles, unaccompanied by increased [Ca2+]i (Himpens & Somlyo, 1988), reflects the time course of Ca2+ sensitization by RhoA/Rho-kinase-mediated inhibition of myosin phosphatase. Identification of physiological and pathological functions of this pathway is greatly facilitated by use of the highly selective, cell permeant Rho-kinase inhibitor Y-27632 (Uehata et al. 1997; Fu et al. 1998; Yoshii et al. 1999).
Rho-kinase regulates myosin II in both smooth muscle and non-muscle by inhibiting dephosphorylation of myosin RLC, but the activity of MLCP can also be modulated by at least two other mechanisms. Protein kinase C (PKC), activated by phorbol esters or diacylglycerol (Jensen et al. 1996; Walker et al. 1998 and references therein), and a constitutively active PKC (Ikebe & Brozovich, 1996) can enhance contraction at constant [Ca2+]i by inhibiting myosin phosphatase directly or by phosphorylating an inhibitor (Somlyo et al. 1989; Itoh et al. 1993). PKC phosphorylates CPI-17, a potent inhibitor (when phosphorylated) of PP-1C (Eto et al. 1995; Kitazawa et al. 1999; Senba et al. 1999). Rho-kinase and PKC can inhibit MLCP, through convergent mechanisms, in both smooth muscle (Jensen et al. 1995) and non-muscle cells (Strassheim et al. 1999), with PKC playing a minor and transient (Iizuka et al. 1999) role in contractile regulation, its extent possibly depending on the agonist and/or cell type involved. Phorbol ester-induced Ca2+ sensitization is not inhibited by Y-27632 (Fu et al. 1998). Conversely, cyclic nucleotide (cAMP or cGMP)-activated kinases, possibly through the activity of phosphorylated telokin, accelerate dephosphorylation of RLC (Wu et al. 1998 and references therein) leading to muscle relaxation. RLC phosphorylation and contraction can be induced in the absence of Ca2+ by phosphatase inhibitors, such as microcystin or calyculin (e.g. Gong et al. 1995; Walker et al. 1998; Kolodney et al. 1999; Weber et al. 1999), and are inhibited by a promiscuous kinase inhibitor, staurosporin, but not by Y-27632 (Kureishi et al. 1999; Iizuka et al. 1999). The Ca2+-independent kinases(s) mediating such phosphorylation remain to be identified (Somlyo, 1999). Direct, in vitro phosphorylation of RLC by Rho-kinase (Amano et al. 1996) plays no significant role in vivo, as in the absence of [Ca2+]i to activate MLCK even massive stimulation of the Rho/Rho-kinase pathway by GTPγS induces minimal and very slow or no RLC phosphorylation and contraction of smooth muscle (Somlyo et al. 1989; Iizuka et al. 1999; Swärd et al. 2000). G-protein-coupled Ca2+ sensitization requires the presence of an active, but not necessarily Ca2+-activated, myosin light chain kinase (Iizuka et al. 1999), but the Ca2+-independent kinase (Weber et al. 1999; reviewed in Somlyo, 1999a) is not Rho-kinase (Kureishi et al. 1999; Iizuka et al. 1999).
Pathological activity of Rho-kinase in smooth muscle has been implicated in experimental hypertension (Uehata et al. 1997) and asthma (Chiba et al. 1999). The Rho-kinase inhibitor Y-27632 reduces blood pressure of hypertensive rats, without affecting normal blood pressure (Uehata et al. 1997). In (experimental) asthma muscarinic Ca2+ sensitization of intrapulmonary bronchial smooth muscle is enhanced and expression of RhoA is increased (Chiba et al. 1999). Tumour necrosis factor, implicated in asthma, also increases Ca2+ sensitivity of bronchial smooth muscles (Parris et al. 1999), but the participation of Rho in this process is not yet known. Thrombin-stimulated vascular smooth muscle migration (Seasholtz et al. 1999) and endothelial contraction (Essler et al. 1999) are also inhibited by C3 and by Y-27632, suggesting that the Rho/Rho-kinase pathway may play a role in atheromatous plaque formation and post-angioplasty re-stenosis.
The importance of Rho family proteins in cytoskeletal organization of non-muscle cells and the specific role of RhoA.GTP in stimulating stress-fibre formation have been known for some time (Paterson et al. 1990; reviewed in Hall, 1994; Lim et al. 1996; Narumiya et al. 1996), although the connection between these effects and inhibition of myosin phosphatase was recognized only as the result of observations on smooth muscle (reviewed in Somlyo & Somlyo, 1994; Somlyo et al. 1999a). An ever increasing literature now documents the important role of the Rho/Rho-kinase-myosin phosphatase inhibition pathway in a variety of non-muscle cells, including platelets (Suzuki et al. 1999), neuronal (Majumdar et al. 1998) and endothelial cells (Vouret-Craviari et al. 1998; Essler et al. 1998). A highly important pathological consequence of inhibition of myosin phosphatase by Rho-kinase is its enhancement of the motility of malignant cells: their migration, transcellular invasion and metastasis, effects that can be inhibited by the Rho-kinase inhibitor Y-27632 (Itoh et al. 1999; Somlyo et al. 1999b).
We conclude that the RhoA/Rho-kinase pathway plays major physiological and pathophysiological roles through modulation of the activity of smooth and non-muscle myosin II, by inhibiting, as originally suggested (Somlyo et al. 1989), the dephosphorylation of myosin II.
Acknowledgments
The authors' research covered in this review was supported by NIH grants HL19242 and HL48807. We thank Dr K. Longenecker for Fig. 2, Dr D. Hartshorne for constructive comments, and Ms B. Nordin for preparation of the manuscript.
References
- Abo A, Webb MR, Grogan A, Segal AW. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochemical Journal. 1994;298:585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein RS, Conti MA. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975;256:597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Alessi D, Macdougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targeting subunits: the major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. European Journal of Biochemistry. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Andrea JE, Walsh MP. Protein kinase C of smooth muscle. Hypertension. 1992;20:585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. Journal of Biological Chemistry. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Bourmeyster N, Stasia M-J, Garin J, Gagnon J, Boquet P, Vignais PV. Copurification of Rho protein and the Rho-GDP dissociation inhibitor from bovine neutrophil cytosol. Effect of phosphoinositides on rho ADP-ribosylation by the C3 exoenzyme of Clostridium botulinum. Biochemistry. 1992;31:12863–12869. doi: 10.1021/bi00166a022. [DOI] [PubMed] [Google Scholar]
- Bradley AB, Morgan KG. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. The Journal of Physiology. 1987;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils J, Chardin P. GEFs: structural basis for their activation of small GTP-binding proteins. Trends in Biological Sciences. 1999;24:306–311. doi: 10.1016/s0968-0004(99)01429-2. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Takada Y, Miyamoto S, Mitsui-Saito M, Karaki H, Misawa M. Augmented acetylcholine-induced, Rho-mediated Ca2+-sensitization of bronchial smooth muscle contraction in antigen-induced airway hypertensive rats. British Journal of Pharmacology. 1999;127:597–600. doi: 10.1038/sj.bjp.0702585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxton TL, Lande B, Hirshman CA. Role of G proteins in agonist-induced Ca2+ sensitization of tracheal smooth muscle. American Journal of Physiology. 1998;275:L748–755. doi: 10.1152/ajplung.1998.275.4.L748. [DOI] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse H-J, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. Journal of Biological Chemistry. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Essler M, Retzer M, Bauer M, Heemskerk JW, Aepfelbacher M, Siess W. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. Journal of Biological Chemistry. 1999;274:30361–30363. doi: 10.1074/jbc.274.43.30361. [DOI] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. Journal of Biochemistry. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on the smooth muscle myosin phosphatase target. Journal of Biological Chemistry. 2000. in the Press. [DOI] [PubMed]
- Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. Journal of Biological Chemistry. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Letters. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- Fujihara H, Walker LA, Gong MC, Lemichez E, Boquet P, Somlyo AV, Somlyo AP. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Molecular Biology of the Cell. 1997;8:2437–2447. doi: 10.1091/mbc.8.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Gong MC, Somlyo AV, Somlyo AP. Possible role of atypical protein kinase C activated by arachidonic acid in Ca2+ sensitization of rabbit smooth muscle. The Journal of Physiology. 1997;500:95–110. doi: 10.1113/jphysiol.1997.sp022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Wu X, Haystead TAJ, Somlyo AP, Cohen PTW, Cohen P, Somlyo AV. Regions of the 100-kDa regulatory subunit M110 required for regulation of myosin-light-chain-phosphatase activity in smooth muscle. European Journal of Biochemistry. 1996;239:326–332. doi: 10.1111/j.1432-1033.1996.0326u.x. [DOI] [PubMed] [Google Scholar]
- Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. Journal of Muscle Research and Cell Motility. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- Gohla A, Offermanns S, Wilkie TM, Schultz G. Differential involvement of Gα12 and Gα13 in receptor-mediated stress fiber formation. Journal of Biological Chemistry. 1999;274:17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- Gong MC, Fuglsang A, Alessi D, Kobayashi S, Cohen P, Somlyo AV, Somlyo AP. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. Journal of Biological Chemistry. 1992;267:21492–21498. [PubMed] [Google Scholar]
- Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of rhoA associated with Ca2+-sensitization of smooth muscle. Journal of Biological Chemistry. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins – ras-family or trimeric or both – in Ca2+ sensitization of smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong MC, Kinter MT, Somlyo AV, Somlyo AP. Arachidonic acid and diacylglycerol release associated with inhibition of myosin light chain dephosphorylation in rabbit smooth muscle. The Journal of Physiology. 1995;486:113–122. doi: 10.1113/jphysiol.1995.sp020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosser YQ, Nomanbhoy TK, Aghazadeh B, Manor D, Combs C, Cerione RA, Rosen MK. C-terminal domain of Rho GDP-dissociation inhibitor directs N-terminal inhibitory peptide to GTPases. Nature. 1997;387:814–819. doi: 10.1038/42961. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annual Review of Cell Biology. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ. Biochemistry of the contractile proteins in smooth muscle. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 423–482. [Google Scholar]
- Hartshorne DJ, Ito M, Erdödi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. Journal of Muscle Research and Cell Motility. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Haystead CMM, Gailly P, Somlyo AP, Somlyo AV, Haystead TAJ. Molecular cloning and functional expression of a recombinant 72.5 kDa fragment of the 110 kDa regulatory subunit of smooth muscle protein phosphatase 1M. FEBS Letters. 1995;377:123–127. doi: 10.1016/0014-5793(95)01318-0. [DOI] [PubMed] [Google Scholar]
- Himpens B, Kitazawa T, Somlyo AP. Agonist dependent modulation of the Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflügers Archiv. 1990;417:21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- Himpens B, Somlyo AP. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. The Journal of Physiology. 1988;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. American Journal of Physiology. 1999;277:L653–661. doi: 10.1152/ajplung.1999.277.3.L653. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. Journal of Biological Chemistry. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Yoshii A, Samizo K, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. A major role for the Rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+-sensitization through inhibition of myosin phosphatase in rabbit trachea. British Journal of Pharmacology. 1999;128:925–933. doi: 10.1038/sj.bjp.0702864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M, Brozovich FV. Protein kinase C increases force and slows relaxation in smooth muscle: evidence for regulation of the myosin light chain phosphatase. Biochemical and Biophysical Research Communications. 1996;225:370–376. doi: 10.1006/bbrc.1996.1182. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO Journal. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Itagaki M, Komori S, Unno T, Syuto B, Ohashi H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of Clostridium botulinum in the regulation of myofilament Ca2+ sensitivity in β-escin skinned smooth muscle of guinea pig ileum. Japanese Journal of Pharmacology. 1995;67:1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- Itoh H, Shimomura A, Okubo S, Ichikawa K, Ito M, Konishi T, Nakano T. Inhibition of myosin light chain phosphatase during Ca2+-independent vasocontraction. American Journal of Physiology. 1993;265:C1319–1324. doi: 10.1152/ajpcell.1993.265.5.C1319. [DOI] [PubMed] [Google Scholar]
- Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Medicine. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Gong MC, Somlyo AV, Somlyo AP. Separate upstream and convergent downstream pathways of G-protein and phorbol ester-mediated Ca2+-sensitization of myosin light chain phosphorylation in smooth muscle. Biochemical Journal. 1996;318:469–475. doi: 10.1042/bj3180469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M. Constitutively active Gα12, Gα13, and Gαq induce Rho-dependent neurite retraction through different signaling pathways. Journal of Biological Chemistry. 1998;273:28700–28707. doi: 10.1074/jbc.273.44.28700. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G-protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proceedings of the National Academy of Sciences of the USA. 1991;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+-sensitization in Triton X-100 demembranated rabbit arterial smooth muscle. The Journal of Physiology. 1999;520:139–152. doi: 10.1111/j.1469-7793.1999.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/ G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. Journal of Cellular Biology. 1999;14:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Gong MC, Somlyo AV, Somlyo AP. Ca2+ channel blockers distinguish between G protein-coupled pharmacomechanical coupling Ca2+-release and Ca2+-sensitization. American Journal of Physiology. 1991;260:C364–370. doi: 10.1152/ajpcell.1991.260.2.C364. [DOI] [PubMed] [Google Scholar]
- Kolodney MS, Thimgan MS, Honda HM, Tsai G, Yee HF., Jr Ca2+-independent myosin II phosphorylation and contraction in chicken embryo fibroblasts. The Journal of Physiology. 1999;515:87–92. doi: 10.1111/j.1469-7793.1999.087ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Ita M, Feng J, Okinaka T, Isaka N, Nakano T. Regulation of Ca2+-independent smooth muscle contraction by alternative staurosporine-sensitive kinase. European Journal of Pharmacology. 1999;376:315–320. doi: 10.1016/s0014-2999(99)00367-2. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. Journal of Biological Chemistry. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. European Journal of Biochemistry. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Loirand G, Cario-Toumaniantz C, Chardin P, Pacaud P. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. The Journal of Physiology. 1999;516:825–834. doi: 10.1111/j.1469-7793.1999.0825u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker K, Read P, Derewenda U, Dauter Z, Liu X, Garrard S, Walker L, Somlyo AV, Nakamoto RK, Somlyo AP, Derewenda ZS. How RhoGDI binds Rho. Acta Crystallographica. 1999;D55:1503–1515. doi: 10.1107/s090744499900801x. [DOI] [PubMed] [Google Scholar]
- Lucius C, Arner A, Steusloff A, Troschka M, Hofmann F, Aktories K, Pfitzer G. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. The Journal of Physiology. 1998;506:83–93. doi: 10.1111/j.1469-7793.1998.083bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar M, Seasholtz TM, Goldstein D, van Lanerolle P, Brown JH. Requirement for Rho-mediated myosin light chain phosphorylation in thrombin-stimulated cell rounding and its dissociation from mitogenesis. Journal of Biological Chemistry. 1998;273:10099–10106. doi: 10.1074/jbc.273.17.10099. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO Journal. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Murányi A, Erdödi F, Ito M, Gergely P, Hartshorne DJ. Identification and localization of myosin phosphatase in human platelets. Biochemical Journal. 1998;330:225–231. doi: 10.1042/bj3300225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Suzuki Y, Khira H, Wada H, Fujioka M, Ito M, Nakano T, Kaibuchi K, Shiku H, Nishikawa M. Regulation of myosin phosphatase through phosphorylation of the myosin-binding subunit in platelet activation. Blood. 1997;90:3936–3942. [PubMed] [Google Scholar]
- Narumiya S. The small GTPase Rho: cellular functions and signal transduction. Journal of Biochemistry. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K, Takuwa Y. Involvement of rho in GTPγS-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Letters. 1995;367:246–250. doi: 10.1016/0014-5793(95)00573-r. [DOI] [PubMed] [Google Scholar]
- Otto B, Steusloff A, Just I, Aktories K, Pfitzer G. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. The Journal of Physiology. 1996;496:317–329. doi: 10.1113/jphysiol.1996.sp021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris JRM, Cobban HJ, Littlejohn AF, Macewan DJ, Nixon GF. Tumour necrosis factor-α activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. The Journal of Physiology. 1999;518:561–569. doi: 10.1111/j.1469-7793.1999.0561p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. Journal of Cellular Biology. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read PW, Liu X, Longenecker K, DiPierro CG, Walker LA, Somlyo AV, Somlyo AP, Nakamoto RK. Human RhoA/RhoGDI complex expressed in yeast: GTP exchange is sufficient for translocation of RhoA to liposomes. Protein Science. 2000. in the Press. [DOI] [PMC free article] [PubMed]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circulation Research. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- Sehr P, Joseph G, Genth H, Just I, Pick E, Aktories K. Glucosylation and ADP ribosylation of Rho proteins: effects on nucleotide binding, GTPase activity, and effector coupling. Biochemistry. 1998;37:5296–5304. doi: 10.1021/bi972592c. [DOI] [PubMed] [Google Scholar]
- Senba S, Eto M, Yazawa M. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. Journal of Biochemistry. 1999;125:354–362. doi: 10.1093/oxfordjournals.jbchem.a022294. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. Journal of Biological Chemistry. 1994;269:30407–304011. [PubMed] [Google Scholar]
- Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo AP, Somlyo AV, Haystead TAJ. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme: The differential effects of the holoenzyme and its subunits on smooth muscle. Journal of Biological Chemistry. 1994;269:31598–31606. [PubMed] [Google Scholar]
- Somlyo AP. Kinases, myosin phosphatase and Rho proteins: curiouser and curiouser. The Journal of Physiology. 1999;516:630. doi: 10.1111/j.1469-7793.1999.0630u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Kitazawa T, Himpens B, Matthijs G, Horiuti K, Kobayashi S, Goldman YE, Somlyo AV. Modulation of Ca2+-sensitivity and of the time course of contraction in smooth muscle: a major role of protein phosphatases? Advances in Protein Phosphatases. 1989;5:181–195. [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Wu X, Walker LA, Somlyo AV. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Review of Physiological and Biochemical Pharmacology. 1999a;134:201–234. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor Y-27632 alters human prostate cancer cell morphology, inhibits migration and reduces myosin phosphorylation. Molecular Biology of the Cell. 1999b;10:416. a suppl. [Google Scholar]
- Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. Journal of Pharmacology and Experimental Therapeutics. 1968;159:129–145. [PubMed] [Google Scholar]
- Strassheim D, May LG, Varker KA, Puhl HL, Phelps SH, Porter RA, Aronstam RS, Noti JD, Williams CL. M3 muscarinic acetylcholine receptors regulate cytoplasmic myosin by a process involving RhoA and requiring conventional protein kinase C isoforms. Journal of Biological Chemistry. 1999;274:18675–18685. doi: 10.1074/jbc.274.26.18675. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yamamoto M, Wada H, Ito M, Nakano T, Sasaki Y, Narumiya S, Skiku H, Nishikawa M. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood. 1999;93:3408–3417. [PubMed] [Google Scholar]
- Swärd K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea pig ileum. The Journal of Physiology. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart MJ, Lee YH, Morgan KG. Cellular redistribution of RKCα, rhoA, and ROKα following smooth muscle agonist stimulation. Experimental Cell Research. 1999;251:92–101. doi: 10.1006/excr.1999.4565. [DOI] [PubMed] [Google Scholar]
- Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Annual Review of Biochemistry. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Tang DC, Kubota Y, Kamm KE, Stull JT. GTP gamma S-induced phosphorylation of myosin light chain kinase in smooth muscle. FEBS Letters. 1993;331:272–275. doi: 10.1016/0014-5793(93)80351-t. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Ichikawa K, Hartshorne DJ, Siegman MJ, Butler TM. Thiophosphorylation of the 130-kDa subunit is associated with a decreased activity of myosin light chain phosphatase in alpha-toxin-permeabilized smooth muscle. Journal of Biological Chemistry. 1995;270:18191–18194. doi: 10.1074/jbc.270.31.18191. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizuki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Boquet P, Pouysségur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Molecular Biology of the Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LA, Gailly P, Jensen PE, Somlyo AV, Somlyo AP. The unimportance of being (protein kinase C) epsilon. FASEB Journal. 1998;12:813–821. doi: 10.1096/fasebj.12.10.813. [DOI] [PubMed] [Google Scholar]
- Weber LP, van Lierop JE, Walsh MP. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. The Journal of Physiology. 1999;516:805–824. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhang Y, Derewenda U, Liu X, Minor W, Nakamoto RK, Somlyo AV, Somlyo AP, Derewenda Z. Crystal structure of RhoA-GDP and its functional implications. Nature Structural Biology. 1997;4:699–703. doi: 10.1038/nsb0997-699. [DOI] [PubMed] [Google Scholar]
- Wu X, Haystead TAJ, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin: synergism with cyclic nucleotide-activated kinase. Journal of Biological Chemistry. 1998;273:11362–11369. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Iizuka K, Dobashi K, Horie T, Harada T, Nakazawa T, Mori M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+-sensitization. American Journal of Respiratory Cell and Molecular Biology. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hirano K, Sakihara C, Nishimura J, Kanaide H. NH2-terminal fragments of the 130 kDa subunit of myosin phosphatase increase the Ca2+ sensitivity of porcine renal artery. The Journal of Physiology. 1999;516:55–65. doi: 10.1111/j.1469-7793.1999.055aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


