Abstract
The effects of increasing intracellular cAMP concentration were studied using photolysis of caged-cAMP in layer II/III neurons recorded intracellularly in visual cortex slices. The recorded neurons exhibited either after-hyperpolarization (AHP) or after-depolarization (ADP) in response to depolarizing current injection. Depending on which afterpotential appeared, the effects of photolysis differed.
In ADP-generating neurons, photolysis of caged-cAMP induced long-lasting depression of postsynaptic potentials (PSPs) evoked by grey matter (GM) stimulation, without altering the size of the ADP. In AHP-generating neurons, photolysis induced long-lasting potentiation of GM-evoked PSPs, with the size of the AHP reduced in the same time course. White matter (WM)-evoked PSPs showed no change.
Extracellular application of bromo-cAMP depressed both GM- and WM-evoked PSPs in ADP- and AHP-generating neurons. This depression may be due to presynaptic effects of cAMP, since photolysis-evoked postsynaptic increase in cAMP concentration never induced depression of PSPs in AHP-generating neurons. This depression was reversible but continued until bromo-cAMP was washed out, while ADP and AHP in the postsynaptic neurons were depressed only temporarily and returned to the pre-application level even in the continued presence of bromo-cAMP.
Bromo-cAMP was applied following photolysis of caged-cAMP. In the neurons in which the photolysis potentiated GM-evoked PSPs this potentiation was cancelled out by bromo-cAMP (depotentiation). In the other neurons, PSPs were depressed only reversibly.
Thus, a postsynaptic increase in cAMP concentration exerts more diverse effects on synaptic plasticity than thus far reported, depending on the difference in neuronal intrinsic excitability and probably on how much, or the way in which, cAMP concentration is increased.
Long-term potentiation (LTP) is often considered to consist of early and late LTPs. In contrast to the early LTP, which lasts maximally for a few hours and requires no protein synthesis, the late LTP has a longer durability, depends on cAMP and protein kinase A and requires protein synthesis (Matthies & Reymann, 1993; Frey et al. 1993). Involvement of cAMP in the early LTP may also be predictable, given the accumulated evidence that cAMP-dependent phosphorylation enhances ligand-gated currents through γ-aminobutyric acid-A (GABAA) and glutamate receptors in central neurons (Greengard et al. 1991; Wang et al. 1991; Keller et al. 1992; Moss et al. 1992; Cerne et al. 1993; Raymond et al. 1993; Blackstone et al. 1994). While the roles of cAMP in early LTP have recently been recognized, the mechanism by which cAMP contributes to the early LTP does not seem to be so simple as a direct modification of receptors. In the hippocampal CA1, increases in cAMP concentration in postsynaptic neurons are reported to not enhance synaptic transmission by itself but to behave as a gate controller, playing a permissive role without which LTP could not be induced by tetanization (Blitzer et al. 1995). In the hippocampal CA3, lateral amygdala and cerebellar cortex, cAMP acts on the presynaptic rather than postsynaptic side (Huang et al. 1994; Weisskopf et al. 1994; Salin et al. 1996; Huang & Kandel, 1998). Thus, the ways in which cAMP is involved in early LTP appear to be diverse at different synapses.
Even at the same synapses, the roles of cAMP in synaptic plasticity seem diverse, since cAMP is implicated in induction of both LTP and its counterpart, long-term depression (LTD), in a number of brain structures. In the hippocampal CA3 where presynaptic involvement of cAMP in LTP is known, LTD has been reported to depend on a type of metabotropic glutamate receptor (mGluR). These receptors are located presynaptically and they inhibit increase in intracellular cAMP concentration (Yokoi et al. 1996). The same type of mGluR is implicated in both LTP and LTD in the dentate gyrus (Huang et al. 1997). In the CA1, LTD as well as LTP has been reported to require cAMP-dependent kinase (Brandon et al. 1995). In the visual cortex, where it has been shown that the same synapses can undergo both LTP and LTD under different conditions (Artola et al. 1990; Kato, 1993a), involvement of cAMP in LTP and LTD was implicated (Kato, 1993b, 1994) and has recently been elucidated more directly in knock-out mice (Hensch et al. 1998).
Thus, recent findings on the roles of cAMP in synaptic plasticity, taken together, point to a diverse array of cAMP actions in synaptic plasticity. To get some idea of how distinctly the same molecule cAMP is involved in different types of synaptic plasticity, we have here studied whether increases in cAMP concentration evoked in the same way have different effects depending on neuron types. This was done by classifying the recorded neurons on the basis of afterpotentials induced by depolarizing current injection. We then checked whether cAMP concentration increased in two distinct ways exerts different effects on the same neurons. For this purpose, intracellular cAMP concentration was increased firstly by photolysing caged-cAMP injected into a postsynaptic neuron, which is presumed to increase the cAMP concentration deep within the neuron. Then, bromo-cAMP, a membrane-permeable analogue of cAMP, was applied extracellularly, in the hope of distributing cAMP more densely near the cell membrane than deeper into the neuron.
METHODS
Wistar rats (110–200 g; 30–50 days old) were rapidly decapitated under ether anaesthesia, and the brains removed and soaked in cold medium (2–5°C for 1–2 min) containing (mM): NaCl 124, KCl 5, KH2PO4 1.3, NaHCO3 26, MgSO4 2.0, CaCl2 2.5 and D-glucose 20, bubbled with 95% O2 and 5% CO2. Slices (350 μm thick) of the visual cortex were prepared with a Microslicer (DTK-1000; Dosaka EM, Kyoto, Japan) and allowed to incubate at room temperature for at least 60 min. The recording chamber was positioned on the stage of an inverted microscope (Nikon TMD) equipped with a lamp house (TMD-EQE, Nikon, 100 W xenon lamp) and was perfused with medium (30°C) at the rate of 6–10 ml min−1. Two pairs of bipolar stimulating electrodes made of parallel tungsten wires insulated except for the tips, were inserted into the slice: one pair into the border region between the white matter (WM) and cortical layer VI for WM stimulation and the other pair into cortical layers II/III for grey matter (GM) stimulation. All experiments were carried out in accordance with the guidelines of the Japanese Physiological Society and had the approval of the Animal Welfare Committee of the Department of Integrative Brain Science (Kyoto University Graduate school of Medicine).
Intracellular recordings were carried out from neurons in layer II/III, which were located right above the WM-stimulating electrodes and about 200–300 μm away from the GM-stimulating electrodes in the tangential direction of the cortex. Neurons were not visually identified under the microscope. We used sharp glass electrodes filled with 3 M potassium acetate (70–100 MΩ), which contained p-1-2-nitrophenyl-ethyl-adenosine-3′,5′-cyclic monophosphate (caged-cAMP, 10 mM) for some experiments. Postsynaptic potentials (PSPs) evoked by subthreshold (60–70% of threshold for a spike) stimulation of the WM at low frequencies (0.06-0.07 Hz) were recorded with a bridge-equipped amplifier (Axoclamp-2A). The PSPs became stable approximately 30 min after the penetration. In the meantime, caged-cAMP was expected to diffuse into the recorded neuron. Then ultra-violet light (UV), excited by a 340 nm filter, was projected through a ×40 objective lens (Nikon Fluor 40) onto the entire microscope field for 1 min, so as to photolyse injected caged-cAMP. Induction of depolarization afterpotentials, after-hyperpolarization (AHP) or after-depolarization (ADP), was attempted by injecting a depolarizing current (+1.0 nA for 1.0 s) before and after the photolysis of caged-cAMP. The current injection usually evoked action potentials at 10–30 Hz, with lower frequencies towards the end of depolarization pulses. The size of ADP was assessed by mean depolarization from the rest averaged over 3 s starting from the termination of the depolarization pulse, and the size of AHP by its peak amplitude. The resting membrane potential and input resistance of the recorded neurons (means ±s.e.m.) were 74.2 ± 1.2 mV and 39.0 ± 2.4 MΩ, respectively, for neurons injected with caged-cAMP, and 75.3 ± 1.3 mV and 39.0 ± 2.4 MΩ, respectively, for the other neurons.
For tetanization, repetitive stimulation (2 s trains at 50 Hz, repeated 5 times every 10 s with intensity 1.5-2 times the threshold for the spike) was given through WM stimulating electrodes. In experiments specified in the text, bromo-cAMP (40 μM) was applied into the bath medium. The drug was washed out with normal medium at the rate of 10–18 ml min−1. The signals were digitized at a rate of 10 kHz. All the sample recordings are averages of five traces. The amplitude of PSPs was measured at the peak. Student's paired or unpaired t test was used for statistics, the level of significance was set at P < 0.05, and values are expressed as means ±s.e.m.
RESULTS
All recordings were made from neurons in layer II/III of the adult rat visual cortex. In the recorded neurons, PSPs were evoked by stimulating the WM or GM. The neurons also generated either AHP or ADP in response to depolarizing current injection. These two types of neuron, classified on the basis of afterpotentials, were intracellularly injected with caged-cAMP. The injection of caged-cAMP itself did not seem to alter the PSP size or shape. The injected caged-cAMP was then photolysed by irradiation with UV light, so as to increase intracellular cAMP concentration. The increases in cAMP concentration thus achieved exerted different effects on GM-evoked PSPs in the two types of neuron. In ADP-generating neurons, the amplitude of GM-evoked PSPs was reduced upon UV irradiation (Fig. 1A and C), without changing the average size of the ADP (Fig. 1B and C). The PSP amplitude 30 min after photolysis was reduced to 73 ± 2% of the amplitude obtained just prior to photolysis, and was significantly smaller than the amplitude of PSPs taken 2–3 min before photolysis (99 ± 1%; P < 0.0002; n = 4). On the other hand, the size of ADP was not significantly different (96 ± 11% at 20 min after photolysis) from the control ADP size. In those four neurons, WM-evoked PSPs were not significantly modified by photolysis.
Figure 1. Effects of photolysis of caged-cAMP on PSPs in ADP-generating neurons.
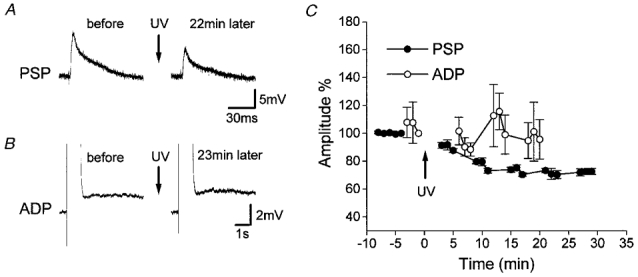
A, in a representative ADP-generating neuron, photolysis of caged-cAMP decreased GM-evoked PSPs. No changes were observed in WM-evoked PSPs (not shown). B, the size of ADP remained unchanged in the same neuron. C, mean time courses (n = 4) of changes in PSPs (•) and ADP (○). Depression of GM-evoked PSPs lasted for 30 min or longer. Photolysis failed to induce consistent changes in ADP. The arrows labelled ‘UV’ indicate the time of photolysis by UV irradiation.
In AHP-generating neurons, GM-evoked PSPs were potentiated and, in parallel, the size of AHP was reduced (Fig. 2). The PSP amplitude 30 min after photolysis (137 ± 15% of the PSP amplitude just prior to photolysis) was significantly smaller than the amplitude taken 4–5 min before photolysis (100 ± 1%; P < 0.04; n = 9). The AHP size was also significantly smaller (57 ± 18% of the pre-photolysis control) than 1–2 min before photolysis (99 ± 2%; P < 0.04; n = 9). The reductions in AHP and PSP sizes were both long lasting and took a similar time course. In those nine neurons again, WM-evoked PSPs were not significantly modified by photolysis.
Figure 2. Effects of photolysis of caged-cAMP on PSPs in AHP-generating neurons.
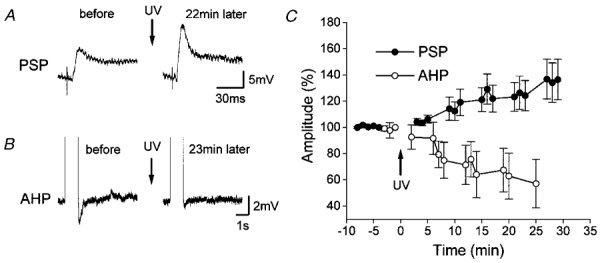
A and B, in a representative AHP-generating neuron, photolysis potentiated GM-evoked PSPs (A), and reduced AHP (B). No changes were observed in WM-evoked PSPs (not shown). C, time courses (n = 9) of changes in PSPs (•) and AHP (○). Potentiation of GM-evoked PSPs and reduction of AHP were both long lasting. The arrows indicate the time of photolysis by UV irradiation.
In neither ADP- nor AHP-generating neurons did the UV irradiation alone alter baseline amplitudes of PSPs elicited by stimulation of WM or GM. In control neurons in which no caged-cAMP was injected, the amplitude of PSPs recorded 30–32 min after the UV irradiation was 99 ± 2% of the pre-irradiation control for WM stimulation and 101 ± 2% for GM stimulation (n = 5; Fig. 3).
Figure 3. Effects of UV irradiation alone on PSPs.
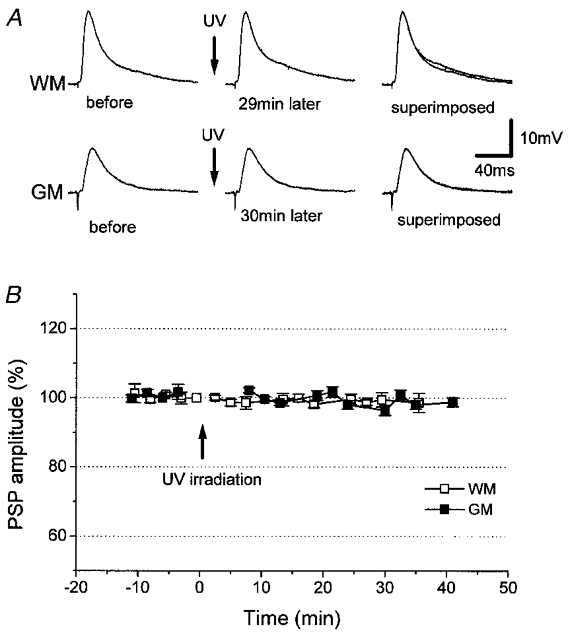
A, UV irradiation alone failed to affect WM- or GM-evoked PSPs in one and the same control neuron. B, time course (n = 5) of the peak amplitude of WM- (□) and GM-evoked PSPs (▪). UV irradiation was at the time indicated by the arrows.
The above findings indicate that there are two distinct types of neuron among layer II/III neurons of the visual cortex: (1) neurons that generate ADP, and (2) those that generate AHP. It appears that PSPs in these different types of neuron responded distinctly to increased cAMP concentration evoked by photolysis, exhibiting either decreases or increases in PSPs. In agreement, various changes in GM-evoked PSPs but not WM-evoked PSPs were induced by photolysis in a separate set of 34 neurons, in which changes in afterpotentials were not recorded. Potentiation and depression in GM-evoked PSPs by more than 10% occurred in 11/34 and 7/34 neurons, respectively. In the other 16/34 neurons, photolysis changed PSPs by less than 10%. In these 16/34 neurons, the photolysis-evoked increase in cAMP concentration was possibly insufficient to induce noticeable modifications of synapses or channels. However, even without any noticeable modification of synaptic efficiency, there might be a latent influence in the affected neurons.
To test the above possibility, we tetanized WM in 6 of the 16/34 neurons in which PSP changes were less than 10% (Fig. 4), because previous findings showed that tetanization of WM in this preparation fails to induce synaptic modification in normal medium (Artola & Singer, 1990; Kato et al. 1991, 1993a). Tetanization induced LTD in the amplitude of WM-evoked PSPs (83 ± 2% of the pre-tetanization control for WM stimulation; at 30 min after tetanization), whereas the amplitude of GM-evoked PSPs showed no change (102 ± 3% of the GM-evoked control PSPs; at 30 min after tetanization). In five normal control neurons (Fig. 5), the amplitudes of WM- and GM-evoked PSPs remained at 102 ± 2 and 104 ± 2%, respectively, at 30 min after tetanization. The depression of WM-evoked PSPs in the six neurons was significant compared with the five normal control neurons (P < 0.0001). This result is consistent with the occurrence of a latent effect of photolysis.
Figure 4. Tetanization-induced LTD in the neurons in which photolysis changed the PSP amplitude by less than 10%.
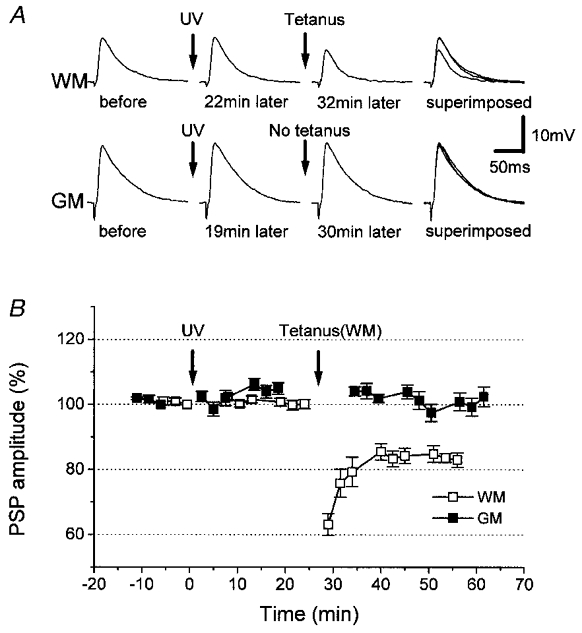
A, tetanic stimulation of WM resulted in LTD in WM-evoked PSPs, but not in GM-evoked PSPs, in a representative neuron. B, time courses (n = 6) of changes in amplitude of WM- (□) and GM-evoked (▪) PSPs. Depression of WM-evoked PSPs lasted for longer than 30 min. Photolysis by UV irradiation and tetanization were performed at the times indicated by the arrows.
Figure 5. Aborted induction of LTD in control neurons.
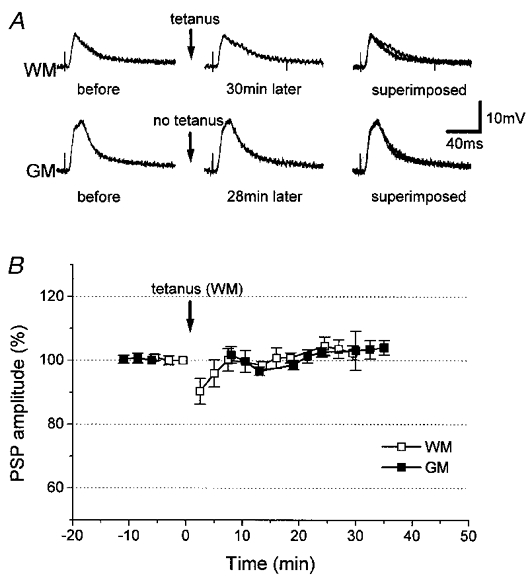
A, in a normal control neuron, neither WM- nor GM-evoked PSPs were depressed by tetanic stimulation of WM. B, time courses (n = 5) of changes in amplitude of WM- (□) and GM-evoked PSPs (▪). WM was tetanized at the time indicated by the arrows.
We then attempted to elevate intracellular cAMP concentration by a different method and compared its effect with the effect of the photolytic elevation of intracellular cAMP. The method we used here was bath application of bromo-cAMP, a phosphodiesterase-resistant, cell-permeable analogue of cAMP. Application of bromo-cAMP into the medium resulted in a reversible depression of PSPs (n = 5; Fig. 6). The amplitude of WM- and GM-evoked PSPs decreased to 87 ± 3 and 84 ± 4% of the respective control amplitudes measured 0–1 min before the bath application, and were significantly smaller than the respective pre-application controls taken 2 min before the application (99 ± 1% for WM stimulation, P < 0.03; 101 ± 1% for GM stimulation, P < 0.01; n = 5), or than the amplitudes of recovered PSPs after washout for 10–15 min with normal medium (99 ± 3% for WM stimulation, P < 0.01; 100 ± 3% for GM stimulation, P < 0.01).
Figure 6. Effects of bath application of bromo-cAMP in normal control neurons.
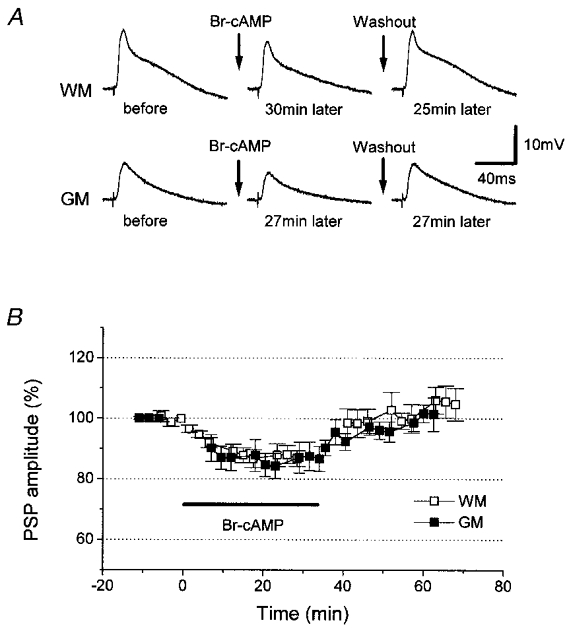
A, in one and the same normal control neuron, WM- and GM-evoked PSPs were depressed by bath application of bromo-cAMP (Br-cAMP), and returned to the initial size by washing out the drug for less than 20 min. B, mean time courses (n = 5) of the amplitude of WM- (□) and GM-evoked PSPs (▪) before and after bromo-cAMP application. The drug was present in the bath during the time indicated by the bar. Suppression of PSPs was completely reversible. Here and in subsequent figures, the duration of bromo-cAMP application is indicated by the bars labelled Br-cAMP.
Effects of bromo-cAMP on afterpotentials (AHP or ADP) were then examined to see how the bromo-cAMP-induced PSP depression is related to its effects on afterpotentials (Fig. 7). Bromo-cAMP application reduced the sizes of AHP (to 66 ± 16% 5 min after the application, n = 3; Fig. 7A) and ADP (to 66 ± 13% 5 min after the application, n = 4; Fig. 7B). However, this reduction was only temporary, and returned to the original level even with bromo-cAMP still in the medium. The depression of PSPs, on the other hand, was maintained in the presence of bromo-cAMP (84 ± 1 and 80 ± 4% for Fig. 7A and B, respectively, at 25–27 min after bromo-cAMP application). Figure 7B also shows that effects of bromo-cAMP on ADP have a faster onset than those on PSPs in ADP-generating neurons.
Figure 7. Reversible decreases in AHP and ADP by bromo-cAMP.
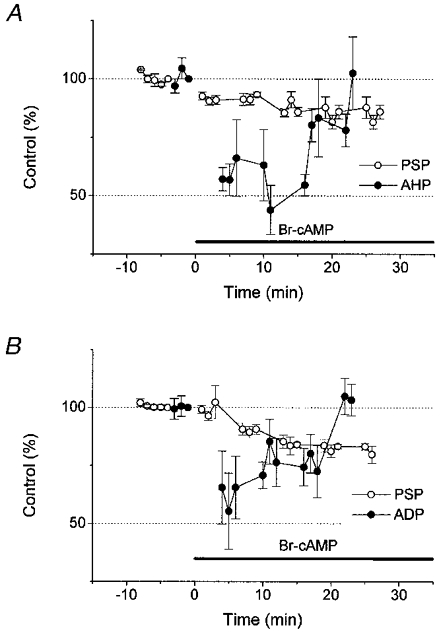
In both AHP-generating neurons (A; n = 3) and ADP-generating neurons (B; n = 4), bromo-cAMP application depressed GM-evoked PSPs, and the depression lasted as long as the drug remained in the bath (○). However, depression of AHP and ADP (• in A and B, respectively) was temporary, and returned to the initial size with the drug still in the bath.
To further clarify the difference in effects of elevation of cAMP concentration by the two different methods, bromo-cAMP was applied following photolysis of caged-cAMP. The results differed depending on how PSPs were modified by the preceding photolysis (Fig. 8). In the experiments described in Fig. 8, we did not record afterpotentials, and are unable to classify the recorded neurons on the basis of afterpotentials. Therefore, PSP changes of greater than 10% were tentatively referred to as ‘potentiation’ and less than 10%‘depression’. In the neurons that showed potentiation of GM-evoked PSPs (presumed AHP-generating neurons), bromo-cAMP application induced depression of both WM- and GM-evoked PSPs (Fig. 8A). The depression of WM-evoked PSPs, which exhibited no changes upon photolysis, was completely reversible by washout of bromo-cAMP, whereas that of GM-evoked PSPs was only partially recovered and the amplitude remained at a level significantly lower (104 ± 3% of the pre-photolysis control; P < 0.03; n = 5) than the post-photolysis level (114 ± 4% of the pre-photolysis control). This indicates depotentiation of PSPs once potentiated by the preceding photolysis. Meanwhile, the amplitude of WM-evoked PSPs was reduced from 101 ± 3 to 76 ± 9% of the pre-photolysis control, and returned to 98.0 ± 3.0% of the pre-photolysis control after the washout for 30 min (Fig. 8A).
Figure 8. Effects of bromo-cAMP in neurons previously subjected to photolysis.
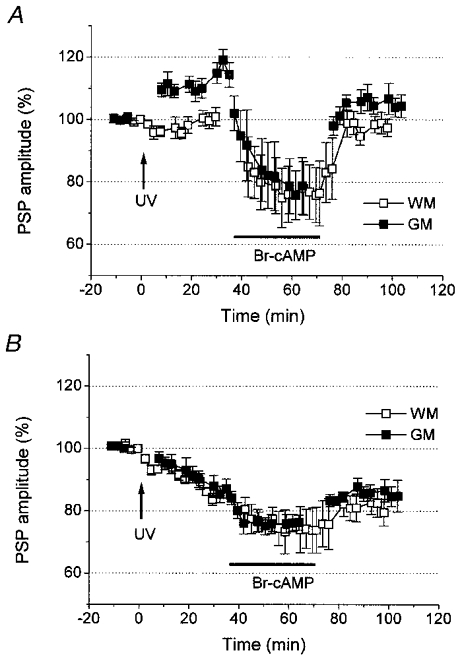
A, mean time course (n = 5) of the amplitude of WM- (□) and GM-evoked (▪) PSPs in the neurons in which GM-evoked PSPs were potentiated by photolysis. Subsequent application of bromo-cAMP decreased the amplitudes of both PSPs. After washout of bromo-cAMP for 30 min, the GM-evoked PSP amplitude remained at a level smaller than that before bromo-cAMP application. Thus, the potentiation induced by photolysis was cancelled out (depotentiated). Meanwhile, the amplitude of WM-evoked PSP was unaffected by photolysis, and subsequent bromo-cAMP application induced a completely reversible depression of PSP. The time of photolysis is indicated by the arrows labelled ‘UV’. B, time course (n = 4) of the amplitude of WM- (□) and GM-evoked PSPs (▪) in the neurons in which PSPs were depressed by photolysis. Depression of PSPs by bromo-cAMP was completely reversible, and the initial depression induced by photolysis survived subsequent application of bromo-cAMP.
In the neurons which exhibited depression of GM-evoked PSPs (presumed ADP-generating neurons; Fig. 8B), a completely reversible depression resulted both in GM- and WM-evoked PSPs. The amplitude of WM-evoked PSPs was reduced from 84 ± 2 to 74 ± 7% of the pre-photolysis control and that of GM-evoked PSPs from 87 ± 3 to 76 ± 2% (n = 4), and returned to 79 ± 4 and 85 ± 5% of the pre-photolysis control after the washout for 30 min, respectively. No significant effects of bromo-cAMP remained after washout of bromo-cAMP.
DISCUSSION
The neurons recorded in the present experiments generate one of two distinct types of afterpotential, AHP or ADP. It has been proposed that ADP- and AHP-generating neurons in the cortex are two distinct populations based on ion channel properties (Nuñez et al. 1993; Kang & Kayano, 1994; Jensen et al. 1996; Kang et al. 1998). This proposal seems to be disputed by the findings that both afterpotentials could occur in the same neurons, depending on extrinsic influences mediated by neurotransmitter receptors such as cholinergic muscarinic receptors, GABAA receptors or mGluRs (Schwindt et al. 1988; Caeser et al. 1993; Greene et al. 1994; Fraser & MacVicar, 1996; Cerne & Spain, 1997). In the present experiments in which no extrinsic neurotransmitters were used, we never came across neurons exhibiting both AHP and ADP, in agreement with the distinct classification of neocortical layer II/III neurons into ADP- and AHP-generating neurons.
The two afterpotentials are considered to originate from different ion channels. Calcium-activated potassium channels are well accepted to underlie AHP (Schwindt et al. 1988; Azouz et al. 1996), whereas calcium-dependent (Fraser & MacVicar, 1996; Kang et al. 1998) or -independent cation channels (Azouz et al. 1996; Cerne & Spain, 1997; Wang, 1999) are considered to be responsible for ADP. AHP- and ADP-generating neurons are thus likely to express different sets of ion channels, or the same channels in different proportions. We have shown that these two types of neuron have distinctive responses to increases in intracellular cAMP concentration. Such cell type specificity may be explained by differential expression of ion channels that are modified cAMP dependently, or possibly by that of cAMP-dependent kinases and phosphatases that mediate cAMP actions.
We showed that an intracellular increase in cAMP concentration depresses and potentiates PSPs in ADP- and AHP-generating neurons, respectively. What is the possible functional meaning of this? ADP-generating neurons are considered to be crucial in generating the rhythmic bursting activity of neocortical neuron circuits (Kang & Kayano, 1994; Gray & McCormick, 1996; Wang, 1999). Such rhythmic bursting, especially that with gamma rhythm, has been regarded as playing an important role in cortical cognitive functions (e.g. Herculano-Houzel et al. 1999). Rhythmic bursting activity is known to be modified by activation of neurotransmitter receptors that mobilize intracellular second messengers, such as acetylcholine receptors (Steriade et al. 1991) and mGluRs (Whittington et al. 1995). Activation of many types of mGluR is known to regulate intracellular cAMP concentration. Interestingly, PSPs in ADP-generating neurons are depressed by increases in intracellular cAMP concentration in the present experiments. Such downregulation of synapses at ADP-generating neurons may help to reduce bursting activity in neocortical neuron circuits. On the other hand, PSPs were potentiated in AHP-generating neurons, which usually show regular spiking activity rather than bursting (Nuñez et al. 1993; Locke & Nerbonne, 1997). PSP potentiation at AHP-generating neurons would therefore serve to reduce bursting activity in a population. Overall, if a monoaminergic or any other diffuse projection system synchronously increases (or decreases) intracellular cAMP concentration in a population of neocortical layer II/III neurons, including both ADP- and AHP-generating neurons, synchronized burst firings would be attenuated (or promoted). In this way, coherence of activities in a neuronal population may be regulated. Indeed, Whittington et al. (1995) reported that mGluRs, which mostly decrease intracellular cAMP and are therefore expected to promote synchronized bursting activity, play an essential role in maintaining synchronized bursting in the gamma frequency range in cortical interneuron circuits.
Besides the cell-specific actions of cAMP, we observed differential effects of cAMP, depending on how cAMP was applied. In both AHP- and ADP-generating neurons, responses to extracellularly applied bromo-cAMP differed from those to photolysed caged-cAMP. In the AHP-generating neurons in which photolysis of caged-cAMP induced long-lasting potentiation of PSPs, subsequent application of bromo-cAMP depressed these potentiated PSPs; hence, depotentiation occurred. In the ADP-generating neurons which showed no change in ADP upon photolysis, there was a temporary depression of ADP in response to bromo-cAMP. These findings may indicate that the same AHP- or ADP-generating neurons possess two or more cAMP-triggered mechanisms of synaptic or channel regulation, and are reminiscent of calcium-dependent regulation of LTP and LTD as follows. One line of theoretical and experimental studies supports the idea that only a small increase in intracellular calcium concentration induces LTD while a large increase leads to LTP, with a moderate increase producing no changes (Lisman, 1989; Cummings et al. 1996; Yasuda & Tsumoto, 1996; Hansel et al. 1997). This concentration model may also work for synaptic or channel regulation by cAMP, and two or more cAMP-dependent mechanisms mentioned above may be triggered concentration dependently. In AHP-generating neurons, bromo-cAMP reduced the size of AHP only temporarily, while photolysed cAMP kept AHP at the reduced level for a much longer time. This may indicate that the increase in cAMP concentration evoked by photolysis is more effective in modifying postsynaptic channels than that by bromo-cAMP application, possibly because of higher cAMP concentration achieved in the former. The weaker effect of bromo-cAMP is also implied by the absence of any long-lasting effects on PSPs in ADP-generating neurons in which the preceding photolysis had induced long-lasting depression of PSPs.
In addition to cAMP concentration, the spatial distribution of cAMP may also explain the difference in effects between caged-cAMP and bromo-cAMP. Membrane-permeable bromo-cAMP is likely to be more densely distributed just beneath the cell membrane, whereas injected caged-cAMP may well remain more densely localized in the depth of the neuron. Also, bromo-cAMP is phosphodiesterase resistant, and could last longer than photolysed cAMP, providing different temporal patterns of intracellular cAMP. Differences in the method of drug application, i.e. photolysis vs. bath application, might also influence such temporal patterns. The significance of the spatiotemporal distribution of cAMP is yet to be studied by imaging related molecules.
A difference in the site of action in terms of pre- or post-synaptic loci should also be considered. Since application of bromo-cAMP necessarily influenced both pre- and postsynaptic structures, presynaptic effects may have shaped the difference between the effects of bromo-cAMP and those of photolysed caged-cAMP which is postsynaptically localized. At the very least, the finding that bromo-cAMP cancelled out the potentiation induced by the postsynaptically injected caged-cAMP appears to indicate that depotentiation occurs postsynaptically. Also, the reversible depression of PSPs induced by bromo-cAMP in AHP-generating neurons may be due to the presynaptic effects of cAMP, since in this study the photolysis-evoked postsynaptic increase in cAMP concentration never induced depression of PSPs in AHP-generating neurons.
WM-evoked PSPs are much less susceptible to cAMP-dependent long-lasting modification than GM-evoked PSPs in the present experiments. WM stimulation is expected to affect both thalamocortical and corticocortical projections, whereas the GM stimulation in the present study is presumed to cause excitation of corticocortical projections almost exclusively. In the motor cortex, corticocortical synapses are shown to be more liable to LTP than thalamocortical synapses (Iriki et al. 1991), which is in line with the present finding. In the adult visual cortex, Kirkwood & Bear (1994) demonstrated that PSPs at synapses of corticocortical projections from layer IV to layer II/III are subject to LTP induction, while Kato et al. (1991) showed that PSPs evoked by WM stimulation are LTP resistant.
In conclusion, a diverse array of cAMP actions in neocortical synaptic plasticity appears to be based partly on the intrinsic responsiveness of neurons. An increase in intracellular cAMP concentration induces synaptic upregulation in AHP-generating neurons and downregulation in ADP-generating neurons in neocortical layer II/III. Moreover, the degree to which cAMP concentration is increased intracellularly, as well as the site of action, may be another determinant of the synaptic effect of increased cAMP concentration.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan to N.K.
References
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. The involvement of N-methyl-D-aspartate receptors in induction and maintenance of long-term potentiation in rat visual cortex. European Journal of Neuroscience. 1990;2:254–269. doi: 10.1111/j.1460-9568.1990.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. The Journal of Physiology. 1996;492:211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. Journal of Neuroscience. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Lyengay R, Landau EM. Postsynaptic cAMP pathway gates early LTP in the hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Zhuo M, Huang YY, Qi M, Gerhold KA, Burton KA, Kandel ER, McKnight GS, Idzerda RL. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeser M, Brown DA, Gähwiler BH, Knöpfel T. Characterization of a calcium-dependent current generating a slow afterdepolarization of CA3 pyramidal cells in rat hippocampal slice cultures. European Journal of Neuroscience. 1993;5:560–569. doi: 10.1111/j.1460-9568.1993.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Cerne R, Rusin KI, Randic M. Enhancement of the N-methyl-D-aspartate response in spinal dorsal horn neurons by cAMP-dependent protein kinase. Neuroscience Letters. 1993;161:124–128. doi: 10.1016/0304-3940(93)90275-p. [DOI] [PubMed] [Google Scholar]
- Cerne R, Spain WJ. GABAA mediated afterdepolarization in pyramidal neurons from rat neocortex. Journal of Neurophysiology. 1997;77:1039–1045. doi: 10.1152/jn.1997.77.2.1039. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang Y-Y, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Greene CC, Schwindt PC, Crill WE. Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. Journal of Neurophysiology. 1994;72:693–704. doi: 10.1152/jn.1994.72.2.693. [DOI] [PubMed] [Google Scholar]
- Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Hansel C, Artola A, Singer W. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. European Journal of Neuroscience. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Gordon JA, Brandon EP, McKnight GS, Idzerda RL, Stryker MP. Comparison of plasticity in vivo and in vitro in the developing visual cortex of normal and protein kinase A RI-deficient mice. Journal of Neuroscience. 1988;18:2108–2117. doi: 10.1523/JNEUROSCI.18-06-02108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. Journal of Neuroscience. 1999;19:3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LQ, Rowan MJ, Anwyl R. mGluR II agonist inhibition of LTP induction, and mGluR II antagonist inhibition of LTD induction, in the dentate gyrus in vitro. NeuroReport. 1997;8:687–693. doi: 10.1097/00001756-199702100-00022. [DOI] [PubMed] [Google Scholar]
- Huang Y-Y, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Huang Y-Y, Li X-C, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and the macromolecular synthesis-dependent late phase. Cell. 1994;79:69–74. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation of thalamic input to the motor cortex induced by coactivation of thalamocortical and corticocortical afferents. Journal of Neurophysiology. 1991;65:1435–1441. doi: 10.1152/jn.1991.65.6.1435. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, Yaari Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. The Journal of Physiology. 1996;492:199–210. doi: 10.1113/jphysiol.1996.sp021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Kayano F. Electrophysiological and morphological characteristics of layer VI pyramidal cells in the cat motor cortex. Journal of Neurophysiology. 1994;72:578–591. doi: 10.1152/jn.1994.72.2.578. [DOI] [PubMed] [Google Scholar]
- Kang Y, Okada T, Ohmori H. A phenytoin-sensitive cationic current participates in generating the afterdepolarization and burst afterdischarge in rat neocortical pyramidal cells. European Journal of Neuroscience. 1998;10:1363–1375. doi: 10.1046/j.1460-9568.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- Kato N. Dependence of long-term depression on postsynaptic metabotropic glutamate receptors in the visual cortex. Proceedings of the National Academy of Sciences of the USA. 1993a;90:3650–3654. doi: 10.1073/pnas.90.8.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N. Mechanisms of β-adrenergic facilitation of LTP in rat visual cortex. NeuroReport. 1993b;4:1087–1090. [PubMed] [Google Scholar]
- Kato N. Long-term depression requiring tACPD-receptor activation and NMDA-receptor blockade. Brain Research. 1994;665:158–160. doi: 10.1016/0006-8993(94)91168-1. [DOI] [PubMed] [Google Scholar]
- Kato N, Artola A, Singer W. Developmental changes in the susceptibility to long-term potentiation of neurons in rat visual cortex slices. Developmental Brain Research. 1991;60:43–50. doi: 10.1016/0165-3806(91)90153-a. [DOI] [PubMed] [Google Scholar]
- Keller BU, Hollmann M, Heinemann S, Konnerth A. Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO Journal. 1992;11:891–896. doi: 10.1002/j.1460-2075.1992.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. Journal of Neuroscience. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences of the USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke RE, Nerbonne JM. Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons. Journal of Neurophysiology. 1997;78:2321–2335. doi: 10.1152/jn.1997.78.5.2321. [DOI] [PubMed] [Google Scholar]
- Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. NeuroReport. 1993;4:712–714. doi: 10.1097/00001756-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABA-A receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Neveu D, Zucker RS. Postsynaptic levels of [Ca2+]i needed to trigger LTD and LTP. Neuron. 1996;16:619–629. doi: 10.1016/s0896-6273(00)80081-1. [DOI] [PubMed] [Google Scholar]
- Nuñez A, Amzica F, Steriade M. Electrophysiology of cat association cortical cells in vivo: intrinsic properties and synaptic responses. Journal of Neurophysiology. 1993;70:418–430. doi: 10.1152/jn.1993.70.1.418. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361:637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:747–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. Journal of Neurophysiology. 1988;59:450–467. doi: 10.1152/jn.1988.59.2.424. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proceedings of the National Academy of Sciences of the USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Salter MW, MacDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Fast burst firing and short-term synaptic plasticity: a model of neocortical chattering neurons. Neuroscience. 1999;89:347–362. doi: 10.1016/s0306-4522(98)00315-7. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo P, Zalutsky R, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Tsumoto T. Long-term depression in rat visual cortex is associated with a lower rise of postsynaptic calcium than long-term potentiation. Neuroscience Research. 1996;24:265–274. doi: 10.1016/0168-0102(95)01001-7. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]


