Abstract
Interleukin-1 is considered a central mediator of cartilage loss in osteoarthritis in several species, however an equine recombinant form of this cytokine is not readily available for in vitro use in equine osteoarthritis research. Equine recombinant interleukin-1β was cloned and expressed and its effects on the expression and activity of selected chondrocytic proteins implicated in cartilage matrix degradation were characterized. Reverse transcriptase polymerase chain reaction methods were used to amplify the entire coding region of the equine IL-1β mRNA, which was cloned into an expression vector, expressed in E. coli, and purified using a Ni2+ chromatographic method. The effects of the recombinant peptide on chondrocyte gene expression were determined by Northern blotting using RNA from equine chondrocyte cultures hybridized to probes for matrix metalloproteinases (MMP 1, MMP 3, MMP 13), tissue inhibitor of matrix metalloproteinases 1 (TIMP 1) and cyclooxygenase 2 (COX 2). Effects on selected mediators of cartilage degradation (nitrite concentrations and MMP activity) were determined using conditioned medium from reIL-1β-treated equine cartilage explant cultures. A recombinant peptide of approximately 21 kd was obtained. Northern blotting analyses revealed a marked up-regulation of expression of all MMPs, TIMP 1, and COX 2 in mRNA from treated chondrocytes. Furthermore, cartilage explants exposed to reIL-1β had augmented collagenase/gelatinase and stromelysin activities as well as increased concentration of nitrite in conditioned media. The development of a biologically active, species-specific IL-1β provides a valuable tool in the study of osteoarthritis pathophysiology and its treatment in horses.
Introduction
Inappropriate induction of a number of proinflammatory cytokines is considered important in the pathophysiologic events of cartilage matrix degradation in rheumatic diseases. Indeed, many of the recent advances in understanding the mechanisms in articular pathobiology in man and domestic animals have been due to the advances in characterizing the effects on cellular metabolism of participating cytokines (1,2). In osteoarthritis (OA), the most studied of the proinflammatory cytokines is interleukin-1, (IL-1), currently considered to play a central role in the disease. The principal cellular source of IL-1 in most tissues is the monocyte/macrophage, however, the peptide is also expressed by connective tissue cells including chondrocytes and synoviocytes (3).
In vitro studies using cartilage from a number of species, have demonstrated that exposure to human recombinant IL-1 (rhIL-1) induces both the inhibition of cartilage matrix synthesis and augmented expression of cartilage degrading proteinases of chondrocytic origin (3). This latter effect is mediated, in large part, by a number of matrix metalloproteinases (MMPs), including collagenases 1 and 3 (MMP 1, MMP 13) and stromelysin 1 (MMP 3) (3,4). The role of IL-1 in the arthritic process is not limited to alterations in protein synthesis; the production of other proinflammatory mediators implicated in OA such as prostaglandin E2 (PGE2) and nitric oxide (NO) is stimulated by this cytokine (5,6).
Recent research supports a role for IL-1 in pathophysiologic processes in equine joints. Elevated levels of IL-1-like biological activity have been identified in the synovial fluid of arthritic horses (7,8) and rhIL-1 has been employed in several in vitro studies using equine cartilage, where it has been demonstrated to enhance cartilage matrix metalloproteinase activity and induce cartilage matrix depletion. Specifically, chondrocytes in explant or monolayer culture exposed to rhIL-1 synthesize MMPs (9,10,11) and cartilage explants exposed to this cytokine undergo matrix degradation (12,13). The potential for inhibition of rhIL-1 effects on equine cartilage has also been the subject of recent research (10,13,14,15).
While a recombinant form of equine IL-1 is not presently available, initial molecular characterization of the protein has been conducted. As for other species, this protein exists in 2 principal forms, IL-1α and IL-1β, and the nucleotide and deduced amino acid sequences for the equine cytokine have recently been reported (16,17). The predicted amino acid sequence of equine IL-1α shows 71.6% and 60.2% identity with that of human and murine IL-1α, respectively, and the corresponding sequence of equine IL-1β shows 66.7% and 61.8% identity with that of human and murine IL-1β, respectively (16). Despite the fact that these 2 forms of IL-1 interact with the same cellular receptors and have analogous functional effects in other species, it is not clear that the sequences are homologous; identity between the deduced amino acid sequences of α and β forms of equine IL-1 is only 26% (17).
The limited sequence identity between equine and human forms of IL-1β raises the possibility that responses of equine tissues to recombinant cytokines of other species may not be entirely representative of those that would be obtained with species-specific ligand-receptor interactions. Activation by rhIL-1β of human synoviocytes and chondrocytes requires only 5% receptor occupancy for half-maximal stimulation of MMP production (18), yet it has been observed that 2 to 3 times the concentration of rhIL-1βnecessary to saturate all receptor sites is required to initiate low levels of MMP secretion in nonprimate target cells (19,20). Moreover, experiments comparing the effects of rhIL-1 and equine mononuclear cell supernatants (a crude source of equine IL-1) have revealed dissimilar and disproportionate levels of stimulation of prostaglandin E2 and MMP synthesis by equine synovial cells and chondrocytes (21,22). These data underscore the importance of developing a species-specific form of IL-1, a molecular biologic tool prerequisite to assure reliable, specific cellular responses by equine target cells.
The objective of the study reported here was to prepare a recombinant IL-1β of equine origin (reIL-1β) and to characterize its effects on the induction of selected putative mediators of cartilage altered cartilage metabolism in equine OA. Specifically, we examined the effect of graduated doses of reIL-1β on 1) the gene expressions of MMP 1, MMP 3, MMP 13, tissue inhibitor of matrix metalloproteinases 1 (TIMP 1), and cyclooxygenase 2 (COX 2) by Northern hybridizations and 2) the activity of MMPs and nitric oxide synthase by substrate digestion and colorometric assays, respectively.
Materials and methods
Cloning and expression of equine IL-1β RNA
Total RNA was extracted from lipopolysaccharide (LPS)-stimulated (1 μg/mL) equine chondrocytes in monolayer culture using a guanidium isothiocyanate-based extraction solvent (Trizol Reagent; Gibco BRL, Grand Island, New York, USA). The IL-1β cDNA was synthesized by using reverse transcriptase-polymerase chain reaction (RT-PCR), with 2 μg of total RNA using a specific set of oligonucleotide primers designed from the published equine cDNA sequence (3). The sequences for the IL-1β primers were 5′-GCA GCC ATG CAT TCA GTG AAC-3′ (sense primer), starting at position 366 bp of the published sequence, and 5′-CTG CCA CCC TTA AGC TTT ATT CAT-3′ (antisense primer) ending at position 880 bp. Amplification by PCR produced a 515 bp cDNA fragment which, after purification, was ligated directly to the hexahistidine- containing expression vector pTRCHis A (Xpress System Protein Expression TrcHis; Invitrogen, Carlsbad, California, USA). Competent E. coli (TOPO TA Cloning Kit; Invitrogen) were transformed with the expression vector and propagated overnight in a microbiologic shaker at 37°C. Expression of reIL-1β was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG).
Purification of reIL-1β on Ni2+ agarose
The hexahistidine-tagged recombinant protein was purified using a commercially available kit (Xpress System Protein Purification; Invitrogen), following the manufacturer's directions. Briefly, the bacterial cell extract was bound to Ni2+ agarose followed by repetitive washes with sodium chloride-sodium phosphate buffers of decreasing pH, followed by elution using an imidazole gradient. Protein quantification was accomplished by using a commercial kit (BioRad Protein Assay; BioRad Laboratories, Hercules, California, USA), with bovine IgG as the standard. Samples of the purified protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the gels were stained with Coomassie blue and silver. The approximate molecular weight of reIL-1β was estimated by interpolation of distance migrated by protein markers of lesser and greater molecular weight than the recombinant protein (23). The theoretical molecular weight of reIL-1β was determined using the Protean program of the DNASTAR package (DNASTAR, Madison, Wisconsin, USA).
Amplification of cDNA and probe preparation
Amplification of cDNA was accomplished, using RT-PCR with 2 μg of total RNA from a chondrocyte culture that had been stimulated (6 h) with 1 μg/mL of LPS using standard protocols. Specific sets of equine oligonucleotide primers for COX 2 and MMP 13 genes were synthesized based on published human cDNA sequences and were used at a final concentration of 5 pM/μL. The cDNA fragments were ligated directly to PCR 2.1 TOPO vector (TOPO TA Cloning Kit; Invitrogen). The sequencing of each insert was performed manually. Clones of MMP 1, MMP 3, and TIMP 1, in pBluescript vector were prepared previously by one of the authors (24).
Digoxigenin-labeled complementary DNA probes were prepared for all MMP fragments and for COX 2 using the protocol outlined in a commercially-available kit (DIG High Prime DNA Labeling and Detection Starter Kit II; Roche Molecular Biochemicals, Indianapolis, Indiana, USA). Briefly, the vector containing the insert of interest was subcloned into competent E. coli (TOPO TA Cloning Kit; Invitrogen), the transformed bacteria were propagated at 37°C in an orbital shaker, and the plasmid was isolated. The cDNA fragment was cleaved from the plasmid via restriction enzyme digestion and purified (QIAquick Gel Extraction Kit; Qiagen, Valencia, California, USA). The fragments were labeled via random primer labeling incorporating digoxigenin-11-dUTP.
Northern blot hybridization
Northern blot hybridization was conducted as previously described (10). Total chondrocyte RNA was resolved on 1.2% agarose-formaldehyde gels, using 3 μg of RNA per lane. After overnight capillary transfer to nylon membranes (Nylon Membranes (positively charged); Roche Molecular Biochemicals), overnight hybridization was conducted at 50°C with 100 ng of labeled probe/mL. After serial post hybridization washes under standard conditions, detection was accomplished using a chemiluminescent method (DIG High Prime DNA Labeling and Detection Starter Kit II; Roche Molecular Biochemicals), as described by the manufacturer. The membranes were then subjected to autoradiography at room temperature for 1 to 30 min.
Stimulation with reIL-1β
To test the influence of reIL-1β on the expression of MMPs and COX 2 in normal equine chondrocytes, confluent cultures of first passage cells were grown to confluence in individual 6-well plates (1 × 106 cells/well). After 3 to 5 d under serum-free conditions, cultures received 1 of 6 different treatments, including control, rhIL-1β (10 ng/mL), reIL-1β (1, 10, 100 ng/mL), and reIL-1β (10 ng/mL) plus dexamethasone (10−5 M). After a 6-hour incubation, total RNA was extracted for Northern blot hybridization. Conditioned medium from similarly treated cultures was harvested after a 24-hour incubation and analyzed for nitrite content and stromelysin activities.
Explant cultures
Equine carpal cartilage explants (40–60 mg/well) were cultured in 24-well plates, as described previously (25). The explants were maintained in basal media for 2 d prior to treatment after which they were exposed to reIL-1β (0, 1, 10, 50, 100 ng/mL) or rhIL-1β (50 ng/mL). Conditioned media were removed after 24 h and analyzed for collagenase/gelatinase activity.
Matrix metalloproteinase assays
Total stromelysin activity was determined by digestion of the azodye substrate azocoll (Calbiochem-Behring, La Jolla, California, USA), using the method of Chavira et al (26). Stromelysin in culture supernatants was activated by the incubation of 200-μL aliquots of medium with the azocoll suspension in the presence of 1 mM aminophenylmercuric acetate. After an incubation of 48 h at 37°C, the tubes were centrifuged and the optical density of the supernatant was read at 520 nm.
Gelatinase/collagenase activity present in conditioned media quantified using a gelatinase/collagenase kit (Molecular Probes Enzchek Gelatinase/Collagenase assay kit; Eugene, Oregon, USA), as described previously (25). Briefly, conditioned medium was incubated with the fluorescent substrate for 1 h and fluorescence was detected using Cytofluor 4000 plate reader.
Nitric oxide assay
Nitrite, a stable end product of nitric oxide metabolism, was measured in conditioned media using the Greiss reaction and sodium nitrite as a standard (27). Briefly, 200-μL aliquots of culture medium were added to an equal volume of a freshly prepared mixture of equal proportions of 1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride. The absorbance of the solution was read at 550 nm after a 30-minute incubation and NO2 concentrations were determined using a standard curve.
Results
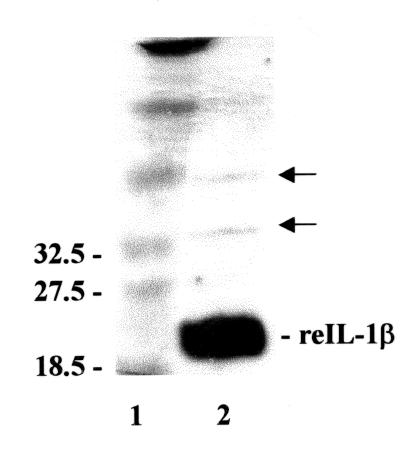
A recombinant equine IL-1β of high purity was obtained from the cloned cDNA by affinity purification using a hexahistidine tag and Ni2+ agarose. Resolution by SDS-PAGE and subsequent Coomassie blue staining revealed a single band migrating at 21 kD, which corresponded with a predicted molecular mass of 22.6 kD. Silver staining failed to reveal substantial quantities of other proteins (Figure 1).
Figure 1. Silver-stained SDS-PAGE gel of purified recombinant equine interleukin-1β. Molecular weights of protein standards of comparable size are indicated to the left of lane 1. The major band (reIL-1β) in lane 2 corresponds to the 21 kD product. Negligible additional staining (arrows) indicates the purity of the protein isolated using the Ni2+ chromatographic method.
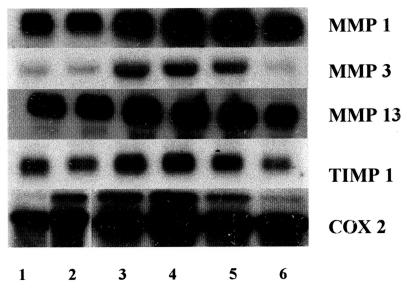
Northern blot analyses using RNA derived from monolayer cultures of equine chondrocytes exposed to graduated nanogram concentrations of the recombinant cytokine revealed marked up- regulation of expression of genes coding for MMP 1, MMP 3, MMP 13, TIMP 1, and COX 2 (Figure 2). Increased gene expression was marked and appeared to be saturable at reIL-1β doses in the 1 to 10 ng/mL range, depending on the specific gene. Further increases (100 ng/mL) did not further stimulate expression and, for all genes studied, reIL-1β stimulation of expression was inhibited by concurrent administration of dexamethasone (10−5 M).
Figure 2. Representative Northern hybridization of matrix metalloproteinase (MMP), tissue inhibitor of matrix metalloproteinase 1 (TIMP 1), and cyclooxygenase 2 (COX 2) expression by normal equine chondrocytes in monolayer culture stimulated with recombinant equine interleukin-1β (reIL-1) or recombinant human interleukin-1β (rhIL-1). Total RNA (4 μg) was resolved on formaldehyde agarose gels. Blots were probed with a digoxigenin-labeled DNA probes. Treatments: lane 1: control; lane 2: 10 ng/mL rhIL-1β; lane 3: 1 ng/mL reIL-1β; lane 4: 10 ng/mL reIL-1β; lane 5: 100 ng/mL reIL-1β; lane 6: 10 ng/mL reIL-1β + 10−5 M dexamethasone.
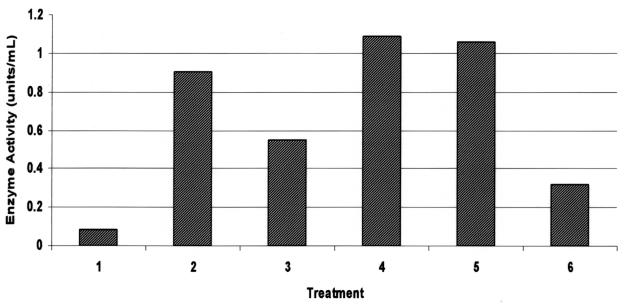
Similar to its effects on MMP gene expression, reIL-1β induced MMP activity (Figures 3, 4). Specifically, a dose-dependent but saturable increase in collagenase/gelatinase and stromelysin activities were noted in conditioned media from cartilage explant and monolayer cultures, respectively, exposed to reIL-1β. Digestion of azocoll by monolayer culture supernatants was maximal at 10 ng/mL (Figure 3) and collagenase/gelatinase activity in conditioned medium of stimulated equine cartilage explants was maximal at 50 ng/mL reIL-1β (Figure 4).
Figure 3. Stromelysin (azocoll digesting) activity in 24-hour conditioned media from chondrocyte monolayer cultures exposed to recombinant equine interleukin-1β (reIL-1) or recombinant human interleukin-1β (rhIL-1). Treatments: lane 1: control; lane 2: 10 ng/mL rhIL-1β; lane 3: 1 ng/mL reIL-1β; lane 4: 10 ng/mL reIL-1β; lane 5: 100 ng/mL reIL-1β; lane 6: 10 ng/mL reIL-1β + 10−5 M dexamethasone.
Figure 4. Collagenase/gelatinase activity in conditioned media from cartilage explants exposed to recombinant equine interleukin-1β (reIL-1) or recombinant human interleukin-1β (rhIL-1) for 24 h. Treatments: lane 1: control; lane 2: 50 ng/mL rhIL-1β; lane 3: 10 ng/mL reIL-1β; lane 4: 50 ng/mL reIL-1β; lane 5: 100 ng/mL reIL-1β.
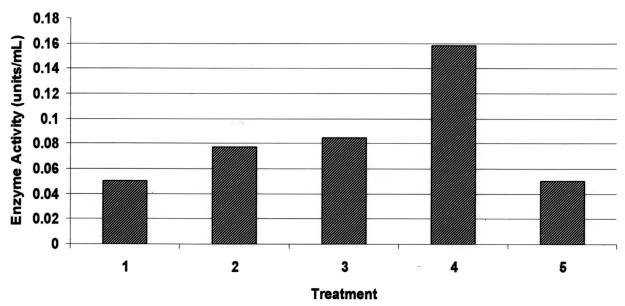
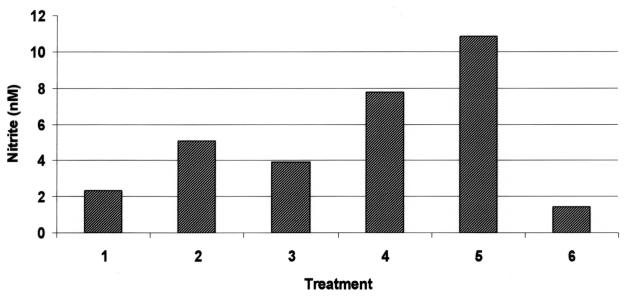
Matrix metalloproteinase activity assay data were paralleled by augmented nitrite content in conditioned medium derived from reIL-1β-treated explants (Figure 5), indicating that the cytokine stimulated the activity of inducible nitric oxide synthase.
Figure 5. Nitrite content in conditioned media from 24-hour chondrocyte monolayer cultures. Treatments: lane 1: control; lane 2: 10 ng/mL rhIL-1β; lane 3: 1 ng/mL reIL-1β; lane 4: 10 ng/mL reIL-1β; lane 5: 100 ng/mL reIL-1β; lane 6: 10 ng/mL reIL-1β + 10−5 M dexamethasone.
Discussion
Using an RT-PCR strategy, with oligonucleotide primers based on a recently reported nucleotide sequence, we were able to successfully amplify, clone, express, and purify a biologically-active recombinant equine IL-1β. Hexahistidine tagging and immobilized metal ion chromatography purification of recombinant proteins have been employed for a number of proteins expressed in bacteria and mammalian cell lines and have proved to be an effective means of one-step purification of reIL-1β. As has been observed elsewhere (28), the presence of the hexahistidine tag did not appear to inhibit the biologic activity of the protein; however, a comparison of the activities of the tagged peptide and a tag-cleaved version was not conducted. Exhaustive testing of the purity of our reIL-1β was not performed; however, subjective analysis of both Coomassie blue and silver stained SDS-PAGE gels of the eluted protein revealed a single band corresponding to the predicted molecular mass of IL-1β, with negligible contamination by other E. coli proteins.
Because the β form of the protein is produced in greater abundance under inflammatory conditions (16), we chose to clone and express reIL-1β. Nonetheless, both α and β forms of IL-1 interact avidly with IL-1 membrane receptors (29), and their effects on chondrocytic metabolism are qualitatively similar (30). They have been used almost interchangeably in numerous studies of cartilage metabolism.
Paralleling previous in vitro reports demonstrating the arthritogenic properties of rhIL-1 in equine cartilage, exposure of equine chondrocytes to reIL-1β markedly induced the expression of MMP 1, MMP 3, and MMP 13, 3 members of a group of metal-dependent proteinases considered important in the degradation of the extracellular matrix of cartilage in osteoarthritis. Induction of MMP gene expression was reflected in increased MMP synthesis and release, as evidenced by the activity assays on conditioned media from stimulated monolayer and explant cultures. While the assays employed cannot be used to evaluate the activity of specific MMPs, this combination of assays reflects collagenase/gelatinase and stromelysin activities and complement the gene expression data.
It has been hypothesized that cartilage matrix degradation in OA may be due, at least in part, to a loss of the normal balance of MMPs and their natural inhibitors the tissue inhibitor of metalloproteinases (TIMPs). This premise is supported by the observation that IL-1 induces stromelysin and collagenase synthesis with no effect (31) or a decline in TIMP secretion by human chondrocytes (32). In the latter study it was also observed that IL-1 had no effect on TIMP messenger RNA levels. Contrary to these findings, we observed increased TIMP expression with exposure to reIL-1β. While these findings are divergent from those of a number of previous studies, IL-1β has been shown to induce TIMP expression (33). Differences in these results may be due to experimental conditions, dose range of reIL-1β employed, or species-related differences in TIMP regulation by IL-1β. Given the importance of TIMP in regulation of MMP activity, this observation warrants further study.
Dexamethasone was used as a control for gene expression studies because of its consistent ability to inhibit expression of MMPs and inducible COX in chondrocytes of other species. Exhaustive characterization of the specific mechanisms of regulation of protein synthesis by corticosteroids has not been accomplished, however they have been shown to modify gene expression by a number of molecular mechanisms, including induction and/or repression of transcription and alteration of mRNA half-life (34,35). In addition to repressing MMP expression, dexamethasone inhibited reIL-1β induction of TIMP as has been recently reported (36). The net effect of parallel inhibition of MMP and TIMP expression by corticosteroids is a phenomenon that requires further study, given the importance of MMP activity in cartilage loss and widespread use of corticosteroids in the treatment of equine OA.
Similar to chondrocytes of other species, exposure of equine chondrocytes to reIL-1β also resulted in enhanced expression of the inducible form of cyclooxygenase, COX 2 (37). Induction of phospholipase A2 and COX 2 activity is a well-recognized effect of IL-1 in human chondrocytes and a number of deleterious effects have been attributed to the presence of elevated levels of PGE2 in the articular environment. Specifically, PGE2 has been implicated in cartilage degradation synovial inflammation, and bone erosion in arthritic joints (38,39,40). As for the studied MMPs, induction of COX 2 expression was inhibited by dexamethasone.
Cartilage explants exposed to reIL-1β had enhanced concentrations of nitrite (NO) released to the media compared to control cultures. Interleukin-1β is a potent inducer of inducible nitric oxide synthase activity in chondrocytes of several species (41,42,43). Nitric oxide is hypothesized to contribute to a number deleterious influences of IL-1 on cartilage metabolism, including augmenting the expression and activation of MMPs (44), reducing synthesis of the natural receptor antagonist protein for IL-1 (45), and the inhibition of proteoglycan and type 2 collagen synthesis (46,47).
Despite a limited sequence identity with the equine protein, recombinant human IL-1β has proved useful in a number of studies of equine articular metabolism (9,10,11,12,13,14,15,25,36,43). Our reIL-1β had effects on chondrocyte biosynthesis similar to those of our rhIL-1β control and parallel those previously reported for rhIL-1. Because neither the quantity nor specific activity of either preparation was stringently quantified, relative potencies cannot be determined from our data. Results of previous in vitro studies using tissues and cytokines of heterologous origin (19,20,21) suggest that equine chondrocytes may be more sensitive to reIL-1β than rhIL-1β, however the receptor binding portion of the ligand may be sufficiently conserved between species that differences may not be marked. Further investigation, with more accurately quantified preparations is required to more accurately characterize differences, if any, in receptor binding kinetics and intracellular signaling pathways.
In summary, using a reported equine cDNA sequence, we were able to construct and express a recombinant equine IL-1β. Preliminary characterization of the recombinant peptide indicates that its effects on the expression and activity of selected proteins of equine chondrocytes are similar to those reported to occur in the cartilage of other species and appear saturable at nanogram quantities. This development provides another valuable tool in the study of disease processes mediated by this cytokine in horses.
Footnotes
Dr. Arnold's present address is the Department of Clinical Studies, New Bolton Center, University of Pennsylvania, Kennett Square, Pennsylvania 19348 USA.
Address correspondence to Dr. J.P. Caron, G-351 Veterinary Medical Center, College of Veterinary Medicine, Michigan State University, East Lansing, Michigan 48824-1314 USA, telephone: 517-353-9710, fax: 517-432-1042, e-mail: caron@cvm.msu.edu
Received May 21, 2001. Accepted November 7, 2001.
Supported by USDA Formula Funds and the Harvey Fiege Genetic Research Fund.
References
- 1.Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci 1999; 4:D694–703. [DOI] [PubMed]
- 2.van den Berg WB. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol 1999;58:136–141. [DOI] [PubMed]
- 3.Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation Rheum Dis Clin North Am 1993;19:545–568. [PubMed]
- 4.Okada Y. Proteinases and matrix degradation. In: Ruddy S, Harris ED, Sledge CB, eds. Textbook of Rheumatology. 6th ed. Philadelphia: WB Saunders, 2001:55–72.
- 5.Campbell IK, Piccoli DS, Hamilton JA. Stimulation of human chondrocyte prostaglandin E2 production by recombinant human interleukin-1 and tumor necrosis factor. Biochim Biophys Acta 1990;1051:310–318. [DOI] [PubMed]
- 6.Maier R, Bilbe G, Rediske J, et al. Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta 1994;1208: 145–150. [DOI] [PubMed]
- 7.Alwan WJ, Carter SD, Dixon JB, et al. Interleukin-1-like activity in synovial fluids and sera of horses with arthritis. Res Vet Sci 1991;51:72–77. [DOI] [PubMed]
- 8.Morris EA, MacDonald BS, Webb AC, Rossenwasser MD. Identification of interleukin-1 in equine osteoarthritic joint effusions. Am J Vet Res 1990;51:59–64. [PubMed]
- 9.Morris EA, Treadwell BV. Effect of interleukin 1 on articular cartilage from young and aged horses and comparison with metabolism of osteoarthritic cartilage. Am J Vet Res 1994;55:138–146. [PubMed]
- 10.Caron JP, Tardif G, Martel-Pelletier J, et al. Modulation of matrix metalloprotease 13 (collagenase 3) gene expression in equine chondrocytes by IL-1 and corticosteroids. Am J Vet Res 1996;57:1631–1634. [PubMed]
- 11.Richardson DW, Dodge GR. Effects of interleukin-1beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res 2000;61:624–630. [DOI] [PubMed]
- 12.MacDonald MH, Stover SM, Willits NH, Benton HP. Regulation of matrix metabolism in equine cartilage explant cultures by interleukin 1. Am J Vet Res 1992;53:2278–2285. [PubMed]
- 13.Frisbie DD, Sandler EA, Trotter GW, McIlwraith CW. Metabolic and mitogenic activities of insulin-like growth factor-1 in interleukin-1-conditioned equine cartilage. Am J Vet Res 2000; 61:436–441. [DOI] [PubMed]
- 14.Frean SP, Gettinby G, May SA, Lees P. Influence of interleukin-1beta and hyaluronan on proteoglycan release from equine navicular hyaline cartilage and fibrocartilage. J Vet Pharmacol Ther 2000;23:67–72. [DOI] [PubMed]
- 15.Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun 2000;274:467–471. [DOI] [PubMed]
- 16.Kato H, Ohashi T, Nakamura N, et al. Molecular cloning of equine interleukin-1 alpha and -beta cDNAs. Vet Immunol Immunopathol 1995;48:221–231. [DOI] [PubMed]
- 17.Howard RD, McIlwraith CW, Trotter GW, Nyborg JK. Cloning of equine interleukin 1 alpha and equine interleukin 1 beta and determination of their full-length cDNA sequences. Am J Vet Res 1998;59:704–711. [PubMed]
- 18.Martel-Pelletier J, McCollum R, DiBattista JA, et al. The interleukin-1 receptor in normal and osteoarthritic human articular chondroctes is the Type-1 receptor: Binding kinetics and biological function. Arthritis Rheum 1992;35:530–540. [DOI] [PubMed]
- 19.Chin JE, Horuk R. Interleukin 1 receptors on rabbit articular chondrocytes: relationship between biological activity and receptor binding kinetics. FASEB J 1990;4:1481–1487. [DOI] [PubMed]
- 20.DiBattista JA, Martel-Pelletier J, Fujimoto N, et al. Prostaglandins E2 and E1 inhibit cytokine induced metalloprotease expression in human synovial fibroblasts. Lab Invest 1994;71:270–278. [PubMed]
- 21.May SA, Hooke RE, Lees P. Interleukin-1 stimulation of equine articular cells. Res Vet Sci 1992;52:342–348. [DOI] [PubMed]
- 22.May SA, Hooke RE, Lees P. Species restrictions demonstrated by the stimulation of equine cells with recombinant human interleukin-1. Vet Immunol Immunopathol 1992;30:373–384. [DOI] [PubMed]
- 23.Southern EM. Measurement of DNA length by gel electrophoresis. Anal Biochem 1979;100:319–323. [DOI] [PubMed]
- 24.Richardson DW, Dodge GR. Molecular characteristics of equine stromelysin and the tissue inhibitor of metalloproteinase 1. Am J Vet Res 1998;59:1557–1562. [PubMed]
- 25.Fenton JI, Chlebek-Brown KA, Peters TA, et al. Glucosamine reduces equine articular cartilage degradation in explant culture. Osteoarthritis Cartilage 2000;8:258–265. [DOI] [PubMed]
- 26.Chavira R Jr, Burnett TJ, Hageman JH. Assaying proteinases with azocoll. Anal Biochem 1984;136:446–450. [DOI] [PubMed]
- 27.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982;126:131–138. [DOI] [PubMed]
- 28.Parwate S, Schey KL, Meier GP, et al. Expression, characterization, and purification of C-terminally hexahistidine-tagged thromboxane A2 receptors. J Biol Chem 1998;273:22753–22760. [DOI] [PubMed]
- 29.Saklatvala J, Bird TA. A common class of receptors for the two types of porcine interleukin-1 on articular chondrocytes. Lymphokine Res 1986;5(Suppl):S99–S104. [PubMed]
- 30.Smith RJ, Rohloff NA, Sam LM, Justen JM, Deibel MR, Cornette JC. Recombinant human interleukin-1 alpha and recombinant human interleukin-1 beta stimulate cartilage matrix degradation and inhibit glycosaminoglycan synthesis. Inflammation 1989;13:367–382. [DOI] [PubMed]
- 31.Yamada H, Toshiyuki K, Nemoto O, et al. Effects of indomethacin on the production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by human articular chondrocytes. J Rheumatol 1996;23:1739–1743. [PubMed]
- 32.Martel-Pelletier J, Zafarullah M, Kodama S, Pelletier JP. In vitro effects of interleukin-1 on the synthesis of metalloproteases, TIMP, plasminogen activators and inhibitors inhuman articular cartilage. J Rheumatol 1991;27 (Suppl):80–84. [PubMed]
- 33.Shingu M, Nagai Y, Isayama T, et al. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol 1993;94:145–149. [DOI] [PMC free article] [PubMed]
- 34.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem 1994;63:451–486. [DOI] [PubMed]
- 35.Krane SM. Some molecular mechanisms of glucocorticoid action. Br J Rheumatol 1993;32 (Suppl):3–5. [DOI] [PubMed]
- 36.Richardson DW, Dodge GR. Dose-dependent effects of corticosteroids on the expression of matrix related genes in normal and cytokine treated articular chondrocytes [Abstract]. Vet Surg 1998;27:515. [DOI] [PubMed]
- 37.Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest 1997;15:1231–1237. [DOI] [PMC free article] [PubMed]
- 38.Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett 1995;372:83–87. [DOI] [PubMed]
- 39.Pelletier JP, Martel-Pelletier J. Evidence for the involvement of interleukin-1 in human osteoarthritic cartilage degradation: protective effect of NSAID. J Rheumatol 1989;16 (Suppl 18):19–27. [PubMed]
- 40.Suda T, Udagawa N, Nakamura I, et al. Modulation of osteoclast differentiation by local factors. Bone 1995;17 (Suppl):87S–91S. [DOI] [PubMed]
- 41.Jarvinen TAH, Moilanen T, Jarvinen TLN, Moilanen E. Nitric oxide mediates interleukin-1 induced inhibition of glycosaminoglycan synthesis in rat articular cartilage. Mediators Inflamm 1995;4:107–111. [DOI] [PMC free article] [PubMed]
- 42.Amin AR, Di Cesare PE, Vyas P, et al. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for an inducible “neuronal-like” nitric oxide synthase. J Exp Med 1995;182:2097–2102. [DOI] [PMC free article] [PubMed]
- 43.Frean SP, Bryant CE, Froling IL, et al. Nitric oxide production by equine articular cells in vitro. Equine Vet J 1997;29:98–102. [DOI] [PubMed]
- 44.Murrell GAC, Jang D, Williams RJ. Nitric oxide activated metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun 1995;206:15–21. [DOI] [PubMed]
- 45.Pelletier JP, Mineau F, Ranger P, et al. The increased synthesis of inducible nitric oxide inhibits IL-1Ra synthesis by human articular chondrocytes; possible role in osteoarthritis cartilage degradation. Osteoarthritis Cartilage 1996;4:77–84. [DOI] [PubMed]
- 46.Oh M, Fukuda K, Asada S, et al. Concurrent generation of nitric oxide and superoxide inhibits proteoglycan synthesis in bovine articular chondrocytes: involvement of peroxynitrite. J Rheumatol 1998;25:2169–2174. [PubMed]
- 47.Cao M, Westerhausen-Larsen A, Niyibizi C, et al. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J 1997;324:305–310. [DOI] [PMC free article] [PubMed]