Abstract
The effects of reinforcement manoeuvres, such as mental computation and the Jendrassik manoeuvre, on muscle spindle sensitivity to passively imposed sinusoidal stretching (1.5 deg, 2 Hz) in relaxed subjects were analysed.
The unitary activity of 26 muscle spindle afferents (23 Ia, 3 II) originating from ankle muscles was recorded using the microneurographic method. Particular care was paid to the subjects' state of physical and mental relaxation.
The results showed that the activity of 54 % of the Ia afferents was modified during mental computation. The modifications took the form of either an increase in the number of spikes (mean, 26 % among 11 Ia fibres) or a shortening in the latency of the response to sinusoidal stretching (mean, 13 ms among 3 Ia fibres), or both. They were sometimes accompanied by an enhanced variability in the instantaneous discharge frequency. The three secondary endings tested exhibited no change in their sensitivity to stretch during mental computation.
The increased sensitivity to passive movements sometimes began as soon as the instructions were given to the subjects and sometimes increased during mental computation. In addition, the increased sensitivity either stopped after the subjects gave the right answer or continued for several minutes.
During the performance of a Jendrassik manoeuvre, the Ia units underwent changes similar to those described above for mental computation.
It was concluded that muscle spindle sensitivity to movement can be modified in relaxed human subjects. The results reinforce the idea that the fusimotor system plays a role in arousal and expectancy, and contribute to narrowing the gap between human and behaving animal data.
Whereas in amphibia, terminal branches of α-motoneurones provide motor innervation to muscle spindles, in mammals, a separate fusimotor supply has evolved, namely γ-motoneurones. These are morphologically different from α-motoneurones, they receive different reflex connections, and they innervate muscle spindles separately and more extensively. Together this suggests that the fusimotor system might, to some extent, act independently of the skeletomotor system and could modify muscle spindle sensitivity selectively in order to make the receptors extract more accurate information about movement. The fusimotor system is indeed better thought of as allowing state-dependent parametric adjustment of length and velocity feedback rather than as simply compensating automatically for muscle shortening, a role devoted to the pre-existing skeleto-fusimotor system (see Prochazka, 1989).
Muscle spindle afferent recordings from behaving animals support this notion of adjustment. Indeed, it has been reported that demanding motor tasks are associated with higher levels of γ-drive than are routine movements, without concomitant changes in skeletomotor activity (Prochazka et al. 1985, 1988; Hulliger et al. 1989; Prochazka, 1989; Gorassini et al. 1993).
Some microneurographic studies on muscle afferents have reported that independent control of fusimotor activity may also occur in humans (Burg et al. 1973, 1974, 1975, 1976; Hagbarth et al. 1975; Burke et al. 1980a, b; Vallbo & Hulliger, 1981; Gandevia & Burke, 1985; Aniss et al. 1990; Gandevia et al. 1994), although this is controversial (see reviews by Vallbo et al. 1979; Prochazka, 1996).
We previously revealed a fusimotor drive in human relaxed muscles more directly by recording γ-efferents that exhibited substantial changes in activity during various reinforcement manoeuvres (Ribot et al. 1986). An increase in the fusimotor activity was induced (1) by cognitive factors, such as listening to instructions about mental computation tasks, performing computations, or focusing attention on the experimental situation; (2) by behavioural factors, such as laughing or talking; (3) by environmental factors, such as hearing or seeing somebody enter the room in which the experiment was taking place or hearing a short auditory stimulus; and (4) by clinical neurophysiological manoeuvres, such as clenching both fists (the Jendrassik manoeuvre). However, these four types of manoeuvre failed to change the muscle spindle response to muscle stretching. Indeed, the muscle spindle responses to ramp and hold stretches recorded with (‘test response’) and without (‘control response’) concomitant manoeuvres did not differ (Ribot et al. 1986).
This lack of change in the muscle spindles' stretch sensitivity may be due to at least two factors. First, any fusimotor discharge, whether spontaneous or induced, was found to be difficult to suppress: only some subjects succeeded in entering a state of deep mental and somatic relaxation that is apparently required to minimise fusimotor activity. Second, the firing rate of the fusimotor neurones that was induced by each of the reinforcement manoeuvres was never greater than the firing rate of their spontaneous discharges. These points suggest that what we had been calling the ‘control response’, when analysing muscle spindle sensitivity to stretch (Ribot et al. 1986), may have been obtained while the γ-system was already discharging and that the performance of any additional manoeuvre may not in fact have increased the fusimotor drive relative to the control situation.
In the present study, we reinvestigated this issue by taking particular care to ensure that the subject was always as physically and mentally relaxed as possible in the control situation. These conditions were fulfilled by using only subjects accustomed to participating in microneurographic experiments. They were helped to relax by listening to a relaxation audiotape, and their level of arousal was monitored throughout the experiments by recording electrodermal activities (Ohman et al. 1993).
Furthermore, since the fusimotor tone might be of the dynamic type (Burg et al. 1976; Ribot et al. 1986; Gandevia et al. 1994), ramp and hold stretches may not be the most appropriate stimulus for investigating whether any corresponding changes occur in the muscle spindle response. We therefore used continuously repeated sinusoidal movements to show up any intermittent or delayed effects.
The results show that reinforcement manoeuvres, such as mental computation and fist clenching, can increase the primary muscle spindle sensitivity to passive movements. This increase indicates that a fusimotor outflow operates on the muscle spindle endings in non-contracting muscles in awake subjects and plays a role in arousal and expectancy, as for behaving animals (see review by Prochazka, 1989).
METHODS
Experiments were performed on 10 healthy volunteers (9 males and 1 female) between 22 and 40 years of age, all of whom gave their written informed consent to the experimental conditions, as required by the Declaration of Helsinki. This study was approved by the local ethics committee (CCPPRB, Marseille I). The subjects were all accustomed to participating in microneurographic experiments and they were selected on the basis of their ability to relax. The physical state of relaxation was verified by the absence of any muscular activity (see below), and mental relaxation was assessed by the recording of electrodermal activity through two surface electrodes placed on each side of the left hand (bandpass: 0.1 Hz to 1 kHz). Any subject who was unable to mentally relax so that a flat electrodermal recording was maintained for about 3 min was excluded from the study.
Recordings
The subjects were seated comfortably in an armchair, with their legs positioned in cushioned grooves so that a standardised relaxed position could be maintained without any muscle activity occurring. The knee joint was at an angle of about 120–130 deg. The right foot lay on a stationary plate and the left foot on a rotating pedal.
The muscle spindle afferent activity was recorded using insulated tungsten microelectrodes (impedance, 500 kΩ to 1 MΩ; tested at 1 kHz with a tip diameter of around 5–10 μm) inserted into the peroneal nerve at the level of the popliteal fossa. Recordings were made from a total of 26 muscle spindle afferents; 23 were classified as primary endings and 3 as secondary endings, depending on whether they fell silent or maintained their activity during imposed muscle shortening, and depending on their dynamic sensitivity to ramp and hold stretches and on their one-to-one driving or absence of driving by an 80 Hz muscle tendon vibration (Roll et al. 1989). Among these afferents, ten innervated the tibialis anterior muscle (TA), four the extensor digitorum muscle (EDL), eight the peroneal lateral muscle (PL) and four the hallucis longus muscle (HL). Note that since particular care was paid to keeping the subject as relaxed as possible, muscle contraction elicited by electrical stimulation was avoided. For that reason, some of the units might have been golgi tendon organs (GTOs). Such misclassification might have occurred, but only for the secondary endings whose sensitivity to vibration is comparable to that of the GTO, i.e. when responsive to vibration, a one-to-one activity may be elicited by low amplitude vibration, generally in the 10–40 Hz range and only rarely at 60 Hz (Roll et al. 1989).
General procedure
Throughout the experiment, the subjects listened to a relaxation audiotape with headphones in order to be as physically and mentally relaxed as possible. The subjects' state of relaxation was monitored throughout the experiment by electrodermal recording. Before any recording, the subjects were requested not to move any part of their bodies, to keep their heads on the headrest with their eyes closed, and to announce the result of the computation as soon as they obtained it, in the knowledge that the most important thing was to give the right result, even if this took a long time.
Once the unitary activity of an identified muscle spindle afferent had been isolated by adjusting the position of the microelectrode, low amplitude sinusoidal movements (1.5 deg) with a frequency of 2 Hz were imposed on the ankle joint. These movements continued to be imposed until the unit was no longer recordable. The response of the unit recorded during the first 3–5 min after the onset of the movements was taken to be the ‘control response’. This long period was chosen in case the subjects were surprised by the onset of the stimulus, since any change in the environmental conditions may increase the fusimotor drive (Ribot et al. 1986). One of the experimenters then approached the subjects, turned off the relaxation tape, and informed them of the mental computation to be performed (e.g. addition of all the odd numbers from 0 to 15). When the result given was wrong, the subjects had to repeat the operation until they succeeded. Afterwards, they were asked to go back to being as mentally relaxed as possible.
When the unitary muscle spindle recording was stable, the procedure was repeated as described above, except that the subjects were asked to clench both fists (Jendrassik manoeuvre) for 10 s instead of performing the mental computation task.
Control of the absence of muscle activity
The absence of muscle activity throughout the experiment was checked by recording both the electromyographic activity of the receptor-bearing muscle and the occurrence of TA tendon displacements.
At the beginning of the experiment, three pairs of surface electrodes were placed as follows: one pair over the TA muscle 2 cm below the tuberosity of the tibia, a second pair over the PL muscle at the same level as that of the first pair, and a third pair over the EDL muscle between the other two pairs. The inter-electrode distance was 4 cm. During the experiment, one pair was selected depending on the receptor-bearing muscle investigated. The EMGs were recorded at high gain (10 000) with a bandpass of 100 Hz to 3.2 kHz; they were sampled at 2000 Hz.
Since numerous studies have shown that the force necessary for a muscle spindle discharge to increase may be as low as 1 % of the maximum voluntary contraction (Kakuda et al. 1996; Gandevia et al. 1997; Wilson et al. 1997; Kakuda & Nagaoka, 1998), the absence of muscle activity was additionally verified by means of a highly sensitive transducer consisting of a photocell positioned about 5 mm in front of the tendon of the TA muscle, which was covered with reflective foil. This set-up enabled detection of any microdisplacements of the muscle tendon (50 μm = 50 mV) accompanying an involuntary contraction. This was an efficient indicator of any diffuse increase in leg muscle tension that might be induced by mental stress or by contraction of remote parts of the body.
The sensitivity of the photocell was independently tested in experiments in which four surface electrodes separated by 4 cm were placed over the TA muscle (one pair proximal, one pair distal). It was thereby possible to detect significant slow increases in the photocell signal when the subject was asked to switch from a profound state of relaxation to a state of preparation to act, without any muscle activity being recorded by any of the two pairs of electrodes. Therefore the photoelectric system was considered to be a more sensitive indicator of muscle tensing than the EMG recording. Figure 1 illustrates this change in the photocell signal and those related to the development of a very tiny voluntary muscle contraction. Whenever such changes in the photocell signal occurred, the recording session was excluded from the analysis. Accidental changes in the photocell trace, such as those appearing in Fig. 2A, were due to slight lateral displacements of the TA tendon because the pedal moved; they were not significant.
Figure 1. Example illustrating the sensitivity of the photocell.
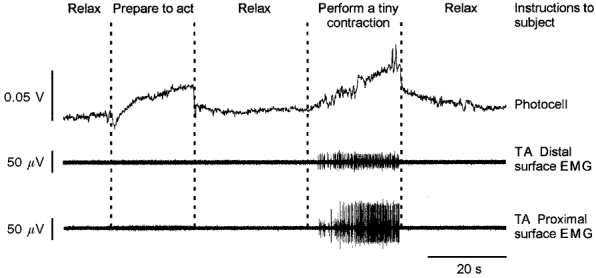
EMG recordings of the proximal (bottom trace) and the distal (middle trace) parts of the tibialis anterior (TA) muscle as well as the photocell recording (top trace). Slow increases in the photocell trace were induced when the subject was asked to switch from a profound state of relaxation to a state of preparation to act (left) or to perform a very tiny voluntary dorsal flexion (right). Note the higher sensitivity of the photoelectric system compared with surface EMG recording in signalling any involuntary increase in leg muscle tension.
Figure 2. Increase in the response of a primary muscle spindle afferent to sinusoidal stretching during the performance of mental computation.
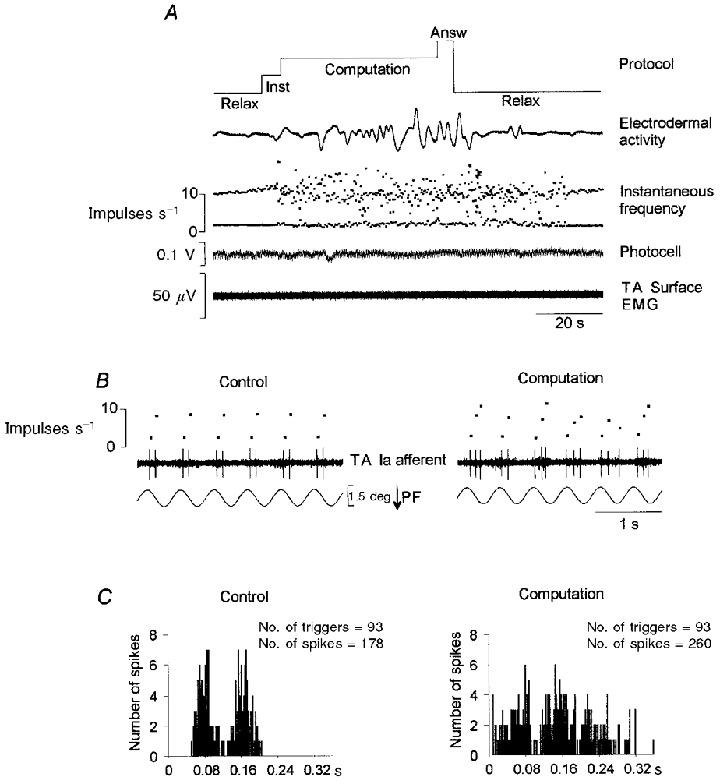
A, the primary afferent activity (TA afferent) recorded during a whole sequence is illustrated by the instantaneous discharge frequency curve. Note the increase in the firing rate, together with an increase in the variability of the instantaneous discharge frequency, both of which occurred shortly after the instructions were given to the subject. In addition, these stretch-sensitivity enhancing effects lasted throughout the period of computation and continued after the subject had been asked to relax, as shown by the flat electrodermal recording. Inst, instruction; Answ, answer. B, expanded time scale recording corresponding to 6 movement cycles during the computation period (right) compared with the control period (left) showing the frequent occurrence of a third spike at various times from one cycle to another during mental computation. PF, plantar flexion. C, cycle histograms were constructed for the whole period of stretching during which mental computation was performed (right), and for the equivalent part of the control period (93 stretch movements combined) (left). Note the increase in the number of spikes and the enlarged histogram obtained while mental computation was being performed.
Data processing
The unitary nerve recordings and the electromyographic, photocell, electrodermal and position data were stored on a digital tape recorder (DTR 1802, Biologic). The data were processed off-line by a computer (CED 1401 interface running the Spike2 software program). Unitary action potentials were converted into TTL (transistor-transistor logic) pulses through a dual time/amplitude window discriminator (DDIS-1, Back Electronics). The nerve spike events recorded were inspected off-line on an expanded time scale.
Cycle histograms giving the responses of the muscle spindle afferents to sinusoidal movements were produced. A trigger point was automatically displayed off-line on one channel at each movement cycle at a time corresponding to 110 ms before the maximum muscle shortening was reached. The cycle histograms were drawn up for the whole period of muscle stretching, i.e. during the 360 ms following the trigger point (see, e.g. Fig. 4). The bin width was 2 ms. The number of cycles cumulated in a cycle histogram varied from one unit to another; it corresponded to the maximum number of cycles imposed during the performance of the manoeuvre (between 60 and 110). There were 93 cycles, for example, if the subject required 47 s to perform the mental computation (see, e.g. Fig. 2). The same number of cycles was used to build up the corresponding cycle histogram characterising the unit's response during the control period immediately prior to the manoeuvre.
Figure 4. The magnitude of the mental computation-induced effects depended on the amplitude of the imposed sinusoidal stretching.
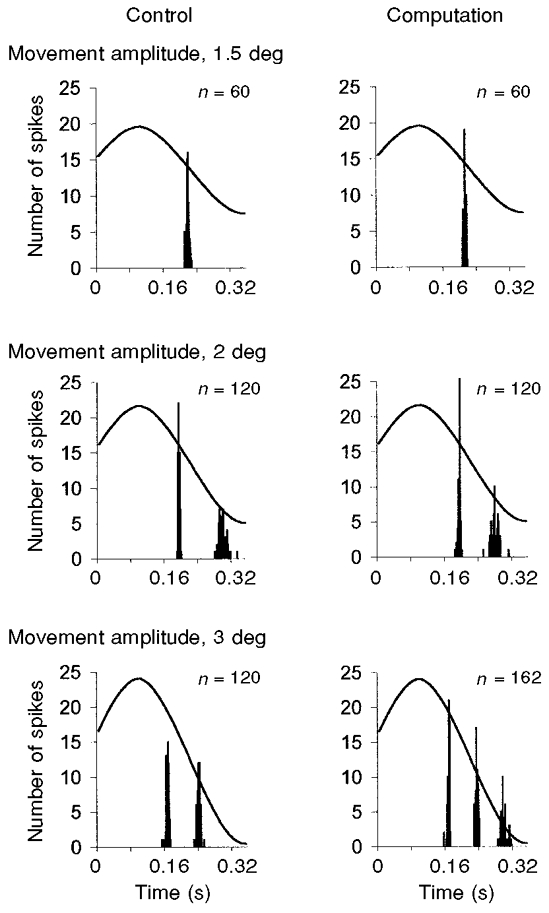
All the cycle histograms shown were drawn up on the basis of 60 cumulated movements. Although mental computation did not affect the unit's response to stretch movements with an amplitude of 1.5 deg (top), a decrease in the second spike latency could be detected with a 2 deg amplitude movement (difference, 14 ms; middle) and an increase in the number of spikes per cycle could be observed with an amplitude of 3 deg (bottom). n, total number of spikes.
RESULTS
Effects of carrying out mental computation on muscle spindle sensitivity to stretch
Recordings were carried out on 26 muscle spindle afferents (23 primary and 3 secondary afferents) belonging to muscles supplied by the peroneal nerve. Six further units were excluded because of variations in the photocell trace, which may have been due to involuntary changes in leg muscle tension, even if no muscle contraction was visible on the EMG recordings (see Methods). The sensory responses to the sinusoidal movements recorded during the control period consisted of one to four spikes per cycle, depending on the unit. The responses of nine primary muscle spindle afferents were not affected by mental computation. It should be noted that, for two of these afferents tested in the same subject, the electrodermal recording was almost flat during both the control and computation periods, whereas it increased during mental computations in all the other subjects. For these two afferents, the lack of effect may have been due to the subject's lack of involvement in the task, reflected by a wrong result being given in both cases.
The response of 14 Ia afferents was modified by mental computation. Eleven Ia fibres exhibited an enhanced sensitivity to stretch, reflected by an increase in the number of spikes per cycle, sometimes with a decrease in the response latency. The percentage increase in the number of spikes during mental computation in comparison with the control period involving the same number of movement cycles (between 60 and 110) varied from 10.5 to 46 % (mean, 26 %). For the remaining three fibres, the sensory spikes occurred earlier during the response to stretching imposed during mental computation than under completely relaxed conditions, so that the only effect of performing the computation was that it reduced the latency of the sensory response from 6 to 20 ms (mean, 13 ms).
Figure 2 shows an example of the computation-induced effects on a primary muscle spindle afferent belonging to the tibialis anterior muscle. Throughout the whole control period, during which the subject was completely relaxed, the unit responded to the imposed sinusoidal movements by producing two spikes per cycle. When mental computation was performed, the sensory response was transformed, as shown by the instantaneous frequency curve (Fig. 2A). These changes in the unit's response continued for 30 s after the subject had given the right response and was asked to go back to being as relaxed as possible; after this period the control response was restored. The expanded time scale recording in Fig. 2B shows that these changes observed during mental computation were due to the frequent occurrence of a third spike in the response per cycle. The increase in the unit's activity (46 %) was confirmed by analysing the firing pattern during the whole stretch period in all the movement cycles imposed during mental computation (93 cycles), compared with the control period, in terms of cycle histograms (Fig. 2C).
In addition to the increased number of spikes, the instantaneous discharge frequency was highly irregular during mental computation, whereas it was constant during the control period (see Fig. 2A). This great variability, observed in four of the affected units, could lead to a nearly decorrelated response. This was the case for the unit shown in Fig. 3. Although the response pattern of this unit (only one spike per cycle) was very stable during the control and post-computation periods, its firing rate was enhanced and highly irregular during mental computation (Fig. 3A). As shown by the raw data and cycle histograms (Fig. 3B and C), spikes occurred during the whole stretch movement and further spikes also occurred during the muscle shortening phases while the subject was performing mental computation. It should be noted that in one unit the variability of the response increased during the mental computation task but the number of spikes did not.
Figure 3. Example of a Ia response decorrelated with sinusoidal movements during the performance of mental computation.
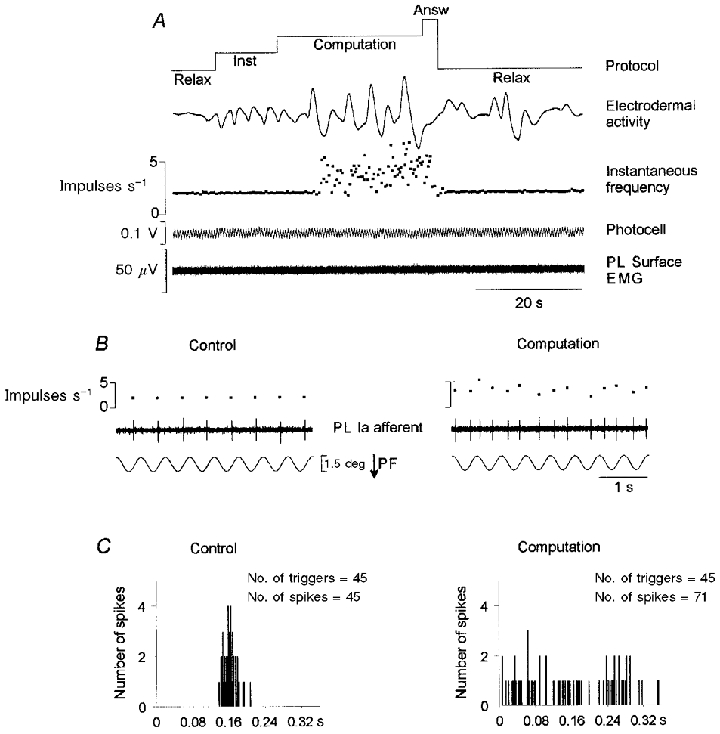
A-C, details as in legend to Fig. 2. Note that spikes could occur during the whole stretch period, as well as during the muscle-shortening phase, during the task. PL, peroneal lateral muscle.
One Ia afferent was recorded for an exceptionally long time (about 2 h), which enabled us to compare the effects of performing mental computation in relation to the amplitude of the sinusoidal movement. The experimental procedure used was exactly the same as that described above, but it was repeated three times with three imposed sinusoidal movement amplitudes (Fig. 4). Although the mental computation task had no effect on the response to a low-amplitude sinusoidal movement (1.5 deg), mental computation affected the primary afferent activity in the case of a higher stretch amplitude (2 deg) in that it reduced the latency of the second spike (difference, 14 ms), thus enhancing the unit's mean discharge frequency. The effects of performing mental computation were most obvious with a stretch amplitude of 3 deg, where the number of spikes per cycle increased relative to the control period. This means that by using a constant, fairly low-amplitude stimulus (i.e. 1.5 deg), we may have prevented even stronger and more widespread effects from occurring among the whole population of units.
The onset of the increase in the Ia sensory activity occurred either while the subject listened to the task instructions (5 units) or during the computation (6 units). In the latter case, the effect either occurred as early as the onset of computation or was delayed (see Fig. 3A).
The increase usually occurred gradually. The percentage increase in the number of spikes was lower in response to the task instructions than during the task. The increase in the sensitivity was also found to develop gradually while mental computations were being performed. The time course of the computation-induced increase in the Ia afferent response is illustrated by the example shown in Fig. 5. The unit shown is the same as that in Fig. 4. Although the unit produced two spikes per cycle in response to a 3 deg amplitude movement during the control period, a third spike sometimes occurred while the subject was being given the instructions, and this spike was usually present during the performance of the task (Fig. 5A). Note that the increase in the number of spikes was concomitant with a decrease in the latency of the second spike composing the unit's response (difference, 8 ms). The activity of this unit was analysed during two successive mental computations (Fig. 5B). Although the effect of mental computation was rather slight during the first computation, at the end of which the subject gave the wrong answer, it became stronger when the subject continued to perform the task and obtained the right answer (2nd computation); in this case, the occurrence of a third spike in the response was much more frequent.
Figure 5. Time course of the computation-induced increase in the Ia afferent response.
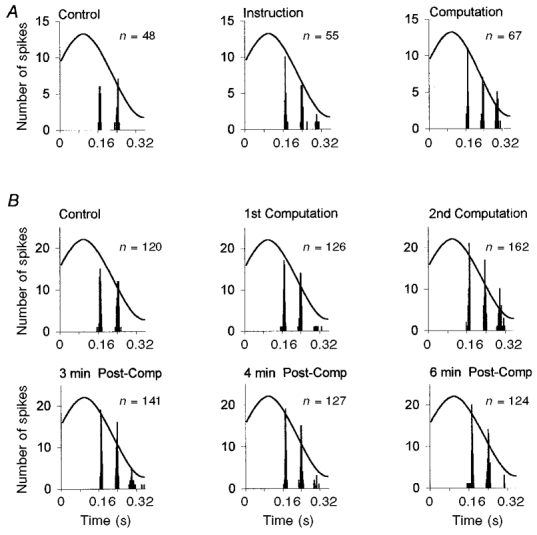
A, the 3 cycle histograms reflect the sensory response to 24 cumulated stretch movements while the subject was relaxed (left), listening to the computation instructions (middle), or performing mental computation (right). Although the two-spike response was stable during the control period (total number of spikes (n) = 48), a third spike occurred in some cycles while the subject was listening to the instructions (55 spikes) and especially during the actual computation (67 spikes). B, the activity of the same primary afferent was analysed during a different recording sequence. The upper 3 cycle histograms show the sensory response during 60 cumulated imposed stretch movements, during the control period and during the performance of 2 successive mental computations. The number of spikes gradually increased during the performance of the mental computation. The 3 lower cycle histograms show the sensory responses 3, 4 and 6 min after the subject had been asked to relax. The control response was gradually restored during the 6 min following the request.
As regards the recovery of the control sensory response, the computation-induced changes either stopped shortly after the subject had been asked to return to being as relaxed as possible (see Fig. 3), or continued despite these instructions (see Fig. 2A). Persistent post-computation effects of this kind were observed in seven of the primary endings, without any clear-cut correlation with the patterns of electrodermal activity. The control response was restored about 1 min after computation in three of these units. Three others underwent such post-effects for up to 2 or 3 min, but the length of recording did not enable us to determine the exact time of recovery. The enhanced activity could persist for up to 6 min (Fig. 5B). The sensitivity of this unit to stretch gradually decreased after the computation task, since the number of third spikes decreased. Within 6 min of carrying out the computation, the response became almost identical to the control response.
Since we previously observed that mental computation did not affect the responses of muscle spindle primary endings to ramp and hold movements (Ribot et al. 1986), movements of this kind were not used in most of the experiments in the present study. Such movements (3 deg, 5 deg s−1) were imposed, however, in the case of three units. In two of these, responses during mental computation differed from those in control conditions. As illustrated in Fig. 6, the change consisted of an increase in the variability of the instantaneous discharge frequency during muscle stretching, which was sometimes accompanied by sporadic spontaneous bursts of firing that were absent during the control period.
Figure 6. Effects of performing mental computation on the primary muscle spindle response to ramp and hold movements.
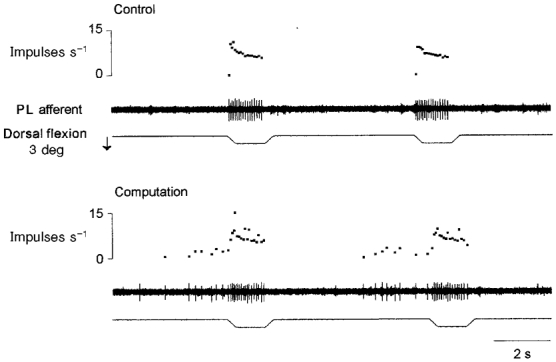
Each panel shows the movement passively imposed on the ankle (3 deg, 5 deg s−1; bottom trace), the Ia afferent activity (peroneal lateral afferent; middle trace) and the instantaneous frequency curve (top trace). Note that the unit's response to stretches was noisier (the variability of the discharge rate increased) when computation was being performed than during the control period. In addition, the sporadic spontaneous discharges observed during the mental computation were not present during the control period.
Lastly, the activity of the three secondary muscle spindle endings tested did not change while mental computations were being carried out.
Effects of the Jendrassik manoeuvre
When stable recordings were maintained (12 units), the subjects were asked to clench both fists (Jendrassik manoeuvre). A displacement in the photocell recording or the development of electromyographic activity in the receptor-bearing muscle led us to discard two of the units tested. Five primary and one secondary muscle spindle ending underwent no change. The firing rates of the remaining four primary muscle spindle afferents increased during sinusoidal stretches, and all these units were also sensitive to mental computation. In two of these units, a stronger effect was observed during fist clenching than during mental computation (Fig. 7). A very stable one spike per cycle activity was transformed into a two spike per cycle activity after a short period of irregular firing shortly after the instructions were given to the subject.
Figure 7. Example of a Ia afferent response to sinusoidal stretching during the performance of a Jendrassik manoeuvre.
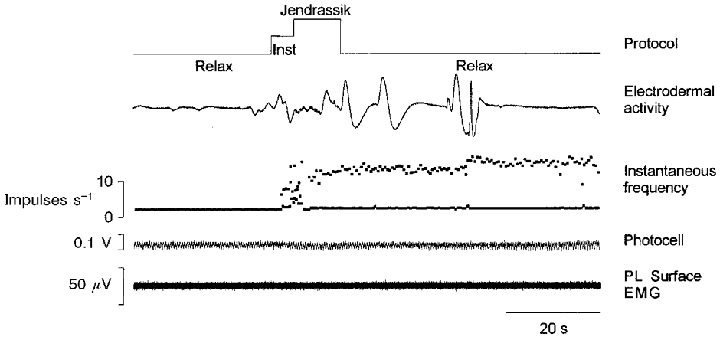
The primary afferent activity (PL afferent) during a whole sequence is illustrated by its instantaneous discharge frequency curve. Although the unit discharged with a constant one-spike response per cycle during the control period, a short period of irregular firing was induced soon after the instructions had been given to the subject, followed by a two-spike response that continued after the performance of the manoeuvre, when the subject had been told to relax. This post-manoeuvre increase in the sensitivity continued for as long as 2 min for this unit. Inst, instruction.
As in the case of mental computation, the increase in muscle spindle sensitivity sometimes lasted for several minutes after the end of fist clenching, and here again there were no obvious correlations with the electrodermal activity. The increased firing continued for as long as 2 min for the unit shown in Fig. 7.
Note that similar changes in the pattern of discharge were induced during each of the two reinforcement manoeuvres. For example, the decorrelated response previously described for the unit illustrated in Fig. 3 was also present during fist clenching.
DISCUSSION
The present study shows that an increase in the responsiveness of primary muscle spindle endings to movement occurred in human subjects performing reinforcement manoeuvres (computational tasks or fist clenching), compared with completely relaxed states. The results indicate that for about half of the tested primary afferents, the firing rate and/or the stretch sensitivity was affected during the task, i.e. either the number of spikes increased or the latency of the response decreased, or both. Furthermore, we sometimes observed an additional increase in the variability of the instantaneous discharge frequency, which in the most extreme cases caused the response to be almost decorrelated from the stimulus. Sometimes these effects took place as soon as the instructions were given to the subjects, and/or increased while they were performing mental computations, and/or continued for several minutes although the subjects were completely relaxed.
Independent fusimotor activity or α-γ-co-activation?
The main purpose of the present study was to explore the potential for the γ-drive to be activated independently of the α-drive. Fusimotor activation was inferred on the basis of an increased muscle spindle activity. It was therefore critical for the increase in sensory activity to be independent of any concomitant activation of the receptor-bearing muscle, bearing in mind that fusimotor effects can be detected for very low levels of increased muscle tension (Kakuda et al. 1996; Gandevia et al. 1997; Wilson et al. 1997; Kakuda & Nagaoka, 1998). Although it is impossible to be sure that a muscle is completely ‘silent’, we believe that the use of the photoelectric system, which could detect very small changes in leg muscle tension even when no muscle activity could be recorded by surface EMG, was an additional means of preventing misinterpretation. Since the sequences were selected on the basis of the absence of change in muscle tension while the subjects were performing the tasks, the present results support the idea that the γ-fusimotor system can be activated independently of the α-motoneuronal system. The present work confirms earlier observations made on activity patterns of single γ-efferents (Ribot et al. 1986) and indicates that changes in the γ-drive can be effectively observed at the level of muscle spindle activity.
Why have these effects on muscle spindle stretch sensitivity rarely been observed? First, one must establish that the subjects are in a completely relaxed state both physically and mentally in the control situation. Indeed, if the γ-system is already discharging because, e.g. the subjects are anxious or they are paying attention to the experimental procedure, or change occurs in the environment, any additional manoeuvre will not affect the γ-drive strongly enough to be detectable indirectly at the level of muscle spindle output. This may be the reason why Vallbo & Al Falahe (1990) reported that some Ia afferents only sometimes exhibited a slight increase in their response when subjects paid attention to an imposed movement in order to actively reproduce it afterwards, compared with when they paid no attention. It remains true, however, that the reinforcement manoeuvres did not enhance sensitivity in the whole population of Ia afferents in our study. We agree with Prochazka et al. (1992) that ‘the fusimotor set is not primarily determined by specific motor tasks and contexts, but rather by a subject's internal attitude to those tasks and contexts … one can identify stimuli which are likely to evoke a particular fusimotor set, but cannot be guaranteed to do so’.
Furthermore, based on animal data, one could have expected stronger effects to occur. For example, the dynamic γ-drive operating during tasks involving difficulty or novelty in the cat greatly increases the responses of primary endings to muscle displacement, in comparison with those recorded during routine movements (Prochazka et al. 1985). Consequently, small changes in the variability of the instantaneous discharge frequency during imposed ramp and hold stretches, such as those observed herein, did not retain our attention in previous experiments (Ribot et al. 1986). From this point of view, it should be noted that muscle spindle firing rates have always been found to be much lower in humans than in animals; this has always been intriguing for neuroscientists working in this field. Such differences may thus also be present in the influence of the fusimotor system on muscle spindle sensitivity. For example, the fusimotor-induced increase in human muscle spindle firing rate during isometric contractions (20 Hz, Wilson et al. 1997) is considerably less than that in awake cats (100–300 Hz, Prochazka et al. 1977).
Finally, a γ-drive independent of the α-drive may be more obvious at the level of the human lower limb muscles (see Aniss et al. 1990), since the human muscles in the distal arm, those most explored by microneurography, are richly supplied with β-innervation (see Kakuda et al. 1997).
The increased sensitivity of the primary muscle spindle afferents is due to an increased γ-drive
The changes in primary muscle spindle sensitivity to stretch that are induced in relaxed subjects by asking them to solve an arithmetic problem can be attributed to two mechanisms: increased fusimotor outflow (see review by Prochazka, 1996) or changes in muscle sympathetic activity (Passatore et al. 1985).
Emotional stress can change the colour and moisture of the skin, indicating that cutaneous sympathetic activity has increased. Responses of this kind were detected in our experiments by recording the subjects' electrodermal activity. Hence, one might be tempted to draw parallels between such responses and the increased muscle spindle stretch sensitivity. However, in contrast to the cutaneous sympathetic activity, the sympathetic outputs to the muscles undergo little change, and sometimes even decrease in contexts of this kind (Delius et al. 1972a, b).
In addition, the persistence of the effects observed (post-effects) seems to argue against a sympathetic origin. The increase in muscle spindle activity could last for a few minutes after the subjects had given the right answer, as long as they were still completely relaxed. In contrast, the increase in the spindle firing rates induced by sympathetic activation in anaesthetised and curarised animals stops at the offset of the stimulation (Passatore et al. 1985).
Furthermore, the increase in the variability of the instantaneous discharge frequency observed here in some units, sometimes leading to an apparently decorrelated spindle response, strongly suggests that this effect is of γ-origin (Inbar et al. 1979; Cordo et al. 1996). Animal recordings have shown, e.g. that muscle spindle afferent responses to sinusoidal stretches were much noisier before than after de-efferentation performed by cutting the cat ventral root (Bergenheim et al. 1995).
Lastly, it should be remembered that such an increase in the muscle spindle sensitivity to stretch in the subject at rest and performing manoeuvres similar to those used in the present study was also deduced by comparing the amplitude of reflexes elicited either by electrical nerve stimulation (H reflex) or by tendon percussion (T reflex). Indeed, Paillard (1959) demonstrated that T reflex amplitude, which depends on the level of the dynamic sensitivity of muscle spindle primary endings, is selectively increased without H reflex change during fist clenching and during mental computation.
The nature of the γ-drive involved is not clear at the moment. The increase in the response of the primary muscle spindle afferents to stretch and the absence of effect on the secondary endings seem to indicate a dynamicγ-drive; however, the additive spikes observed during the shortening phases in some primary afferents and the increase in the variability of the instantaneous discharge frequency indicate a staticγ-drive (Hulliger et al. 1977). Studies need to be conducted with different parameters of stimulation to characterise the nature of the γ-induced effects.
Functional significance
The present study supports the view that the γ-fusimotor system plays a role independent of the α-motoneurone system, associated with arousal and expectancy (Prochazka, 1989). Switching from a state of deep relaxation to an active state, either physical or mental, prepares the muscle spindles to better play their role in information and/or regulation of movement. In particular, the enhanced muscle spindle activity together with the noise introduced by the fusimotor drive will enable the receptors to extract more accurate information about movement, as suggested by both animal (Bergenheim et al. 1995) and human (Cordo et al. 1996) studies.
Acknowledgments
This work was supported by CNRS, MRES and INSERM grants.
References
- Aniss AM, Diener HC, Hore J, Burke D, Gandevia SC. Reflex activation of muscle spindles in human pretibial muscles during standing. Journal of Neurophysiology. 1990;64:671–679. doi: 10.1152/jn.1990.64.2.671. [DOI] [PubMed] [Google Scholar]
- Bergenheim M, Johansson H, Pedersen J. The role of the γ-system for improving information transmission in populations of Ia afferents. Neuroscience Research. 1995;23:207–215. doi: 10.1016/0168-0102(95)00941-l. [DOI] [PubMed] [Google Scholar]
- Burg D, Szumski AJ, Struppler A, Velho F. Afferent and efferent activation of human muscle receptors involved in reflex and voluntary contraction. Experimental Neurology. 1973;41:754–768. doi: 10.1016/0014-4886(73)90066-6. [DOI] [PubMed] [Google Scholar]
- Burg D, Szumski AJ, Struppler A, Velho F. Assessment of fusimotor contribution to reflex reinforcement in humans. Journal of Neurology Neurosurgery and Psychiatry. 1974;37:1012–1021. [Google Scholar]
- Burg D, Szumski AJ, Struppler A, Velho F. Observations on muscle receptor sensitivity in the human. Electromyography and Clinical Neurophysiology. 1975;15:15–28. [PubMed] [Google Scholar]
- Burg D, Szumski AJ, Struppler A, Velho F. Influence of a voluntary innervation on human muscle spindle sensitivity. In: Shahani M, editor. Motor Systems: Neurophysiology and Muscle Mechanisms. Amsterdam: Elsevier; 1976. pp. 95–110. [Google Scholar]
- Burke D, McKeon B, Skuse NF, Westerman RA. Anticipation and fusimotor activity in preparation for a voluntary contraction. The Journal of Physiology. 1980a;306:337–348. doi: 10.1113/jphysiol.1980.sp013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, McKeon B, Westerman RA. Induced changes in the thresholds for voluntary activation of human spindle endings. The Journal of Physiology. 1980b;302:171–181. doi: 10.1113/jphysiol.1980.sp013236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo P, Inglis JT, Verschueren S, Collins JJ, Merfeld DM, Rosenblum S, Buckley S, Moss F. Noise in human muscle spindles [letter] Nature. 1996;383:769–770. doi: 10.1038/383769a0. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiologica Scandinavica. 1972a;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiologica Scandinavica. 1972b;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D. Effect of training on voluntary activation of human fusimotor neurons. Journal of Neurophysiology. 1985;54:1422–1429. doi: 10.1152/jn.1985.54.6.1422. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Wilson LR, Cordo PJ, Burke D. Fusimotor reflexes in relaxed forearm muscles produced by cutaneous afferents from the human hand. The Journal of Physiology. 1994;479:499–508. doi: 10.1113/jphysiol.1994.sp020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Wilson LR, Inglis JT, Burke D. Mental rehearsal of motor tasks recruits α-motoneurones but fails to recruit human fusimotor neurones selectively. The Journal of Physiology. 1997;505:259–266. doi: 10.1111/j.1469-7793.1997.259bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Prochazka A, Taylor JL. Cerebellar ataxia and muscle spindle sensitivity. Journal of Neurophysiology. 1993;70:1853–1862. doi: 10.1152/jn.1993.70.5.1853. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Wallin G, Burke D, Löfstedt L. Effects of the Jendrassik manoeuvre on muscle spindle activity in man. Journal of Neurology Neurosurgery and Psychiatry. 1975;38:1143–1153. doi: 10.1136/jnnp.38.12.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Dürmüller N, Prochazka A, Trend P. Flexible fusimotor control of muscle spindle feedback during a variety of natural movements. Progress in Brain Research. 1989;80:87–101. doi: 10.1016/s0079-6123(08)62202-5. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Matthews PBC, Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. The Journal of Physiology. 1977;267:811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar G, Madrid J, Rudomin P. The influence of the gamma system on cross-correlated activity of Ia muscle spindles and its relation to information transmission. Neuroscience Letters. 1979;13:73–78. doi: 10.1016/0304-3940(79)90078-8. [DOI] [PubMed] [Google Scholar]
- Kakuda N, Nagaoka M. Dynamic response of human muscle spindle afferents to stretch during voluntary contraction. The Journal of Physiology. 1998;513:621–628. doi: 10.1111/j.1469-7793.1998.621bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N, Vallbo Å B, Wessberg J. Fusimotor and skeletomotor activities are increased with precision finger movement in man. The Journal of Physiology. 1996;492:921–929. doi: 10.1113/jphysiol.1996.sp021358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N, Wessberg J, Vallbo Å B. Is human muscle spindle afference dependent on perceived size of error in visual tracking? Experimental Brain Research. 1997;114:246–254. doi: 10.1007/pl00005633. [DOI] [PubMed] [Google Scholar]
- Ohman A, Esteves F, Flykt A, Soares JJF. Gateways to consciousness: Emotion, attention and electrodermal activity. In: Roy JC, Boucsein W, Fowles DC, Gruzelier JH, editors. Progress in Electrodermal Research. New York and London: Plenum Press; 1993. pp. 137–159. [Google Scholar]
- Paillard J. Functional organization of afferent innervation studied in man by monosynaptic testing. American Journal of Physical Medicine. 1959;38:239–247. [PubMed] [Google Scholar]
- Passatore M, Grassi C, Filippi GM. Sympathetically-induced development of tension in jaw muscles: the possible contraction of intrafusal muscles fibres. Pflügers Archiv. 1985;405:297–304. doi: 10.1007/BF00595681. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Progress in Neurobiology. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Sheperd T, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. section 12, part 1. [Google Scholar]
- Prochazka A, Gorassini M, Taylor J. Adaptive control of proprioception. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 129–136. [Google Scholar]
- Prochazka A, Hulliger M, Trend P, Dürmüller N. Dynamic and static fusimotor set in various behavioural contexts. In: Hnik P, Soukup T, Vejsada R, Zelena J, editors. Mechanoreceptors. New York: Plenum Press; 1988. pp. 417–430. [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, Appenteng K. ‘Fusimotor set’: new evidence for α-independent control of γ-motoneurones during movement in the awake cat. Brain Research. 1985;339:136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Westerman RA, Ziccone SP. Ia afferent activity during a variety of voluntary movements in the cat. The Journal of Physiology. 1977;268:423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot E, Roll J-P, Vedel J-P. Efferent discharges recorded from single skeletomotor and fusimotor fibres in man. The Journal of Physiology. 1986;375:251–268. doi: 10.1113/jphysiol.1986.sp016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J-P, Vedel J-P, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Experimental Brain Research. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B, Al Falahe NA. Human muscle spindle response in a motor learning task. The Journal of Physiology. 1990;421:553–568. doi: 10.1113/jphysiol.1990.sp017961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo Å B, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B, Hulliger M. Independence of skeletomotor and fusimotor activity in man? Brain Research. 1981;223:176–180. doi: 10.1016/0006-8993(81)90819-2. [DOI] [PubMed] [Google Scholar]
- Wilson LR, Gandevia SC, Burke D. Discharge of human muscle spindle afferents innervating ankle dorsiflexors during target isometric contractions. The Journal of Physiology. 1997;504:221–232. doi: 10.1111/j.1469-7793.1997.221bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


