Abstract
The cell volume of suspended C6 glioma cells and primary cultured rat astrocytes was measured at normothermia (37 °C), and at mild (32 °C) and moderate (27 °C) hypothermia by flow cytometry with electrical cell sizing.
Under control conditions (37 °C), C6 glioma cells had a volume of 809 ± 29 μm3. Moderate hypothermia (27 °C) led to rapid cell swelling, with a maximum volume of 113.1 ± 1.3 % of control being achieved after 50 min. After rewarming to 37 °C, cell volume recovered very slowly and incompletely (to 107.2 ± 0.4 % of control). Less severe hypothermia (32 °C) led to a smaller increase in cell volume (108.7 ± 0.5 % of control).
The maximal cell swelling response and the kinetics of swelling were similar in C6 glioma cells and primary cultured astrocytes.
Hypothermia-induced cell swelling was dependent on the presence of extracellular Na+ and was reduced by the Na+–H+ antiporter inhibitor EIPA.
The underlying mechanisms of hypothermia-induced cell swelling are an intracellular accumulation of Na+ by (1) differential effects of hypothermia on the membrane permeabilities of Na+ and K+ and (2) activation of the Na+–H+ antiporter by a shift of its activation curve to a more alkaline value.
The maintenance of a normal cell volume is indispensable for proper cell function and survival (Macknight, 1987). Under physiological conditions, the cell volume is kept constant within a narrow range by a variety of highly efficient and well characterised regulatory mechanisms, including the Na+–H+ antiporter (Rotin & Grinstein, 1989), the K+-Cl− cotransporter (Hallows & Knauf, 1994), the Na+-K+-2Cl− cotransporter (Tas et al. 1987) and the Cl−-HCO3− exchanger (Kimelberg et al. 1979) (for a review see Strange, 1994). However, during various pathological circumstances these mechanisms are overwhelmed, and alterations of the cell volume, most often cell swelling, are observed. One example of such a pathological condition is deep hypothermia of the brain. From experiments performed by Brendel and colleagues in the 1960s (Thauer & Brendel, 1962), it is known that deep brain hypothermia of less than 10°C induces massive, even fatal, swelling of the brain. This swelling was associated with the accumulation of Na+ in the brain parenchyma. However, additional pathophysiological details were unknown at that time as further studies at the molecular and cellular level were not performed.
Scientific interest in hypothermia resurfaced after it was shown that not only deep hypothermia (10–15°C), as has been known for a long time, but also mild hypothermia (∼32°C), affords neuroprotection after cerebral ischaemia and trauma (Morikawa et al. 1992). This finding has far reaching clinical consequences, since mild hypothermia has the advantage of being associated with fewer and less serious side effects than deep hypothermia (Ginsberg et al. 1992). However, it is not known whether, in addition to its neuroprotective effect, mild hypothermia also leads to swelling of brain tissue, as seen during deep hypothermia. Hypothermia-induced brain tissue swelling could partially mask positive effects of mild hypothermia on the injured brain. We have therefore investigated the effect of mild (32°C) to moderate (27°C) hypothermia on the cell volume of glial cells, as these cells appear to be specifically involved in the swelling in the brain after trauma and ischaemia (Gerschenfeld et al. 1959). The results of this study might be of even wider interest, since, most surprisingly, to our knowledge the effects of these levels of hypothermia on the volume homeostasis of mammalian cells in general have not yet been systematically studied.
METHODS
Cell culture
C6 glioma cells (American Tissue Culture Collection, Rockville, MA, USA) or primary cultured rat astrocytes were used in all experiments. Cells for primary culture were obtained from rat cortex prepared according to Frangakis & Kimelberg (1984). Briefly, 1- to 3-day-old Sprague-Dawley rat pups (Charles River, Kisslegg, Germany) were killed by decapitation following deep CO2 anaesthesia. This procedure is in accordance with the national guidelines of Upper Bavaria for the conduct of animal experiments. The brains were dissected from the meninges and blood vessels, minced, and incubated with dispase (3 mg ml−1; Boehringer Mannheim, Germany) at 37°C until complete dissociation of the tissue had occurred. The resulting cell suspension was washed, seeded into 250 ml culture flasks (Falcon, Oxnard, USA) and cultured under standard conditions (humidified room air, 5 % CO2, 37°C; bicarbonate-buffered Dulbecco's modified Eagle's medium (DMEM; 25 mm bicarbonate, pH 7.4; Seromed), supplemented with 10 % fetal calf serum, 100 i.u. ml−1 penicillin and 50 μg ml−1 streptomycin; Sigma). GFAP (glial fibrillary acidic protein) immunohistochemistry revealed the cell culture to consist of > 95 % astrocytes. For experiments, cells were harvested by trypsinisation (0.05 % trypsin and 0.02 % EGTA in PBS; Sigma) and resuspended in 15 ml serum-free bicarbonate-buffered DMEM (25 mm) at a density of 1 × 106-2 × 106 cells ml−1. Identical conditions to those described above were used for the culture, harvesting and resuspension of C6 glioma cells. The cell suspension (either C6 cells or astrocytes) was transferred to an acrylic glass chamber, described in detail previously by Kempski and co-workers (Kempski et al. 1983). pH and temperature were recorded continuously by electrodes (Mettler-Toledo, Steinbach, Germany) and adjusted to physiological values (pH 7.35 and 37°C) when necessary. PO2, PCO2 and the osmolarity of the culture medium were monitored every 20 min, using a blood gas analyser (Radiometer Copenhagen, Copenhagen, Denmark) and an osmometer (Osmomat 030, Gonotec, Berlin, Germany), respectively. Cell viability was assessed by Trypan Blue exclusion during the control period and every 30 min during hypothermia.
Measurement of cell volume
Cell volumes were determined by flow cytometry based on an advanced Coulter system (Metricell, MIT, Munich, Germany) utilising hydrodynamic focusing (Kachel et al. 1982). The system was calibrated using latex beads (Dynospheres, Praesel & Lorei, Hanau, Germany) of known diameter. The accuracy of the method enables the detection of cell volume changes of ∼2 %. For a single measurement, 1 × 104-2 × 104 cells were used. The design of the flow cytometer allows detection of cell volume independently from temperature changes of the cell suspension. This was confirmed by control measurements using latex beads (Dynospheres) suspended at 37 and 32°C. Another potential error may arise from the influence of the temperature on the actual shape of the cells during their passage through the Coulter aperture. Some cells, such as erythrocytes, are subject to a deformation of their normally spherical shape when they pass through the Coulter aperture, due to the hydro-mechanical shear stress at that location (Kachel et al. 1982). This longitudinal deformation, which would lead to an underestimation of the actual cell volume, can be quantified and used for the establishment of a correction factor. This factor can then be used to calculate the correct volume of the cells under investigation. Since hypothermia decreases the fluidity, and thus the stiffness, of the cell membrane (Lehninger, 1987), the deformation of the cells in the aperture might be impaired, thus leading to an erroneous increase in cell volume, as calculated by a correction factor derived at normothermia. Therefore, we attempted to establish correction factors for both hypo- and normothermic conditions by visualising the cells in the Coulter aperture using a novel flow cytometrical system developed in our laboratory (Wietzorrek et al. 1999). However, the measurements revealed that glial cells, in contrast to erythrocytes, did not change their shape either at 37 or at 27°C (data not shown). Consequently, alterations in membrane fluidity by hypothermia do not affect the system for cell volume quantification used in this study.
Experimental protocol
Values of the pH, PO2, PCO2, osmolarity and temperature of the medium as well as cell viability and volume under baseline conditions were obtained during a 30 min control period preceding the experiment. Preparations in which these parameters were unstable during the control period were rejected.
Hypothermia of the cell suspension was achieved by adjusting the thermostat of the water bath attached to the cell incubation chamber to the desired temperature level (27, 29.5 or 32°C). To facilitate rapid temperature equilibration, crushed ice was added to the water bath. Hypothermia of 27, 29.5 or 32°C was reached in less than 4 min without overshoot and maintained for 60 min within a range of 0.1°C.
In most experiments, the cell suspension was reheated and maintained at 37°C for another 30 min in order to study the recovery from hypothermia of the measured parameters.
In experiments in Na+- or Cl−-free medium, the ions were isotonically replaced by choline chloride (Plesnila et al. 1999) or sodium gluconate (Shrode & Putnam, 1994), respectively. After being harvested, the cells were washed twice with standard medium, then suspended in Na+- or Cl−-free medium and transferred to the incubation chamber. After adjustment of the temperature and pH of the medium to physiological values, the first measurement was performed.
For inhibition of the Na+–H+ antiporter, 50 μM 5-(N-ethyl-N-isopropyl)amiloride (EIPA) (Boyarsky et al. 1993) was added to the cell suspension 20 min prior to the induction of hypothermia.
Statistical analysis
The data are reported as means ± s.e.m. The findings of each group were analysed for significance against baseline (37°C) using Friedman's ANOVA on ranks followed by Dunn's post hoc test. For evaluation of significant differences between two groups, the Mann-Whitney rank sum test was used. For more than two groups the Kruskal-Wallis test and Mann-Whitney rank sum procedures with Bonferroni's correction were used. All calculations were carried out using SigmaStat 2.0 (Jandel Scientific, Erkrath, Germany), except for that of the Spearman correlation coefficient, which was calculated using Statistica 5.1 (StatSoft, Tulsa, USA). Differences between measurements or groups were considered significant at P < 0.05.
RESULTS
At 37°C, the volumes of C6 glioma cells and primary cultured astrocytes were 809 ± 29 and 1464 ± 19 μm3, respectively, and remained stable during the 30 min of the control phase. The volume of C6 glioma cells had increased significantly (103.9 ± 0.7 % of control), 10 min after the induction of hypothermia (32°C). At the end of the observation period (30 min), the volume of the C6 glioma cells was 107.0 ± 0.4 % of control (data not shown).
In order to analyse the kinetics of the cell volume increase and the relationship between the swelling response and the temperature decrease, experiments were carried out for longer periods (60 min), with cooling also to 29.5 and 27°C (Figs 1 and 2). At the end of the hypothermic period of most experiments, the ambient temperature was restored to 37°C in order to determine whether cell swelling induced by hypothermia was reversible. The kinetics of the hypothermia-induced cell swelling showed an initial (5–40 min) rapid rise and a levelling off thereafter (40–60 min) (Fig. 1). It could be described by a single time constant. The duration and velocity of the cell volume increase appeared to be dependent on the degree of hypothermia. At 32°C, cell volume rapidly increased to 107.9 ± 0.7 % during the first 20 min. Subsequently (20–60 min), a plateau was reached with only minimal further swelling, i.e. from 107.9 to 108.7 % of control. The volume kinetics of the glial cells suspended at 27°C had similar properties, although the rapid swelling phase was of longer duration. The maximum volume increase at 27°C was 113.1 ± 1.3 %, indicating a dose dependency of glial cell swelling on the degree of hypothermia. After restoration of the ambient temperature to 37°C, the cell volume recovered very slowly, compared with the initial swelling response immediately after the induction of hypothermia. Longer observation periods were necessary to obtain further cell volume recovery, as seen in experiments with a recovery period of 60 min (n = 2, data not shown). Cell viability was ≥ 95 % under control conditions and was not affected by hypothermia.
Figure 1. Volume response of C6 glioma cells at different levels of hypothermia.
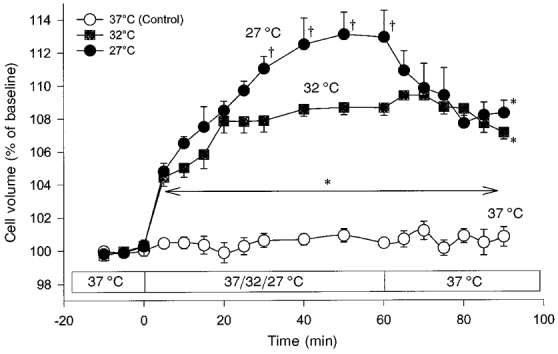
The cell volume of suspended cells was measured by flow cytometry with electrical cell sizing. The cell volume response of the cells is given as a percentage of the cell volume under baseline conditions, which was set at 100 % (means ± s.e.m.; n = 5). Under baseline conditions, the cells had an absolute volume of 809 ± 29 μm3. Whereas at 37 °C no increase in the cell volume occurred, a significant swelling response developed during hypothermia. Cell swelling due to hypothermia was only partially reversible upon restoration of the ambient temperature to 37 °C. *P < 0.05vs. 37 °C (Control); †P < 0.05vs. 32 °C.
Figure 2. Correlation of the cell volume response and the degree of hypothermia.
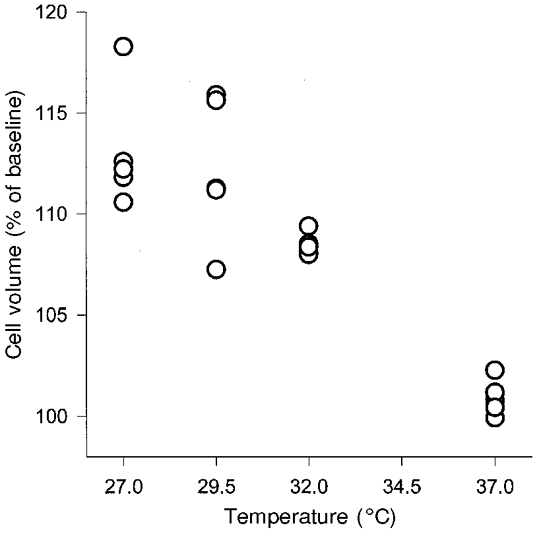
The maximal cell volume response (at 50 min) obtained from each experiment is plotted against the degree of hypothermia applied. The Spearman correlation coefficient (rs = −0.90, P < 0.001, n = 20) demonstrates the close relationship between the two parameters.
The extent of cell swelling at 50 min and the degree of hypothermia were subjected to a regression analysis for calculation of the Spearman correlation coefficient (Fig. 2). A highly significant (P < 0.001) coefficient of correlation (rs) of −0.90 was obtained. This reflects the close interdependency of cell swelling and the degree of hypothermia.
In order to test the validity of the findings based on C6 glioma cells, experiments were also performed on primary cultured astrocytes (Fig. 3). The degree of cell swelling of primary cultured astrocytes induced by hypothermia (32°C) was comparable to that observed in C6 glioma cells. At 50 min, an almost identical maximal cell volume was reached, i.e. 107.7 ± 1.2 % of control in astrocytes, compared with 107.9 ± 0.7 % in C6 glioma cells. The kinetics of the initial cell volume increase and the subsequent plateau phase were also very similar in both cell types under study.
Figure 3. Cell volume response of primary cultured astrocytes to hypothermia of 32 °C.
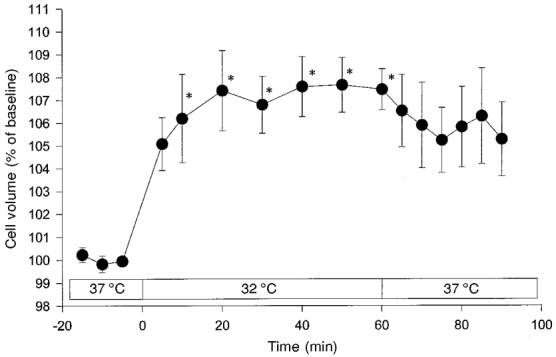
Exposure of primary cultured astrocytes to hypothermia (32 °C) also elicited cell swelling of comparable intensity to that observed in C6 glioma cells (cf. Fig. 1). As for the C6 glioma cells, cell volume recovered slowly following a return to 37 °C. Values are means ± s.e.m. (n = 5). *P < 0.05vs. baseline at 37 °C.
Since deep hypothermia-induced brain oedema is known to be associated with accumulation of Na+ in the brain parenchyma (Brendel et al. 1966), the influence of extracellular Na+ on cell swelling induced by mild hypothermia (32°C) was also studied. When Na+ in the suspension medium was isotonically replaced by choline, hypothermia-induced cell swelling was completely eliminated (Fig. 4).
Figure 4. Influence of Na+-free medium on hypothermia-induced cell swelling (32 °C).
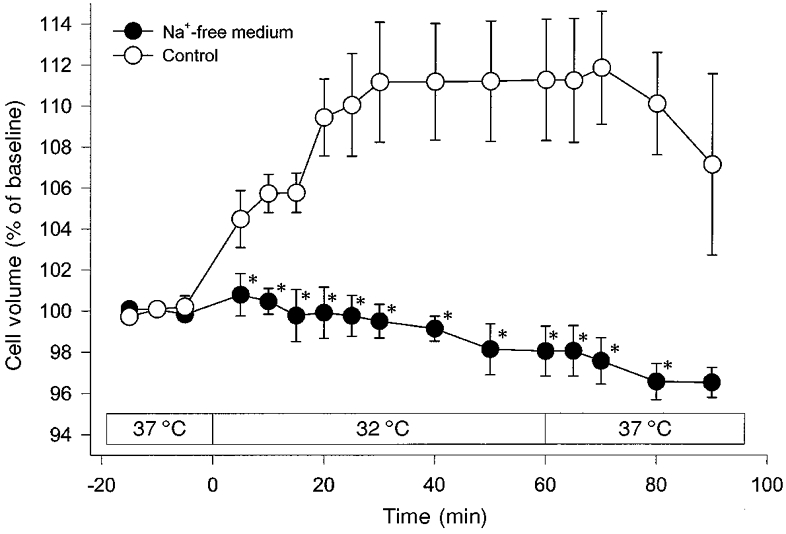
Omission of Na+ from the suspension medium abolished hypothermia-induced cell swelling in C6 glioma cells completely. Values are means ± s.e.m. (n = 5). *P < 0.008vs. Control.
For evaluation of the role of Cl− in cooling-induced cell swelling, the cells were incubated in Cl−-free medium. The swelling response to hypothermia was not significantly different in the presence or absence of extracellular Cl− (Fig. 5).
Figure 5. Influence of Cl−-free medium on hypothermia-induced cell swelling (32 °C).
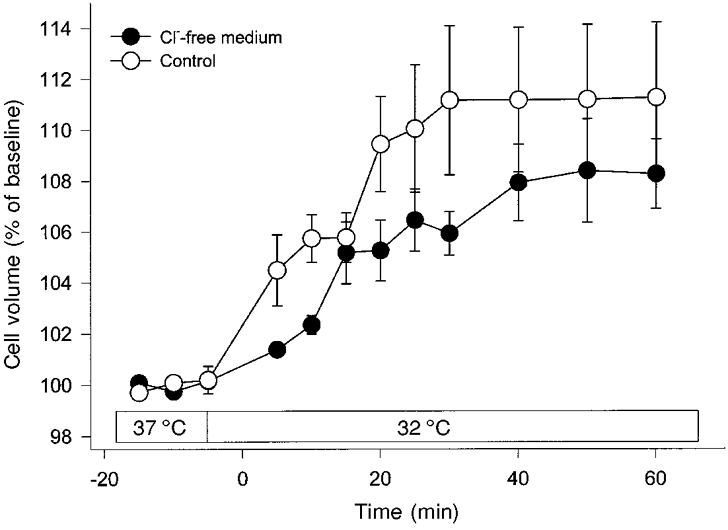
Omission of Cl− from the suspension medium had no significant influence on hypothermia-induced swelling in C6 glioma cells. Values are means ± s.e.m. (n = 5).
In order to test the hypothesis that the Na+–H+ antiporter is responsible for part of the hypothermia-induced Na+ influx, the transporter was inhibited by the addition of 50 μM EIPA to the cell suspension. Under these conditions, hypothermia-induced cell swelling was reduced by ∼45 %. Rewarming the medium to 37°C restored normal cellular volume (Fig. 6). This indicates that the Na+–H+ antiporter is active during hypothermia.
Figure 6. Inhibition of the Na+–H+ antiporter during hypothermia-induced cell swelling (32 °C).
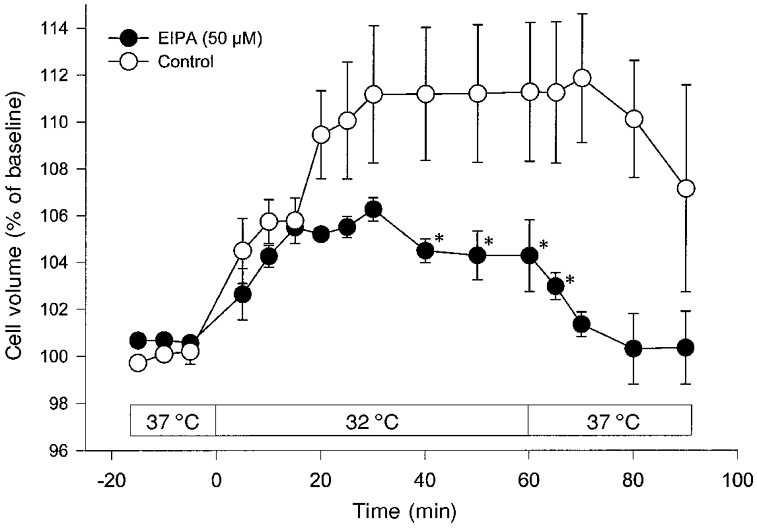
Inhibition of the Na+–H+ antiporter in C6 glioma cells by EIPA (50 μM) significantly reduced hypothermia-induced cell swelling by ≈45 %. Cell volume recovery during rewarming of the cells to 37 °C was complete after this treatment. Values are means ± s.e.m. (n = 5). *P < 0.016vs. Control.
DISCUSSION
The present results show that glial cells swell when the physiological ambient temperature is lowered by only 5°C. Obviously, mild hypothermia interferes with the cell volume control of these cells under otherwise physiological conditions. The analysis of underlying mechanisms of this phenomenon is of great interest, because the maintenance of a normal cell volume has high biological priority and is highly regulated under various conditions, as demonstrated by the regulatory volume decrease (RVD) or regulatory volume increase (RVI) phenomenon upon anisotonic exposure of suspended cells (Hoffmann & Simonsen, 1989).
The actual volume of a mammalian cell is determined by the osmolyte and water distribution across the cell membrane (assuming that water is freely diffusable across the cell membrane and follows the movement of osmolytes, water movements will not be mentioned in particular). For a rapid volume increase as described here, the main osmolytes involved are ions, e.g. Na+, Cl− and K+, which under steady-state conditions are distributed across the cell membrane as described by the Gibbs-Donnan relationship (Hallows & Knauf, 1994). The intracellular-extracellular ion distribution, however, is altered during a variety of cellular processes, such as by changes in the membrane potential or by activation of membrane transporters utilising the intracellular-extracellular Na+ gradient for translocation of solutes against their concentration gradient. To avoid a dependency of the cell volume on these basic cellular functions and to regulate the cell volume when the extracellular ion composition is altered, the cells are furnished with a variety of pumps, coupled transporters and channels for this purpose (see below). According to these considerations, hypothermia-induced cell swelling may result from either the direct inhibition of ion extrusion, e.g. by impairing the Na+,K+-ATPase, or the indirect stimulation of ion uptake, e.g. by activation of the Na+–H+ antiporter.
Our data clearly demonstrate that the main ion which accumulates in the cell during hypothermia-induced cell swelling is Na+ (Fig. 4). This observation is supported by the early work of Senft (1967) and Dipolo & Latorre (1972) who, respectively, investigated the effects of hypothermia on the membrane potential of lobster axons and barnacle muscle fibres. Both groups demonstrated that hypothermia leads to the depolarisation of cells as a result of an increased influx of Na+. Because ATP production is depressed during hypothermia (Singer & Bretschneider, 1990), the Na+,K+-ATPase was thought to be involved in this process. Therefore it was believed that sodium ions, which leak along their concentration gradient into the cytoplasm, are less effectively removed by the Na+,K+-ATPase during hypothermia. Consequently, the accumulation of Na+ would result in loss of membrane potential. However, the direct involvement of the Na+,K+-ATPase in hypothermia-induced depolarisation was ruled out by experiments with its inhibitor strophanthidin. This drug, which mimics the inhibitory effect of hypothermia on the Na+,K+-ATPase, did not influence the membrane potential either under resting conditions at 37°C or during hypothermia. The same is true for astrocytes. Inhibition of the Na+,K+-ATPase by ouabain did not alter cell volume, indicating a lack of a net osmolyte accumulation inside the cells. Further analysis revealed, however, that administration of ouabain to glial cells leads to an intracellular accumulation of Na+ which is counterbalanced by the efflux of K+ in a 1:1 ratio (Kempski et al. 1983). Finally, the hypothermia-induced Na+ influx was largely attributed to a differential effect of cooling on Na+ and K+ permeabilities. In fact, Na+ influx exceeded the efflux of K+ by a factor of 2.6 when the temperature was lowered by 13°C (Dipolo & Latorre, 1972). Therefore astrocytic cell swelling from hypothermia may be attributed to an imbalance between the passive movement of Na+ and K+ across the cell membrane. The differential effect of hypothermia on Na+ and K+ permeabilities also explains why the inhibition of the Na+,K+-ATPase (by ouabain or hypothermia) does not have any effect on cell volume or membrane potential. At 37°C, the inhibition of the Na+,K+-ATPase leads to the described movement of Na+ and K+ in a 1:1 ratio, resulting in no change in the cell volume. During hypothermia, when the Na+,K+-ATPase is also inhibited, the movement of Na+ and K+ no longer occurs in a 1:1 ratio, but rather in a manner that is in favour of an influx of Na+ (Dipolo & Latorre, 1972). The result is an intracellular accumulation of Na+ which leads to membrane depolarisation and cell swelling (Fig. 7).
Figure 7. Schematic drawing of the mechanisms responsible for hypothermia-induced cell swelling.
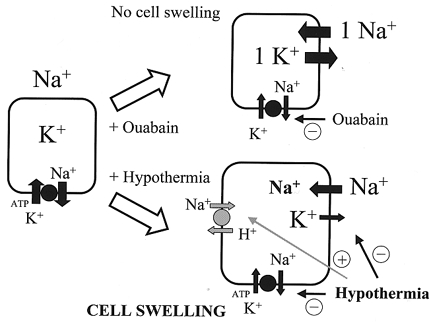
Hypothermia and ouabain both inhibit the Na+,K+-ATPase. In the case of ouabain, the cells maintain their cell volume and their membrane potential because the subsequent compensatory Na+ influx and K+ efflux occur in a 1:1 ratio. Hypothermia changes this ratio in favour of the influx of Na+. The evolving intracellular Na+ accumulation leads to cell swelling. In addition, hypothermia activates the Na+–H+ exchanger leading to additional influx of Na+.
Hypothermia may also influence various ion exchangers and cotransporters involved in cell volume regulation, including the Na+–H+ antiporter (Rotin & Grinstein, 1989), the K+-Cl− cotransporter (Hallows & Knauf, 1994), the Na+-K+-2Cl− cotransporter (Tas et al. 1987), as well as the Cl−-HCO3− exchanger (Kimelberg et al. 1979). In fact, the attenuation of hypothermia-induced cell swelling by the specific inhibition of the Na+–H+ antiporter (Fig. 6) attests to this. The underlying mechanisms of this activation are currently under discussion. However, on the basis of the present knowledge about the Na+–H+ antiporter, several possibilities are conceivable. In addition to its involvement in cell volume regulation, the Na+–H+ antiporter is also the most important membrane transporter for the regulation of the intracellular pH (pHi) (Kimelberg & Ricard, 1982). Therefore, it is plausible that by interfering with pHi regulation, hypothermia may also activate the Na+–H+ antiporter. In normothermia, baseline pHi regulation in astrocytes is not dependent on the Na+–H+ antiporter (Plesnila et al. 1999) and is predominantly maintained by a H+-ATPase (Volk et al. 1998) and the Na+-dependent Cl−-HCO3− exchanger (Shrode & Putnam, 1994). One possible explanation for the activation of the Na+–H+ antiporter is that hypothermia suppresses the active H+-ATPase leading to accumulation of protons in the cytoplasm with subsequent activation of the passive Na+–H+ antiporter. Consequently, protons are transported out of the cells and Na+ is accumulated intracellularly, thus increasing the osmotic load of the cell resulting in subsequent water influx and cell swelling. This scenario requires intracellular acidification that would lower the pH to the level necessary for the activation of the Na+–H+ antiporter. However, this is unlikely, considering that hypothermia induces intracellular alkalisation rather than acidification (Aickin & Thomas, 1977).
The most likely explanation for activation of the Na+–H+ antiporter by hypothermia is via a shift of its activation curve to more alkaline values, a mechanism that was demonstrated recently in a very elegant way for frog skeletal muscle (Marjanovic et al. 1998). As a result, the antiporter is already activated at normal pHi, inducing cytoplasmic alkalisation and an increase in the intracellular Na+ concentration. This would explain the hypothermia-induced intracellular alkalisation and cell swelling. In addition, activation of the Na+–H+ antiporter by an alkaline shift of its set point is, like hypothermia-induced cell swelling, self limiting, since the evolving alkalisation increasingly inhibits the exchanger.
Most interestingly, the inhibition of the Na+–H+ antiporter seems to affect only a quite late (> 15 min) phase of the cellular swelling response to hypothermia (Fig. 6). The reason for that observation is as yet unclear, but a possible explanation might be that the two mechanisms described to mediate hypothermia-induced cell swelling act in a parallel manner, but that their temporal and quantitative contributions to the swelling process are different. Therefore inhibition of the Na+–H+ antiporter, which might show delayed activation, due to conformational changes of the protein (Marjanovic et al. 1998), eliminates only one part of the swelling response to hypothermia, thus leaving only the first, immediately activated part visible (Fig. 6).
To our knowledge, the present study demonstrates for the first time that mild hypothermia causes a significant swelling of cells obtained from mammalian brain. The underlying mechanisms of hypothermia-induced cell swelling are an intracellular accumulation of Na+ by (1) differential effects of hypothermia on the membrane permeabilities of Na+ and K+ and (2) an activation of the Na+–H+ antiporter by a shift of its activation curve to a more alkaline value (Fig. 7).
The clinical relevance of these findings derived from suspended cells in vitro needs to be further clarified, in light of the potential hypothermia-induced swelling effect on normal, uninjured cells in the brain parenchyma of patients with increased intracranial pressure following traumatic or ischaemic cerebral injury.
Acknowledgments
The authors would like to thank H. Kleylein and E. Martin for excellent secretarial assistance and Elisabeth Pereira, Christina Fleet and Sunu Thomas for critical reading of the manuscript. This work was supported by a grant from Deutsche Forschungsgemeinschaft (DFG) to N.P. (Pl-249/2–2) and by Friedrich-Baur-Stiftung.
References
- Aickin CC, Thomas RC. Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. The Journal of Physiology. 1977;267:791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G, Ransom B, Schlue WR, Davis MB, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- Brendel W, Reulen HJ, Aigner P, Messmer K. Changes in electrolytes during deep hypothermia. IV. Cold edema of the brain in hybernators. Pflügers Archiv für die Gesamte Physiologie des Menschen und der Tiere. 1966;292:83–89. [PubMed] [Google Scholar]
- Dipolo R, Latorre R. Effect of temperature on membrane potential and ionic fluxes in intact and dialysed barnacle muscle fibres. The Journal of Physiology. 1972;225:255–273. doi: 10.1113/jphysiol.1972.sp009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangakis MV, Kimelberg HK. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochemical Research. 1984;9:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, Wald F, Zadunaisky JA, de Robertis EDP. Function of astroglia in the water-ion metabolism of the central nervous system: An electronmicroscope study. Neurology. 1959;9:412–424. doi: 10.1212/wnl.9.6.412. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovascular and Brain Metabolism Reviews. 1992;4:189–225. [PubMed] [Google Scholar]
- Hallows KR, Knauf PA. Principles of cell volume regulation. In: Strange K, editor. Cellular and Molecular Physiology of Cell Volume Regulation. Boca Raton, Ann Arbor, London, Tokyo: CRC Press; 1994. pp. 3–29. [Google Scholar]
- Hoffmann EK, Simonsen LO. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiological Reviews. 1989;69:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Kachel V, Glossner E, Schneider H. Advanced instrumentation for electrical cell sizing in flow. Progress in Clinical and Biological Research. 1982;102:83–93. [PubMed] [Google Scholar]
- Kempski O, Chaussy L, Gross U, Zimmer M, Baethmann A. Volume regulation and metabolism of suspended C6 glioma cells: an in vitro model to study cytotoxic brain edema. Brain Research. 1983;279:217–228. doi: 10.1016/0006-8993(83)90180-4. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Biddlecome S, Bourke RS. SITS-inhibitable Cl− transport and Na+-dependent H+ production in primary astroglial cultures. Brain Research. 1979;173:111–124. [PubMed] [Google Scholar]
- Kimelberg HK, Ricard C. Control of intracellular pH in primary astrocyte cultures by external Na+ Transactions of the American Society for Neurochemistry. 1982;13:112. [Google Scholar]
- Lehninger AL. Prinzipien der Biochemie. Berlin, New York: Walter de Gruyter; 1987. [Google Scholar]
- Macknight ADC. Volume maintenance in isoosmotic conditions. In: Kleinzeller A, editor. Current Topics in Membrane Transport. Cell Volume Control: Fundamental and Comparative Aspects in Animal Cells. New York: Academic Press; 1987. pp. 3–29. [Google Scholar]
- Marjanovic M, Elliott AC, Dawson MJ. The temperature dependence of intracellular pH in isolated frog skeletal muscle: lessons concerning the Na+–H+ exchanger. Journal of Membrane Biology. 1998;161:215–225. doi: 10.1007/s002329900328. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Ginsberg MD, Dietrich WD, Duncan RC, Kraydieh S, Globus MY, Busto R. The significance of brain temperature in focal cerebral ischemia: histopathological consequences of middle cerebral artery occlusion in the rat. Journal of Cerebral Blood Flow and Metabolism. 1992;12:380–389. doi: 10.1038/jcbfm.1992.55. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Haberstok J, Peters J, Chang RCC, Kölbl I, Baethmann A, Staub F. Effect of lactacidosis on cell volume and intracellular pH of astrocytes. Journal of Neurotrauma. 1999;16:831–841. doi: 10.1089/neu.1999.16.831. [DOI] [PubMed] [Google Scholar]
- Rotin D, Grinstein S. Impaired cell volume regulation in Na+–H+ exchange-deficient mutants. American Journal of Physiology. 1989;257:C1158–1165. doi: 10.1152/ajpcell.1989.257.6.C1158. [DOI] [PubMed] [Google Scholar]
- Senft JP. Effects of some inhibitors on the temperature-dependent component of resting potential in lobster axon. Journal of General Physiology. 1967;50:1835–1848. doi: 10.1085/jgp.50.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- Singer D, Bretschneider HJ. Metabolic reduction in hypothermia: pathophysiological problems and natural examples – Part 1. Thoracic and Cardiovascular Surgeon. 1990;38:205–211. doi: 10.1055/s-2007-1014020. [DOI] [PubMed] [Google Scholar]
- Strange K. Cellular and Molecular Physiology of Cell Volume Regulation. Boca Raton, Ann Arbor, London, Tokyo: CRC Press; 1994. [Google Scholar]
- Tas PW, Massa PT, Kress HG, Koschel K. Characterization of an Na+/K+/Cl− co-transport in primary cultures of rat astrocytes. Biochimica et Biophysica Acta. 1987;903:411–416. doi: 10.1016/0005-2736(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Thauer R, Brendel W. Hypothermie. Progress in Surgery. 1962;2:73–271. doi: 10.1159/000386265. [DOI] [PubMed] [Google Scholar]
- Volk C, Albert T, Kempski OS. A proton-translocating H+-ATPase is involved in C6 glial pH regulation. Biochimica et Biophysica Acta. 1998;1372:28–36. doi: 10.1016/s0005-2736(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Wietzorrek J, Plesnila N, Baethmann A, Kachel V. A new multiparameter flow cytometer: optical and electrical cell analysis in combination with video microscopy in flow. Cytometry. 1999;35:291–301. doi: 10.1002/(sici)1097-0320(19990401)35:4<291::aid-cyto1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]


