Abstract
The signalling pathway underlying histamine activation of non-selective cation channels was investigated in single equine tracheal myocytes. Application of histamine (100 μM) activated the transient calcium-activated chloride current (ICl(Ca)) and sustained, low amplitude non-selective cation current (ICat). The H1 receptor antagonist pyrilamine (10 μM) blocked activation of ICl(Ca) and ICat. Simultaneous application of histamine (100 μM) and caffeine (8 mm) during H1 receptor blockade activated ICl(Ca), but not ICat. Neither the H2 receptor antagonist cimetidine (20 μM) nor the H3 receptor antagonist thioperamide (20 μM) prevented activation of ICl(Ca) and ICat.
Intracellular dialysis of anti-Gαi/Gαo antibodies completely blocked activation of ICat by histamine, whereas ICl(Ca) was not affected. By contrast, anti-Gαq/Gα11 antibodies greatly inhibited ICl(Ca), but did not alter activation of ICat.
1-Oleoyl-2-acetyl-sn-glycerol (OAG, 20–100 μM) did not induce any current or affect currents activated by histamine or methacholine (mACH). Simultaneous application of OAG and caffeine activated ICl(Ca), but not ICat, indicating that a rise in [Ca2+]i and stimulation of diacylglycerol-sensitive protein kinase C (PKC) is not sufficient to activate ICat. The phospholipase C inhibitor U73122 (2 μM) blocked histamine activation of ICl(Ca) and ICat, but simultaneous exposure of myocytes to histamine and caffeine restored both ICl(Ca) and ICat in the presence of U73122.
Histamine and mACH activated currents with equivalent I–V relationships. The currents activated by these agonists were not additive; following activation of ICat by mACH, histamine failed to induce an additional membrane current. Similarly, mACH did not induce an additional current after full activation of ICat by histamine.
We conclude that H1 histamine receptors activate ICat through coupling to Gi/Go proteins. Activation of ICat also requires intracellular calcium release, mediated by H1 receptors coupling to Gq/G11 proteins. This coupling is analogous to the activation of ICat by co-stimulation of M2 and M3 receptors.
Histamine has long been recognized as an important inflammatory mediator in the lung and other tissues. Its release by mast cells in response to inflammatory stimuli contributes to acute bronchoconstriction and vasospasm, as well as other vascular effects such as vascular permeability and endothelium-dependent vasodilatation. The cellular mechanisms underlying histamine-induced contraction of smooth muscle are incompletely understood. Exposure of smooth muscle cells to histamine results in the stimulation of phospholipase C, generation of inositol 1,4,5-triphosphate, and subsequent Ca2+ release from the sarcoplasmic reticulum (Kotlikoff et al. 1987; Fujiwara et al. 1988; Komori et al. 1992; Janssen & Sims, 1993; Helliwell et al. 1994), as well as the activation of metabotropic, non-selective cation channels (Komori et al. 1992). The signalling mechanism for activation of the non-selective cation current (ICat) by histamine in smooth muscle cells is uncertain. Experiments by Komori et al. (1992) indicated an essential similarity between the ICat activated by histamine and acetylcholine. That is, both currents were blocked by exposure of myocytes to pertussis toxin, whereas calcium release (indicated by the calcium-activated potassium current) was unaffected by this exposure. Since the muscarinic ICat requires the simultaneous activation of pertussis-sensitive (M2) receptors and calcium release through phospholipase C (M3)-linked receptors (Inoue & Isenberg, 1990b,c; Pacaud & Bolton, 1991; Wang et al. 1997; Bolton & Zholos, 1997), we wondered whether the ICat activated by histamine receptor binding also involved the simultaneous stimulation of phospholipase C and a process coupled by pertussis-sensitive G proteins. Here we describe the receptor specificity and signalling process for histamine activation of non-selective cation channels in airway smooth muscle cells, and the relationship between histamine receptor and muscarinic receptor currents.
METHODS
Cell preparation
Single equine tracheal myocytes were isolated as described previously (Wang et al. 1997). Briefly, equine tracheas were obtained post mortem from horses with normal airways donated for euthanasia; euthanasia was performed by i.v. adminstration of sodium pentobarbital, under an approved protocol by the Animal Care and Use Committee at the University of Pennsylvania. The trachealis was dissected free of connective tissue and cartilage, and cut into pieces of about 2 cm × 2.5 cm. A cannula was inserted between the mucosa and smooth muscle and the tissue was superfused under pressure through the cannula at a rate of 1 ml min−1 with medium M199 (Gibco) containing: 300 U ml−1 collagenase (Type D, Boehringer Mannheim), 8 U ml−1 elastase (Worthington), and 5 mg soybean trypsin inhibitor (Type I, Sigma). After perfusion for 15–20 min the digested tissue was gently triturated with a large bore pipette to release single cells. The solution containing single cells was centrifuged at 500 g for 3 min, and the cells resuspended in medium M199 and stored at 4°C for up to 8 h.
Membrane current recording
Voltage clamp experiments were performed using the nystatin perforated and standard whole-cell patch clamp techniques, as previously described (Fleischmann et al. 1996). Patch pipettes were pulled from borosilicate capillary glass (TW 150F-4, WPI) using a Flaming/Brown micropipette puller (P-87, Shutter Instruments). For the perforated patch clamp experiments, pipettes filled with intracellular solution had a resistance of 3–5 MΩ and nystatin was included in the pipette solution at a final concentration of 200–300 mg ml−1. When electrical access was detected cells were clamped at a holding potential of −60 mV. Membrane capacitance and series resistance were continuously monitored and compensated, and experiments initiated following a decrease in the access resistance to below 40 MΩ (usually 6–10 min after gigaohm-seal formation). If a sudden drop in series resistance occurred during or after seal formation, experiments were terminated. In some experiments the standard whole-cell technique was used to dialyse cells, using 1–3 MΩ pipettes. Voltage-command protocols were generated by an EPC-9 amplifier (Heka Electronik) and data were recorded on a Macintosh computer and VHS tape for off-line analysis.
Fura-2 fluorescence measurement
Simultaneous measurements of intracellular Ca2+ concentration ([Ca2+]i) in voltage-clamped cells were made using single-excitation fluorescence measurements (Neher & Augustine, 1992), as previously described (Fleischmann et al. 1996). Cells were loaded with 2 μM fura-2 AM (Molecular Probes, Inc.) for 10 min at 35°C, and then transferred to the recording chamber; after a brief period to allow adhesion to the chamber, the cells were continuously perfused with pre-warmed bath solution. Recordings were made after 15 min of perfusion to washout extracellular fura-2 AM and to allow the de-esterification of the calcium indicator. Fura-2 was initially excited at 340 and 380 nm wavelengths (xenon 75 W arc lamp) at 2 Hz to calculate the initial [Ca2+]i, and subsequently continually exposed to 380 nm excitation light during the experiment. The emitted fluorescence above 510 nm was detected by a photomultiplier tube (Thorn EMI Electron Tubes). Values of Rmax (maximum 340/380 nm fluorescence ratio), Rmin (minimum 340/380 nm fluorescence ratio), and Sf380/Sb380 (ratio of 380 nm fluorescence in calcium-free and saturating calcium concentrations) were determined using 10 μM ionomycin and 10 mm calcium or 10 μM ionomycin and 10 mm EGTA for saturating and calcium-free conditions, respectively. For single wavelength determination, [Ca2+]i during experiments was calculated using the following equation:
where F (0) is the pre-stimulus 380 nm fluorescence, ΔF(t) the 380 nm fluorescence during experiments, [Ca2+]i,0 the initial [Ca2+]i calculated from the dual wavelength measurement; [Ca2+]i,t the time dependent [Ca2+]i during the experiment, KD the dissociation constant for calcium binding to fura-2, and K'D the adjusted value where K'D = KD(Rmax/Rmin). Fluorescence and electrophysiological signals were simultaneously recorded on VCR tape and then re-digitized using an A/D converter (TL-125, Scientific Solutions, USA) for analysis.
Solutions and reagents
The composition of the normal bath solution was (mm): 125 NaCl, 5 KCl, 1.8 CaCl2, 1 MgSO4, 10 Hepes, 10 glucose (pH 7.4). The fura-2 loading solution contained (mm): 115 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 15 glucose, 25 Hepes (pH 7.4), with 2 μM fura-2 AM. The pipette solution contained (mm): 130 CsCl, 5 MgCl2, 3 EGTA, 1 CaCl2, 10 Hepes (pH 7.3) for the nystatin perforated patch clamp recording. Nystatin (300 μg ml−1) was added to the intracellular solution just before experiments. For standard whole-cell recording measurements, the composition of pipette solution was (mm): 130 CsCl, 1.2 MgCl2, 0.1 EGTA, 1 ATP-Mg, 10 Hepes (pH 7.3). In some experiments, NaCl in the bath solution was partially replaced by caesium acetate to change the chloride equilibrium potential or TrisCl to shift the cation equilibrium potential. All external and internal solutions were filtered (0.2 μm Acrodisc, Gelman, USA) before use.
Caffeine, cimetidine, histamine, methacholine, nystatin, pyrilamine and thioperamide were purchased from Sigma. Antibodies directed against the α subunit of Gαi1/Gαi2 and Gαi3/Gα0, fura-2 AM, and 1-oleoyl-2-acetyl-sn-glycerol (OAG) were obtained from Calbiochem. Anti-Gαq/Gα11 antibody was from DuPont. Reageants were applied through a puffer pipette connected to a pressure ejection device (Picospritzer). The pipette was placed 60–80 μm from the cell of interest. The time of agonist application was recorded using a circuit that delivered an offset to the command voltage signal. In some experiments agonists were applied through two application pipettes mounted on a double holder and controlled by separate valves.
Data analysis
All values presented are expressed as means ± s.e.m. Student' paired t test was used to determine the significance of differences between observations within groups. One-way ANOVA (analysis of variance) for repeated measurements was used to determine the statistical significance of differences between groups.
RESULTS
Histamine activates both calcium-activated chloride currents and non-selective cation currents
The effect of histamine on [Ca2+]i and inward membrane currents was examined in freshly dispersed equine tracheal myocytes at 35°C using the perforated (nystatin) patch clamp method. Figure 1A shows a typical biphasic [Ca2+]i and membrane current response during the application of histamine (100 μM) to a cell voltage clamped at −60 mV and dialysed with caesium ions to block potassium currents. Sustained application of histamine for 40 s evoked a transient (rapidly inactivating), low noise current with a large amplitude, followed by a noisy sustained current with a small amplitude. In a group of six cells the mean peak amplitude of the transient current was 972 ± 79 pA and the half-time of current decay (t½) was 3.1 ± 0.4 s. The mean amplitude of the sustained inward current was 16 ± 3 pA (measured at the end of histamine application). The histamine-induced [Ca2+]i response consisted of an initial transient rise and a sustained [Ca2+]i elevation. The mean peak of [Ca2+]i was 796 ± 46 nM from a resting level of 149 ± 32 nM, and the sustained [Ca2+]i level was 244 ± 38 nM (measured at the end of histamine application). As shown in Fig. 1B, muscarinic stimulation produced equivalent current and [Ca2+]i responses. Previous studies have identified these currents as a transient calcium-activated chloride current (ICl(Ca)) and a sustained non-selective cation current (ICat) (Benham et al. 1985; Inoue & Isenberg, 1990b,c; Janssen & Sims, 1992; Fleischmann et al. 1997; Wang et al. 1997). As summarized in Fig. 1B, histamine (100 μM) and methacholine (50 μM) evoked transient and sustained current and [Ca2+]i responses of similar magnitude.
Figure 1. Histamine (His) and methacholine (mACH) induce similar biphasic [Ca2+]i and current responses.
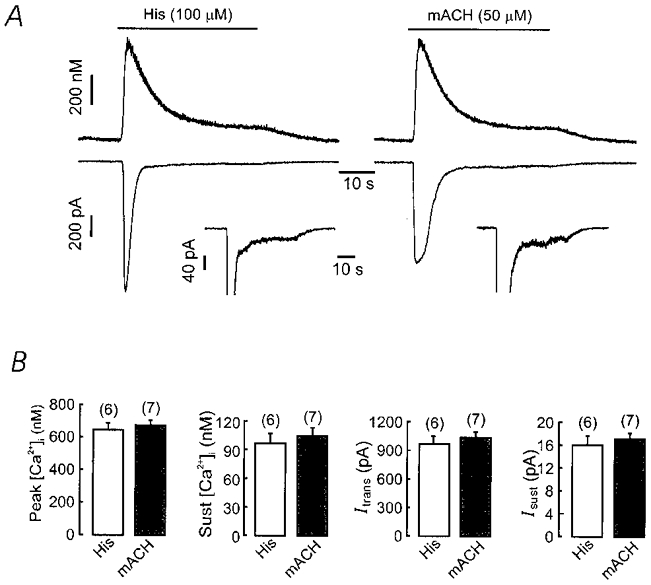
A, simultaneous recordings of [Ca2+]i (top) and membrane current (bottom) in separate equine trachealis cells exposed to histamine (left) or mACH (right). Both agonists induced a biphasic rise in [Ca2+]i and a biphasic inward current. The inset below the current trace shows the current at a magnified scale to illustrate the sustained inward current. Both cells were loaded with fura-2 AM and clamped at −60 mV using the perforated (nystatin) patch clamp technique. B, results from a series of experiments as in A. The magnitude of the transient and sustained increase in [Ca2+]i and inward currents was quite similar between histamine and methacholine. Numbers in parentheses indicate the number of cells tested.
We confirmed the ionic nature of the histamine-activated currents by selectivity experiments. As shown in Fig. 2B, replacing chloride ions with acetate ions (93.5 mm caesium acetate substituted for NaCl, calculated 34 mV positive shift in ECl) resulted in a shift in the reversal potential of the initial transient current to more positive values (Erev = 2 ± 1 and 28 ± 3 mV for NaCl and caesium acetate bath solutions, respectively; n = 7), without affecting the reversal potential of the sustained current (Erev = 2 ± 1 and 3 ± 3 mV, respectively; n = 6), indicating that the transient current is predominately carried by chloride ions, as previously described (Janssen & Sims, 1993). After 10 s of histamine application, the current reversal potential approached 0 mV in these experiments, indicating complete inactivation of the transient ICl(Ca), and sustained activation of a non-selective cation current, as previously reported for muscarinic currents (Fleischmann et al. 1997). Figure 2C plots the current reversal potential for ramp currents in the experiment shown in Fig. 2A and B as a function of time after histamine application. To confirm the ionic nature of the histamine-activated sustained current, we replaced Na+ with Tris (105 mm TrisCl substituted for 105 mm NaCl), and myocytes were clamped at −60 mV. Under these conditions, application of histamine still activated a biphasic current response. The transient ICl(Ca) reversed close to 0 mV as expected for a chloride current, whereas the reversal potential of the sustained current was markedly shifted to negative potentials. In a total of five similar experiments, the mean reversal potential of the sustained current was shifted from −2 ± 1 mV with NaCl to −32 ± 3 mV with TrisCl; the theoretical monovalent cation equilibrium potential in TrisCl would be −44 mV. The shift to more negative potentials when Tris is substituted for Na+ indicates that the current is predominately carried by monovalent cations. The finding that the shift was less negative than predicted for a current carried only by caesium and sodium ions could be explained if a significant permeability to divalent cations is assumed, as is observed with muscarinic activation of non-selective cation channels (Inoue & Isenberg, 1990a,b,c; Loirand et al. 1991; Pacaud & Bolton, 1991; Inoue & Kuriyama, 1993; Lee et al. 1993; Fleischmann et al. 1997; Wang et al. 1997; Kim et al. 1998a). Thus histamine activates a non-selective cation current with a low amplitude, that is sustained throughout agonist exposure.
Figure 2. Histamine activates calcium-activated chloride and non-selective cation currents.
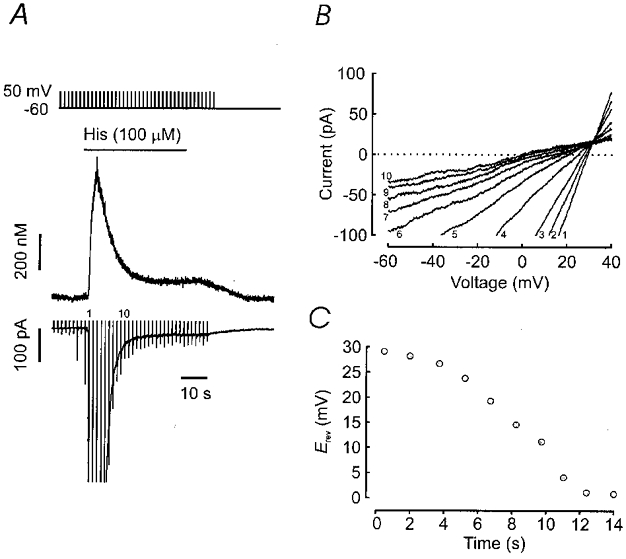
A, [Ca2+]i and inward current responses to histamine were recorded under conditions in which the chloride equilibrium potential was shifted to 34 mV by replacing extracellular NaCl (125 mm, Fig. 1) with NaCl and caesium acetate (31.5 and 93.5 mm, respectively). Ramp pulses from −60 to +50 mV for 150 ms were applied at 1.5 s intervals. Numbers indicate sequential ramp currents shown in B and C. B, difference currents for sequential ramp pulses during histamine application. At the peak of the transient current the instantaneous current reversal potential (Erev; intersection of current trace with dotted zero current line) was 29 mV, indicating a predominant chloride conductance. As the transient current decayed, the reversal potentials progressively approached 0 mV (monovalent cation reversal potential = 2 mV). C, a plot of reversal potentials obtained from the ramp pulses shown in Bversus the time after histamine activation of currents indicates a progressive decay of the chloride current.
Histamine activation of both ICl(Ca) and ICat is mediated by H1 receptors
Although histamine has been demonstrated to induce calcium release from the sarcoplasmic reticulum and subsequently to activate ICl(Ca) through H1 receptors in smooth muscle cells (Janssen & Sims, 1993; Wang & Large, 1993; Helliwell et al. 1994), the receptor subtype(s) mediating activation of ICat is unknown. In order to address this question experiments were performed in the presence of H1, H2, or H3 receptor antagonists. As shown in Fig. 3A, after pretreatment of myocytes with the H1 receptor antagonist pyrilamine (10 μM) for 5 min, application of histamine did not activate either the transient ICl(Ca) or sustained ICat. Similar results were observed in six myocytes. Since muscarinic activation of ICat in these cells requires stimulation of both M2 receptors and intracellular Ca2+ release mediated by M3 receptors (Wang et al. 1997), by analogy failure of histamine to activate ICat could be due to loss of the facilitory effect of Ca2+ release on channel opening associated with H1 receptor blockade. If so, simultaneous application of caffeine to trigger Ca2+ release would be expected to restore the histamine ICat, as is observed with the muscarinic ICat (Wang et al. 1997). As shown in Fig. 3B, however, simultaneous application of histamine and caffeine (8 mm) failed to activate ICat in the presence of pyrilamine, although the transient ICl(Ca) was observed in each cell tested (n = 6 cells). Moreover, selective H2 and H3 receptor antagonists failed to block histamine-activated currents. As shown in Fig. 3C, exposure of myocytes to the H2 antagonist cimetidine (20 μM) for 5 min did not alter the transient ICl(Ca) or the sustained ICat (n = 5), and Fig. 3D shows that the H3 receptor antagonist thioperamide (20 μM) was similarly without effect on either current (n = 4). These data indicate that stimulation of H1 receptors is sufficient to activate both ICl(Ca) and ICat.
Figure 3. H1 receptors mediate coupling to ICl(Ca) and ICat.
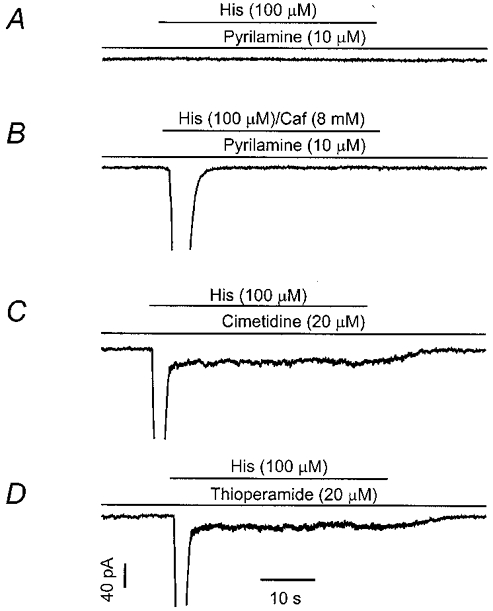
A, pretreatment of a myocyte with the selective H1 receptor antagonist pyrilamine (10 μM) for 5 min prevented the activation of ICl(Ca) and ICat. B, following incubation (5 min) with pyrilamine (10 μM), simultaneous application of histamine and caffeine evoked ICl(Ca), but not ICat.C, in a third cell, the H2 receptor antagonist cimetidine did not block histamine-activated ICl(Ca) or ICat. D, similarly, the H3 receptor antagonist thioperamide (20 μM, for 5 min) had no significant effect on histamine-activated ICl(Ca) or ICat. All cells were voltage clamped at −60 mV under perforated patch conditions.
Gi/G0 proteins mediate histamine activation of ICat
It has been reported that pertussis toxin (PTX) blocks histamine activation of ICat, but not ICl(Ca), in guinea-pig ileal myocytes (Komori et al. 1992). To extend these findings the activation of ICl(Ca) and ICat was examined in myocytes dialysed with antibodies directed against specific Gα proteins, which functionally disrupt G protein signalling (Komwatana et al. 1996; Wang et al. 1997; Wang & Kotlikoff, 1997; Kim et al. 1998b). Control experiments consisted of dialysis of cells for 5 min with the same intracellular solution without antibodies, followed by puffer application of histamine. As shown in Fig. 4A, under conditions of cell dialysis, histamine induced both ICl(Ca) and ICat equivalent to the currents observed in the permeabilized patch preparation. In five cells the mean amplitudes of ICl(Ca) and ICat were 1048 ± 74 and 14 ± 3 pA, respectively. As shown in Fig. 4B, however, application of histamine to cells dialysed with anti-Gαi1/Gαi2 antibodies (1:35) for 5 min activated ICl(Ca) to the same level as in control cells (1006 ± 55 pA; n = 6), but failed to activate ICat. Similarly, dialysis with anti-Gαi3/Gα0 antibodies (1:35; n = 5 cells) for 5 min also blocked sustained ICat without altering ICl(Ca) (Fig. 4C). Conversely dialysis with anti-Gqα/G11α antibodies (1:35) substantially attenuated ICl(Ca), without altering ICat (Fig. 4D). In six experiments the mean amplitude of ICl(Ca) was 326 ± 25 pA, compared to the control value of 1048 ± 74 pA (P < 0.05), whereas the amplitude of ICat in these cells was 15 ± 4 pA, compared to the control value of 14 ± 3 pA.
Figure 4. H1 receptors couple to ICat through Gi/Go antibodies.
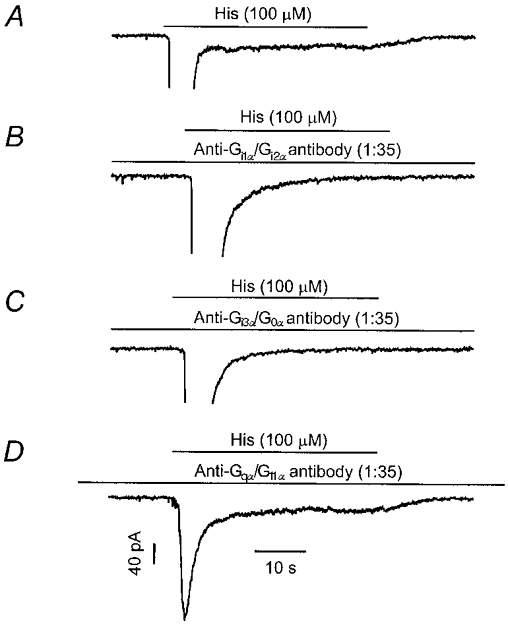
A, activation of ICl(Ca) (partially shown) and ICat (sustained inward current) in a control cell after dialysis for 5 min with control internal solution. B, intracellular dialysis of anti-Gαi1/Gαi2 (1:35) antibodies blocked histamine-activated sustained ICat, without affecting ICl(Ca) (partially shown). Recordings were made 5 min after break-in. C, intracellular dialysis of anti-Gαi3/Gα0 antibodies (1:35) also inhibited histamine activation of ICat, but not ICl(Ca). D, dialysis of anti-Gαq/Gα11 antibodies (1:35) for 5 min had no effect on ICat, but blunted ICl(Ca), indicating an inhibition of calcium release (peak ICl(Ca) was > 1000 pA for panels A–C). All cells were voltage clamped at −60 mV.
Diacylglycerol formation does not mediate activation of ICat by histamine
α-Adrenoceptor activation of ICat is mediated by diacylglycerol in rabbit portal vein myocytes (Helliwell & Large, 1997). As shown in Fig. 5A, application of the cell-permeable diacylglycerol analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG, 20 μM) failed to activate any membrane current, whereas subsequent application of histamine or mACH induced a typical current response (n = 6). OAG did not induce either transient ICl(Ca) or sustained ICat when applied at 100 μM. Since intracellular Ca2+ release greatly facilitates activation of ICat (Inoue & Isenberg, 1990b; Pacaud & Bolton, 1991; Komori et al. 1993; Bolton & Zholos, 1997; Fleischmann et al. 1997; Wang et al. 1997), we used caffeine to effect Ca2+ release during OAG application. Figure 5B shows an example of five similar experiments, in which simultaneous application of OAG (20 μM) and caffeine (8 mm) failed to evoke a sustained ICat, although the transient ICl(Ca) was observed in each cell tested. These data indicate that, unlike in portal vein smooth muscle cells, ICat activation is not mediated by OAG. Inhibition of phospholipase C activity, and thereby endogenous diacylglycerol formation, did not block histamine-activated ICat. As shown in Fig. 5C, after pretreatment of myocytes with U73122 (2 μM) for 5 min, application of histamine did not activate either transient ICl(Ca) or sustained ICat. Since inhibition of phospholipase C prevents histamine from generating inositol trisphosphate, we reasoned that the failure of histamine to activate ICat was probably due to inhibition of Ca2+ release in this experiment. Consistent with this interpretation, simultaneous application of histamine and caffeine evoked both ICl(Ca) and ICat in the presence of U73122 (Fig. 5C, right panel; n = 5). As shown in Fig. 5D, U73122 also blocked muscarinic activation of ICl(Ca) and ICat, which was restored by simultaneous application of mACH and caffeine. These findings indicate that diacylglycerol generation is not required for activation of ICat and suggest different signalling pathways for ICat linked to adrenoceptor stimulation rather than histamine receptor or muscarinic receptor stimulation.
Figure 5. The H1 receptor-ICat coupling pathway is independent of diacylglycerol.
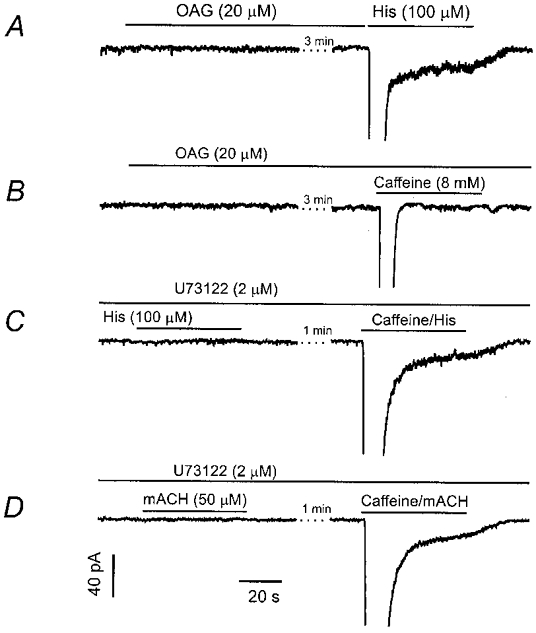
A, addition of OAG to the bath did not induce a current in a patch-clamped equine trachealis myocyte, whereas subsequent application of histamine induced ICl(Ca) and ICat. B, the failure of OAG to stimulate ICat did not result from a requirement for calcium facilitation, since subsequent exposure to caffeine evoked ICl(Ca), but not ICat. C, pre-treatment with the phospholipase C inhibitor U73122 (2 μM) for 5 min prevented activation of both ICl(Ca) and ICat by histamine. The inhibition of ICat resulted from the requirement for calcium facilitation, as shown by the reconstitution of both currents following release of calcium with caffeine. D, an increase in [Ca2+]i is also required for activation of ICat by methacholine. All four separate cells (A–D) were clamped at −60 mV using the perforated patch clamp method.
Histamine and methacholine activate equivalent non-selective cation currents
The I–V relationships of metabotropic non-selective cation currents in smooth muscle are variable. The muscarinic ICat in guinea-pig ileal myocytes displays a characteristic, time-dependent current decay (relaxation) at hyperpolarizing potentials (Benham et al. 1985; Inoue & Isenberg, 1990a; Zholos & Bolton, 1994, 1995), whereas the I–V relationship for adrenoceptor ICat in rabbit portal vein myocytes is S-shaped (Helliwell & Large, 1996, 1997). We next examined the I–V relationship of the histamine ICat and compared it to the muscarinic current. Following activation of ICat, 1 s step pulses from −60 mV to voltages of between −120 and 80 mV were imposed. Figure 6B shows the difference current obtained by subtracting the background current before application of histamine from the test current after full activation of sustained ICat. Current relaxations were not observed at hyperpolarizing or depolarizing potentials and the muscarinic ICat showed an equivalent I–V relationship (Fig. 6C; n = 5 for both conditions). Thus the metabotropic currents activated by histamine and methacholine display an equivalent I–V relationship, which differs from the acetylcholine-activated ICat in guinea-pig ileal smooth muscle.
Figure 6. The voltage dependence of ICat activated by histamine and methacholine is equivalent.
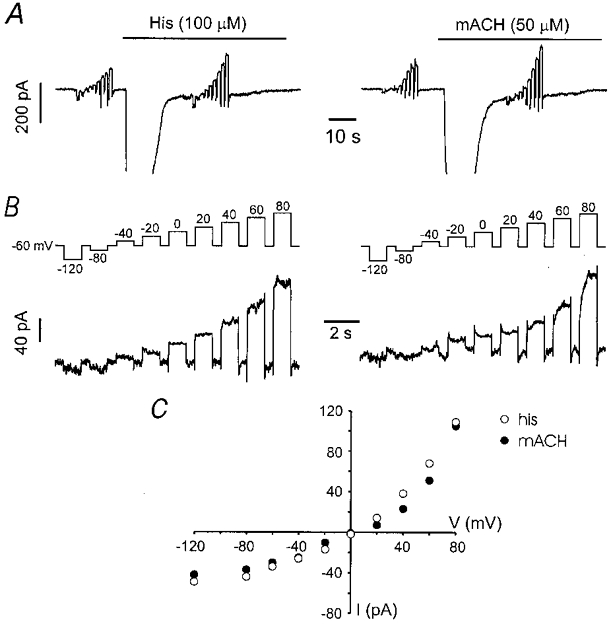
A, traces show activation of ICl(Ca) (partially shown) and ICat by histamine (left) and methacholine (right). Voltage pulses of 1 s duration from −60 mV to potentials from −120 to 80 mV were applied before and during activation of ICat. Note that ICl(Ca) had decayed completely before test potentials were imposed. Both cells were recorded using the perforated patch clamp method. B, difference currents were obtained from the voltage clamp protocol shown (top trace) by subtraction of the current before agonist application from the currents obtained during ICat activation. Note that current relaxations of either the histamine- or methacholine-induced ICat were not observed at hyperpolarizing potentials. C, the I–V relationship for the histamine- and methacholine-activated cation currents shown in above. The currents were taken from the end of the voltage step and are not normalized. Note the slight outward rectification of both currents.
To gain further insight as to whether histamine and muscarinic receptors are coupled to the same non-selective cation channels, we determined the additivity of the currents, or whether application of histamine could induce an additional ICat after full activation of the current by mACH. As shown in Fig. 7 (top trace), following activation of ICat by mACH (100 μM), cells were exposed to histamine (200 μM) in the continued presence of mACH by means of a second puffer pipette containing both agonists. Application of histamine (200 μM) did not induce any additional transient ICl(Ca) or sustained ICat in the continued presence of mACH (100 μM) in six such experiments. Moreover, when myocytes were first exposed to histamine (200 μM) and then additionally to mACH (100 μM) using the same protocol, mACH failed to evoke either ICl(Ca) or ICat (Fig. 7, bottom trace, n = 5). Thus the ICat coupled to histamine and muscarinic receptors do not summate, suggesting that the same channels are activated by either stimulus.
Figure 7. Histamine- and methacholine-activated currents are not additive.
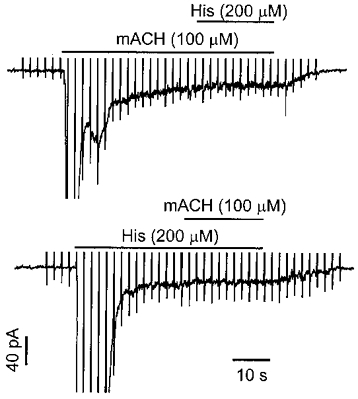
Following activation of ICat by mACH, histamine failed to induce an additional current (top). Similarly, methacholine was unable to evoke an additional current following activation of ICat by histamine (bottom). Both cells were clamped at −60 mV and recorded using the perforated patch clamp method.
DISCUSSION
We have shown that sustained application of histamine induces an initial transient increase in [Ca2+]i and associated ICl(Ca), as well as a sustained rise in [Ca2+]i, and a sustained metabotropic non-selective cation current. Muscarinic stimulation of tracheal myocytes results in an identical biphasic [Ca2+]i and current response, which is mediated by two distinct receptors (M2 and M3) and associated G proteins (Wang et al. 1997; Wang & Kotlikoff, 1997). Since histamine stimulates phospholipase C and Ca2+ release by H1 receptors coupled to PTX-insensitive Gq/G11 proteins (Raymond et al. 1991) and activation of ICat is known to be mediated by pertussis-sensitive G proteins (Komori et al. 1992), we wondered whether histamine activates ICat through the stimulation of multiple receptors or results from diverse G protein coupling of H1 receptors. Our data (Fig. 3) indicate that H1 receptors mediate both current responses, since: (1) the H1 receptor antagonist pyrilamine blocked both ICl(Ca) and ICat currents; (2) application of histamine and caffeine restored ICl(Ca), but not ICat in the continued presence of pyrilamine; and (3) H2 and H3 receptor antagonists (cimetidine and thioperamide, respectively) were without effect on either current. Moreover, similar to the muscarinic ICat, phospholipase C-mediated Ca2+ release is necessary but not sufficient for histamine activation of ICat. Thus phospholipase C inhibition by U73122 blocks activation of ICat (as well as ICl(Ca)), but histamine activation of the current is reconstituted by the simultaneous application of caffeine (Fig. 5). Phospholipase C activation by H1 receptors is not sufficient, however, since intracellular dialysis with anti-Gαi1/Gαi2 or anti-Gαi3/Gα0 antibodies blocked ICat without altering histamine activation of ICl(Ca) (Fig. 4). Conversely, anti-Gαq/Gα11 antibodies blunted ICl(Ca), but did not affect ICat (Fig. 4), indicating that activation of H1 receptors coupled to Gi/Go proteins is an upstream signalling requirement for the activation of ICat by histamine. This hypothesis is further supported by the finding that histamine activates ICat in the presence of dialysed anti-Gαq/Gα11 antibodies, since under these circumstances Gi/Go coupling is unaffected, and the anti-Gαq/Gα11 antibodies decrease, but do not completely block, Ca2+ release (Fig. 4; Wang et al. 1997), providing sufficient release to mediate full activation of the ICat current. These findings are consistent with a previous report that histamine activation of ICat is mediated by PTX-sensitive G proteins (Komori et al. 1992), and are quite similar to our previous findings for the muscarinic ICat (Wang et al. 1997). We have also shown that diacylglycerol formation is not required for activation of either histamine or muscarinic receptor ICat (Fig. 5), unlike the adrenoceptor ICat in vascular myocytes (Helliwell & Large, 1997), since exposure to OAG does not activate the current, even when simultaneous Ca2+ release is provoked. Taken together, these data indicate that the post-receptor signalling requirements for activation of ICat metabotropic cation channels in airway myocytes by histamine and acetylcholine are indistinguishable, and suggest that H1 receptors are physiologically coupled to both PTX-sensitive and PTX-insensitive G proteins.
The equivalent upstream signalling requirements for cation channels activated by histamine and acetylcholine suggests that both stimuli open the same metabotropic channels. Consistent with this interpretation, we have shown that the I–V relationship of the currents activated by both stimuli is equivalent (Fig. 6). Interestingly, the I–V relationship (Benham et al. 1985; Inoue & Isenberg, 1990a; Zholos & Bolton, 1994, 1995) and the requirement for intracellular Ca2+ release (Inoue & Isenberg, 1990a; Pacaud & Bolton, 1991; Zholos & Bolton, 1994, 1995) differ for the muscarinic ICat in ileal and tracheal myocytes, although the muscarinic receptor signalling requirements (simultaneous activation of M2 and M3 receptors) appear to be equivalent (Zholos & Bolton, 1997), suggesting that functionally distinct ICat channels are expressed in smooth muscle. A further indication that the cation channels activated by histamine and muscarinic receptor stimulation are equivalent was provided by evidence that these stimuli do not summate at the level of the ICat current. Thus additional current could not be obtained following full activation of ICat by the separate stimuli (Fig. 7).
In summary our data suggest that histamine and acetylcholine couple to the same channels. While definitive proof must await molecular identification of the target channels and channel-receptor heterologous expression studies, our data indicate that the separate stimuli activate channels with equivalent properties by identical post-receptor signalling processes.
Acknowledgments
We thank Mr Mario Brenes for excellent technical assistance. This work was supported by NIH grants HL 45239 and HL 41084 (M.I.K.).
References
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Zholos AV. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sciences. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- Fleischmann BK, Wang YX, Kotlikoff MI. Muscarinic activation and calcium permeation of nonselective cation currents in airway myocytes. American Journal of Physiology. 1997;272:C341–349. doi: 10.1152/ajpcell.1997.272.1.C341. [DOI] [PubMed] [Google Scholar]
- Fleischmann BK, Wang YX, Pring M, Kotlikoff MI. Voltage-dependent calcium currents and cytosolic calcium in equine airway myocytes. The Journal of Physiology. 1996;492:347–358. doi: 10.1113/jphysiol.1996.sp021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Sumimoto K, Itoh T, Suzuki H, Kuriyama H. Relaxing actions of procaterol, a beta 2-adrenoceptor stimulant, on smooth muscle cells of the dog trachea. British Journal of Pharmacology. 1988;93:199–209. doi: 10.1111/j.1476-5381.1988.tb11422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Dual effect of external Ca2+ on noradrenaline-activated cation current in rabbit portal vein smooth muscle cells. The Journal of Physiology. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. The Journal of Physiology. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Wang Q, Hogg RC, Large WA. Synergistic action of histamine and adenosine triphosphate on the response to noradrenaline in rabbit pulmonary artery smooth muscle cells. Pflügers Archiv. 1994;426:433–439. doi: 10.1007/BF00388307. [DOI] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. The Journal of Physiology. 1990a;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. The Journal of Physiology. 1990b;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. American Journal of Physiology. 1990c;258:C1173–1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- Inoue R, Kuriyama H. Dual regulation of cation-selective channels by muscarinic and α1-adrenergic receptors in the rabbit portal vein. The Journal of Physiology. 1993;465:427–448. doi: 10.1113/jphysiol.1993.sp019685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ, Sims SM. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal smooth muscle cells. The Journal of Physiology. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ, Sims SM. Histamine activates Cl− and K+ currents in guinea-pig tracheal myocytes: convergence with muscarinic signalling pathway. The Journal of Physiology. 1993;465:661–677. doi: 10.1113/jphysiol.1993.sp019699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Koh EM, Kang TM, Kim YC, So I, Isenberg G, Kim KW. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. The Journal of Physiology. 1998a;513:749–760. doi: 10.1111/j.1469-7793.1998.749ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Kim SJ, Sim JH, Cho CH, Juhnn YS, Suh SH, So I, Kim KW. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflügers Archiv. 1998b;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- Komori S, Kawai M, Pacaud P, Ohashi H, Bolton TB. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflügers Archiv. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- Komori S, Kawai M, Takewaki T, Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. The Journal of Physiology. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komwatana P, Dinudom A, Young JA, Cook DI. Cytosolic Na+ controls and epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proceedings of the National Academy of Sciences of the USA. 1996;93:8107–8111. doi: 10.1073/pnas.93.15.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff MI, Murray RK, Reynolds EE. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. American Journal of Physiology. 1987;253:C561–566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- Lee HK, Bayguinov O, Sanders KM. Role of nonselective cation current in muscarinic responses of canine colonic muscle. American Journal of Physiology. 1993;265:C1463–1471. doi: 10.1152/ajpcell.1993.265.6.C1463. [DOI] [PubMed] [Google Scholar]
- Loirand G, Pacaud P, Baron A, Mironneau C, Mironneau J. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. The Journal of Physiology. 1991;437:461–475. doi: 10.1113/jphysiol.1991.sp018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. The Journal of Physiology. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P, Bolton TB. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. The Journal of Physiology. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JR, Albers FJ, Middleton JP, Lefkowitz RJ, Caron MG, Obeid LM, Dennis VW. 5-HT1A and histamine H1 receptors in HeLa cells stimulate phosphoinositide hydrolysis and phosphate uptake via distinct G protein pools. Journal of Biological Chemistry. 1991;266:372–379. [PubMed] [Google Scholar]
- Wang Q, Large WA. Action of histamine on single smooth muscle cells dispersed from the rabbit pulmonary artery. The Journal of Physiology. 1993;468:125–139. doi: 10.1113/jphysiol.1993.sp019763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Fleischmann BK, Kotlikoff MI. M2 receptor activation of nonselective cation channels in smooth muscle cells: calcium and Gi/G(o) requirements. American Journal of Physiology. 1997;273:C500–508. doi: 10.1152/ajpcell.1997.273.2.C500. [DOI] [PubMed] [Google Scholar]
- Wang YX, Kotlikoff MI. Muscarinic signaling pathway for calcium release and calcium-activated chloride current in smooth muscle. American Journal of Physiology. 1997;273:C509–519. doi: 10.1152/ajpcell.1997.273.2.C509. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. The Journal of Physiology. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. The Journal of Physiology. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Bolton TB. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. British Journal of Pharmacology. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]


