Abstract
In response to a hyposmotic stress cells undergo a regulatory volume decrease (RVD) by losing osmotically active solutes and obliged water. During RVD, trout red cells lost taurine, K+ and Cl− but gained Na+ and Cl−. Over the full time course of RVD the chloride concentration in the cell water remained remarkably constant. Thus membrane potential and cell pH, which depends on the ratio of internal to external chloride concentration ([Cl−]i:[Cl−]o), remained fixed.
When cell volume decreases it is only possible to keep the chloride concentration in the cell water constant if an equal percentage of the cell chloride pool and of the cell water pool are lost simultaneously. Quantitative analysis of our data showed that this requirement was fulfilled because, over the full time course of RVD, cells lost osmotically active solutes with a constant stoichiometry: 1 Cl−:1 positive charge:2.35 taurine. Any change in taurine permeability, by modifying the stoichiometric relationship, would affect the amount of water lost and consequently cell chloride concentration.
Experiments carried out with different cations as substitutes for external Na+ suggest that the constancy of the chloride concentration is not finely tuned by some mechanism able to modulate the channel transport capacity, but results in part from the fact that the swelling-dependent channel constitutively possesses an adequately fixed relative permeability for cations and taurine. However, as a significant fraction of K+ and Cl− loss occurs via a KCl cotransporter, the contribution of the cotransport to the stochiometric relationship remains to be defined.
The large amount of taurine released during RVD (50 % of all solutes) was shown to be transported as an electroneutral zwitterion and not as an anion. How the channel can accommodate the zwitterionic form of taurine, which possesses a high electrical dipole, is considered.
Most cells respond to an acute increase in volume by releasing osmotically active cytoplasmic solutes via specific volume-dependent pathways, allowing the cell to undergo a regulatory volume decrease (RVD). The functional properties of these pathways as well as the mechanisms by which they are regulated are still poorly defined.
Fish red blood cells have proved to be a useful model for studying these pathways. They possess two swelling-sensitive transport systems, a KCl cotransporter and a 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS)-sensitive, broadly selective channel (Kirk et al. 1992; Bursell & Kirk, 1996), and they respond to volume increase by releasing three different osmotically active solutes, K+, Cl− and taurine (Garcia-Romeu et al. 1991). A volume increase can be induced either by suspending cells in a hypotonic saline or by promoting an uptake of salts and osmotically obliged water. Curiously, for swelling of similar magnitudes, the trout red cell adopts different regulatory patterns depending on how its volume has been altered (Motais et al. 1991). This observation has been explained by the fact that the KCl cotransporter and the channel are activated by different and specific stimuli: the cotransporter is activated by cell volume increase but repressed by a decrease in cell ionic strength; conversely, the channel is activated by a decrease in cell ionic strength but appears to be insensitive to cell volume increase (Guizouarn & Motais, 1999). The significance of such a complex regulatory pattern remains unclear. However, it becomes physiologically relevant if we consider that changes in cell solute concentration, occurring during RVD, are not expected to drastically alter intracellular pH, cell membrane potential, enzyme activities or other cell processes.
When the volume increase is due to a net uptake of salts and obliged water (e.g. after hormonal stimulation of Na+–H+ exchange), resulting in an increase in cell electrolyte concentration, it makes sense that, to recover volume and a normal electrolyte content, the cell specifically extrudes excess salts and avoids using the organic compound taurine as an osmoregulatory solute. Indeed, it has been observed that in such a situation, termed isosmotic swelling, RVD occurs exclusively by a KCl loss mediated by the KCl cotransport (Borgese et al. 1987; Motais et al. 1991).
Conversely, when swelling is due to an entry of water not accompanied with salts, as when cells are facing an hypotonic medium, a dilution of cell electrolytes occurs. Then, at first sight, it could be considered that the best way to recover cell volume and electrolyte content is to extrude excess water by the loss of the organic compound taurine and to avoid the use of electrolytes, already diluted, as osmoregulatory solutes. However, that strategy would lead to a large alteration of the [Cl−]i:[Cl−]o ratio: after removal of excess water, the cell will recover its volume and the concentration of electrolytes in the cell, mainly [Cl−]i, will be the concentration of cell electrolytes before swelling; at the same time the external chloride in the hypotonic saline, [Cl−]o, will remain diluted. Thus the ratio [Cl−]i:[Cl−]o will be greater after RVD than before and for a dilution of one-third of the external medium, the membrane potential would be shifted from about −22 to −11 mV and the intracellular pH from 7.40 to 7.60. Volume recovery also cannot be obtained by a loss of KCl exclusively; this would induce a large decrease in the intracellular chloride pool and would greatly alter the [Cl−]i:[Cl−]o ratio, but in this case promoting hyperpolarisation and acidification of the cell. Consequently, in response to hypotonic swelling, it makes sense that the cell loses both taurine and electrolytes, as experimentally observed (Garcia-Romeu et al. 1991; Motais et al. 1991; Guizouarn & Motais, 1999). Clearly the movements of Cl− and taurine have to be co-ordinated if variations in membrane potential and cell pH are to be limited. It is noteworthy that the Cl− loss is necessarily linked with a loss of cation (K+). Furthermore, in such hypotonic swelling, the loss of KCl occurs not exclusively through the KCl cotransporter as proposed previously (Guizouarn et al. 1993) but via both the KCl cotransporter and the swelling-activated, DIDS-sensitive channel (Bursell & Kirk, 1996; Lewis et al. 1996). Thus co-ordination between Cl− and taurine permeability would imply a complex regulation between the different pathways.
The purpose of the present study was therefore to follow the chloride concentration in cell water as a function of time during RVD and to analyse whether some co-ordination between Cl− and taurine permeability exists. Moreover, at physiological pH, taurine is present both as an anion and as a zwitterion (e.g. an electroneutral but strongly polarised molecule). The osmotic change must be close to electrogenically neutral. Therefore we also studied what form(s) of taurine was transferred across the cell membrane. Experiments were carried out by measuring both cell water content and cell solute content as a function of time during RVD. We provide evidence that (1) taurine played a pivotal role in maintaining constant cell chloride concentration and consequently membrane potential and cell pH and (2) taurine moved as a zwitterion via the DIDS-sensitive channel.
METHODS
Cell preparation
Rainbow trout (Onchorhynchus mychiss; 200–250 g) were obtained from a commercial hatchery and kept for 1 week in the laboratory in tanks provided with running tap water (15°C). They were killed by a sharp blow to the head and blood was obtained by caudal venipuncture using heparinised syringes. This procedure is in accordance with the European Union Animal Welfare Committee. The red blood cells were washed four times in standard saline solution. They were then suspended at 20 % haematocrit and incubated overnight at 4°C with 5 mm glucose, to ensure that they reached a steady state with respect to ion and water content before experimentation.
Solutions
The basic solution used throughout these experiments contained (mm): 145 NaCl, 4 KCl, 5 CaCl2, 1 MgSO4, 15 N-[2-hydroxyethyl]piperazine-N'-[3-propane-sulphonic acid] (EPPS), pH 7.85, 320 mosmol (kg H2O)−1. In some experiments Na+ was replaced by N-methyl-D-glucamine (NMDG), potassium, choline or tetramethylammonium. All solutions contained ouabain to a final concentration of 10−4 M.
Experimental protocol
A sudden decrease in osmolality was induced by adding deoxygenated buffered water (15 mm EPPS, pH 7.85) to the red cell suspension at time zero. All the experiments were performed in solution flushed with N2 and under a N2 atmosphere since it has been demonstrated that oxygenation of cells per se activates a Cl−-dependent K+ pathway (Borgese et al. 1991; Nielsen et al. 1992).
Determination of water content
Just before swelling and at various time intervals afterwards, samples of cell suspension were poured into three nylon tubes and centrifuged for 10 min at 30 000 g in a refrigerated centrifuge. The red cell pellet was separated from the supernatant by slicing the tube with a razor blade. It was extracted with a close fitting plastic rod onto a piece of weighed aluminium foil and after being weighed wet, the pellet was dried to a constant weight for 10 h at 80°C and reweighed. Cell water content is expressed as grams of water per gram of dry cell solids (g (g dcs)−1). The extracellular space was measured after a very short exposure of cells to 22Na+ (45 s), and a correction of 3.5 % was applied for this to all calculations.
Ion content and concentration
The dry cells were suspended in 5 ml distilled water overnight. Perchloric acid (100 μl of 70 % v/v) was then added to the suspension. After centrifugation at 30 000 g for 10 min the clear supernatant was saved for analysis of cations, Cl− and amino acids. Measurements of ions and amino acids were made as previously described (Garcia-Romeu et al. 1991). A trapping correction of 3.5 % was routinely applied to the final calculation. Ion contents were expressed as micromoles per gram of dry cell solids (μmol (g dcs)−1). Ion concentrations in cell water were calculated from ion contents and cell water contents and expressed as millimoles per litre of cell water.
RESULTS
Characteristics of taurine loss when hypotonic swelling is induced in physiological saline
Figure 1 presents the mean results of 21 experiments showing the effect of hypotonic shock on the water and solute content of ouabain-treated red blood cells. At time zero the isotonic saline (320 mosmol (kg H2O)−1) was made hypotonic (215 mosmol (kg H2O)−1) by addition of buffered water (see Methods). Figure 1A shows that after a rapid osmotic swelling to 136 % of the initial cell volume, the volume of the cells began to reduce; within 1 h the cell volume returned to 116 % of its initial value.
Figure 1. Changes in cell content of trout erythrocytes undergoing RVD.
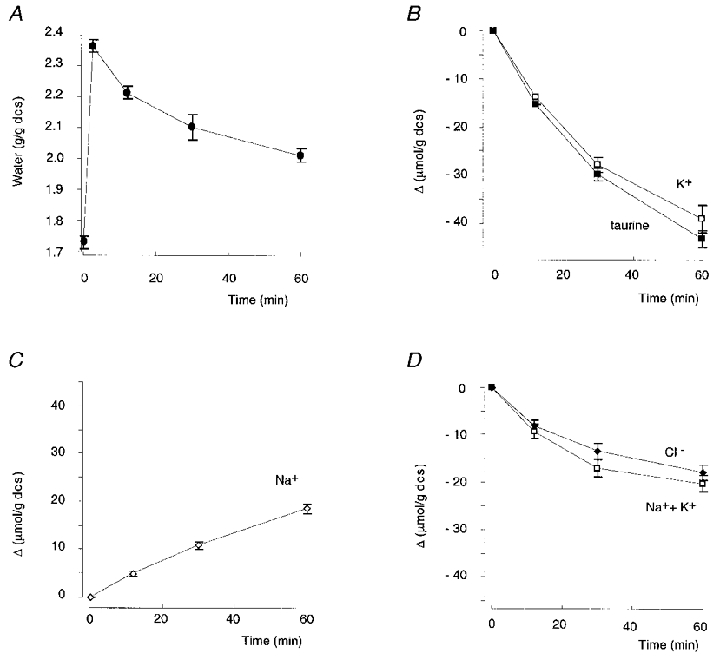
A-D, changes in water, K+, Na+, Cl− and taurine content (expressed per gram of dry cell solids) of trout erythrocytes undergoing regulatory volume decrease after hypotonic swelling (from 320 to 215 mosmol (kg H2O)−1) in the presence of 10−4 M ouabain. D shows comparative evolution of Cl− and Na++ K+ content. Mean values of 21 experiments ± s.e.m. The initial intracellular concentrations were (mm): K+, 156.6 ± 1.7; Cl−, 66.4 ± 2.3; Na+, 7.1 ± 0.9; taurine, 52.9 ± 2.0.
The regulatory volume decrease (RVD) is dependent on a net loss of osmotically active cellular solutes which drag water out. As previously shown (Guizouarn & Motais, 1999), Na+, K+, Cl− and taurine account for up to 90 % of trout erythrocyte osmolality, with the remaining osmolality due to the presence of greatly or totally impermeant solutes such as ATP, ADP, PO43− and proteins (mainly haemoglobin). Thus RVD of trout erythrocytes is expected to be achieved only by the loss of K+, Cl− and taurine down their electrochemical gradients. Consequently, if only these three solutes are involved in RVD: (1) the cell water calculated to be dragged by the measured net loss of K+, Cl− and taurine must correspond to the measured loss of water and (2) the amount of positive charge leaving the cell (i.e. K+) must be electrically balanced by an equivalent amount of negative charge, i.e. either only Cl− if taurine is lost as the electrically neutral zwitterion or the sum (Cl−+ taurine) if taurine is lost as an anion.
However, the RVD of trout erythrocytes following an hypotonic shock is more complex (Garcia-Romeu et al. 1991). As expected the cells lost K+, Cl− and taurine but also gained Na+ (Fig. 1B–D). Na+ uptake by the cell promotes water uptake and thus partially counteracts RVD. Table 1 (first line) shows the mean values of solute and water changes measured after 60 min in the 21 experiments presented in Fig. 1. An analysis of these data allows the nature of the transported taurine to be evaluated.
Table 1.
Changes in water (observed/expected) and solute (ion, taurine) content at 60 min in erythrocytes exposed to hypotonic medium in which Na+ was present or totally replaced by K+ or choline
| External cations | Na+ | K+ | Cl− | Taurine | Total osmotic particles | Water measured | Water expected |
|---|---|---|---|---|---|---|---|
| μmol (g dry cell solids)−1 | g (g dry cell solids)−1 | ||||||
| Na+ (n = 21) | +18.61 ± 0.99 | −38.86 ± 1.94 | −17.85 ± 1.59 | −42.97 ± 1.87 | −80.69 ± 3.73 | −0.37 ± 0.02 | −0.38 ± 0.02 |
| K+ (n = 3) | −0.89 ± 0.39 | +28.21 ± 1.94 | +26.84 ± 1.47 | −37.30 ± 0.92 | +16.86 ± 3.10 | +0.06 ± 0.02 | +0.08 ± 0.02 |
| Choline (n = 3) | −0.34 ± 0.40 | −47.91 ± 2.33 | +1.00 ± 0.93 | −34.95 ± 1.22 | — | −0.14 ± 0.02 | — |
Values are means ± s.e.m.; −, loss of substance; +, gain of substance. The hypotonic medium being 215 mosmol (i.e. contains 215 mmol of osmolyte per litre of water) and the loss of osmolytes being accompanied by an isosmotic loss of water, 4.65 ml water is lost for each mmol of osmolyte leaving the cell.
As shown in Table 1, the cells simultaneously lost K+, Cl− and taurine but gained Na+. The net amount of these osmotically active solutes lost in 1 h, 80.69 ± 3.73 μmol (g dcs)−1, is calculated to drag out of the cells an amount of water (0.38 ± 0.02 g H2O (g dcs)−1) corresponding closely with the experimentally measured loss of water (0.37 ± 0.02 g H2O (g dcs)−1). In other words no solute, other than Na+, K+, Cl− and taurine, contributed significantly to RVD.
Considering now the movement of electrical charges across the cell membrane, it appears that the cells lost 38.86 ± 1.94 μequiv (g dcs)−1 of positive K+ charges but simultaneously gained 18.61 ± 0.99 μequiv (g dcs)−1 of Na+. The net cellular loss of positive charges is then −20.24 ± 1.74 μequiv (g dcs)−1. During the same period of time the net loss of Cl− represents −17.85 ± 1.59 μequiv (g dcs)−1. Thus the loss of positive charges (Δ(Na++ K+)) and the loss of Cl− were not significantly different (Δ(Na++ K+) –ΔCl− = +2.39 ± 2.19 μequiv (g dcs)−1), as expected if both Na+ and K+ moved accompanied with Cl−. Therefore the large amount of taurine lost in 1 h (-42.97 ± 1.87 μmol (g dcs)−1) cannot be electrically balanced if this solute moves as an anion. Clearly taurine was transported as an electrically neutral compound, i.e. a zwitterion.
Effects of [Na+]o replacement on the characteristics of taurine loss
It has been shown previously (Garcia-Romeu et al. 1991) that, in response to a hypotonic shock, the putative volume-activated channel not only carried Ki+, Cli−, taurinei and Nao+ down their electrochemical gradients, but also mediated the downhill movement of different cations used as substitutes for external Na+. As illustrated in Fig. 2A, the magnitude of the regulatory volume decrease is dependent on the capacity of the external cation to penetrate into the cell. RVD is partially or totally inhibited when the external cation is more permeant than Na+ and therefore drags more water into the cell than Na+ (e.g. K+, choline, tetramethylammonium). By contrast RVD is greatly accelerated when the external cation is less permeant than Na+ and thus drags less water into the cell (e.g. NMDG). The following experiments were designed to analyse whether the type of cation present in the extracellular medium in place of Na+ could affect the characteristics of taurine loss.
Figure 2. Effects of [Na+]o replacement on cell H2O and Cl− content.
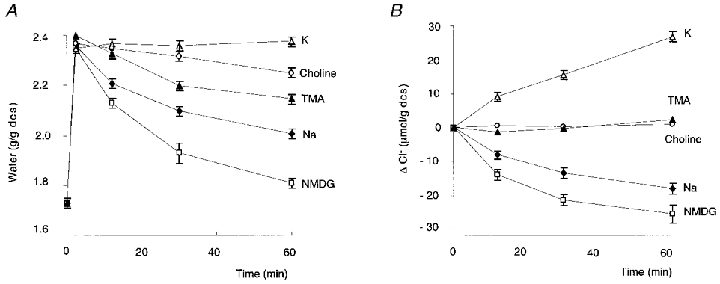
Changes in water (A) and Cl− (B) content of trout erythrocytes undergoing RVD after being suspended in hypotonic medium (215 mosmol (kg H2O)−1) containing different cations: N-methyl-d-glucamine (NMDG), n = 4; Na+, n = 21; K+, n = 3; choline, n = 3; tetramethylammonium (TMA), n = 3. Mean values ± s.e.m.
Substitution of K+ for Na+
When red cells were transferred to a hypotonic medium in which Na+ was totally replaced by K+, RVD was completely blocked and the red cell volume even increased slightly over the 60 min of the experiment (Fig. 2A). Thus, in response to swelling, the cells did not lose water as expected, but on the contrary some water entered into the cells (0.06 g H2O (g dcs)−1). This is explained by the fact that the cells gained both K+ and Cl− (Table 1; Fig. 2B) and that the net amount of (K++ Cl−) uptake into the cells was greater than the amount of taurine leaving the cells (Table 1).
Table 1 also shows that the measured entry of water corresponded to the calculated amount of water expected to be dragged by the changes in cellular content of Na+, K+, Cl− and taurine (+0.06 ± 0.02 and +0.08 ± 0.02 g H2O (g dcs)−1, respectively), indicating that no other additional osmotically active solute was involved. Under the conditions of this experiment, the net change in cations (Na++ K+ = +27.32 ± 1.95 μequiv (g dcs)−1) was electrically balanced by the Cl− change (+26.84 ± 1.47 μequiv (g dcs)−1), showing clearly that the large loss of taurine (-37.3 ± 0.92 μmol (g dcs)−1) corresponds to the loss of an electrically neutral solute.
Substitution of choline for Na+
As illustrated in Fig. 2A, when red cells were transferred to a Na+-free hypotonic medium with choline as a substitute for Na+, RVD was partially inhibited. It has been shown previously that choline, which does not enter the cell in isotonic conditions, penetrates into the cell after hypotonically induced swelling via a DIDS-sensitive pathway at a rate similar to that of K+ leaving the cell (Garcia-Romeu et al. 1991). The constant cellular content of Cl− observed during RVD in choline medium (Fig. 2b; Table 1) reflects the fact that K+ loss was counterbalanced by an equal choline uptake. Hence the osmotic effect of K+ loss was also erased by choline uptake. In such a situation RVD will only result from the loss of taurine, either moving as an anion (accompanied by an equal amount of an undetermined, osmotically active cation) or moving alone as an electrically neutral solute. The experimentally measured value of water lost during 1 h of RVD as shown in Table 1 (0.14 ± 0.02 g H2O (g dcs)−1), closely fits the calculated amount of water expected to be dragged by taurine moving alone as a zwitterion (0.16 g). If taurine was moving as an anion accompanied by an osmotically active cation, the amount of water dragged out would be doubled (0.32 g). Thus again, the loss of taurine corresponds to the loss of an electrically neutral solute.
Changes in cellular chloride concentration during RVD
When erythrocytes were suspended in a hypotonic physiological (i.e. Na+-containing) saline, cells lost chloride continuously over the full time of volume recovery (Fig. 2B). It must be emphasised that the net loss of Cl− resulted from a loss of Cl− as KCl minus an entry of Cl− as NaCl. Thus in the absence of NaCl uptake, the net loss of chloride would be greater. Conversely, in the presence of more permeant cations than Na+, the net loss of chloride would be reduced or even a gain of chloride would be observed, depending on the rate of cation permeation. Thus, as illustrated in Fig. 2B, in the presence of choline no net loss of chloride occurred, whereas in the presence of external K+ the cells gained chloride.
The changes in cell chloride concentration, however, depend not only on the amount of chloride lost but also on water movement resulting from each osmolyte leaving the cell or penetrating into the cell. A comparison of Fig. 2A and B indicates that in the Na+-containing saline, cells lost Cl− but also water, whereas in high K+ saline cells gained Cl− but also gained water. The changes in Cl− concentration of cell water induced by suspending cells in the different hypotonic media are shown in Fig. 3. When erythrocytes were suspended in the Na+-containing saline (Fig. 3A), osmotic swelling promoted a rapid dilution of internal chloride from 66.33 ± 1.94 to 46.89 ± 1.42 mm. In red blood cells, membrane potential and intracellular pH depend on the Donnan equilibrium. The chloride distribution across the red cell membrane, [Cl−]i:[Cl−]o, allows calculation of the Donnan equilibrium membrane potential, ECl (ECl = 58 × log[Cl−]i:[Cl−]o) and the intracellular pH, [pH]i ([pH]i = [pH]o+ log[Cl−]i:[Cl−]o). A calculation of chloride distribution ratios indicates that the cell dilution due to osmotic swelling could induce a very slight decrease in membrane potential (from −22.14 ± 0.69 to −19.62 ± 0.91 mV) and an increase in cell pH (from 7.40 ± 0.02 to 7.45 ± 0.01). However, subsequently, over the full time course of volume recovery, the intracellular chloride concentration remained remarkably constant despite the large decrease in chloride content occurring during RVD (Fig. 3A), indicating that the membrane potential and the internal pH of the red blood cells also remained remarkably constant. This conclusion is fully confirmed by previous direct measurements of the membrane potential of trout red cells impaled with microelectrodes, showing that in response to an osmotic-induced swelling, the membrane potential remained constant during volume regulation (Guizouarn et al. 1993). Conversely, as shown in Fig. 3B, for cells suspended in saline containing K+ or choline in place of Na+, the chloride concentration in cell water continuously increased during RVD, the increase being greater in the K+ than in the choline medium.
Figure 3. Effects of [Na+]o replacement on cell chloride concentration.
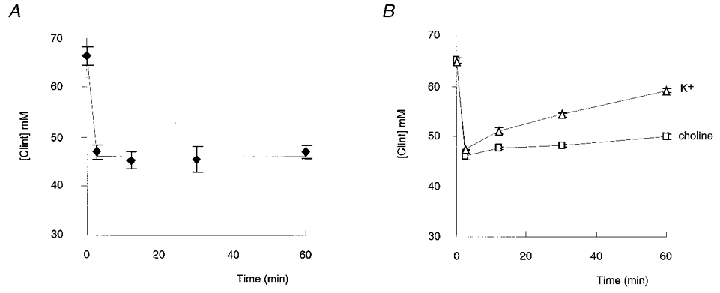
Changes in chloride concentration of cell water when red blood cells were suspended at time 0 in hypotonic medium (215 mosmol (kg H2O)−1) containing Na+, n = 21 (A) and in hypotonic medium in which external Na+ (145 mm) was replaced by choline (□; n = 3) or K+ (▵; n = 3) (B). Mean values ± s.e.m.
In conclusion, in physiological situations, i.e. when Na+ was present in the external medium, cells maintained a constant chloride concentration during RVD and consequently their membrane potential and internal pH were not affected. By contrast, when external Na+ was replaced by more permeant cations, such a constant intracellular Cl− concentration was no longer observed.
DISCUSSION
In this study we show firstly that, when the hypotonically induced swelling occurred in a physiological (Na+-containing) medium, the chloride concentration in the cell water remained remarkably constant over the full time course of RVD. This means that, despite variable losses of cell electrolytes and changes in cell volume, both membrane potential and cell pH remained fixed. Conversely when external Na+ was replaced by K+ or choline the chloride concentration in the cell water varied significantly during RVD.
Secondly, during RVD, taurine was released as an electroneutral zwitterion whatever the nature of the cation present in the hypotonic medium (Na+, K+ or choline).
The role of taurine in maintaining cell chloride concentration and consequently membrane potential and cell pH
When swelling was induced by suspending cells in a Na+-containing hypotonic medium, the intracellular chloride concentration remained remarkably constant over the full time course of RVD (Fig. 3A). It is only possible to keep the chloride concentration in the cell water constant when the cell volume decreases if an equal fraction of the cell chloride content and of the cell water content are lost simultaneously. Indeed, it was observed that the hypotonically swollen cell, which contained 2.36 g H2O (g dcs)−1 and 115 μmol Cl− (g dcs)−1, lost in 1 h 0.37 g H2O, i.e. 15.7 % of its water pool and 17.85 μmol Cl−, i.e. 15.5 % of its chloride pool (Table 1). In other words, when cells lose both 1.15 μmol Cl− (g dcs)−1 and 0.0236 g H2O (g dcs)−1 (i.e. 1 % of the respective pools), the chloride concentration in cell water remains constant. However, to extrude 0.0236 g H2O, 5 μmol of osmotically active particles have to be lost and chloride accounts for only 1.15 μmol. Thus the constancy of the Cl− concentration in the cell water can be observed if the loss of 1.15 μmol of Cl− is always accompanied by the loss of 3.85 μmol of other osmotically active solutes (termed ‘osmolytes’), giving a stoichiometry of 1 Cl−‘cotransported’ with 3.35 osmolytes.
In a physiological, Na+-containing saline the net loss of 20 μequiv positive charges (39 μequiv K+ minus 19 μequiv Na+) was electrically balanced by Cl− (Table 1). Then to keep [Cl−]i constant, each Cl− was accompanied by 1 cation plus 2.35 neutral taurine (42.97 μmol taurine vs. 17.85 μequiv Cl−; Table 1). This is in agreement with the expected stochiometric ratio stated above. It remains unclear how the loss of Cl−, positive charges and taurine are so strictly related to maintain a constant stoichiometry of 1:1:2.35. The swelling-activated channel, which mediates the movements of Cl−, K+, Na+ and taurine, has been shown to possess a marked selectivity for Cl− over cations (Lewis et al. 1996). Then the net movement of Cl− will be determined solely by the net movement of cations. Thus, it could be assumed that the observed stochiometry reflects an adequately fixed relative permeability of the channel for K+, Na+ and taurine. However, it has been shown that in response to hypotonic shock, a significant fraction of the KCl loss occurs via the KCl cotransporter (Bursell & Kirk, 1996). This suggests that there must be some complex cross-talk between the two pathways. As graded changes in intracellular ionic strength control channel activity and cotransport activity in opposite directions (Motais et al. 1991; Guizouarn & Motais, 1999), intracellular ionic strength could be part of the cross-talk system.
Figure 3B shows that it was sufficient to replace external Na+ by more permeant cations such as K+ or choline for the cell to lose the ability to keep [Cl−]i constant during RVD. These results indicate that the constancy of the chloride concentration is not finely tuned by some mechanism able to modulate the channel transport capacity. They suggest that the chloride concentration in the cell water simply results from the net movements of osmolytes through a channel which constitutively possesses a fixed relative permeability for each solute.
In conclusion, in a physiological situation, the membrane potential and cell pH are maintained constant during RVD as long as taurine loss is stoichiometrically related to cation (K+, Na+) movements and Cl− loss. This stoichiometric relationship is dependent on the fixed, relative permeability characteristics of the osmolyte channel, but the parallel contribution of the KCl cotransport remains to be defined.
Taurine permeation via the swelling-activated pathway
Taurine, or 2-aminoethane sulphonic acid, differs from more familiar amino acids in being a sulphonic rather than a carboxylic amino acid and in being a β- rather than an α-amino acid. The sulphonic group is a strong acid (pK1, 1.5), making taurine almost completely zwitterionic at physiological pH. Thus under our experimental conditions (intracellular pH 7.4), with a pK2 of 8.82, only about 3.8 % of taurine molecules (i.e. 1.5 mm) are negatively charged, the remaining taurine (i.e. 37.5 mm) bearing both a negative and a positive charge. Note that, if the negatively charged form of taurine were released from the cell by the swelling-activated pathway, the concentration of anionic taurine in the cell water would immediately be replenished from the zwitterionic pool according to the mass-action law.
The question then arises whether taurine moves via the swelling-activated pathway in the anionic or in the zwitterionic form. There is compelling evidence from a range of vertebrate cell types, including fish erythrocytes, supporting the hypothesis that the volume-sensitive release of taurine is mediated by a swelling-activated anion channel (Strange & Jackson, 1995; Strange et al. 1996; Kirk, 1997). Electrophysiological studies carried out at highly alkaline pH, pH 8.2, and using very high concentrations of taurine (the purpose of both manoeuvres being to ensure sufficient concentration of the negatively charged form to produce a measurable current) indicate that swelling-activated anion channels possess a significant permeability to taurine in the anionic form (Banderali & Roy, 1992; Jackson & Strange, 1993; Roy, 1995). To our knowledge, our results give the first experimental evidence that under physiological conditions, taurine is released via the swelling-activated channel as a zwitterion and not as an anion. The taurine anion probably did not significantly move via the channel because its intracellular concentration was too low.
The present findings then prompt the question: how can the anion channel accommodate the zwitterionic form of taurine which has a high electrical dipole? There is increasing evidence that the swelling-activated anion channels which mediate taurine transport also have a significant permeability to a wide variety of inorganic and organic solutes which bear charges (anions, amino acids) or are uncharged like polyols (sorbitol, inositol) (Kirk et al. 1992; Goldstein et al. 1994; Kirk, 1997). In several cases, electrical studies (Chan et al. 1994; Verdon et al. 1995; Jackson et al. 1996) as well as flux studies (Garcia-Romeu et al. 1991; Thoroed & Fugelli, 1994; Hall et al. 1996; Bursell & Kirk, 1996) suggest that these anion channels have a substantial permeability to both inorganic (K+, Na+) and organic (choline) cations, though, in most cases, a low cation permeability has been observed (Strange et al. 1996). It is thus widely accepted that a single broadly selective anion channel provides a common pathway for a range of structurally unrelated compounds. The selectivity properties of the channel remain unclear. The size of the substrate is certainly an important factor, with an estimated pore diameter of 0.8–0.9 nm. However, the rate of polyol permeation is also influenced by the presence and arrangement of hydroxyl groups on the solute, suggesting significant interaction between the channel protein and the permeating solute (Napathorn & Spring, 1994). Similarly, it has been observed that swelling-activated channels have a higher permeability to β-amino acids than to similarly sized α-amino acids (Pasantes-Morales et al. 1994; Roy, 1995). In the erythrocytes of at least one fish (flounder), taurine, a β-amino acid, is much more permeant than alanine or even glycine (Wolowyck et al. 1989). Such a difference would not be expected if zwitterionic amino acids diffuse freely in a water filled pore. It is also inconsistent with the fact that no measurable water transport (Fiévet et al. 1998) is associated with the appearance of a taurine channel activity resulting from expression in Xenopus oocytes of the trout red cell band 3 protein (Fiévet et al. 1995; Garcia-Romeu et al. 1996; Motais et al. 1996). Therefore, it is possible that the facilitated permeability of β- versusα-amino acids can be attributed to a different spacing between positive and negative charges on the amino acid which have to fit with fixed counter-ions localised on the channel protein.
Acknowledgments
We thank Dr Mortimer Civan for reviewing the manuscript.
References
- Banderali U, Roy G. Anion channels for amino acids in MDCK cells. American Journal of Physiology. 1992;263:C1200–1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- Borgese F, Garcia-Romeu F, Motais R. Control of cell volume and ion transport by β-adrenergic catecholamines in erythrocytes of rainbow trout Salmo gairdneri. The Journal of Physiology. 1987;382:123–144. doi: 10.1113/jphysiol.1987.sp016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F, Motais R, Garcia-Romeu F. Regulation of Cl− dependent K transport by oxy-deoxyhemoglobin transitions in trout red cells. Biochimica et Biophysica Acta. 1991;1066:252–256. doi: 10.1016/0005-2736(91)90194-d. [DOI] [PubMed] [Google Scholar]
- Bursell JDH, Kirk K. Swelling-activated K+ transport via two functionally distinct pathways in eel erythrocytes. American Journal of Physiology. 1996;270:R61–70. doi: 10.1152/ajpregu.1996.270.1.R61. [DOI] [PubMed] [Google Scholar]
- Chan HC, Fu WO, Chung YW, Huang SJ, Chan PSF, Wong PYD. Swelling-induced anion and cation conductances in human epithelial cells. The Journal of Physiology. 1994;478:449–460. doi: 10.1113/jphysiol.1994.sp020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiévet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO Journal. 1995;14:5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiévet B, Perset F, Gabillat N, Guizouarn H, Borgese F, Ripoche P, Motais R. Transport of uncharged organic solutes in Xenopus oocytes expressing red cell anion exchangers (AE1s) Proceedings of the National Academy of Sciences of the USA. 1998;95:10996–11001. doi: 10.1073/pnas.95.18.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu F, Borgese F, Guizouarn H, Fiévet B, Motais R. A role for the anion exchanger AE1 (band 3 protein) in cell volume regulation. Cellular and Molecular Biology. 1996;42:985–994. [PubMed] [Google Scholar]
- Garcia-Romeu F, Cossins AR, Motais R. Cell volume regulation by trout erythrocytes: characteristics of the transport systems activated by hypotonic swelling. The Journal of Physiology. 1991;440:547–567. doi: 10.1113/jphysiol.1991.sp018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L, Davis EM. Taurine, betaine and inositol share a volume-sensitive transporter in skate erythrocyte cell membrane. American Journal of Physiology. 1994;267:R426–431. doi: 10.1152/ajpregu.1994.267.2.R426. [DOI] [PubMed] [Google Scholar]
- Guizouarn H, Harvey BJ, Borgese F, Gabillat N, Garcia-Romeu F, Motais R. Volume-activated Cl−-independent and Cl−-dependent K+ pathways in trout red blood cells. The Journal of Physiology. 1993;462:609–626. doi: 10.1113/jphysiol.1993.sp019572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizouarn H, Motais R. Swelling activation of transport pathways in erythrocytes: effects of Cl−, ionic strength and volume changes. American Journal of Physiology. 1999;276:C210–220. doi: 10.1152/ajpcell.1999.276.1.C210. [DOI] [PubMed] [Google Scholar]
- Hall JA, Kirk J, Potts JR, Rae C, Kirk K. Anion channel blockers inhibit swelling-activated anion, cation and non electrolyte transport in HeLa cells. American Journal of Physiology. 1996;271:C579–588. doi: 10.1152/ajpcell.1996.271.2.C579. [DOI] [PubMed] [Google Scholar]
- Haynes JK, Goldstein L. Volume-sensitive amino acid transport in erythrocytes of the little skate, Raja erinacea. American Journal of Physiology. 1993;265:R173–179. doi: 10.1152/ajpregu.1993.265.1.R173. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Churchwell K, Ballatori N, Boyer JL, Strange K. Swelling-activated anion conductance in skate hepatocytes: regulation by cell Cl and ATP. American Journal of Physiology. 1996;270:C57–66. doi: 10.1152/ajpcell.1996.270.1.C57. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. American Journal of Physiology. 1993;265:C1489–1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Kirk K. Swelling-activated organic osmolyte channels. Journal of Membrane Biology. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- Kirk K, Ellory JC, Young JD. Transport of organic substrates via a volume-activated channel. Journal of Biological Chemistry. 1992;267:23475–23478. [PubMed] [Google Scholar]
- Lewis RA, Bursell JDH, Kirk K. Anion-selectivity of the swelling-activated osmolyte channel in eel erythrocytes. Journal of Membrane Biology. 1996;149:103–111. doi: 10.1007/s002329900011. [DOI] [PubMed] [Google Scholar]
- Motais R, Fiévet B, Borgese F, Garcia-Romeu F. Association of the band 3 protein with a volume-activated anion and amino acid channel: a molecular approach. Journal of Experimental Biology. 1996;200:361–367. doi: 10.1242/jeb.200.2.361. [DOI] [PubMed] [Google Scholar]
- Motais R, Guizouarn H, Garcia-Romeu F. Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochimica et Biophysica Acta. 1991;1075:169–180. doi: 10.1016/0304-4165(91)90248-f. [DOI] [PubMed] [Google Scholar]
- Napathorn S, Spring KR. Further characterization of the sorbitol permease in PAP-HT25 cells. American Journal of Physiology. 1994;267:C514–519. doi: 10.1152/ajpcell.1994.267.2.C514. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Lykkeboe G, Cossins AR. Oxygenation-activated K fluxes in trout red blood cells. American Journal of Physiology. 1992;263:C1057–1064. doi: 10.1152/ajpcell.1992.263.5.C1057. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Sanchez-Olea R, Moran J. Regulatory volume decrease in cultured astrocytes. Permeability pathway to amino acids and polyols. American Journal of Physiology. 1994;266:C172–178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- Roy G. Amino acid current through anion channels in cultured human glial cells. Journal of Membrane Biology. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Strange K, Jackson PS. Swelling-activated organic osmolyte efflux: a new role for anion channels. Kidney International. 1995;48:994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- Thoroed SM, Fugelli K. The Na-independent taurine influx in flounder erythrocytes and its association with the volume regulatory taurine efflux. Journal of Experimental Biology. 1994;186:245–268. doi: 10.1242/jeb.186.1.245. [DOI] [PubMed] [Google Scholar]
- Verdon B, Winnpenny JP, Whitfeld KJ, Argent BE, Gray MA. Volume-activated chloride currents in pancreatic duct cells. Journal of Membrane Biology. 1995;147:173–183. doi: 10.1007/BF00233545. [DOI] [PubMed] [Google Scholar]
- Wolowyck MW, Fincham DA, Young JD. The effects of furosemide, piretanide and MK-196 on volume sensitive solute transport in fish erythrocytes. Proceedings of the Western Pharmacological Society. 1989;32:309–311. [PubMed] [Google Scholar]


