Abstract
A hypotonic challenge, but not cAMP stimulation, was found to induce release of ATP measured by the luciferin-luciferase assay from both the murine mammary carcinoma cell line C127i and C127 cells stably transfected with the cDNA for human cystic fibrosis transmembrane conductance regulator (CFTR) protein (C127/CFTR). CFTR expression augmented swelling-induced ATP release by 10–20 times under hypotonic conditions (≤ 80 % osmolality).
Glibenclamide failed to suppress swelling-induced ATP release from C127/CFTR cells. In contrast, whole-cell patch-clamp recordings showed that both the cAMP-activated ohmic Cl− currents and volume-sensitive outwardly rectifying (VSOR) Cl− currents were prominently suppressed by glibenclamide.
Gd3+ markedly blocked swelling-induced ATP release but failed to suppress both cAMP- and swelling-activated Cl− currents in the CFTR-expressing cells. Even after pretreatment and during treatment with Gd3+, VSOR Cl− currents were activated normally.
The continuous presence of an ATP-hydrolysing enzyme, apyrase, in the bathing solution did not prevent activation of VSOR Cl− currents in C127/CFTR cells.
The rate of regulatory volume decrease (RVD) in C127/CFTR cells was much faster than that in C127i cells. When apyrase was added to the bathing solution, the RVD rate was retarded in C127/CFTR cells.
On balance, the following conclusions can be deduced. First, swelling-induced ATP release is augmented by expression of CFTR but is not mediated by the CFTR Cl− channel. Second, swelling-induced ATP release is not mediated by the VSOR Cl− channel. Third, the released ATP facilitated the RVD process but is not involved in the activation of VSOR Cl− channels in C127/CFTR cells.
Cystic fibrosis transmembrane conductance regulator (CFTR) belongs to a superfamily of ATP-binding cassette (ABC) transporters (Hyde et al. 1990), most members of which form ATP-dependent pumps for a wide variety of substances (Higgins, 1992). Unlike other members of the ABC transporter family, CFTR functions as a cAMP/protein kinase A-activated Cl− channel regulated by intracellular ATP (Andersen et al. 1991). Evidence has accumulated in support of the concept that CFTR is a regulator of other ion channels (Devidas & Guggino, 1997), such as the outwardly rectifying Cl− channel (ORCC) (Egan et al. 1992; Gabriel et al. 1993; Jovov et al. 1995; Schwiebert et al. 1995), the volume-sensitive Cl− channel (Vennekens et al. 1999), the amiloride-sensitive epithelial Na+ channel (ENaC) (Stutts et al. 1995) and the ATP-dependent inwardly rectifying K+ channel (ROMK) (McNicholas et al. 1996).
Some investigators have suggested that CFTR may mediate the plasma membrane permeability (Schwiebert et al. 1995; Prat et al. 1996; Jiang et al. 1998) or conductance of ATP (Reisin et al. 1994; Schwiebert et al. 1995; Cantiello et al. 1998). However, the ‘CFTR = ATP channel hypothesis’ has recently been challenged by the observation that the ATP permeability or conductance is not specifically associated with CFTR using a variety of methods and preparations (Takahashi et al. 1994; Reddy et al. 1996; Li et al. 1996; Grygorczyk et al. 1996; Grygorczyk & Hanrahan, 1997) and that the pore properties of the CFTR Cl− channel and the ATP channel are distinct (Sugita et al. 1998). Thus, a precise relationship between CFTR and ATP release is still a matter for investigation (Devidas & Guggino, 1997).
CFTR expression has also been found to enhance ATP release induced by cell shrinkage upon removal of extracellular Cl− (Rotoli et al. 1996) or by osmotic cell swelling (Hazama et al. 1998b; Taylor et al. 1998). Swelling-induced ATP release has been shown to be coupled to activation of a volume-sensitive Cl− conductance which is involved in regulatory shrinkage (regulatory volume decrease, RVD) after osmotic swelling in rat hepatoma cells (Wang et al. 1996; Roman et al. 1997) and in rat and human biliary epithelial cells (Roman et al. 1999). Thus, there is a possibility that swelling-induced ATP release from CFTR-expressing cells is associated with the activity of CFTR Cl− channels and/or volume-sensitive Cl− channels. Therefore, in the present study, this possibility was examined by measuring ATP release and Cl− conductances in a murine mammary carcinoma cell line, C127i, and in C127 cells stably transfected with CFTR cDNA (C127/CFTR). Here we have provided clear evidence that swelling-induced ATP release is mediated neither by the CFTR Cl− channel nor by the volume-sensitive outwardly rectifying (VSOR) Cl− channel, and that the mechanism of activation of the VSOR Cl− channel is independent of the released ATP in the CFTR-expressing cell.
METHODS
Cells
A mouse mammary adenocarcinoma cell line, C127i, was obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium (DMEM) containing 10 % fetal calf serum (FCS). A stable transfectant of C127i with the cDNA for human CFTR (C127/CFTR) was a kind gift of Dr M. J. Welsh (University of Iowa), and was cultured in DMEM with 10 % FCS and 200 μg ml−1 geneticin.
Luminometric ATP assay
Using the luciferin-luciferase method (ATP Luminescence Kit; AF-2L1, Toa Electronics, Tokyo, Japan), the extracellular bulk ATP concentration was measured with an ATP analyser (AF-100, Toa Electronics) at room temperature (24–26°C) after the cells had been incubated for 60 min in the control isotonic solution and for 30 min in the test solutions at 37°C. In some experiments, the ATP assay was performed after the cells had been incubated for 15 min in a hypotonic solution at 37 or 23°C. Since ATP release is known to be stimulated dramatically by mechanical perturbation (Grygorczyk & Hanrahan, 1997; Watt et al. 1998), replacement and collection of the extracellular solution were made without tilting or touching the cell monolayer. The isotonic PBS solution contained (mm): 137 NaCl, 2.7 KCl, 1 CaCl2, 1 MgCl2, 8.1 Na2HPO4 and 1.5 KH2PO4 (pH 7.4, 300 mosmol (kg H2O)−1). The hypotonic solutions of a variety of osmotic levels (56 % unless otherwise noted) were prepared by mixing the isotonic PBS with a solution containing (mm): 5 KCl, 1 MgCl2, 1 CaCl2 and 10 Hepes-NaOH (pH 7.4, 35 mosmol (kg H2O)−1). In several experiments, hypotonic stress (56 % osmolality) was applied whilst maintaining the ionic strength constant by reducing the mannitol concentration in the following isotonic mannitol solution (mm): 280 mannitol, 2.7 KCl, 1 CaCl2, 1 MgCl2, 8.1 Na2HPO4 and 1.5 KH2PO4 (pH 7.4, 300 mosmol (kg H2O)−1). cAMP stimulation was performed by adding 1 mm dibutyryl cAMP (db-cAMP) with or without 5 μM forskolin (FSK) to the isotonic or hypotonic PBS. When required, 500 μM glibenclamide, 30 μM GdCl3 or 0.3 U ml−1 apyrase was added to the hypotonic PBS. The data for each drug were compared with the data collected from separate control experiments (without drug) performed with the same batch of cells on the same day.
Cell viability assay
Cell viability was determined by a colorimetric assay (Mossman, 1983) based on the ability of viable cells, but not dead cells, to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; the reaction generates a dark blue formazan product), using a Cell Counting Kit (Dojindo, Kumamoto, Japan). A hypotonic challenge or cAMP stimulation was made as described above.
Patch-clamp recordings
Whole-cell Cl− current recordings were performed, as described previously (Liu et al. 1998; Zhou et al. 1998), using wide-tipped electrodes (around 2 MΩ) at room temperature. Series resistance (< 5 MΩ) was compensated (to 60–75 %) to minimize voltage errors. The time course of current activation and recovery was monitored by repetitively applying (every 15 s) ramp pulses (1.5 s duration) to ± 100 mV for cAMP-activated Cl− currents or alternating step pulses (2 s duration) to ± 40 mV for swelling-activated Cl− currents from a holding potential of 0 mV. To monitor the voltage and time dependence of swelling-activated Cl− current, step pulses (2 s duration) were applied from a pre-potential at −100 mV (0.6 s duration) to test potentials of −80 to +100 mV (or +60 mV) in 20 mV increments. The time and voltage dependence of cAMP-activated Cl− currents was examined by applying step pulses (1 s duration) from −100 to +100 mV in 20 mV increments. The amplitude of instantaneous current was measured 2.5 ms after the step pulse onset.
Currents were recorded using an Axopatch 200A amplifier (Axon Instruments). Current signals were filtered at 1 kHz using a four-pole Bessel filter and digitized at 4 kHz. pCLAMP software (version 6.0.2; Axon Instruments) was used for command pulse control, data acquisition and analysis.
CFTR Cl− currents were activated by applying a cAMP-stimulating cocktail containing 1 mm db-cAMP and 5 μM FSK. Volume-sensitive Cl− currents were activated by changing the bath perfusate from an isotonic to a hypotonic CsCl solution. Isotonic CsCl bathing solution (osmolarity, 320 mosmol (kg H2O)−1) contained (mm): 110 CsCl, 12 Hepes, 5 MgSO4, 100 mannitol and 8 Tris or an appropriate amount of CsOH to titrate to pH 7.5. Hypotonic (260 mosmol (kg H2O)−1) CsCl solution was prepared by reducing the concentration of mannitol to 40 mm. Electrophysiological data were indistinguishable between Tris-titrated and CsOH-titrated solutions. However, the CsOH-titrated solution was exclusively employed when the Gd3+ effect was examined to rule out a possible interaction of Tris with the lanthanide. Anion selectivity of both cAMP- and swelling-activated currents had been testified by a positive shift of reversal potential by 25–28 mV upon reduction of the extracellular Cl− concentration to 30 mm by replacing CsCl with mannitol (data not shown, n = 3 each). Glibenclamide (500 μM), GdCl3 (30 μM) or apyrase (0.3 or 2 U ml−1) was added to the bathing solution when required. The intracellular CsCl solution contained (mm): 110 CsCl, 2 MgSO4, 1 Na2ATP, 15 NaHepes, 10 Hepes, 1 EGTA and 50 mannitol (pH 7.3, 300 mosmol (kg H2O)−1).
Cell volume measurements
The mean cell volume was measured at room temperature using a Coulter counter (CDA-500, Sysmex, Kobe, Japan), as reported previously (Hazama & Okada, 1988). Isotonic or hypotonic solution consisted of (mm): 95 NaCl, 4.5 KCl, 1 MgCl2, 1 CaCl2, 110 or 0 mannitol and 5 Hepes-NaOH (pH 7.3; 310 or 200 mosmol (kg H2O−1)). Apyrase (0.29 U ml−1) was dissolved directly in hypotonic solution.
Data are given as means ± s.e.m. Statistical differences of the data were evaluated by Student's t test between two groups or by ANOVA and Scheffé's method among multiple groups, and considered significant at P < 0.05.
RESULTS
Effects of CFTR expression on swelling-induced ATP release
The chemiluminescence measurements showed virtually no basal constitutive ATP release from C127i cells under isotonic conditions (Fig. 1, ○). Application of 1 mm db-cAMP and 5 μM FSK never induced ATP release from C127i cells (data not shown, n = 5). However, a severalfold increase in the extracellular bulk ATP concentration was observed after a 30 min exposure of C127i cell monolayers to hypotonic solutions (Fig. 1, •).
Figure 1. Osmolality dependence of ATP release from C127/CFTR and C127i cells.
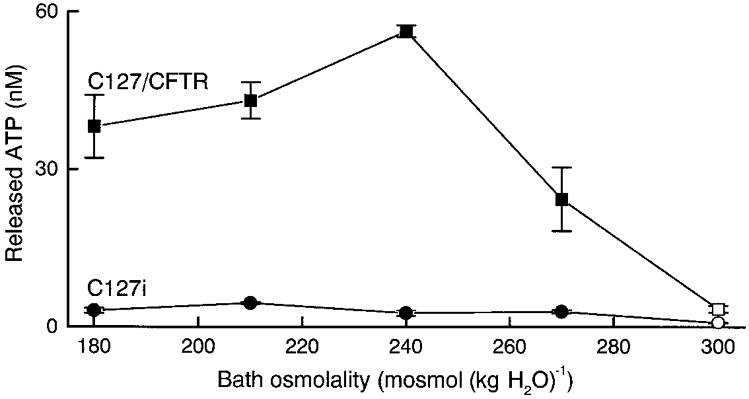
The data represent the mean values of 4 observations (bars, s.e.m.). Data at 300 mosmol (kg H2O)−1 are significantly different from those at 270, 240, 210 and 180 mosmol (kg H2O)−1 in both C127/CFTR and C127i cells. Data for C127/CFTR cells are significantly different from those for C127i at each osmolality.
By stable CFTR expression, the basal constitutive ATP release under isotonic conditions was increased from 0.798 ± 0.096 to 3.398 ± 0.618 nM (Fig. 1, □). This is in good agreement with a previous finding in mouse NIH-3T3 fibroblasts (Takahashi et al. 1994). Osmotic swelling induced by a hypotonic challenge has been reported to lead to dramatic enhancement of ATP release from C127/CFTR cells (Hazama et al. 1998b) and human airway epithelial cells (Taylor et al. 1998). The present data confirmed these observations and demonstrated that the effect of hypotonic stress was dose dependent in C127/CFTR cells (Fig. 1, ▪).
When an ATP-hydrolysing enzyme, apyrase, was added to the hypotonic solution, the extracellular ATP level markedly decreased under hypotonic conditions to a level (0.024 ± 0.0009 nM, n = 12) significantly below the basal level under isotonic conditions in the absence of apyrase (1.122 ± 0.228 nM, n = 12) for C127/CFTR cells. A similar effect of apyrase was also observed for C127i cells (data not shown, n = 8).
However, stimulation with 1 mm db-cAMP (for 30 min) neither raised the extracellular ATP concentration above the background level under isotonic conditions nor affected ATP release induced by a hypotonic challenge (Fig. 2A). Similarly, stimulation with 1 mm db-cAMP plus 5 μM FSK failed to induce ATP release from C127/CFTR cells under isotonic conditions (data not shown, n = 5). This is in good agreement with previous results in other cell lines (Takahashi et al. 1994; Grygorczyk & Hanrahan, 1997) but is in sharp contrast to a previous observation of cAMP-induced ATP release from C127/CFTR cells (Prat et al. 1996).
Figure 2. Effects of stimulation with db-cAMP (1 mm) and/or hypotonic stress (56 % osmolality) on ATP release and cell viability in C127/CFTR cells.

A, ATP release. B, cell viability assessed by the colorimetric MTT method. Viability-dependent absorbance measured in cells stimulated with db-cAMP or hypotonic solution was normalized to that in control cells under isotonic conditions. * Significantly different from each other.
Neither hypotonic perturbation nor cAMP stimulation was associated with changes in cell viability (Fig. 2B). Thus, non-specific cell lysis was not significantly involved in the ATP release.
The bulk ATP concentration in 400 μl of extracellular solution bathing 4 × 105 C127/CFTR cells reached around 30 nM during 30 min incubation with a hypotonic solution of 56 % osmolality (Fig. 2A). This result corresponds to a mean ATP release rate of around 2 × 104 s−1 from a single C127/CFTR cell. The value should represent an underestimate, because cells would have ecto-ATPase on the cell surface (Plesner, 1995).
Sizable ATP release (to 17.6 ± 2.8 nM, n = 5) was observed from C127/CFTR cells even 15 min after incubation in hypotonic solution. At room temperature (23°C), similar levels of ATP release (to 15.7 ± 3.1 nM, n = 8, after 15 min) were observed from swollen C127/CFTR cells. Swelling-induced ATP release (to 15.2 ± 1.8 nM, n = 5) was similarly observed in C127/CFTR cells when a hypotonic challenge (37°C for 15 min) was made whilst maintaining ionic strength constant by reducing the extracellular mannitol concentration.
Effects of glibenclamide and Gd3+ on swelling-induced ATP release
The sulfonylurea glibenclamide has been reported to inhibit ATP release induced by cAMP stimulation (Schwiebert et al. 1995) or by mechanical stress (Sprague et al. 1998; Pedersen et al. 1999) in a number of CFTR-expressing cells. At a high concentration (500 μM), however, glibenclamide never suppressed the swelling-induced ATP release from C127/CFTR cells. This is in good agreement with a recent observation of glibenclamide insensitivity of swelling-induced ATP release from rabbit ciliary epithelial cells (Mitchell et al. 1998). As reported previously (Taylor et al. 1998), glibenclamide itself was found to suppress (by 20–30 % at 500 μM) the luciferase reaction in both isotonic and hypotonic conditions. After correcting for this artifact using a calibration curve obtained by applying known amounts of ATP (Fig. 3, inset), glibenclamide was found to have no significant effect on the ATP release (Fig. 3).
Figure 3. Effects of glibenclamide (500 μM) on ATP release from C127/CFTR cells under isotonic or hypotonic (56 % osmolality) conditions.
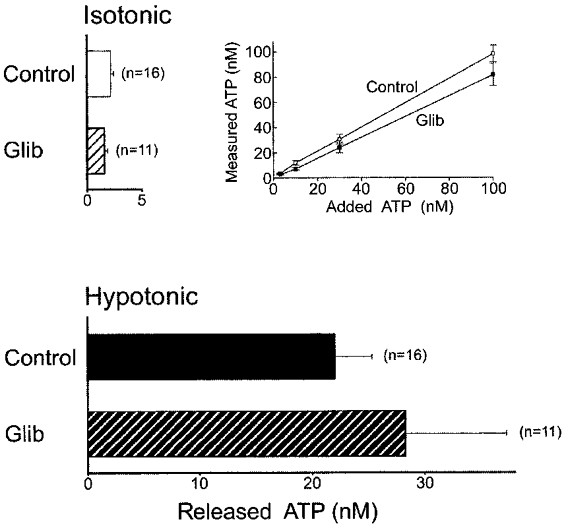
Inset, calibration curve of luciferin-luciferase reactions in the absence (Control, □) and presence (Glib, ▪) of 500 μM glibenclamide in hypotonic solution.
The trivalent lanthanide Gd3+ is a known blocker of mechanostress-gated ion channels (Hamill & McBride, 1996) and has recently been found to inhibit swelling-induced ATP release from human airway epithelial cells (Taylor et al. 1998). In C127/CFTR cells, similar sensitivity of swelling-induced (but not of constitutive basal) ATP release to 30 μM Gd3+ was observed, as shown in Fig. 4.
Figure 4. Effects of Gd3+ (30 μM) on ATP release from C127/CFTR cells under isotonic or hypotonic (56 % osmolality) conditions.
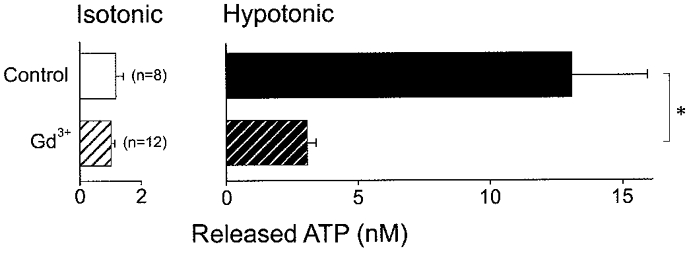
* Significantly different from each other.
Effects of glibenclamide and Gd3+ on CFTR Cl− currents
No appreciable Cl− current was observed in either C127i or C127/CFTR cells without cAMP stimulation under isotonic conditions. In C127/CFTR cells cAMP stimulation consistently activated whole-cell Cl− currents (Fig. 5A). Cell volume was never observed to change during cAMP stimulation under a light microscope. The currents exhibited time-independent kinetics in response to step voltage pulses (Fig. 5B a) and a virtually linear current-voltage (I–V) relationship (Fig. 5C, ○). In contrast, C127i cells failed to respond to cAMP stimulation (data not shown, n = 3).
Figure 5. Effects of glibenclamide (500 μM) on whole-cell cAMP-activated Cl− currents in C127/CFTR cells.
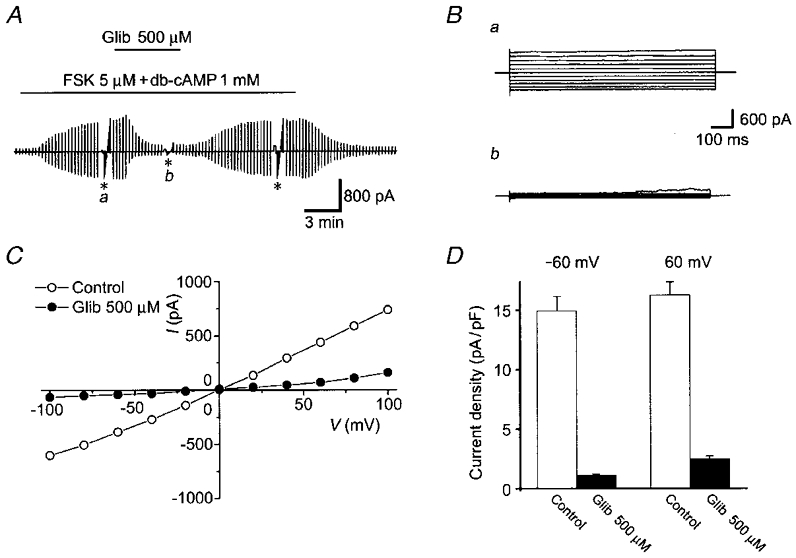
A, representative record before and after cAMP stimulation in the presence (Glib) and absence of 500 μM glibenclamide in the bath solution during application of ramp pulses from −100 to +100 mV or of step pulses from −100 to +100 mV in 20 mV increments (asterisks). B, expanded traces of current responses (a and b in A) to step pulses in the absence (a) and presence (b) of glibenclamide. C, instantaneous I–V relationships in the absence (○, from Ba) and presence (•, from Bb) of glibenclamide. D, mean current densities (n = 5) at ± 60 mV in the absence (Control) and presence (Glib) of glibenclamide.
Since glibenclamide is known to inhibit cAMP-activated ohmic CFTR Cl− currents (Sheppard & Welsh, 1992; Tominaga et al. 1995), the sensitivity of cAMP-activated whole-cell Cl− currents in C127/CFTR cells to 500 μM glibenclamide was examined. As shown in Fig. 5, extracellular application of glibenclamide actually blocked the Cl− current.
In contrast to glibenclamide, Gd3+ (30 μM) never suppressed cAMP-activated Cl− currents (Fig. 6). Although the current was sometimes increased by Gd3+ (Fig. 6A), the mean current density at −40 mV with Gd3+ (-15.0 ± 1.7 pA pF−1, n = 12) was not significantly different from that without Gd3+ (-12.3 ± 1.7 pA pF−1, n = 12). After application of Gd3+, the currents still exhibited time-independent kinetics (Fig. 6B) and a linear I–V relationship (Fig. 6C).
Figure 6. Effects of Gd3+ (30 μM) on whole-cell cAMP-activated Cl− currents in C127/CFTR cells.
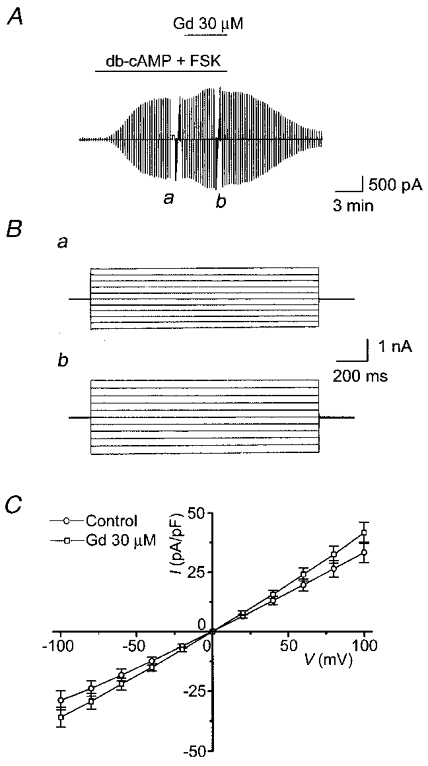
A, representative record before and after cAMP stimulation in the presence and absence of 30 μM Gd3+ in the bath solution during application of ramp pulses from −100 to +100 mV or of step pulses from −100 to +100 mV in 20 mV increments (a and b). B, expanded traces of current responses (a and b in A) to step pulses in the absence (a) and presence (b) of Gd3+. C, I–V relationships for the mean current densities (n = 7) in the absence (○) and presence (□) of Gd3+.
Even after pretreatment (for 30–60 min) and during treatment with 30 μM Gd3+, cAMP stimulation induced activation of Cl− current in C127/CFTR cells (Fig. 7A). The mean Cl− current was 9.6 ± 1.7 pA pF−1 (n = 8) at −40 mV and not significantly different from that without Gd3+ pretreatment (Fig. 6). In the presence of Gd3+, the currents again exhibited time-independent kinetics (Fig. 7B) and a linear I–V relationship (Fig. 7C).
Figure 7. Effects of pretreatment (30–60 min) with Gd3+ (30 μM) on cAMP-induced activation of Cl− currents in C127/CFTR cells.
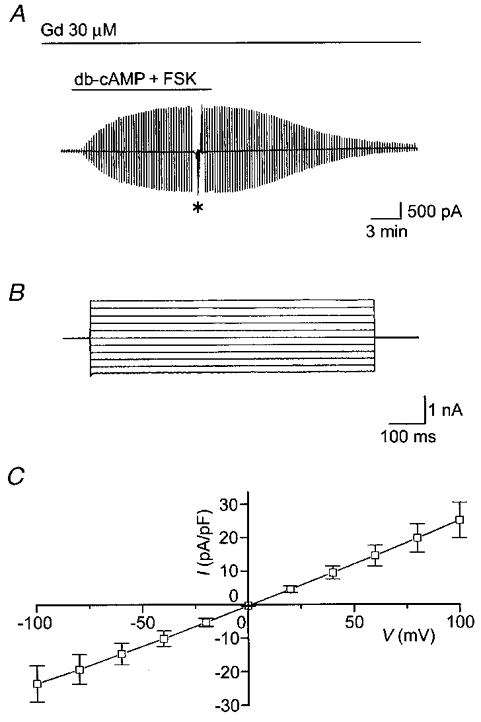
A, representative record in the presence of Gd3+ in the bath solution during application of ramp pulses from −100 to +100 mV or of step pulses from −100 to +100 mV in 20 mV increments (asterisk) from a cell pretreated with Gd3+ for 45 min. B, expanded traces of current responses to step pulses (*, A). C, I–V relationship for the mean current density (n = 8) in the presence of Gd3+.
Effects of glibenclamide, Gd3+ and apyrase on swelling-activated Cl− currents
During exposure to hypotonic solution (81 % osmolality) under whole-cell voltage clamp, a large activation of Cl− current was found in C127/CFTR cells (Fig. 8A). Current activation was always associated with cell swelling. The swelling-induced Cl− currents showed time-dependent inactivation kinetics at positive potentials larger than +80 mV (Fig. 8B) and outward rectification (Fig. 8C), both of which are phenotypic characteristics of VSOR Cl− channels (Strange et al. 1996; Nilius et al. 1997; Okada, 1997). Similar Cl− currents were also activated by a hypotonic challenge in C127i cells (data not shown, n = 3) but were not studied in detail in the present study.
Figure 8. Effects of glibenclamide (200 μM) on whole-cell VSOR Cl− currents in C127/CFTR cells.

A, representative record before and after hypotonic challenge (81 % osmolality) in the presence and absence of 200 μM glibenclamide (Glib) in the bath solution during application of alternating pulses from 0 to ± 40 mV or of step pulses from −100 to +100 mV (or +60 mV) in 20 mV increments (asterisks). In the presence of glibenclamide, positive pulses were applied only up to +60 mV, because the giga-seal was disrupted at larger positive potentials. B, expanded traces of current responses (a and b in A) to step pulses from −100 to +100 mV (a) and from −100 to +60 mV (b) in the absence (a) and presence (b) of glibenclamide. C, I–V relationships for the mean current densities (n = 6) in the absence (○) and presence (•) of glibenclamide.
As observed previously in human epithelial Intestine 407 cells (Liu et al. 1998), 200 μM glibenclamide inhibited VSOR Cl− currents preactivated in C127/CFTR cells (Fig. 8). The mean Cl− current density was significantly suppressed after application of glibenclamide (from 108.4 ± 10 to 52.2 ± 4.5 pA pF−1 at −40 mV, n = 6).
However, 30 μM Gd3+ had no appreciable effect on VSOR Cl− currents when applied either after swelling-induced activation (Fig. 9A and B) or prior to and during a hypotonic challenge (Fig. 9C). The mean Cl− current density at −40 mV in the absence of Gd3+ (110.3 ± 12.1 pA pF−1, n = 10) was not significantly different from that 7–10 min after application of Gd3+ (102.6 ± 11.9 pA pF−1, n = 10) or from that after pretreatment and during treatment with Gd3+ (133.8 ± 11.1 pA pF−1, n = 10).
Figure 9. Effects of application of and pretreatment with Gd3+ (30 μM) on whole-cell VSOR Cl− currents in C127/CFTR cells.
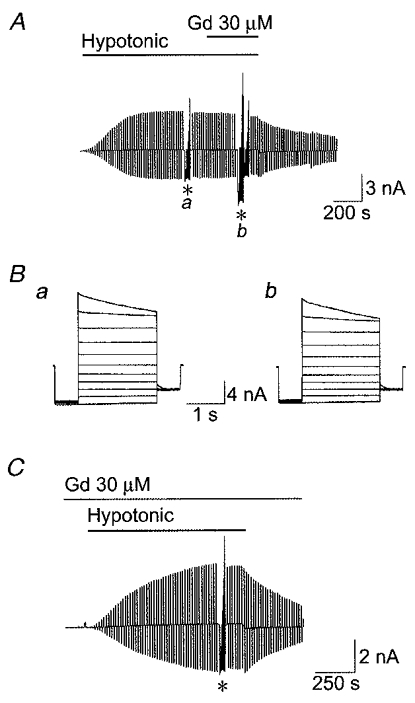
A, effect of Gd3+ applied after activation of the Cl− current. Representative record before and after hypotonic challenge (81 % osmolality) in the presence and absence of 30 μM Gd3+ in the bath solution during application of alternating pulses from 0 to ± 40 mV or of step pulses from −100 to +100 mV in 20 mV increments (asterisks). B, expanded traces of current responses (a and b in A) to step pulses in the absence (a) and presence (b) of Gd3+. C, effect of pretreatment with Gd3+. The record was made in the presence of Gd3+ in the bath solution during application of alternating pulses from 0 to ± 40 mV or of step pulses from −100 to +100 mV in 20 mV increments (asterisk) from a cell pretreated with Gd3+ for 15 min.
Even in the continuous presence of apyrase at 0.3 U ml−1, a hypotonic challenge induced activation of VSOR Cl− currents. The mean Cl− current density at −40 mV was 115.7 ± 12.7 pA pF−1 (n = 5) after 10 min pretreatment and during treatment with apyrase, which was not significantly different from the control value obtained from the same batch of cells (96.5 ± 8.3 pA pF−1, n = 5). No significant effect was also observed by applying apyrase at 2 U ml−1 (data not shown, n = 3). These results are in contrast to previous observations of the inhibiting effects of apyrase on swelling-activated Cl− currents in rat hepatoma cells (Wang et al. 1996; Roman et al. 1997) and in rat and human biliary epithelial cells (Roman et al. 1999).
Effects of CFTR expression on RVD
C127i cells exhibited very slow volume regulation after osmotic swelling during a hypotonic challenge (Fig. 10A, ▵). In contrast, the rate of RVD was much faster in C127/CFTR cells (Fig. 10B, ○). However, removal of released ATP by apyrase markedly retarded the RVD in C127/CFTR cells (Fig. 10B, •) but had little effect on the RVD rate in C127i cells (Fig. 10A, ▴).
Figure 10. Volume regulation of C127i (A) and C127/CFTR cells (B) after osmotic swelling in the absence (open symbols) and presence (filled symbols) of apyrase.
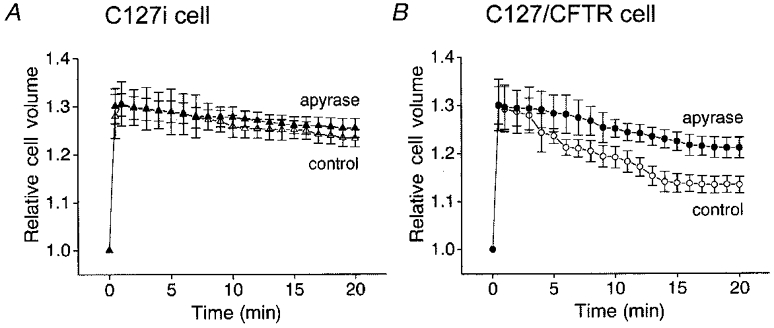
A hypotonic challenge (65 % osmolality) was applied at time 0. Cell volume was normalized to that before hypotonic challenge (3556 ± 223 fl for C127i and 2865 ± 111 fl for C127/CFTR cells). Data represent the means of 10–16 observations. At 20 min after hypotonic challenge, the mean relative cell volume with apyrase was significantly different from that without apyrase in C127/CFTR cells but was not significantly different in C127i cells.
DISCUSSION
What is the physiological role of swelling-induced, CFTR-augmented ATP release?
Extracellular ATP is known to serve as the signal for a variety of cell functions, including skeletal, cardiac and smooth muscle contraction, neurotransmission, endocrine secretion, epithelial secretion, platelet aggregation, inflammatory reaction, etc. (Burnstock, 1981; Gordon, 1986). ATP may be released by exocytosis of ATP-containing secretory granules (e.g. Hazama et al. 1998a) or by non-specific cytolysis. Recently, evidence of non-cytolytic ATP release across the plasma membrane has been obtained from many cell types that lack secretory granules. One of most effective stimuli for non-cytolytic ATP release is mechanical membrane perturbation (Grygorczyk & Hanrahan, 1997; Sprague et al. 1998; Watt et al. 1998) or osmotic cell swelling (Wang et al. 1996; Hazama et al. 1998b; Mitchell et al. 1998). Swelling-induced ATP release was found to be enhanced by expression of the ABC protein CFTR (Hazama et al. 1998b; Taylor et al. 1998). The present study confirmed this finding (Fig. 1). It is possible that CFTR-lacking C127 cells express a higher activity of ecto-ATPase, thereby apparently inducing a smaller amount of ATP release. However, the level of swelling-induced ATP release was essentially not affected by removal of extracellular Ca2+, which is a cofactor for ecto-ATPase, or by addition of 0.1 mm EGTA (S. Tanaka & Y. Okada, unpublished observations). The fact that swelling-induced, CFTR-augmented ATP release is not due to cell lysis was evident from its Gd3+ sensitivity (Fig. 4) and from cell viability measurements (Fig. 2B).
Since impaired cell volume regulation under hypotonic conditions was found in intestinal crypt epithelia of CFTR (-/-) mice (Valverde et al. 1995), CFTR may somehow be implicated in the RVD mechanism. In the present study, CFTR-expressing C127 cells were shown to exhibit a much faster RVD than the parental C127i cells (Fig. 10). In addition, the facilitating effect of CFTR expression was largely abolished by an ATP-hydrolysing enzyme, apyrase (Fig. 10B). These observations are in good agreement with previous observations in rat HTC hepatoma cells (Wang et al. 1996), HTC cells overexpressing MDR proteins (Roman et al. 1997) and rat and human biliary epithelial cells (Roman et al. 1999). Thus, it is evident that swelling-induced, CFTR-augmented ATP release is involved in RVD regulation in an autocrine manner, presumably via stimulation of P2-purinergic receptors, which are stimulated by ATP concentrations as high as 10 nM (Dubyak & El-Moatassim, 1993).
Do CFTR Cl− channels serve as the pathway for swelling-induced, CFTR-augmented ATP release?
The original hypothesis that the CFTR Cl− channel itself acts as the ATP channel (Reisin et al. 1994; Schwiebert et al. 1995; Cantiello et al. 1998) has become questionable since the emergence of a large number of conflicting observations (see Reddy et al. 1996; Devidas & Guggino, 1997). The present study has provided three additional lines of evidence against the ‘CFTR = ATP channel’ hypothesis. In C127/CFTR cells, (1) cAMP stimulation activated CFTR Cl− currents but did not induce significant ATP release (Fig. 2), (2) glibenclamide inhibited the CFTR Cl− current (Fig. 5) but, in contrast, failed to suppress swelling-activated ATP release (Fig. 3), and (3) Gd3+ inhibited swelling-activated ATP release (Fig. 4) without significantly inhibiting cAMP-activated Cl− currents (Figs 6 and 7). However, there remains the possibility that some domain of the CFTR protein other than the Cl−-conducting pore may serve the ATP release pathway or that the CFTR protein regulates a separate ATP-releasing channel/transporter. Further studies are needed to elucidate the precise mechanism by which CFTR expression enhances swelling-induced ATP release.
Do VSOR Cl− channels serve as the pathway for swelling-induced, CFTR-augmented ATP release?
Osmotic swelling activates both VSOR Cl− channels and ATP release. The VSOR Cl− channel is known to exhibit considerable permeability to large anions and organic osmolytes (Strange et al. 1996; Nilius et al. 1997; Okada, 1997). Thus, it is possible that ATP can permeate through the swelling-activated Cl− channel under some regulation by CFTR proteins. However, the following two observations in the present study are at variance with this possibility: (1) glibenclamide never inhibited swelling-induced ATP release (Fig. 3) but markedly inhibited VSOR Cl− currents in C127/CFTR cells (Fig. 8), and (2) Gd3+ blocked swelling-induced ATP release (Fig. 4) but, in contrast, failed to inhibit VSOR Cl− currents in C127/CFTR cells (Fig. 9).
What other candidates are there for the ATP release pathway?
Since the other outwardly rectifying chloride channel, ORCC, is known to be inhibited by glibenclamide in other epithelial cells (Rabe et al. 1995; Volk et al. 1995), ORCC could be excluded from the candidate proteins for the ATP channel. Thus, there may be unidentified ATP-permeable channels. However, the present study showed that the mean ATP release rate during 30 min stimulation with a hypotonic solution is rather low (2 × 104 s−1 cell−1) compared with the transporting rate of many types of ion-selective channels (106-108 s−1 channel−1). Alternatively, it is also possible that ATP is released from the cytoplasm by a non-channel-mediated mechanism such as by a transporter or exocytosis.
Does released ATP trigger activation of VSOR Cl− channels?
Although VSOR Cl− channels cannot serve as the pathway for ATP release, there remains the possibility that ATP release is related to VSOR Cl− channels in another way. For example, the released ATP may be directly or indirectly involved in the activation of VSOR Cl− channels. Recently, this attractive hypothesis has actually been put forward by Fitz and his collaborators (Wang et al. 1996; Roman et al. 1997, 1999), based on their observations that the ATP hydrolase, apyrase, and putative blockers for P2 receptors, suramin and Reactive Blue 2, prevented activation of swelling-induced Cl− currents. However, suramin and Reactive Blue 2 were shown to directly block preactivated volume-sensitive Cl− currents in human tracheal cells (Galietta et al. 1997). Extracellular ATP was previously found to inhibit (but never enhance) the outward component of volume-sensitive Cl− currents at submillimolar to millimolar concentrations in other cell types (Jackson & Strange, 1995; Tsumura et al. 1996). Our preliminary data have shown that extracellular application of 100 μM ATP never induced activation of Cl− currents under isotonic conditions and never augmented swelling-activated Cl− currents under hypotonic conditions in C127/CFTR cells (I. Abdullaev, R. Z. Sabirov & Y. Okada, unpublished observations). Also, the present study has provided the following three lines of evidence against this hypothesis. (1) Under isotonic conditions, C127/CFTR cells never showed sizable Cl− currents, although they exhibited a considerable amount of constitutive ATP release (Fig. 1), (2) pretreatment of C127/CFTR cells with Gd3+, which blocked swelling-induced ATP release (Fig. 4), never prevented swelling-induced activation of Cl− currents (Fig. 9), and (3) removal of released ATP by apyrase never affected activation of VSOR Cl− currents.
In summary, we have shown that swelling-induced ATP release is augmented by CFTR expression in mammary epithelial C127 cells. It is concluded that, in the CFTR-expressing cells, swelling-induced ATP release is mediated neither by the CFTR Cl− channel nor by the VSOR Cl− channel. It is also concluded that swelling-induced ATP release is not involved in the activation of the VSOR Cl− channel, whereas released ATP is involved in the regulation of RVD in an autocrine manner.
Acknowledgments
The authors are grateful to M. J. Welsh (University of Iowa) for providing C127/CFTR cells and to A. F. James (King's College London) for reading the manuscript. Thanks are also given to R. Z. Sabirov and S. Morishima for discussion and to A. Miwa, M. Ohara, A. Hattori and K. Shigemoto for technical assistance. This work was, in part, supported by Grant-in-Aid for Scientific Research for Priority Areas of ‘ABC Proteins’ from the Ministry of Education, Science and Culture of Japan, and by a grant from Houansha Foundation.
A. Hazama, H.-T. Fan and I. Abdullaev contributed equally to this study.
References
- Andersen MP, Gregory RJ, Thompson S, Souzd DW, Paul S, Mulligan RC, Smith AE, Welsh NJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–204. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Neurotransmitters and trophic factors in the autonomic nervous system. The Journal of Physiology. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello HF, Jackson GR, Jr, Grosman CF, Prat AG, Borkan SC, Wang Y, Reisin IL, O'Riordan CR, Ausiello FA. Electrodiffusional ATP movement through the cystic fibrosis transmembrane conductance regulator. American Journal of Physiology. 1998;274:C799–809. doi: 10.1152/ajpcell.1998.274.3.C799. [DOI] [PubMed] [Google Scholar]
- Devidas S, Guggino WB. The cystic fibrosis transmembrane conductance regulator and ATP. Current Opinion in Cell Biology. 1997;9:547–552. doi: 10.1016/s0955-0674(97)80032-4. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Egan M, Flotte T, Afione S, Solow R, Zeitlin PL, Carter BJ, Guggino WB. Defective regulation of outwardly rectifying Cl− channels by protein kinase A corrected by insertion of CFTR. Nature. 1992;358:531–536. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993;363:263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- Galietta LJV, Falzoni S, DiVirgilio F, Romeo G, Zegarra-Moran O. Characterization of volume-sensitive taurine- and Cl−-permeable channels. American Journal of Physiology. 1997;273:C57–66. doi: 10.1152/ajpcell.1997.273.1.C57. [DOI] [PubMed] [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochemical Journal. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. American Journal of Physiology. 1997;272:C1058–1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. Journal of Membrane Biology. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacological Reviews. 1996;48:231–252. [PubMed] [Google Scholar]
- Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic β cells using a novel biosensor technique. Pflügers Archiv. 1998a;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- Hazama A, Miwa A, Miyoshi T, Shimizu T, Okada Y. ATP release from swollen or CFTR-expressing epithelial cells. In: Okada Y, editor. Cell Volume Regulation: the Molecular Mechanism and Volume Sensing Machinery. Amsterdam: Elsevier; 1998b. pp. 93–98. [Google Scholar]
- Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. The Journal of Physiology. 1988;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annual Review of Cell Biology. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. Journal of General Physiology. 1995;105:661–677. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach WR, Guggino WB, Foskett JK, Engelhardt JF. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. Journal of Cell Biology. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovov B, Ismailov II, Benos DJ. Cystic fibrosis transmembrane conductance regulator is required for protein kinase A activation of an outwardly rectified anion channel purified from bovine tracheal epithelia. Journal of Biological Chemistry. 1995;270:1521–1528. doi: 10.1074/jbc.270.4.1521. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Bear CE. Purified cystic fibrosis transmembrane conductance regulator (CFTR) does not function as an ATP channel. Journal of Biological Chemistry. 1996;271:11623–11626. doi: 10.1074/jbc.271.20.11623. [DOI] [PubMed] [Google Scholar]
- Liu Y, Oiki S, Tsumura T, Shimizu T, Okada Y. Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. American Journal of Physiology. 1998;275:C343–351. doi: 10.1152/ajpcell.1998.275.2.C343. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Guggino WB, Schwiebert EM, Hebert SC, Giebisch GH, Egan ME. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound, glibenclamide, is enhanced by co-expression with the ATP-binding cassette transporter CFTR. Proceedings of the National Academy of Sciences of the USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proceedings of the National Academy of Sciences of the USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman T. A rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Progress in Biophysics and Molecular Biology. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Pedersen SF, Nilius B, Lambert IH, Hoffmann EK. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochimica et Biophysica Acta. 1999;1416:271–284. doi: 10.1016/s0005-2736(98)00228-4. [DOI] [PubMed] [Google Scholar]
- Plesner L. Ecto-ATPases: Identities and functions. International Review of Cytology. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- Prat AG, Reisin IL, Ausiello DA, Cantiello HF. Cellular ATP release by the cystic fibrosis transmembrane conductance regulator. American Journal of Physiology. 1996;270:C538–545. doi: 10.1152/ajpcell.1996.270.2.C538. [DOI] [PubMed] [Google Scholar]
- Rabe A, Disser J, Froemter E. Cl− channel inhibition by glibenclamide is not specific for the CFTR-type Cl− channel. Pflügers Archiv. 1995;429:659–662. doi: 10.1007/BF00373986. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Grygory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. Journal of Biological Chemistry. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Roman RM, Feranchak AP, Salter KD, Wang Y, Fitz JG. Endogenous ATP release regulates Cl− secretion in cultured human and rat biliary epithelial cells. American Journal of Physiology. 1999;276:G1391–1400. doi: 10.1152/ajpgi.1999.276.6.G1391. [DOI] [PubMed] [Google Scholar]
- Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz JG. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. Journal of Biological Chemistry. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- Rotoli BM, Bussolati O, Dall'Asta V, Hoffmann EK, Cabrini G, Gazzola GC. CFTR expression in C127 cells is associated with enhanced cell shrinkage and ATP extrusion in Cl−-free medium. Biochemical and Biophysical Research Communications. 1996;227:755–761. doi: 10.1006/bbrc.1996.1581. [DOI] [PubMed] [Google Scholar]
- Schwiebert EK, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. Journal of General Physiology. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. American Journal of Physiology. 1998;275:H1726–1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Sugita M, Yue Y, Foskett JK. CFTR Cl− channel and CFTR-associated ATP channel: distinct pores regulated by common gates. EMBO Journal. 1998;17:898–908. doi: 10.1093/emboj/17.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Matsushita K, Welsh MJ, Stokes JS. Effect of cAMP on intracellular and extracellular ATP content of Cl−-secreting epithelia and 3T3 fibroblasts. Journal of Biological Chemistry. 1994;269:17853–17857. [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. American Journal of Physiology. 1998;275:C1391–1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Horie M, Sasayama S, Okada Y. Glibenclamide, an ATP-sensitive K+ channel blocker, inhibits cardiac cAMP-activated Cl− conductance. Circulation Research. 1995;77:417–423. doi: 10.1161/01.res.77.2.417. [DOI] [PubMed] [Google Scholar]
- Tsumura T, Oiki S, Ueda S, Okuma M, Okada Y. Sensitivity of volume-sensitive Cl− conductance in human epithelial cells to extracellular nucleotides. American Journal of Physiology. 1996;271:C1872–1878. doi: 10.1152/ajpcell.1996.271.6.C1872. [DOI] [PubMed] [Google Scholar]
- Valverde MA, O'Brien JA, Sepulveda FV, Ratcliff RA, Evans MJ, Colledge WH. Impaired cell volume regulation in intestinal crypt epithelia of cystic fibrosis mice. Proceedings of the National Academy of Sciences of the USA. 1995;92:9038–9041. doi: 10.1073/pnas.92.20.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Trouet D, Vankeerberghen A, Voets T, Cuppens H, Eggermont J, Cassiman J-J, Droogmans G, Nilius B. Inhibition of volume-regulated anion channels by expression of the cystic fibrosis transmembrane conductance regulator. The Journal of Physiology. 1999;515:75–85. doi: 10.1111/j.1469-7793.1999.075ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Rabe A, Korbmacher C. Glibenclamide inhibits an outwardly rectifying chloride channel in M-1 mouse cortical collecting duct cells. Cellular Physiology and Biochemistry. 1995;5:222–231. [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proceedings of the National Academy of Sciences of the USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. Journal of Biological Chemistry. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Zhou S-S, Hazama A, Okada Y. Tyrosine kinase-independent extracellular action of genistein on the CFTR Cl− channel in guinea pig ventricular myocytes and CFTR-transfected mouse fibroblasts. Japanese The Journal of Physiology. 1998;48:389–396. doi: 10.2170/jjphysiol.48.389. [DOI] [PubMed] [Google Scholar]


