Abstract
Intracellular pH (pHi) plays an important role in regulating fluid and electrolyte secretion by salivary gland acinar cells. The pH-sensitive, fluorescent dye 2′,7′-bis(carboxyethyl)-5(6)-carboxylfluorescein (BCECF) was used to characterize the mechanisms involved in regulating pHi during muscarinic stimulation in mouse sublingual mucous acinar cells.
In the presence of HCO3−, muscarinic stimulation caused a rapid decrease in pHi (0.24 ± 0.02 pH units) followed by a slow recovery rate (0.042 ± 0.002 pH units min−1) to the initial resting pHi in sublingual acinar cells. The muscarinic receptor-induced acidification in parotid acinar cells was of a similar magnitude (0.25 ± 0.02 pH units), but in contrast, the recovery rate was ≈4-fold faster (0.181 ± 0.005 pH units min−1).
The agonist-induced intracellular acidification was inhibited by the anion channel blocker niflumate, and was prevented in the absence of HCO3− by treatment with the carbonic anhydrase inhibitor methazolamide. These results indicate that the muscarinic-induced acidification is due to HCO3− loss, probably mediated by an anion conductive pathway.
The Na+–H+ exchange inhibitor 5-(N-ethyl-N-isopropyl)amiloride (EIPA) amplified the magnitude of the agonist-induced acidification and completely blocked the Na+-dependent pHi recovery.
To examine the molecular nature of the Na+–H+ exchange mechanism in sublingual acinar cells, pH regulation was investigated in mice lacking Na+–H+ exchanger isoforms 1 and 2 (NHE1 and NHE2, respectively). The magnitude and the rate of pHi recovery in response to an acid load in acinar cells isolated from mice lacking NHE2 were comparable to that observed in cells from wild-type animals. In contrast, targeted disruption of the Nhe1 gene completely abolished pHi recovery from an acid load. These results demonstrate that NHE1 is critical for regulating pHi during a muscarinic agonist-stimulated acid challenge and probably plays an important role in regulating fluid secretion in the sublingual exocrine gland.
In NHE1-deficient mice, sublingual acinar cells failed to recover from an acid load in the presence of bicarbonate. These results confirm that the major regulatory mechanism involved in pHi recovery from an acid load is not Na+−HCO3− cotransport, but amiloride-sensitive Na+–H+ exchange via isoform 1.
The regulation of intracellular pH (pHi) in epithelial cells is critical for maintaining normal enzyme activity as well as for modulating fluid and electrolyte absorption and secretion (Aronson, 1985). There are several ion transport pathways involved in epithelial pHi regulation including Na+–H+ exchangers, Cl−−HCO3− exchangers and Na+−HCO3− cotransporters (Geibel et al. 1990; Kopito, 1990; Steward et al. 1996). In salivary acinar cells, Na+–H+ exchange plays a significant role in regulating Cl−- and HCO3−-dependent fluid secretion during muscarinic stimulation via at least two mechanisms. Upregulation of Na+–H+ exchanger activity maintains a neutral intracellular pH, thereby enhancing the production of HCO3− (Turner, 1993) and the activity of the intracellular pH-sensitive anion channel (Arreola et al. 1995). Moreover, Na+–H+ and Cl−−HCO3− exchangers act in concert to drive NaCl uptake in exchange for H+ and HCO3− loss across the basolateral membrane, thereby, increasing the intracellular [Cl−] and enhancing Cl− efflux through apical anion channels (Case et al. 1984; Melvin et al. 1988; Brown et al. 1989; Lau et al. 1989).
The magnitude and duration of the resulting stimulation-induced cytosolic acidification are thus regulated by Na+–H+ exchanger activity. Four distinct isoforms of Na+–H+ exchangers (NHE1-NHE4) with different kinetics and pharmacological properties have been identified in epithelial tissues (Orlowski et al. 1992; Wang et al. 1993; Bookstein et al. 1994b). NHE1 is ubiquitously expressed and is thought to be involved in maintaining the intracellular pH homeostasis and cell volume (Noel & Pouyssegur, 1995), whereas NHE2-NHE4 show a more limited tissue distribution and are thought to be involved in organ-specific functions such as NaCl absorption (Biemesderfer et al. 1993; Bookstein et al. 1994a; Schultheis et al. 1998a,b). More recently, the cloning and expression of NHE5 (Attaphitaya et al. 1999; Baird et al. 1999) and NHE6 (Numata et al. 1998) have also been described. High level expression of NHE5 is restricted to the brain, whereas NHE6 expression appears to be restricted to mitochondria.
Multiple NHE isoforms are expressed in a salivary gland-specific manner (He et al. 1997; Lee et al. 1998; Park et al. 1999). It appears that NHE1 is the major isoform mediating recovery from an intracellular acid challenge in both rat (Robertson et al. 1997; Park et al. 1999) and mouse parotid serous acinar cells (Evans et al. 1999). Subsequent to the muscarinic agonist-induced acidification, a rapid NHE-dependent pHi recovery occurs in parotid and submandibular acinar cells (Lau et al. 1989; Soltoff et al. 1989; Steward et al. 1989), and in mouse parotid this recovery has been directly linked to NHE1 expression (Evans et al. 1999). In contrast, rat sublingual mucous acini show little pHi recovery in response to an agonist-induced acidification (Zhang et al. 1992).
The mechanism for the observed variability in response to stimulation in different salivary glands is not known, but it probably reflects either a different acidification mechanism or the expression of different Na+–H+ exchanger isoforms. To address this issue, we investigated the pHi regulatory mechanisms activated during stimulation in mouse sublingual mucous acinar cells. We show that the intracellular acidification induced by muscarinic stimulation is due to HCO3− loss mediated by an anion conductive pathway. The magnitude and the duration of this acidification correlate with the activity of an EIPA-sensitive Na+–H+ exchanger, consistent with NHE1 or NHE2 expression (Park et al. 1999), but not NHE3 or NHE4 expression (Chambrey et al. 1997; Park et al. 1999). Targeted disruption of the Nhe1 and Nhe2 genes demonstrate that NHE1, but not NHE2, is essential for regulating pHi during muscarinic stimulation and, therefore, is important for regulating fluid secretion in the mouse sublingual gland. Furthermore, the lack of pHi recovery in HCO3−-containing medium demonstrates the absence of Na+-HCO3− contransporter activity. Some aspects of this work have been previously reported in abstract form (Nguyen & Melvin, 1999).
METHODS
Materials and solutions
The acetoxymethyl ester form of 2′-7′-bis(carboxyethyl)-5-carboxyfluorescein (BCECF-AM) and 5-(N-ethyl-N-isopropyl) amiloride (EIPA) were purchased from Molecular Probes (Eugene, OR, USA). Collagenase P was from Boehringer-Mannheim GmbH, (Penzberg, Germany), and all other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA).
HCO3−-free solutions contained (mm): 135 NaCl, 5.4 KCl, 0.4 KH2PO4, 0.33 NaH2PO4, 0.8 MgSO4, 1.2 CaCl2, 10 glucose, 20 Hepes, pH 7.4 with Tris-Base. HCO3−-containing solutions contained (mm): 110 NaCl, 5.4 KCl, 0.4 KH2PO4, 0.33 NaH2PO4, 0.8 MgSO4, 1.2 CaCl2, 10 glucose, 20 Hepes and 25 NaHCO3 (pH 7.4 with NaOH). When NH4Cl was used to induce an acid load, 30 mm NaCl was replaced with NH4Cl. Solutions containing HCO3− were equilibrated with 5 % CO2 and 95 % O2, whereas HCO3−-free solutions were gassed with 100 % O2. The high K+ solution used to calibrate the fluorescence signals contained (mm): 120 KCl, 20 NaCl, 0.8 MgCl2, 20 Hepes and 0.005 nigericin, and the pH adjusted to the required value between 5.6 and 8.
Preparation of sublingual acinar cells
C57Bl/6 male and transgenic mice were fed ad libitum on a standard diet and water. Targeted disruptions of the murine Nhe1 and Nhe2 genes were previously performed as described by Bell et al. (1999) and Schultheis et al. (1998a), respectively. Heterozygous offspring were used to establish breeding colonies in the University of Rochester vivarium. Mice were rendered unconscious by exposure to a rising concentration of CO2 gas and killed by exsanguination. The sublingual glands were quickly removed, trimmed of connective tissues, and finely minced in digestion medium (Eagle's modified essential medium, Biofluids, Inc., Rockville, MD, USA) containing collagenase P (0.3 mg per 7.5 ml per animal). The minced glands were incubated at 37°C in a shaker with continuous agitation (100 cycles min−1). After the first 20 min interval the minced sublingual glands were dispersed by gentle pipetting (10 times) with a 10 ml plastic pipette and centrifuged (210g for 15 s). The supernatant was discarded and the pellet was re-suspended in 7.5 ml collagenase digestion medium for 40 min, at the end of which time the acinar cells were rinsed and harvested by centrifugation. The resulting sublingual acinar cell preparation was loaded with pH-sensitive fluoroprobe by incubation for 30 min at room temperature with BCECF-AM at a concentration of 2 μM. The BCECF-loaded acinar cells were continuously gassed with 100 % O2.
Fluorescence measurement of pHi
BCECF-loaded acinar cells were allowed to adhere to the base of a superfusion chamber mounted on a Nikon Diaphot 200 microscope interfaced with an Axon Imaging Workbench system (Foster City, CA, USA). Cells were excited at 490 and 440 nm and emitted fluorescence was measured at 530 nm. Intracellular pH was estimated by in situ calibration of the ratio of fluorescence at 490nm to that at 440 nm (F490/F440) performed using the nigericin-high K+ method of Thomas et al. (1979). The relationship between F490/F440 and pHi was linear over the pH range 6.4–7.6 (n = 5). Data presented in the figures are from single representative experiments. Values quoted are the means ± s.e.m. for the number of acinar aggregates examined. All experiments were performed with three or more separate preparations.
RESULTS
Muscarinic agonist stimulation induces HCO3−-dependent intracellular acidification in sublingual acinar cells
The data presented in Fig. 1A show the effects of stimulation with the muscarinic agonist carbachol (Cch) on the pHi of mouse mucous sublingual and serous parotid acini in HCO3−-containing medium. Stimulation with 10 μM Cch resulted in a rapid (half-time, ∼40 s, n = 7) decrease in pHi (-0.24 ± 0.02 pH units), which slowly recovered towards the pre-stimulation level in sublingual acinar cells. This is considerably slower (0.042 ± 0.002 pH units min−1) than the recovery rate seen in acinar cells from other types of salivary glands (Lau et al. 1989; Soltoff et al. 1989; Steward et al. 1989), including mouse parotid acinar cells (Fig. 1A; 0.181 ± 0.005 pH units min−1, n = 7). This transient decrease in pHi in response to muscarinic stimulation has been attributed to HCO3− efflux in the rat parotid, rat sublingual and rabbit mandibular glands (Melvin et al. 1988; Nauntofte & Dissing, 1988; Lau et al. 1989; Steward et al. 1989; Zhang et al. 1992). To investigate the HCO3− dependence of the muscarinic agonist-induced acidification, mouse sublingual acinar cells were stimulated in a HCO3−-free medium. Figure 1B shows that the acidification was not completely abolished; however, the magnitude of the carbachol-induced acidification was significantly reduced in HCO3−-free medium (> 50 % less compared with that observed in the presence of HCO3−; pHi, −0.10 ± 0.04 pH units, n = 5). The residual acidification in the absence of extracellular HCO3− may be due to the increased production of metabolic acid or to the efflux of HCO3− generated by intracellular carbonic anhydrase. To test this latter possibility, the effect of the carbonic anhydrase inhibitor methazolamide was examined. Figure 1B demonstrates that the acidification induced by carbachol was totally abolished when acini (n = 7) were perfused in HCO3−-free medium containing 1 mm methazolamide in all cases.
Figure 1. HCO3−-dependent muscarinic receptor-induced acidification of sublingual and parotid acinar cells.
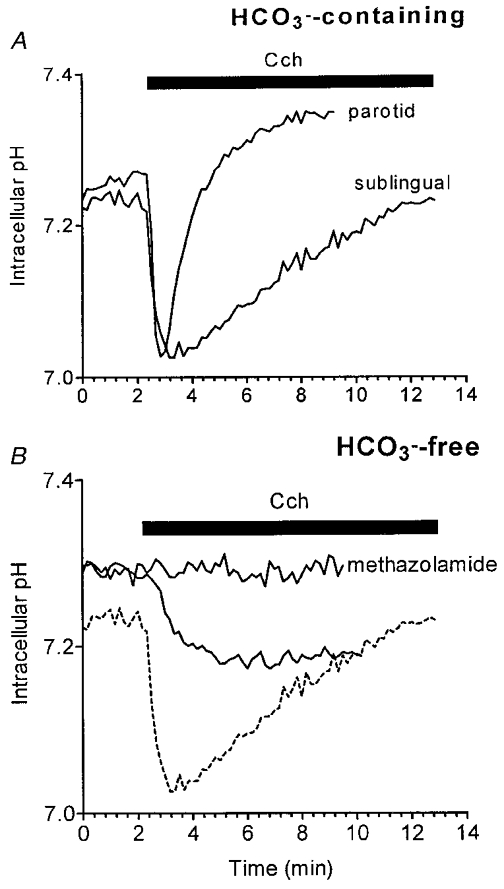
Acinar cells were isolated and loaded with BCECF as described in Methods. A, the pHi response of sublingual and parotid acinar cells in the presence of 25 mm HCO3− to 10 μM carbachol (Cch) during the time period indicated by the filled bar. B, the effect of Cch on the pHi in sublingual acini perfused with HCO3−-free medium in the absence or presence of the carbonic anhydrase inhibitor methazolamide (1 mm). For comparison to the response of acinar cells in HCO3−-containing medium, the sublingual acini trace from A is also shown (dashed line).
In other salivary gland acinar cells, the agonist-induced HCO3− efflux is thought to occur primarily via an anion conductance channel (Melvin et al. 1988; Steward et al. 1996). To test this possibility in mouse sublingual acini, the effect of the anion channel inhibitor niflumate on the HCO3−-dependent acidification was determined. Figure 2A shows that repetitive 30 s exposures to Cch produced intracellular acidifications of similar rate and magnitude, whereas 50 μM niflumate inhibited the drop in pHi by >95 % (n = 7).
Figure 2. Inhibitory effects of niflumate on agonist-induced HCO3− efflux and EIPA-sensitive pHi recovery.
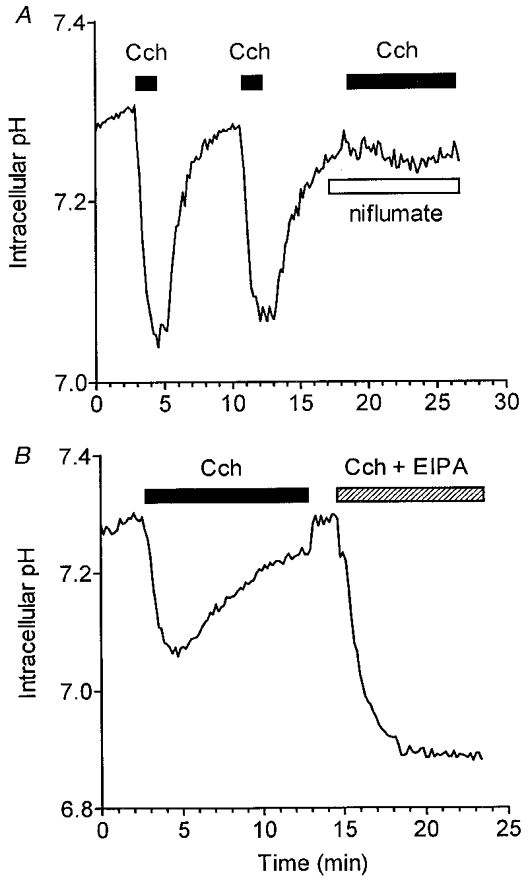
BCECF-loaded acini were perfused with HCO3−-containing medium. A, acini were stimulated with 10 μM Cch for two 30 s periods as indicated by the filled bars, and then with Cch after the addition of 50 μM niflumate during the period indicated by the open bar. B, acini were stimulated with 10 μM Cch as indicated by the filled bar. After removal of Cch for 2 min, acini were again stimulated by Cch in the presence of 10 μM EIPA during the interval indicated by the hatched bar.
During sustained carbachol stimulation, the niflumate-sensitive, HCO3−-dependent acidification slowly recovers to the original unstimulated pHi. Recovery from an acid load in most mammalian cells is mediated by a Na+–H+ exchange mechanism (Alpern, 1990). Addition of 10 μM of the Na+–H+ exchange inhibitor EIPA (or removal of extracellular Na+, data not shown) to carbachol-stimulated acini resulted in a nearly 2-fold amplification of the agonist-induced decrease in pHi, and completely blocked pHi recovery (Fig. 2B). These results suggest that an amiloride-sensitive Na+–H+ exchanger, probably NHE1 or NHE2, is the primary transport mechanism involved in the recovery of pHi during muscarinic stimulation in sublingual mucous acinar cells.
Loss of pHi recovery in NHE1-deficient sublingual mucous acinar cells
The strong EIPA sensitivity (see Fig. 2) of the intracellular pH recovery in sublingual acinar cells indicates that either NHE1 or NHE2 is involved in the alkalinization process (Chambrey et al. 1997; Park et al. 1999). To directly test the involvement of NHE1 and NHE2 in pHi regulation, sublingual acinar cells from NHE1- and NHE2-deficient mice were acid loaded by a short exposure to NH4Cl. Figure 3A shows that in cells from wild-type mice, the intracellular pH rapidly recovered towards the initial pH level (initial recovery rate, 0.23 ± 0.01 pH units min−1, comparable to the rate seen in sublingual acinar cells isolated from C57Bl/6 mice, 0.25 ± 0.01 pH units min−1, data not shown). This pHi recovery was absent under the same experimental conditions in acini from mice lacking NHE1 (Fig. 3B), but was present in acini isolated from NHE2 -/- mice, and recovered with an initial rate of 0.22 ± 0.01 pH units min−1 (Fig. 3B). Furthermore, when acini were stimulated with 10 μM carbachol in the presence of HCO3−, an initial rapid cytosolic acidification (pHi 7.05 ± 0.03) was observed, followed by a slow pHi recovery (0.039 ± 0.001 pH units min−1) in acini from wild-type animals (Fig. 4A; n = 6). In contrast, the initial cytosolic acidification was greater (6.98 ± 0.02 pH units), and the pHi recovery was abolished in acini from NHE1 -/- mice (Fig. 4B; n = 5). Therefore, these data demonstrate that NHE1 is the major Na+–H+ exchanger isoform regulating pHi in sublingual mucous acinar cells during a muscarinic agonist-induced acid challenge.
Figure 3. Loss of pHi recovery in sublingual mucous acinar cells from NHE1-deficient mice.
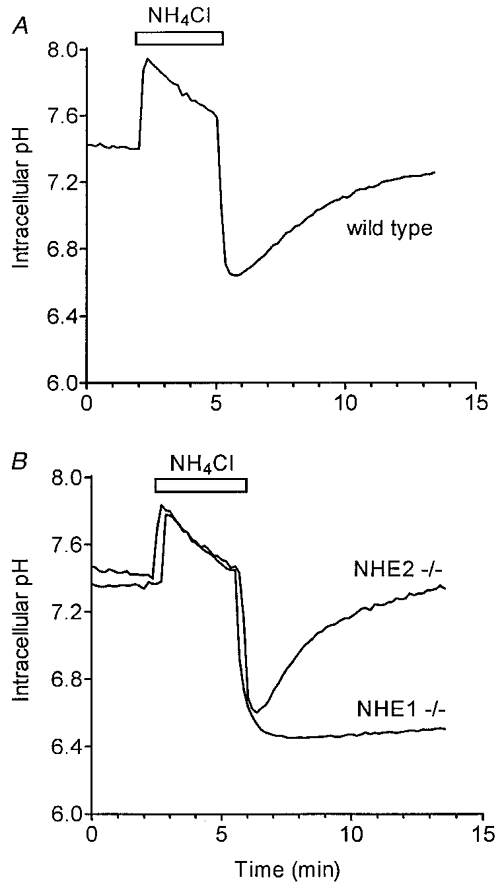
BCECF-loaded acini were perfused with HCO3−-free, Na+-containing medium and then acid loaded by a 3 min exposure to NH4Cl. A, pHi recovery in acini isolated from NHE1 wild-type mice. B, loss of pHi recovery in acini from NHE1-deficient mice (NHE1 -/-); and pHi recovery in acinar cells from mice lacking NHE2 expression (NHE2 -/-) was comparable to that seen in wild-type mice (A).
Figure 4. Intracellular pHi recovery from Cch-stimulated acidification is absent in acini from NHE1-deficient mice.
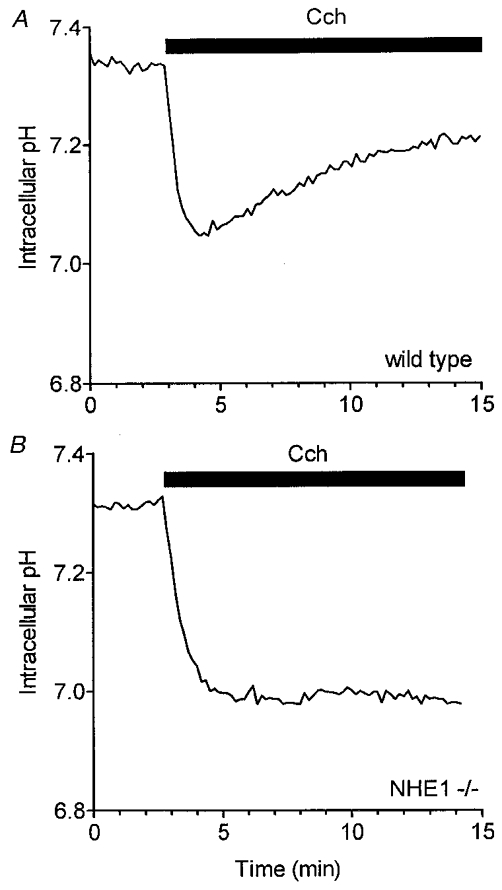
Sublingual acinar cells were loaded with BCECF. A, pHi response of acinar cells isolated from wild-type mice in the presence of 25 mm HCO3− to 10 μM Cch during the time period indicated by the filled bar. B, loss of the pHi recovery in acini from mice lacking expression of NHE1 (NHE1 -/-).
Absence of Na+-HCO3− cotransport in mucous sublingual acinar cells
Although Na+–H+ exchange is the dominant pHi regulatory mechanism in mouse sublingual acinar cells, it is not clear whether Na+-HCO3− cotransport activity may also contribute under physiological conditions. To test this possibility, acinar cells were acid loaded in the presence of HCO3−. Figure 5A shows that acinar cells from wild-type mice displayed an EIPA-sensitive pHi recovery (n = 7), whereas acinar cells from mice lacking NHE1 activity (NHE1 -/-) failed to respond to an acid load (Fig. 5B; n = 8). These results demonstrate that mouse sublingual acinar cells lack Na+- HCO3− cotransporter activity.
Figure 5. Lack of HCO3−-dependent pHi recovery in mucous sublingual acinar cells.
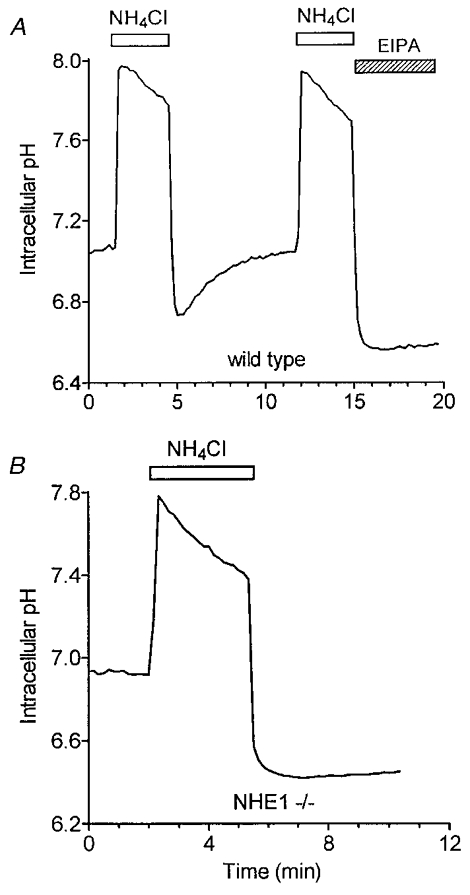
BCECF-loaded acini were perfused in HCO3−-containing solutions (see Methods). Acidification in acini was induced by exposure to 30 mm NH4Cl for approximately 3 min. A, after recovery, acini isolated from NHE1 wild-type mice were acid loaded again in the presence of 10 μM EIPA. B, lack of pHi recovery in acini from NHE1-deficient mice in the presence of HCO3−.
DISCUSSION
Saliva formation is dependent upon the coordinated activity of multiple ion transport mechanisms including pHi regulatory proteins such as Na+–H+ and Cl−-HCO3− exchangers (Turner, 1993; Cook et al. 1994) and Na+- HCO3− cotransporters (Steward et al. 1996). Intracellular pH regulatory proteins in most types of exocrine glands are generally similar, although there are distinct differences as well. For example, Cl−-HCO3− exchange is expressed in the acinar cells of parotid and submandibular salivary glands of several species (Turner & George, 1988; Lee et al. 1999), but not in rat sublingual (Zhang et al. 1992) or human labial gland acini (Valdez et al. 1994). All exocrine glands appear to acidify in response to muscarinic stimulation (Nauntofte & Dissing, 1988; Lau et al. 1989; Soltoff et al. 1989; Steward et al. 1996), including the mucous sublingual gland (Zhang et al. 1992). However, unlike other exocrine glands (Nauntofte & Dissing, 1988; Lau et al. 1989; Soltoff et al. 1989; Steward et al. 1996), the rate and magnitude of pHi recovery from this acid challenge are substantially less in sublingual gland acinar cells (Zhang et al. 1992).
HCO3− efflux upon muscarinic stimulation
The intracellular acidification evoked by muscarinic stimulation in salivary gland acinar cells has been attributed to HCO3− flux through non-specific anion channels (Melvin et al. 1988; Brown et al. 1989; Lau et al. 1989). In the present study, the agonist-induced intracellular acidification was significantly reduced in the absence of HCO3−, and the residual acidification observed in HCO3−-free medium was abolished by the carbonic anhydrase inhibitor methazolamide (Fig. 1). These observations indicate, as in other exocrine glands, that HCO3− efflux underlies the acidification in sublingual acinar cells. Furthermore, HCO3− apparently exits the cells via a non-selective, niflumate-sensitive, anion channel (Fig. 2), consistent with the results found in other exocrine gland acinar cells (Melvin et al. 1988; Nauntofte & Dissing, 1988; Lau et al. 1989; Steward et al. 1996). Thus, it appears that the blunted pHi recovery observed in sublingual acinar cells does not reflect a unique acidification mechanism, but more probably represents a difference in the mechanism involved in the extrusion of acid equivalents.
Na+–H+ exchange mediates the agonist-induced pHi recovery
Recovery from an acid challenge in sublingual acinar cells required extracellular Na+, was blocked by EIPA, and was independent of HCO3−. Our initial interpretation of these results is that Na+-HCO3− cotransport does not play a significant role in pHi in this gland, but that the predominant alkalinization mechanism is an EIPA-sensitive Na+–H+ exchanger. This is in agreement with reports documenting the presence of a Na+–H+ exchange mechanism in the acinar cells of other exocrine glands (Melvin et al. 1988; Muallem & Loessberg, 1990; Robertson et al. 1997). The absence of a Na+-HCO3− cotransporter in mouse sublingual glands is not surprising since the composition of rodent saliva is Cl− rich and the intracellular HCO3− is apparently derived exclusively from the action of carbonic anhydrase on intracellular CO2 and H2O (Turner, 1993). This contrasts with the ovine parotid, where a basolateral Na+-HCO3− cotransporter is responsible for the uptake of HCO3− and the production of a HCO3−-rich saliva (Steward et al. 1996). Although an unlikely alternative mechanism, an EIPA-sensitive, DIDS-insensitive Na+-HCO3− cotransporter, NBC3, has been recently described (Pushkin et al. 1999) that could potentially contribute to the pHi recovery. However, the lack of pHi recovery in response to an acid load under physiological conditions (in the presence of HCO3−) in sublingual acinar cells isolated from NHE1-deficient mice indicates that Na+-HCO3−cotransport contributes little, if any, to intracellular pH regulation in this cell type (see Fig. 5).
Intracellular pH recovery in sublingual acinar cells isolated from mice lacking NHE1 or NHE2
NHE1 is expressed in the basolateral membrane of both ductal and acinar cells of rat parotid and submandibular glands (Robertson et al. 1997; Lee et al. 1998; Park et al. 1999), whereas NHE2 and NHE3 are seen in the apical membranes of duct cells (Lee et al. 1998; Park et al. 1999). The localization of the different NHE isoforms has not been reported for mouse salivary glands, nevertheless, the EIPA sensitivity of Na+–H+ exchanger activity in sublingual acinar cells (Fig. 2) predicts that either NHE1 or NHE2 is dominant. Therefore, sublingual acinar cells from NHE1- and NHE2-deficient mice were acid loaded to test directly the involvement of NHE1 and NHE2 in pHi regulation. The pHi recovery was absent in acini from mice lacking NHE1 but was present in acini isolated from NHE2 -/- mice. These data demonstrate that NHE1 is the major Na+–H+ exchanger isoform for regulating pHi in sublingual mucous acinar cells during a muscarinic agonist-induced acid challenge, an important process required for driving Cl−- and HCO3−-dependent secretion via the apical, niflumate-sensitive anion channel.
In conclusion, our results determined that the muscarinic receptor-induced acidification in mouse sublingual acinar cells is mediated by a HCO3−-dependent, niflumate-sensitive mechanism, most probably an anion channel (Zhang et al. 1995). Furthermore, we directly demonstrated that Na+-HCO3− cotransporter activity is absent in these cells, and that the major intracellular pH regulating mechanism responsible for the recovery from an agonist-induced acid challenge is the Na+–H+ exchanger isoform NHE1. Thus, both the agonist-induced acidification and the pHi recovery processes in mouse sublingual acinar cells are comparable to those previously described in other exocrine glands. What then is the mechanism responsible for the marked difference in the pHi recovery response in sublingual gland acinar cells? The simplest explanation is that less NHE1 protein is expressed in mouse sublingual acinar cells, resulting in the 3- to 5-fold slower pHi recovery rate (0.042 ± 0.001 and 0.181 ± 0.005 pH units min−1 for sublingual and parotid acinar cells, respectively). Alternatively, a dramatic upregulation of NHE1 activity occurs in mouse (Evans et al. 1999) and rat parotid acinar cells (Melvin et al. 1988; Lau et al. 1989; Soltoff et al. 1989) that is absent in sublingual cells. Thus, differential regulation of NHE1 may be involved in the observed differences in rates of pHi recovery. We are currently investigating such mechanisms.
Acknowledgments
We thank Dr W. Scott for providing the NHE1 knockout mice used to establish a breeding colony in Rochester and L. Richardson for technical assistance with genotyping animals. This work was supported in part by National Institutes of Health Grants DK50594 (G.E.S.), DE08921 and DE09692 (J.E.M.).
References
- Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiological Reviews. 1990;70:79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- Aronson PS. Properties of the renal Na+/H+ exchanger. Annals of the New York Academy of Sciences. 1985;456:220–228. doi: 10.1111/j.1749-6632.1985.tb14867.x. [DOI] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Inhibition of Ca2+-dependent Cl− channels from secretory epithelial cells by low internal pH. Journal of Membrane Biology. 1995;147:95–104. doi: 10.1007/BF00235400. [DOI] [PubMed] [Google Scholar]
- Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. Journal of Biological Chemistry. 1999;274:4383–4388. doi: 10.1074/jbc.274.7.4383. [DOI] [PubMed] [Google Scholar]
- Baird NR, Orlowski J, Szabo EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. Journal of Biological Chemistry. 1999;274:4377–4382. doi: 10.1074/jbc.274.7.4377. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. American Journal of Physiology. 1999;276:C788–795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. American Journal of Physiology. 1993;265:F736–742. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. Journal of Clinical Investigation. 1994a;93:106–113. doi: 10.1172/JCI116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein C, Musch MW, DePaoli A, Xie Y, Villereal M, Rao MC, Chang EB. A unique sodium-hydrogen exchange isoform (NHE-4) of the inner medulla of the rat kidney is induced by hyperosmolarity. Journal of Biological Chemistry. 1994b;269:29704–29709. [PubMed] [Google Scholar]
- Brown PD, Elliott AC, Lau KR. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. The Journal of Physiology. 1989;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Hunter M, Novak I, Young JA. The anionic basis of fluid secretion by the rabbit mandibular salivary gland. The Journal of Physiology. 1984;349:619–630. doi: 10.1113/jphysiol.1984.sp015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambrey R, Achard JM, Warnock DG. Heterologous expression of rat NHE4: a highly amiloride-resistant Na+/H+ exchanger isoform. American Journal of Physiology. 1997;272:C90–98. doi: 10.1152/ajpcell.1997.272.1.C90. [DOI] [PubMed] [Google Scholar]
- Cook DI, van Lennep EW, Roberts ML, Young JA. Secretion by the major salivary glands. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. NY: Raven Press; 1994. pp. 1061–1117. [Google Scholar]
- Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic-induced upregulation of Na+/H+ exchange in mouse parotid acinar cells. Journal of Biological Chemistry. 1999;274:29025–29030. doi: 10.1074/jbc.274.41.29025. [DOI] [PubMed] [Google Scholar]
- Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+/H+ exchange and Na+/HCO3−cotransport in the rabbit proximal tubule. Proceedings of the National Academy of Sciences of the USA. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflügers Archiv. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- Kopito RR. Molecular biology of the anion exchanger gene family. International Review of Cytology. 1990;123:177–199. doi: 10.1016/s0074-7696(08)60674-9. [DOI] [PubMed] [Google Scholar]
- Lau KR, Elliott AC, Brown PD. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. American Journal of Physiology. 1989;256:C288–295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Lee MG, Choi JY, Luo X, Strickland E, Thomas PJ, Muallem S. Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. Journal of Biological Chemistry. 1999;274:14670–14677. doi: 10.1074/jbc.274.21.14670. [DOI] [PubMed] [Google Scholar]
- Lee MG, Schultheis PJ, Yan M, Shull GE, Bookstein C, Chang E, Tse M, Donowitz M, Park K, Muallem S. Membrane-limited expression and regulation of Na+/H+ exchanger isoforms by P2 receptors in the rat submandibular gland duct. The Journal of Physiology. 1998;513:341–357. doi: 10.1111/j.1469-7793.1998.341bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin JE, Moran A, Turner RJ. The role of HCO3− and Na+/H+ exchange in the response of rat parotid acinar cells to muscarinic stimulation. Journal of Biological Chemistry. 1988;263:19564–19569. [PubMed] [Google Scholar]
- Muallem S, Loessberg PA. Intracellular pH-regulatory mechanisms in pancreatic acinar cells. I. Characterization of H+ and HCO3− transporters. Journal of Biological Chemistry. 1990;265:12806–12812. [PubMed] [Google Scholar]
- Nauntofte B, Dissing S. Cholinergic-induced electrolyte transport in rat parotid acini. Comparative Biochemistry and Physiology. 1988;90:739–746. doi: 10.1016/0300-9629(88)90693-7. [DOI] [PubMed] [Google Scholar]
- Nguyen H-V, Melvin JE. Characterization of intracellular pH-regulatory mechanisms in mouse sublingual acinar cells. The Journal of Physiology. 1999;517.P:62. P. [Google Scholar]
- Noel J, Pouyssegur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. American Journal of Physiology. 1995;268:C283–296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- Numata M, Petrecca K, Lake N, Orlowski J. Identification of a mitochondrial Na+/H+ exchanger. Journal of Biological Chemistry. 1998;273:6951–6959. doi: 10.1074/jbc.273.12.6951. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na+/H+ exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na+/H+ exchanger NHE-1 and two structurally related proteins. Journal of Biological Chemistry. 1992;267:9331–9339. [PubMed] [Google Scholar]
- Park K, Olschowka JA, Richardson LA, Bookstein C, Chang EB, Melvin JE. Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. American Journal of Physiology. 1999;276:G470–478. doi: 10.1152/ajpgi.1999.276.2.G470. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. Journal of Biological Chemistry. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Robertson MA, Woodside M, Foskett JK, Orlowski J, Grinstein S. Muscarinic agonists induce phosphorylation-independent activation of the NHE-1 isoform of the Na+/H+ antiporter in salivary acinar cells. Journal of Biological Chemistry. 1997;272:287–294. [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. Journal of Clinical Investigation. 1998a;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Harline M, Riddle T, Duffy J, Doetschman T, Wang T, Giebisch G, Aronson P, Lorenz J, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nature Genetics. 1998b;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, McMilliam MK, Cantley LC, Cragoe EJ, Jr, Talamo BR. The effects of muscarinic, alpha-adrenergic, and substance P agonists and ionomycin on ion transport mechanisms in the rat parotid acinar cell. Journal of General Physiology. 1989;93:285–319. doi: 10.1085/jgp.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MC, Poronnik P, Cook DI. Bicarbonate transport in sheep parotid secretory cells. The Journal of Physiology. 1996;494:819–830. doi: 10.1113/jphysiol.1996.sp021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MC, Seo Y, Case RM. Intracellular pH during secretion in the perfused rabbit mandibular salivary gland measured by 31P NMR spectroscopy. Pflügers Archiv. 1989;414:200–207. doi: 10.1007/BF00580964. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Turner RJ. Mechanisms of fluid secretion by salivary glands. Annals of the New York Academy of Sciences. 1993;694:24–35. doi: 10.1111/j.1749-6632.1993.tb18339.x. [DOI] [PubMed] [Google Scholar]
- Turner RJ, George JN. Cl−/HCO3− exchange is present with Na+/K+/Cl− cotransport in rabbit parotid acinar basolateral membranes. American Journal of Physiology. 1988;254:C391–396. doi: 10.1152/ajpcell.1988.254.3.C391. [DOI] [PubMed] [Google Scholar]
- Valdez IH, Paulais M, Fox PC, Turner RJ. Microfluorometric studies of intracellular Ca2+ and Na+ concentrations in normal human labial gland acini. American Journal of Physiology. 1994;267:G601–607. doi: 10.1152/ajpgi.1994.267.4.G601. [DOI] [PubMed] [Google Scholar]
- Wang Z, Orlowski J, Shull GE. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na+/H+ exchanger. Journal of Biological Chemistry. 1993;268:11925–11928. [PubMed] [Google Scholar]
- Zhang GH, Arreola J, Melvin JE. Inhibition by thiocyanate of muscarinic-induced cytosolic acidification and Ca2+ entry in rat sublingual acini. Archives of Oral Biology. 1995;40:111–118. doi: 10.1016/0003-9969(94)00151-z. [DOI] [PubMed] [Google Scholar]
- Zhang GH, Cragoe EJ, Jr, Melvin JE. Regulation of cytoplasmic pH in rat sublingual mucous acini at rest and during muscarinic stimulation. Journal of Membrane Biology. 1992;129:311–321. doi: 10.1007/BF00232912. [DOI] [PubMed] [Google Scholar]


