Abstract
The relative contributions of the rapid and slow components of the delayed rectifier potassium current (IKr and IKs, respectively) to dog cardiac action potential configuration were compared in ventricular myocytes and in multicellular right ventricular papillary muscle and Purkinje fibre preparations. Whole-cell patch-clamp techniques, conventional microelectrode and in vivo ECG measurements were made at 37°C.
Action potential duration (APD) was minimally increased (less than 7%) by chromanol 293B (10 μM) and L-735,821 (100 nM), selective blockers of IKs, over a range of pacing cycle lengths (300–5000 ms) in both dog right ventricular papillary muscles and Purkinje fibre strands. D-Sotalol (30 μM) and E-4031 (1 μM), selective blockers of IKr, in the same preparations markedly (20–80%) lengthened APD in a reverse frequency-dependent manner.
In vivo ECG recordings in intact anaesthetized dogs indicated no significant chromanol 293B (1 mg kg−1 i.v.) effect on the QTc interval (332.9 ± 16.1 ms before versus 330.5 ± 11.2 ms, n = 6, after chromanol 293B), while D-sotalol (1 mg kg−1 i.v.) significantly increased the QTc interval (323.9 ± 7.3 ms before versus 346.5 ± 6.4 ms, n = 5, after D-sotalol, P < 0.05).
The current density estimated during the normal ventricular muscle action potential (i.e. after a 200 ms square pulse to +30 mV or during a 250 ms long ‘action potential-like’ test pulse) indicates that substantially more current is conducted through IKr channels than through IKs channels. However, if the duration of the square test pulse or the ‘action potential-like’ test pulse was lengthened to 500 ms the relative contribution of IKs significantly increased.
When APD was pharmacologically prolonged in papillary muscle (1 μM E-4031 and 1 μg ml−1 veratrine), 100 nM L-735,821 and 10 μM chromanol 293B lengthened repolarization substantially by 14.4 ± 3.4 and 18.0 ± 3.4% (n = 8), respectively.
We conclude that in this study IKs plays little role in normal dog ventricular muscle and Purkinje fibre action potential repolarization and that IKr is the major source of outward current responsible for initiation of final action potential repolarization. Thus, when APD is abnormally increased, the role of IKs in final repolarization increases to provide an important safety mechanism that reduces arrhythmia risk.
The delayed rectifier potassium current (IK) is a major outward current responsible for ventricular muscle action potential repolarization (Carmeliet, 1993; Sanguinetti & Keating, 1997). This current was first described by Noble & Tsien (1969) using the two-microelectrode voltage-clamp technique in multicellular sheep cardiac Purkinje fibre strands. Since its discovery it has been examined in single isolated myocytes obtained from various regions of the heart in several mammalian species (Noble & Tsien, 1969; Sanguinetti & Jurkiewicz, 1990; Follmer & Colatsky, 1990; Varróet al. 1993; Gintant, 1996; Salata et al. 1996a). In most species, IK can be separated into rapid and slow components (IKr and IKs, respectively) that differ from one another in terms of their sensitivity to drugs, rectification characteristics, and kinetic properties (Sanguinetti & Jurkiewicz, 1990; Carmeliet, 1992; D. W. Liu & Antzelevitch, 1995; Gintant, 1996; Heath & Terrar, 1996a,b). Specific IKr blockers (e.g. D-sotalol, dofetilide and E-4031) greatly lengthen cardiac action potential duration (APD) (Singh & Vaughan Williams, 1970; Strauss et al. 1970; Lathrop, 1985; Jurkiewicz & Sanguinetti, 1993) and thus provide anti-arrhythmic benefit by increasing the refractory wavelength. However, these drugs also increase risk for development of bradycardia-induced polymorphic ventricular tachyarrhythmias (Hohnloser & Woosley, 1994). The APD increase induced by selective IKr blockade displays ‘reverse use dependency’ (Hondeghem & Snyders, 1990) that is especially pronounced in Purkinje fibres (Varróet al. 1985). Thus, with a premature impulse, when the time between successive depolarizations is short and an increase in APD would provide the most anti-arrhythmic benefit, the actual APD increase due to IKr block is the least. Conversely, when the time between successive action potentials is long, as with slow heart rates, selective IKr block produces a far greater increase in APD. Long APDs such as these are often associated with the development of early afterdepolarizations that are probably responsible for induction of Torsade de Pointes ventricular arrhythmias.
The absence of selective IKs blockers until recently has made it impossible to evaluate directly the physiological role of this current in determining action potential configuration. Nevertheless, selective IKs block has generally been assumed to increase APD and refractoriness in a frequency-independent manner. On this basis, there has been an effort to develop selective IKs blockers as potential anti-arrhythmic agents devoid of the risk of Torsade de Pointes arrhythmia induction. Propofol, thiopenthone (Heath & Terrar, 1996a) and indapamide (Turgeon et al. 1994) were first used as pharmacological tools to block IKs and thereby separate IKs from IKr in guinea-pig ventricular myocytes. These compounds, however, effectively block IKs at concentrations higher than 100 μM, which calls into question their IKs selectivity. Two compounds, chromanol 293B (Busch et al. 1996) and L-735,821 (Salata et al. 1996b; Cordeiro et al. 1998) have recently been reported to selectively block IKs, but their effects on cardiac action potential configuration have not been examined in detail. Moreover, available results obtained with chromanol 293B and with L-735,821 are contradictory. Cordeiro et al. (1998), for instance, found L-735,821 to markedly increase APD in single, isolated rabbit Purkinje fibre myocytes. Bosch et al. (1998) similarly found chromanol 293B to increase APD in guinea-pig and human ventricular myocytes. However, conventional microelectrode recordings in guinea-pig right papillary muscle showed that chromanol 293B only slightly lengthened APD in the absence of isoproterenol (isoprenaline) (Schreieck et al. 1997). These contradictory findings may have several explanations. For one, APD measurements in single myocytes inherently show relatively large beat-to-beat variations that make identification of the effects of selective ion channel block on action potential configuration uncertain at best. In addition, the relative expression of IKr and IKs exhibits considerable species variation (Jurkiewicz & Sanguinetti, 1993; Varróet al. 1993; Li et al. 1996; Salata et al. 1996a). Regional differences in ion channel expression within the ventricle (Antzelevitch et al. 1991; Bryant et al. 1998) probably also confound interpretation of results and lead to differences in the effects of selective ion channel blockade in myocytes isolated from whole hearts.
Because IKs activation occurs at around 0 mV and this voltage is more positive than the normal Purkinje fibre action potential plateau voltage, IKs block should not be expected to increase Purkinje fibre APD. Conversely, in ventricular muscle, action potential plateau voltage is more positive (∼+20 mV) allowing IKs to be substantially more activated. Thus IKs block in ventricular muscle would be expected to increase APD markedly. Such a difference in the effects of IKs block might, therefore, be expected to produce anti-arrhythmic benefit. This is because lengthening ventricular muscle APD with little or no change in Purkinje fibre APD would cause less drug-induced dispersion in repolarization and limit arrhythmogenesis.
The main goal of this study was to compare the magnitude and extent of changes in ventricular muscle APD produced by selective block of IKr and IKs with those effects produced in Purkinje fibres. The results from such studies would establish the role of IKs in producing normal cardiac action potential repolarization. Thus, we compared the effects of two purported IKs blockers (chromanol 293B and L-735,821) with the effects produced by two recognized, selective IKr blockers (E-4031and D-sotalol) in both single myocytes and multicellular cardiac preparations.
METHODS
All experiments were approved by the Hungarian National Research Foundation (OTKA) and conducted in compliance with the Guide for the Care and Use of Laboratory Animals (USA NIH publication No. 85–23, revised 1985).
Conventional microelectrode measurements
Adult mongrel dogs of either sex weighing 8–16 kg were used. Following anaesthesia induced by sodium pentobarbital (30 mg kg−1i.v.), each heart was rapidly removed through a right lateral thoracotomy and immediately rinsed in oxygenated modified Locke's solution containing (mm): Na+ 140, K+ 4, Ca2+ 1.0, Mg2+ 1, Cl− 126, HCO3− 25 and glucose 11. The solution pH ranged from 7.35 to 7.45 when gassed with 95% O2-5% CO2 at 37°C. Purkinje strands obtained from either ventricle and right ventricular papillary muscle tips were mounted individually in a tissue chamber (volume ∼40 ml). Each preparation was stimulated (HSE stimulator type 215/II) initially at a constant cycle length of 1000 ms (frequency 1 Hz) using rectangular constant current pulses of 2 ms in duration. The current pulses were isolated from ground and delivered through bipolar platinum electrodes in contact with the preparations. At least 1 h was allowed for each preparation to equilibrate while continuously superfused with modified Locke's solution warmed to 37°C before experimental measurements commenced. Transmembrane potentials were recorded using conventional 5–20 MΩ, 3 M KCl-filled microelectrodes connected to the input of a high impedance electrometer (Biologic Amplifier VF 102, Claix, France). In addition, the first derivative of transmembrane voltage with respect to time (Vmax) was electronically obtained (Biologic Differentiator DV 140, Claix, France) and, along with the transmembrane voltage amplifier outputs, continuously monitored on a dual beam storage oscilloscope (Tektronix model 2230).
The maximum diastolic potential, action potential amplitude and action potential durations at 50% and 90% of repolarization (APD50 and APD90) were automatically measured using software developed in our laboratory (Hugo Sachs Elektronik, March-Hugstetten, Germany; action potential evaluation system) running on a 386 microprocessor based, IBM compatible computer, containing an ADA 3300 analog-to-digital data acquisition board (Real Time Devices Inc., PA, USA) with a maximum sampling frequency of 40 kHz. In each experiment, baseline action potential characteristics were first determined during continuous pacing at 1 Hz, and then when pacing cycle length was sequentially varied from 300–5000 ms. The 25th action potential was measured at each cycle length, and the cycle length was then changed so that ‘quasi’ steady-state frequency response relations could be generated rapidly. Preparations were then superfused for 40–60 min with either drug before repeating the pacing protocol and measuring the same parameters. Attempts were made to maintain the same impalement throughout each experiment. If an impalement was, however, dislodged, electrode adjustment was attempted, and if the action potential characteristics of the re-established impalement deviated by less than 5% from the previous measurement, the experiment continued. When this 5% limit was exceeded, the experiment was terminated and all data were excluded from analyses.
Patch-clamp measurements
Cell isolation
Ventricular myocytes were enzymatically dissociated from hearts which were removed from mongrel dogs of either sex weighing 10–20 kg following anaesthesia (sodium pentobarbital, 30 mg kg−1i.v.). The hearts were immediately placed in cold (4°C) normal Tyrode solution. A portion of the left ventricular wall containing an arterial branch large enough to cannulate was then perfused in a modified Langendorff apparatus at a pressure of 60 cmH2O with solutions in the following sequence: (1) normal Tyrode solution (10 min), (2) Ca2+-free solution (10 min), and (3) Ca2+-free solution containing collagenase (type I, 0.66 mg ml−1, Sigma) and bovine serum albumin (fraction V, fatty acid free, 2 mg ml−1, Sigma) (15 min). Protease (type XIV, 0.12 mg ml−1, Sigma) was added to the final perfusate and another 15–30 min of digestion was allowed. Portions of the left ventricular wall judged to be well digested were diced into small pieces and placed either in Kraft-Brühe (KB) solution or in Ca2+-free solution supplemented with CaCl2 (1.25 mm) for 15 min. Next, these tissue samples were gently agitated in a small beaker to dislodge single myocytes from the extracellular matrix. All cell suspensions resulting from this dissociation procedure contained a mixture of subepicardial, midmyocardial and subendocardial myocytes. During the entire isolation procedure, solutions were gassed with 100% O2 while their temperatures were maintained at 37°C. Myocytes were allowed to settle to the bottom of the beaker for 10 min, and then half of the supernatant was replaced with fresh solution. This procedure was repeated three times. Myocytes placed in KB solution were stored at 4°C; those placed in Tyrode solution were maintained at 12–14°C prior to experimentation. Cells that were stored in KB solution or immediately placed in 1.25 mm calcium containing solution had the same appearance and there were no discernible differences in their characteristics.
Compositions of solutions used for cell isolation
Normal Tyrode solution (mm): NaCl 135, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, Hepes 10, NaHCO3 4.4, glucose 10 and CaCl2 1.0 (pH 7.2 adjusted with NaOH). Ca2+-free solution (mm): NaCl 135, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, Hepes 10, NaHCO3 4.4, glucose 10 and taurine 20 (pH 7.2 adjusted with NaOH). KB solution (mm): KOH 90, L-glutamic acid 70, taurine 15, KCl 30, KH2PO4 10, MgCl2 0.5, Hepes 10, glucose 11 and EGTA 0.5 (pH 7.3 adjusted with KOH).
Experimental procedure, drugs and solutions
One drop of cell suspension was placed within a transparent recording chamber mounted on the stage of an inverted microscope (TMS, Nikon, Tokyo, Japan), and individual myocytes were allowed to settle and adhere to the chamber bottom for at least 5 min before superfusion was initiated. Only rod-shaped cells with clear striations were used. Cell capacitance (199.3 ± 13.7 pF, n = 69) was measured by applying 10 mV hyperpolarizing pulse from a holding potential of −10 mV. The capacity was measured by integration of the capacitive transient divided by the amplitude of the voltage step (10 mV). Hepes-buffered Tyrode solution served as the normal superfusate. This solution contained (mm): NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, glucose 5.5 and Hepes 5.0 at pH 7.4.
E-4031 (Institute for Drug Research, Budapest, Hungary) and D-sotalol (Bristol-Arzneimittel, Troisdorf, Germany) were diluted from a 1 mm or 10 mm aqueous stock solution, respectively, at the time of the experiment. Chromanol 293B (obtained as a gift from Hoechst AG, Frankfurt, Germany) was similarly diluted at the time of use from a 10 mm stock solution containing 100% DMSO. DMSO at this concentration did not produce discernible effects either on APD or measured currents. L-735,821 (obtained as a gift from Merck-Sharpe & Dohme Laboratories, Rathway, NJ, USA) was diluted in superfusate from a 100 μM stock solution containing 10% DMSO. Patch-clamp micropipettes were fabricated from borosilicate glass capillaries (Clark, Reading, UK) using a P-97 Flaming-Brown micropipette puller (Sutter Co., Novato, CA, USA). These electrodes had resistances between 1.5 and 2.5 MΩ when filled with pipette solution containing (mm): potassium aspartate 100, KCl 45, K2ATP 3, MgCl2 1, EGTA 10 and Hepes 5. The pH of this solution was adjusted to 7.2 with KOH. Nisoldipine (1 μM) (obtained as a gift from Bayer AG, Leverkusen, Germany) was placed in the external solution to eliminate inward Ca2+ current (ICa), and sodium current (INa) was inactivated by applying a holding potential of −40 mV which also largely inactivated transient outward current (Ito). Membrane currents were recorded with an Axopatch-1D amplifier (Axon Instruments, Foster City, CA, USA) using the whole-cell configuration of the patch-clamp technique. After establishing a high (1–10 GΩ) resistance seal by gentle suction, the cell membrane beneath the tip of the electrode was disrupted by suction or by application of 1.5 V electrical pulses for 1–5 ms. The series resistance was typically 4–8 MΩ before compensation (50–80%, depending on the voltage protocols). Experiments where the series resistance was high, or substantially increased during measurement, were discarded. Membrane currents were digitized using a 333 kHz analog-to-digital converter (Digidata 1200, Axon Instruments) under software control (pCLAMP 6.0, Axon Instruments). Analyses were performed using pCLAMP 6.0 software after low-pass filtering at 1 kHz. All patch-clamp data were collected at 37°C.
ECG measurements in intact anaesthetized dogs
Adult mongrel dogs of either sex weighing 8–16 kg were anaesthetized using sodium pentobarbital (30 mg kg−1i.v.) with subsequent bolus i.v. injections (6 mg kg−1) administered as needed. These dogs were ventilated with room air at a rate and tidal volume sufficient to maintain arterial O2, CO2 and pH within normal limits (Végh et al. 1992). Catheters were inserted into the right and left femoral veins for drug and anaesthetic administration. The dose of each drug applied was 1 mg kg−1. Drugs were administered slowly (over a period of 1 min) in a volume equivalent to 0.5 ml kg−1. Surface electrocardiographic (ECG) leads I, II and III were continuously monitored and recorded after 1, 3 and 5 min and every subsequent 5 min during drug administration for up to 30 min. After completion of the experiments, animals were killed by i.v. overdose of pentobarbital.
Statistical analyses
Results were compared using Student's t tests for paired and unpaired data. When P < 0.05, results were considered significant. Data are expressed as means ± s.e.m.
RESULTS
Effects of E-4031 and D-sotalol on IKr
The effects of E-4031 and D-sotalol on IKr were examined in isolated dog ventricular myocytes. Test pulses of 1000 ms in duration to between −20 mV and +50 mV were applied from a holding potential of −40 mV. The decaying tail current at −40 mV after the test pulse was assessed as IKr. L-735,821 (100 nM) or chromanol 293B (30 μM) were used to block IKs completely. Under these conditions, E-4031 (1 μM) completely abolished and D-sotalol (30 μM) attenuated (not shown) IKr tail currents (Fig. 1).
Figure 1. E-4031-sensitive current (IKr) in dog ventricular myocytes.
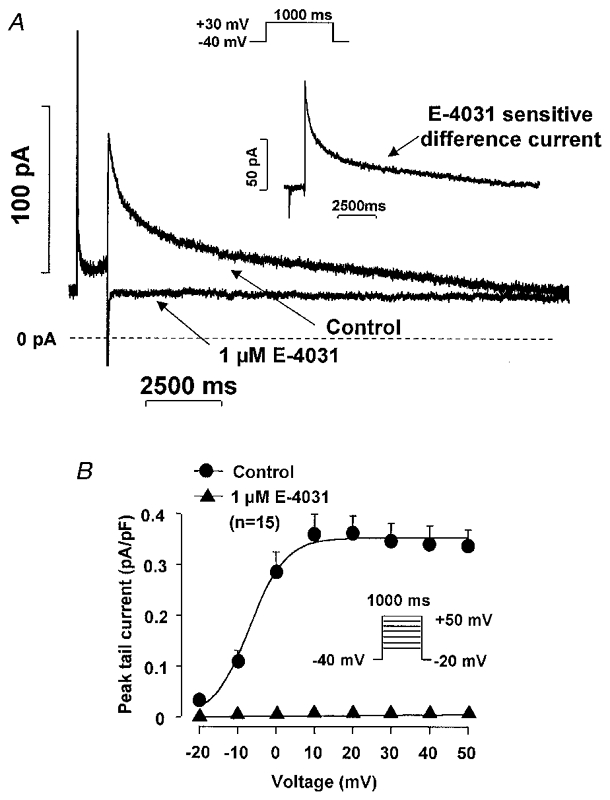
A, recording of IKr in the absence and presence of 1 μM E-4031. The inset presents the E-4031 (1 μM)-sensitive difference current at +30 mV. B, the peak IKr tail current amplitude-voltage relationship in the absence and presence of 1 μM E-4031. Nisoldipine (1 μM) was used to block inward ICa and L-735,821 (100 nM) to block IKs. Holding potential (Vh) was −40 mV, pulse duration was 1000 ms, and pulse frequency was 0.05 Hz.
Effects of chromanol 293B and L-735,821 on IKs
The effects of chromanol 293B and L-735,821 on IKs were examined using long (5000 ms) test pulses to between 0 mV and +50 mV from a holding potential of −40 mV in the presence of 1–5 μM E-4031 to inhibit IKr. The decaying tail current at −40 mV following each test pulse was assessed as IKs. Chromanol 293B (10 μM) greatly reduced and L-735,821 (100 nM) completely abolished IKs (Fig 2 and Fig 3).
Figure 2. E-4031-insensitive current (IKs) in dog ventricular myocytes: effect of chromanol 293B.
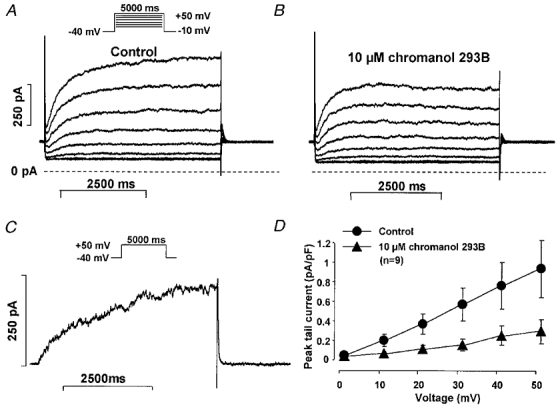
A and B, recordings in the absence and presence, respectively, of 10 μM chromanol 293B. C, the chromanol 293B (10 μM)-sensitive difference current at +50 mV. D, peak IKs tail current amplitude- voltage relationship in the absence and presence of 10 μM chromanol 293B. Nisoldipine (1 μM) was used to block inward ICa and E-4031 (5 μM) to block IKr. Vh was −40 mV, pulse duration was 5000 ms, and pulse frequency was 0.1 Hz.
Figure 3. E-4031-insensitive current (IKs) in dog ventricular myocytes: effect of L-735,821.
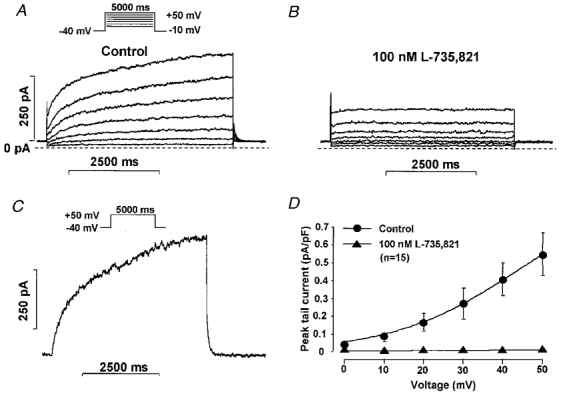
A and B, recordings in the absence and presence, respectively, of 100 nM L-735,821. C, the L-735,821 (100 nM)-sensitive difference current at +50 mV. D, peak IKs tail current amplitude-voltage relationship in the absence and presence of 100 nM L-735,821. Nisoldipine (1 μM) was used to block inward ICa and E-4031 (5 μM) to block IKr. Vh was −40 mV, pulse duration was 5000 ms, and pulse frequency was 0.1 Hz.
Possible contribution of IKs and IKr to action potential repolarization in dog ventricular muscle and Purkinje fibres
The effects on dog ventricular muscle and Purkinje fibre action potential configuration produced by equipotent concentrations of chromanol 293B (10 μM) and L-735,821 (100 nM) that blocked IKs (Fig. 4) were examined and compared with those of D-sotalol (30 μM) and E-4031 (1 μM) that blocked IKr (Fig. 5). Conventional microelectrode techniques were used and the effects of these compounds that completely or markedly blocked either IKs or IKr were examined in both dog ventricular muscle and Purkinje fibre strands over a wide range of stimulation cycle lengths (300–5000 ms). Chromanol 293B and L-735,821 produced small changes in APD amounting to less than a 7% increase over baseline measurements, and these unremarkable effects of IKs demonstrated little frequency dependence in both ventricular muscle and Purkinje fibre strands (Fig. 6). In contrast, D-sotalol and E-4031 markedly lengthened both dog papillary muscle and Purkinje fibre APD (Fig. 6). In addition, the increase in APD following IKr block occurred in a reverse frequency-dependent fashion so that the increase in APD was always greater at long cycle lengths than at short ones (Fig. 6). These results clearly show that IKr block lengthens APD greatly while selective IKs block in dog has little effect on normal cardiac APD in both ventricular muscle and Purkinje fibres.
Figure 4. Effect of IKs block on the action potential in dog ventricular right papillary muscle and Purkinje fibre.
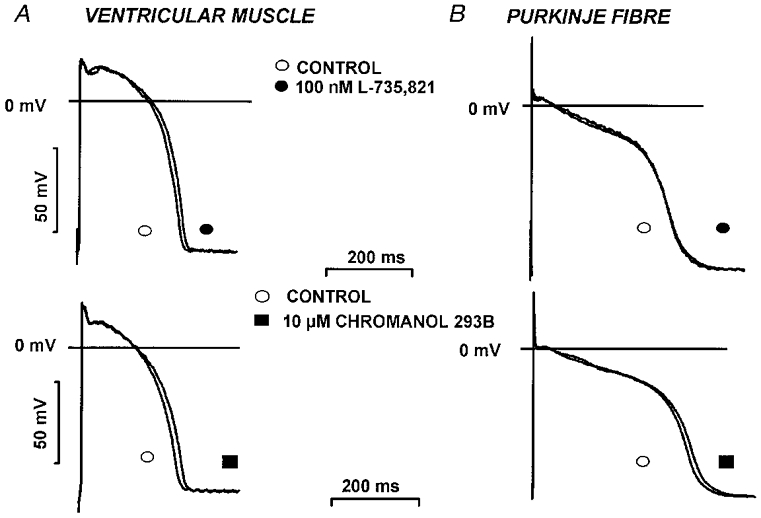
Action potential recordings from canine ventricular papillary muscles (A) and Purkinje fibre strands (B) before and after 40 min superfusion with 100 nM L-735,821 (top) or 10 μM chromanol 293B (bottom). Stimulation frequency was 1 Hz.
Figure 5. Effect of IKr block on the action potential in dog ventricular right papillary muscle and Purkinje fibre.
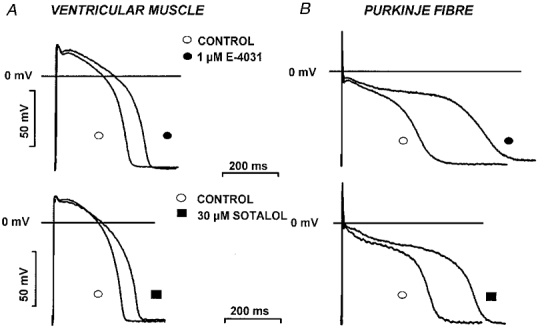
Action potential recordings from canine ventricular papillary muscles (A) and Purkinje fibre strands (B) before and after 40 min superfusion with 1 μM E-4031 (top) or 30 μM D-sotalol (bottom). Stimulation frequency was 1 Hz.
Figure 6. Frequency-dependent effect of IKr and IKs block on action potential duration.
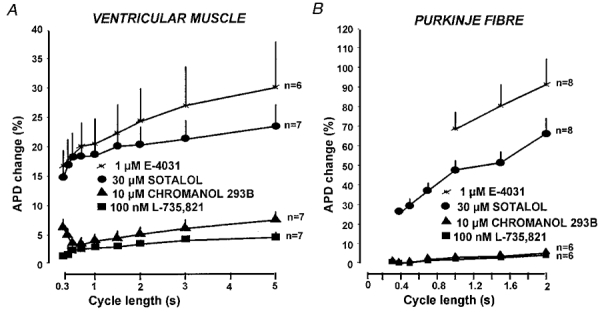
Frequency-dependent effect of IKr (by 1 μM E-4031 or 30 μM sotalol) and IKs block (by 10 μM chromanol 293B or 100 nM L-735,821) on action potential duration (APD) in canine ventricular papillary muscles (A) and Purkinje fibre strands (B). Pacing cycle length (1/frequency) is plotted on the abscissa and the ordinate indicates percentile changes in APD90. Bars represent s.e.m.
Because IKs is modulated by changes in intracellular cAMP, we also examined the effects of IKs block on APD in the presence of 1 μM forskolin to activate adenylcyclase and increase intracellular cAMP. Forskolin (1 μM) alone (n = 17) markedly shortened APD in dog right papillary muscle paced at cycle lengths ranging between 300 and 5000 ms (i.e. from 190.2 ± 4.4 to 157.1 ± 3.3 ms and 258.2 ± 5.7 to 212.5 ± 4.2 ms at cycle lengths of 300 and 5000 ms, respectively). Addition of L-735,821 (100 nM) or chromanol 293B (10 μM) in the continuous presence of forskolin had little effect on APD (150.2 ± 2.2 versus 153.2 ± 2.6 ms and 207.5 ± 3.4 versus 209.0 ± 4.5 ms following L-735,821 and 164.1 ± 4.3 versus 176.0 ± 4.2 ms and 217.6 ± 5.1 versus 234.9 ± 9.1 ms following chromanol 293B at pacing cycle lengths of 300 and 5000 ms, respectively). These results again show that selective IKs block only slightly lengthened APD over a wide range of stimulation frequencies, even in the presence of elevated intracellular cAMP.
Estimation of IKs and IKr activation during the plateau phase of the action potential
Earlier results suggested that IKr activates rapidly during action potentials but deactivates slowly, while IKs activates slowly at more positive potentials (Gintant, 1996). In addition, IKs accumulation over successive depolarization is not likely since its deactivation is fast with respect to diastolic intervals occurring at physiological heart rates. IKr and IKs kinetics such as these may account for the small effect of chromanol 293B and L-735,821 on APD at concentrations that completely or markedly blocked IKs in the present study. To examine this phenomenon further, we carefully evaluated the kinetics of IKr and IKs at depolarized potentials (+30 mV) corresponding to the action potential plateau.
In our study, IKr indeed activated rapidly in dog ventricular myocytes (Fig. 7A). Using gradually increasing test pulse durations from a holding potential of −40 mV to +30 mV in the presence of 100 nM L-735,821 to block IKs, the activation time constant (τ) for IKr was 53.8 ± 5.8 ms (n = 15) with an amplitude (A) of 69.7 ± 6.4 pA (n = 15). Deactivation of IKr on return to −40 mV from +30 mV was slow (Fig. 7B), and it was best fitted by a double exponential relation where the parameters were: τ1 = 360.3 ± 26.3 ms; τ2 = 3310 ± 280 ms; A1 = 31.8 ± 0.7 pA and A2 = 34.1 ± 3.14 pA (n = 15).
Figure 7. Activation and deactivation kinetics of IKr and IKs in dog ventricular myocytes.
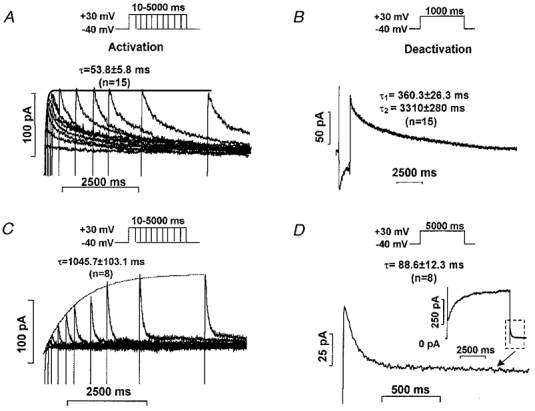
A and C, activation kinetics of IKr and IKs, respectively, measured as tail currents at −40 mV after test pulses to +30 mV with duration gradually increasing between 10 and 5000 ms. B and D, deactivation kinetics of IKr and IKs outward tail current, respectively, at −40 mV after a 1000 or 5000 ms, respectively, long test pulse to +30 mV. The inset in D shows IKs tail current at higher resolution.
IKs kinetics were also assessed but in the presence of 5 μM E-4031 to eliminate IKr. IKs activation under these conditions in dog ventricular myocytes was slow (Fig. 7C) (τ = 1045.7 ± 103.1 ms, A = 61.1 ± 8.3 pA, n = 8). IKs deactivation in these myocytes was fast (Fig. 7D) (τ = 88.6 ± 12.3 ms, n = 8).
To estimate the magnitude of IKs and IKr activated during the cardiac action potential, we compared the amplitudes of the L-735,821-sensitive (IKs) and E-4031-sensitive (IKr) currents at the end of a 150 ms long test pulse to +30 mV and their tail currents on return to −40 mV. Using this protocol we assessed IKr and IKs at voltages corresponding to the plateau and repolarization phases of the action potential. Because deactivation of IKr is slow in comparison to its recovery from inactivation (Spector et al. 1996), tail currents measured at −40 mV do not accurately reflect the magnitude of IKr activated during the test pulse. The opposite situation may be expected with IKs because this current does not appear to inactivate and the driving force for K+ is larger at positive than at negative voltages. We, therefore, measured IKs and IKr by subtracting membrane currents before and after 4–5 min of exposure to L-735,821 and E-4031, respectively. The E-4031-sensitive current (IKr) amplitude at the end of the 150 ms long test pulse was 25.8 ± 3.2 pA (n = 16), or about 29% (86.5 ± 10.5 pA, n = 16) of the tail current amplitude measured after the test pulse returned to −40 mV (Fig. 8A, left panel). The L-735,821-sensitive current (IKs) during the test pulse to +30 mV was larger than its tail current on return to −40 mV (Fig. 8A, right panel). The magnitude of IKs tail current during the test pulse was 12.5 ± 0.8 pA at +30 mV versus 3.5 ± 0.5 pA at −40 mV (n = 18) still approximately an order of magnitude less than the IKr tail current.
Figure 8. Comparison of the magnitude of IKr and IKs after short and long voltage pulses.
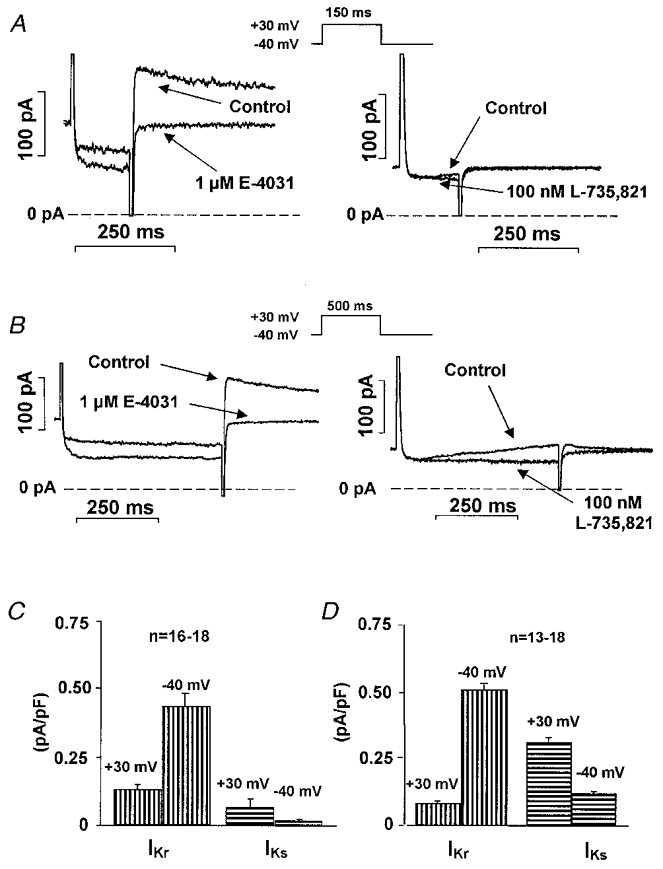
A, recordings of E-4031 (IKr, left)- and L-735,821 (IKs, right)-sensitive currents after application of a short (150 ms) depolarizing test pulse to +30 mV from a holding potential of −40 mV. B, recordings of E-4031 (IKr, left)- and L-735,821 (IKs, right)-sensitive currents after a long (500 ms) depolarizing test pulse to +30 mV from a holding potential of −40 mV. C, average IKr and IKs currents at the end of a short (150 ms, right panel) and a long (500 ms, left panel) depolarizing test pulse to +30 mV, and peak tail current at −40 mV. Bars represent s.e.m.
We also compared IKs and IKr magnitudes during ‘action-potential-like’ test pulses. These test pulses were obtained by digitizing representative right ventricular dog action potentials recorded with conventional microelectrodes. A 40 ms long prepulse to −40 mV was added at the beginning of the idealized action-potential-like test pulse (Fig. 9A). Under these conditions the IKr difference current (i.e. the E-4031-sensitive current) during the action potential plateau phase was small with its magnitude increasing as the test voltage became more negative (Fig. 9A). In contrast, the IKs difference current (i.e. the L-735,821-sensitive current) remained small throughout all phases of the action potential-like test pulse (Fig. 9A). These results indicate that the outward current carried through IKr channels during the action potential is more than 10 times greater than through IKs channels (Figs 8A and 9A). These results agree well with the failure to increase ventricular and Purkinje fibre APD by blocking IKs, while IKr block caused marked lengthening.
Figure 9. E-4031 (IKr)- and L-735,821 (IKs)-sensitive difference currents during short and long ‘action potential-like’ test pulse.
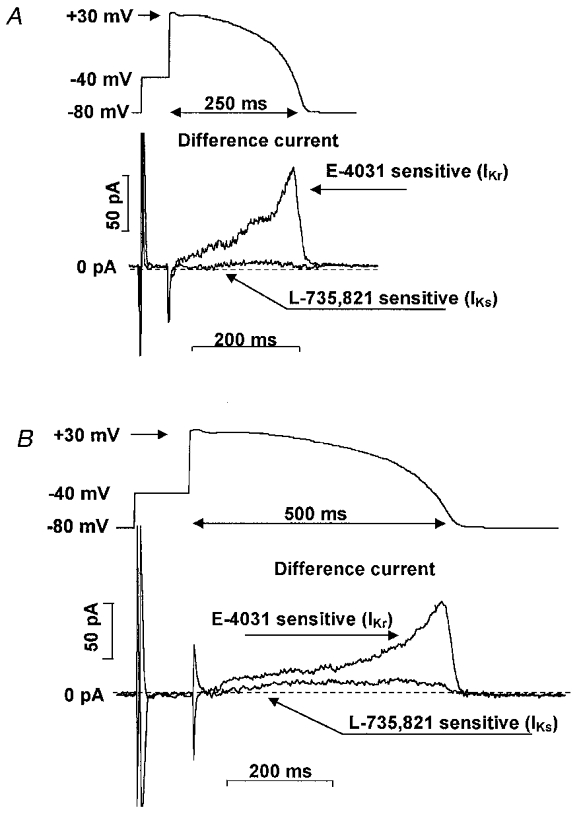
A, E-4031-sensitive (1 μM) (IKr) and L-735,821-sensitive (100 nM) (IKs) difference currents recorded during an ‘action-potential-like’ test pulse in canine ventricular myocytes. The ‘action-potential-like’ test pulse was obtained by recording a normal canine ventricular action potential with a conventional microelectrode in a multicellular papillary muscle preparation and adding a 50 ms prepulse from −80 to −40 mV. B, recordings of E-4031-sensitive (IKr) and L-735,821-sensitive (IKs) currents when the action-potential-like test pulse duration was increased by a factor of 2 (i.e. to ≈500 ms). Recordings in A and B were obtained in the same myocyte. Similar results to those illustrated were obtained in 4–7 additional myocytes.
Because we found IKs to have little role in normal action potential repolarization, we also examined its possible role when action potential duration was artificially increased. In these experiments we either applied long (500 ms) step-wise, rectangular test pulses to +30 mV (Fig. 8B) or an ‘action-potential-like’ test pulse having a duration of 500 ms (Fig. 9B). IKr was not substantially changed due to its fast activation when the duration of the test pulse was increased from 150 to 500 ms (IKr magnitude was 21.3 ± 1.9 pA at +30 mV and 101.0 ± 14.7 pA at −40 mV, n = 13) (Fig. 8B, left panel, and Fig. 9B). However, the magnitude of IKs was significantly increased to 61.5 ± 5.7 pA at +30 mV and 22.7 ± 2.5 pA (n = 18) when test pulse durations were increased to 500 ms (Fig. 8B, right panel, and Fig. 9B).
The effects of L-735,821 and chromanol 293B on pharmacologically lengthened action potentials
The effects of both L-735,821 and chromanol 293B were tested in dog ventricular papillary muscle action potentials, lengthened pharmacologically by exposure to 1 μM E-4031 (to block IKr) and 1 μg ml−1 veratrine (a recognized sodium channel agonist). In these experiments, performed while continuously pacing at 1 Hz, recordings were taken every 5 min after initiating superfusion with 1 μM E-4031 + 1 μg ml−1 veratrine until a ‘quasi’ steady-state was attained (Fig. 10A and B). Then, in the continued presence of E-4031 and veratrine that pharmacologically lengthened APD, the effects of IKs block were examined by either applying 100 nM L-735,821 or 10 μM chromanol 293B. L-735,821 markedly lengthened APD under these conditions from 383.5 ± 25.2 to 442.1 ± 32.3 ms (P < 0.01, n = 7) (Fig. 10). This effect was in sharp contrast to the negligible effect of L-735,821 on normal APD (Figs 4 and 6). Comparable effects on APD were obtained with chromanol 293B in the continuous presence of E-4031 and veratrine (APD was 366.1 ± 13.1 ms before chromanol 293B versus 429.5 ± 23.5 ms after its addition, P < 0.01, n = 8). These results indicate that the effect of IKs on APD is substantially increased when APD is abnormally lengthened.
Figure 10. Effect of 100 nM L-735,821 on dog ventricular action potentials recorded in the presence of 1 μM E-4031 and 1 μg ml−1 veratrine.

A, the time course of a representative experiment. At 0 min 1 μM E-4031 and 1 μg ml−1 veratrine were added and measurements were taken every 5 min until a ‘quasi’ steady state was achieved. Then 100 nM L-735,821 was added to the bath in the continuous presence of E-4031 and veratrine. The relation prior to addition of L-735,821 was fitted by the equation Y = A+Bexp(-X/C) to estimate the time-dependent changes that would have occurred in the absence of the IKs blocker (continuous line) so that the magnitude of its effect at 140 min is indicated by the arrow. B, representative action potentials recorded at baseline (0 min), after exposure to E-4031 and veratrine alone (70 min), and following addition of L-735,821 (130 min). C, comparison of the effect of L-735,821 on ‘short’ (open bar) and on ‘long’ (filled bar) dog ventricular action potentials, respectively, recorded in the absence or presence of E-4031 and veratrine. Small asterisks represent significant changes from baseline measurements (i.e. at 0 min). The filled star represents significant changes between the bars (P < 0.01 in both cases). Columns and error bars indicate means and s.e.m.
Influence of IKs and IKr inhibitions on the QTc interval in anaesthetized closed chest dogs
To examine further whether IKs block lengthens APD and increases QT interval, we examined the effects of chromanol 293B (1 mg kg−1i.v.) and D-sotalol (1 mg kg−1i.v.) in anaesthetized dogs (Table 1). In agreement with our invitro observations, chromanol 293B did not significantly affect QTc interval, while D-sotalol markedly lengthened it.
Table 1.
The effect of i.v. 1 mg kg−1 chromanol 293B and i.v. 1 mg kg−1 d-sotalol on the ECG interval durations in intact anaesthetized dogs
| Chromanol 293B (n = 6) | d-Sotalol (n = 5) | |||
|---|---|---|---|---|
| Interval durations | Control | Chromanol 293B | Control | d-Sotalol |
| PP (ms) | 463.3 ± 52.2 | 463.3 ± 39.4 | 360 ± 21.9 | 450.0 ± 19.5 * |
| PQ (ms) | 98.3 ± 10.5 | 95.0 ± 8.8 | 92.0 ± 10.2 | 96.0 ± 7.5 |
| QRS (ms) | 45.0 ± 2.2 | 48.3 ± 4.8 | 38.0 ± 2.0 | 42.0 ± 3.7 |
| QT (ms) | 223.3 ± 12.0 | 223.3 ± 9.5 | 194.0 ± 7.5 | 232.0 ± 3.7 * |
| QTc (ms) | 332.9 ± 16.1 | 330.5 ± 11.2 | 323.9 ± 7.3 | 346.5 ± 6.4 * |
QTc, QT was corrected using the Bazett equation.
P < 0.05.
DISCUSSION
Summary of the main results
Our results indicate that both chromanol 293B and L-735,821, purportedly selective IKs blockers, did not substantially lengthen APD in either dog right ventricular papillary muscle or Purkinje fibre preparations. Equivalent concentrations of both compounds, however, substantially blocked IKs in isolated dog ventricular myocytes. Adenylcyclase stimulation by forskolin, known to increase IKs (Walsh et al. 1989), did not substantially enhance the small increase in APD induced by either chromanol 293B or L-735,821 in dog papillary muscle. In contrast, E-4031 and D-sotalol (recognized IKr blockers) markedly lengthened dog ventricular muscle and Purkinje fibre APD. In agreement with these invitro results, QTc was increased invivo by D-sotalol but not by chromanol 293B in anaesthetized dogs. However, in papillary muscle preparations where APD was prolonged by E-4031 and veratrine both chromanol 293B and L-735,821 increased repolarization considerably.
Choice of drug concentrations
The concentrations of drugs used in this study are comparable to those previously described in the literature (Lathrop, 1985; Sanguinetti & Jurkiewicz, 1990; Salata et al. 1996b; Busch et al. 1996). D-Sotalol at a concentration of 30 μM, inhibited IKr by 30–50%; 1 and 5 μM E-4031 caused complete block. This amount of IKr block made examination of the effects of D-sotalol and E-4031 on Purkinje fibre APD difficult. E-4031-induced Purkinje fibre APD lengthening was, for example, so pronounced that recordings at pacing cycle lengths shorter than 1000 ms could not be achieved because these stimuli fell within the total refractory period.
The concentrations of L-735,821 (100 nM) and chromanol 293B (10 μM and 30 μM) were also comparable to those used by others (Salata et al. 1996b; Busch et al. 1996). This L-735,821 concentration completely blocked IKs as previously reported (Salata et al. 1996b). Chromanol 293B at 10 μM blocked IKs by 70% in agreement with findings in guinea-pig ventricular myocytes (Busch et al. 1996). Higher chromanol 293B concentrations, however, notably affected other repolarizing currents (Bosch et al. 1998). Although the application of a higher concentration of chromanol 293B made interpretation of its effect on action potential repolarization uncertain at best; we used 30 μM chromanol 293B in order to block IKs completely, during the assessment of IKr. At this chromanol 293B concentration, we observed marked Ito depression in good agreement with earlier reports from Bosch et al. (1998). High chromanol 293B concentrations may also block IKr; however, the results of this current study do not address or confirm this speculation.
The 1 mg kg−1i.v. dose of chromanol 293B in the in vivo experiments was chosen because both chromanol 293B and D-sotalol have similar molecular weights (324.4 versus 309, respectively) and both compounds were assumed to have similar potencies for channel block. Although chromanol 293B proved to be a potent IKs blocker in the patch-clamp measurements, the possibility that the applied dose of 1 mg kg−1i.v. chromanol 293B did not completely block IKs cannot be ruled out.
Comparison of the results with earlier findings
IKs and IKr are both generally accepted as having important roles during normal cardiac action potential repolarization (Sanguinetti & Jurkiewicz, 1990; D. W. Liu & Antzelevitch, 1995; Singh, 1998). However, selective IKs blockers have only recently been available (Salata et al. 1996b; Busch et al. 1996). With the development of such IKs blockers, it is possible to determine directly the effect of IKs on APD.
The few published studies that have examined the effect of IKs on cardiac APD were performed in guinea-pig papillary muscle (Schreieck et al. 1997) as well as in isolated guinea-pig and human ventricular myocytes (Bosch et al. 1998; Bryant et al. 1998) and in rabbit Purkinje cardiocytes (Cordeiro et al. 1998). The results obtained often contradict one another. Schreieck et al. (1997) for example using conventional microelectrodes, did not observe a significant APD increase after exposing multicellular guinea-pig papillary muscle preparations to 10 μM chromanol 293B. This lack of effect has been argued to result from the absence of adrenergic stimulation (Schreieck et al. 1997). In contrast, Bosch et al. (1998), using the whole-cell patch-clamp technique in single isolated guinea-pig and human myocytes, reported that APD increased following chromanol 293B exposure. In that study, a relatively small number of cells (5–8 cells) were examined and measurements in the absence or presence of chromanol 293B were made in different myocyte groups. It is also notable that APD measurements in single, isolated myocytes show enormous beat-to-beat variability probably due to loss of electrotonic influences among electrically coupled myocytes or the run-down of currents affecting repolarization. Nevertheless, our results using conventional microelectrode recordings in dog papillary muscles agree, in part, with those of Schreieck et al. (1997) in guinea-pig papillary muscle; i.e. 10 μM chromanol 293B did not lengthen APD in the absence of forskolin. However, in dog papillary muscle, we found no increase in APD after adenylcyclase stimulation as Schreieck et al. (1997) did following isoproterenol exposure in guinea-pig. This deviation from the findings of Schreieck et al. (1997) might be due to species differences. Certainly IKs amplitude is relatively large in the guinea-pig (Sanguinetti & Jurkiewicz, 1990) compared with that in the dog and other species (Gintant, 1996). In addition, we applied 1 μM forskolin while Schreieck et al. (1997) used 100 nM isoproterenol to activate adenylcyclase. Because other currents are also modulated by cAMP (e.g. ICa and ICl) that also affect APD (Harvey & Hume, 1989), the observations in the two studies may not be directly due to IKs block.
IKs block, in our study, produced substantially different effects in multicellular dog cardiac Purkinje fibre strands than previously reported by Cordeiro et al. (1998) using L-735,821 in four single, isolated rabbit cardiac Purkinje fibre cells. These investigators reported marked APD lengthening after superfusion with only 20 nM L-735,821. The reason for this discrepancy in findings is unknown. However, some investigators have suggested that because of its physical and/or chemical properties, L-735,821 poorly penetrates multicellular preparations but easily enters single myocytes (J. J. Salata, personal communication). Be that as it may, the action potential plateau voltage in the rabbit Purkinje fibre cells illustrated by Cordeiro et al. (1998, Fig. 9) is approximately −20 mV, while in the same study (Cordeiro et al. 1998, Fig. 11) these authors show that activation of the L-735,821-sensitive current (presumably IKs) occurs at voltages positive to 0 mV. These facts make it unlikely that the observed increase in APD reported by Cordeiro et al. (1998) was due to IKs block.
The effects of D-sotalol and E-4031 on Purkinje fibre APD in our study are in excellent agreement with those previously published (Strauss et al. 1970; Lathrop, 1985; Varróet al. 1986; Sanguinetti & Jurkiewicz, 1990).
Estimation of the amount of IKs and IKr activated during the action potential
We estimated IKr and IKs during normal ventricular action potentials. Currents measured during and after 200 ms rectangular and artificial action-potential-like test pulses indicated that IKr is several times greater than IKs. Consistent with these findings, the recent papers of Hancox et al. (1998) and Zhou et al. (1998) have also confirmed that IKr plays a crucial role in the action potential repolarization under physiological conditions. On the other hand, our finding suggests that IKs, unlike IKr, plays little role during normal action potential repolarization. Such a conclusion is well supported by the negligible effect of IKs block on isolated ventricular muscle and Purkinje fibre APD as well as on intact dog QTc.
When the duration of the rectangular or action potential-like test pulse was increased, however, IKs was more fully activated. Thus, IKs is expected to limit excessive APD lengthening when repolarization is abnormally lengthened. This speculation is supported by our experiments where APD was substantially increased pharmacologically by augmenting inward (INa) and decreasing outward (IKr) currents (Fig. 10).
Potential significance of the results
Prior to this study, IKs was believed vital to normal cardiac action potential repolarization. As such, IKs was thought to control normal APD and refractoriness (D. W. Liu & Antzelevitch, 1995; Sanguinetti & Keating, 1997; Singh, 1998). In addition, based on experiments performed in guinea-pig ventricular myocytes, selective IKs block was believed to increase APD without producing the undesired, reverse use-dependent APD lengthening which is characteristic of IKr block (Jurkiewicz & Sanguinetti, 1993). This expectation was based on the finding that IKs deactivates slowly in guinea-pig so that reduction in outward current due to its block would be expected to be greater at fast heart rates (short diastolic intervals) than at slow heart rates or long intervals between subsequent action potentials. More recently, however, both in dog ventricular myocytes (Gintant, 1996) and human ventricular myocytes (Iost et al. 1998), IKr has been demonstrated to deactivate slowly while IKs deactivates relatively rapidly. This is quite unlike the situation in the guinea-pig and brings the speculation originally presented by Jurkiewicz & Sanguinetti (1993) into question. It is also notable that Heath & Terrar (1996b) have recently reported rather rapid deactivation of IKs also in guinea-pig myocytes. Our finding that IKs block does not remarkably increase APD in either normal dog ventricular muscle or Purkinje fibres over a wide range of pacing frequencies directly contradicts the Jurkiewicz & Sanguinetti (1993) hypothesis. Our findings, however, must be examined in perspective with other recent observations. Shimizu & Antzelevitch (1998) have, for example, recently reported that chromanol 293B lengthened APD in wedge-perfused canine left ventricular muscle preparations. In these experiments, 1–10 μM chromanol 293B produced only a slight increase in APD, as in the present study. However, in that study (Shimizu & Antzelevitch, 1998) chromanol 293B concentrations greater than 30 μM substantially increased APD. Such concentrations are greater than those required to block IKs fully, and these chromanol 293B concentrations probably affect other outward currents involved in the control of APD (Bosch et al. 1998).
IKs block in the presence of sympathetic stimulation is also believed to selectively prevent APD shortening associated with cAMP-dependent augmentation of IKs (Vanoli et al. 1995). Such an effect could potentially provide anti-arrhythmic benefit and represent an innovative approach to arrhythmia treatment. In support of this speculation L-768,673 (a structural analogue of L-735,821) provides anti-arrhythmic efficacy following coronary artery ligation and sympathetic stimulation (Billman et al. 1998a,b). Our results in the presence of forskolin do not support such a speculation. As such, additional research is needed to clarify the effect of sympathetic stimulation on IKs and their combined role in arrhythmogenesis.
Although IKs may have little role in normal action potential repolarization, it probably plays a vital role when cardiac APD is abnormally lengthened by other means (e.g.by reductions in IKr or Ik1 or increases in INa or ICa). As such, pharmacological block of IKs might be expected to have severe detrimental consequences when this protective mechanism is eliminated. For example, if repolarization is excessively lengthened due to drug-induced IKr block, hypokalaemia, genetic abnormality, or bradycardia, the subsequent increase in APD would favour IKs activation and provide a negative feedback mechanism to limit further APD lengthening. Without such a mechanism, excessive APD lengthening might lead to enhanced regional repolarization dispersion (Surawicz, 1989) and increase propensity for development of early afterdepolarization (El-Sherif, 1992) associated with Torsade de Pointes induction. Such a role for IKs in limiting excessive APD lengthening was first postulated by Ito & Surawicz (1981), and if IKs plays such a role, anti-arrhythmic agents producing non-selective block of IKr and IKs (e.g. quinidine and azimilide) might be associated with a greater pro-arrhythmic risk than ‘pure’ (selective) IKr blockers (e.g. sotalol and dofetilide). In agreement with this speculation, Salata et al. (1998) have recently recommended IKs activation for prevention of pro-arrhythmic complications due to excessive potassium channel block.
Some forms of inherited long QT syndrome (LQT) probably represent situations where loss of the protective effect of IKs is detrimental. For example, LQT1 is an inherited disorder where fewer IKs channels are expressed than in normal individuals. Our results in dog indicating that IKs plays little role in normal action potential repolarization suggest that its absence alone would not result in a prolonged APD and a long QT interval. Thus, with the presence of the LQT1 phenotype in man associated with reduced IKs expression it is difficult to reconcile our findings. This discrepancy between observations may have two explanations: (1) IKs is more abundantly expressed in man than in dog, or (2) reduction in IKs in both dog and man increases the likelihood that reduction in other outward currents (or an increase in inward current) results in LQT. Preliminary results in man showing that IKs is similar to that in dog and that its block does not affect normal papillary muscle APD (Varróet al. 1999) supports the second possibility. Thus, it may be that the absence of IKs in these individuals simply limits their ability to restrict excessive APD lengthening due to other causes (e.g. hypokalaemia or bradycardia). This explanation would account for the recent finding that the penetrance of genetic defects involving reduction in IKs channel expression (LQT1) is rather low compared with other forms of LQT (Swan et al. 1998; Priori et al. 1998). Some of these authors report that only about 25% of patients with genetic defects encoding for IKs channels actually had abnormally long QT intervals (Priori et al. 1998).
Marked gender differences have recently been described in the prevalence of inherited and acquired LQT that may be due to differences in potassium current expression (X. K. Liu et al. 1998). This is an important area of research interest and significant differences may exist in IKs expression in males and females. However, in the present studies no attempt was made to differentiate between results obtained in myocytes or preparations isolated from animals of different gender.
D. W. Liu & Antzelevitch (1995) showed in isolated dog ventricular myocytes that M cells express a lower density of IKs channels than do subendocardial or subepicardial cells. These investigators postulate on this basis that the longer M cell APD was due to less repolarizing current flowing through IKs channels. Our present data, however, indicate that an 80–100%IKs block failed to lengthen APD substantially in dog subendocardial papillary muscle; i.e. substantial IKs block did not cause subendocardial cells to resemble M cells. Thus, differences in other membrane currents probably account for the differences in M cell and subendocardial ventricular muscle cell action potential configurations. Differences in endocardial and M cell sodium window currents (or slowly inactivating INa) density, for example, may help account for APD differences in these two cell types.
Conclusions
This study indicates that in normal dog ventricular muscle IKs plays a minor role in control of APD. This current, however, could provide an important means of limiting excessive APD lengthening when action potentials are increased beyond normal by other mechanisms.
Acknowledgments
This work was supported by grants from the Hungarian National Research Foundation (OTKA T-16651, OTKA T-020604), Hungarian Ministry of Health (ETT T-06125/POT97 and T-037/98), Hungarian Ministry of Education (FKFP 1025/1997) and from the Hungarian Academy of Sciences. Thanks are also extended to Mrs Zsuzsa Molnár for the technical assistance helping with the conventional microelectrode experiments and to Ms Ágnes Krajevszky for the technical assistance she provided in the ECG measurements. D.A.L. participated in the writing of this paper in his capacity at Georgetown University, and no official support or endorsement by NHLBI/NIH is intended or should be inferred.
References
- Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, van Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circulation Research. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Billman GE, Houle MS, Lynch J. Selective IKs blockade protects against ventricular fibrillation induced by myocardial ischaemia. European Heart Journal. 1998a;19:17. Abstract suppl. [Google Scholar]
- Billman GE, Houle MS, Lynch J. Selective IKs but not IKr blockade protects against ventricular fibrillation induced by myocardial ischaemia. Circulation. 1998b;98:I-52. [Google Scholar]
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of the chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current on repolarisation in human and guinea pig ventricular myocytes. Cardiovascular Research. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovascular Research. 1998;40:322–331. doi: 10.1016/s0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- Busch AE, Suessbrich H, Waldegger S, Sailer E, Greger R, Lang H, Lang F, Gibson KJ, Maylie JG. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Archiv. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. Journal of Pharmacology and Experimental Therapeutics. 1992;262:809–817. [PubMed] [Google Scholar]
- Carmeliet E. Mechanism and control of repolarisation. European Heart Journal. 1993;14(suppl. H):3–13. doi: 10.1093/eurheartj/14.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Spitzer KW, Giles WR. Repolarising K+ currents in rabbit heart Purkinje cells. The Journal of Physiology. 1998;508:811–823. doi: 10.1111/j.1469-7793.1998.811bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherif N. The proarrhythmic mechanism of drugs that prolong repolarisation. Role of early afterdepolarisation. New Trends in Arrhythmias. 1992;8:617–626. [Google Scholar]
- Follmer CH, Colatsky TJ. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- Gintant GA. Two components of delayed rectifier current in canine atrium and ventricle. Does IKs play a role in the reverse rate dependence of Class III agents? Circulation Research. 1996;78:26–37. doi: 10.1161/01.res.78.1.26. [DOI] [PubMed] [Google Scholar]
- Hancox JC, Levi AJ, Witchel HJ. Time course and voltage dependence of expressed HERG current compared with native ‘rapid’ delayed rectifier K current during the cardiac ventricular action potential. Pflügers Archiv. 1998;436:843–853. doi: 10.1007/s004240050713. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Hume JR. Autonomic regulation of a chloride current in heart. Science. 1989;244:983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Heath BM, Terrar DA. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Experimental Physiology. 1996a;81:587–603. doi: 10.1113/expphysiol.1996.sp003961. [DOI] [PubMed] [Google Scholar]
- Heath BM, Terrar DA. The deactivation kinetics of the delayed rectifier components IKr and IKs in guinea-pig isolated ventricular myocytes. Experimental Physiology. 1996b;81:605–621. doi: 10.1113/expphysiol.1996.sp003962. [DOI] [PubMed] [Google Scholar]
- Hohnloser SH, Woosley RL. Sotalol. New England Journal of Medicine. 1994;331:31–38. doi: 10.1056/NEJM199407073310108. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Snyders DJ. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990;81:686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- Iost N, Virág L, Opincariu M, Szécsi J, Varró A, Papp JG. Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovascular Research. 1998;40:508–515. doi: 10.1016/s0008-6363(98)00204-1. [DOI] [PubMed] [Google Scholar]
- Ito S, Surawicz B. Effect of tetraethylammonium chloride on action potential in cardiac Purkinje fibers. American Journal of Physiology. 1981;241:H139–144. doi: 10.1152/ajpheart.1981.241.2.H139. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circulation Research. 1993;71:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Lathrop DA. Electromechanical characterization of the effects of racemic sotalol and its optical isomers on isolated canine ventricular trabecular muscles and Purkinje strands. Canadian The Journal of Physiology and Pharmacology. 1985;63:1506–1512. doi: 10.1139/y85-248. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circulation Research. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circulation Research. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M, Woosley RL. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. Journal of Pharmacology and Experimental Therapeutics. 1998;285:672–679. [PubMed] [Google Scholar]
- Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. The Journal of Physiology. 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Bloise R, Schwartz PJ. Low penetrance in the long QT syndrome: the importance of molecular diagnosis. European Heart Journal. 1998;19:424. Abstract suppl. [Google Scholar]
- Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ, Raynor B, Swanson R, Fermini B. IK of rabbit ventricle is composed of two currents: evidence for IKs. American Journal of Physiology. 1996a;271:H2477–2489. doi: 10.1152/ajpheart.1996.271.6.H2477. [DOI] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Sanguinetti MC, Siegl DK, Claremon DA, Remy DC, Eliott JM, Libby BE. The novel Class III antiarrhythmic agent, L-735821 is a potent and selective blocker of IKs in guinea pig ventricular myocytes. Circulation. 1996b;94:I-529. [Google Scholar]
- Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Molecular Pharmacology. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. Journal of General Physiology. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Keating MT. Role of delayed rectifier potassium channels in cardiac repolarisation and arrhythmias. News in Physiological Sciences. 1997;12:152–158. [Google Scholar]
- Schreieck J, Wang Y, Gjini V, Korth M, Zrenner B, Schömig A, Schmitt C. Differential effect of beta-adrenergic stimulation on the frequency-dependent electrophysiologic actions of the new class III antiarrhythmics dofetilide, ambasilide, and chromanol 293B. Journal of Cardiovascular Electrophysiology. 1997;8:1420–1430. doi: 10.1111/j.1540-8167.1997.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the Long-QT syndrome. Effects of β-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarisation and Torsade de Pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Singh BN. Antiarrhythmic drugs: a reorientation in light of recent developments in the control of disorders of rhythm. American Journal of Cardiology. 1998;81:3–13D. doi: 10.1016/s0002-9149(98)00147-7. [DOI] [PubMed] [Google Scholar]
- Singh BN, Vaughan Williams EM. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. British Journal of Pharmacology. 1970;39:675–687. doi: 10.1111/j.1476-5381.1970.tb09893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. Journal of General Physiology. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss HC, Bigger JT, Hoffman BF. Electrophysiological and β-receptor blocking effects of MJ 1999 on dog and rabbit cardiac tissue. Circulation Research. 1970;26:661–678. doi: 10.1161/01.res.26.6.661. [DOI] [PubMed] [Google Scholar]
- Surawicz B. Electrophysiologic substrate of Torsade de Pointes: Dispersion of repolarisation or early afterdepolarisation? Journal of American College of Cardiology. 1989;14:172–184. doi: 10.1016/0735-1097(89)90069-7. [DOI] [PubMed] [Google Scholar]
- Swan H, Saarinen K, Kontula K, Toivonen L, Viitasalo M. Evaluation of QT interval duration and dispersion and proposed clinical criteria in diagnosis of long QT syndrome in patients with a genetically uniform type of LQT1. Journal of American College of Cardiology. 1998;32:486–491. doi: 10.1016/s0735-1097(98)00248-4. [DOI] [PubMed] [Google Scholar]
- Turgeon J, Daleau P, Bennett PB, Wiggins SS, Selby L, Roden DM. Block of IKs, the slow component of the delayed rectifier K+ current, by the diuretic agent indapamide in guinea pig myocytes. Circulation Research. 1994;75:879–886. doi: 10.1161/01.res.75.5.879. [DOI] [PubMed] [Google Scholar]
- Vanoli E, Priori SG, Nakagawa H, Hirao K, Napolitano C, Diehl L, Lazzara R, Schwartz PJ. Sympathetic activation, ventricular repolarisation and IKr blockade: implications for the antifibrillatory efficacy of potassium channel blocking agents. Journal of American College of Cardiology. 1995;25:1609–1614. doi: 10.1016/0735-1097(95)00046-7. [DOI] [PubMed] [Google Scholar]
- Varró A, Iost N, Virág L, Opincariu M, Szécsi J, Papp JG. Does IKs play an important role in the repolarization in normal human ventricular muscle? Circulation. 1999;100:I-495. [Google Scholar]
- Varró A, Lathrop DA, Hester SB, Nánási PP, Papp JG. Ionic currents and action potentials in rabbit, rat, and guinea pig ventricular myocytes. Basic Research in Cardiology. 1993;88:93–102. doi: 10.1007/BF00798257. [DOI] [PubMed] [Google Scholar]
- Varró A, Nakaya Y, Elharrar V, Surawicz B. Effect of antiarrhythmic drugs on the cycle length-dependent action potential duration in dog Purkinje and ventricular muscle fibers. Journal of Cardiovascular Pharmacology. 1986;8:178–185. doi: 10.1097/00005344-198601000-00026. [DOI] [PubMed] [Google Scholar]
- Végh A, Komori S, Szekeres L, Parratt JR. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovascular Research. 1992;26:487–495. doi: 10.1093/cvr/26.5.487. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Begenisch TB, Kass RS. Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. Journal of General Physiology. 1989;93:841–854. doi: 10.1085/jgp.93.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophysical Journal. 1998;74:230–241. doi: 10.1016/S0006-3495(98)77782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


