Abstract
Activity of amino acid transport and relative abundance of mRNAs encoding related transporters have been studied in parallel either before or following in vitro culture of explants of human placental chorionic villi.
Amino acid transport activities through systems L (1.9-fold), y+L (2.6-fold) and y+ (3.2-fold) were markedly enhanced following culture for 48 h.
Relative mRNA abundance (determined by reverse transcription-polymerase chain reaction) for the heavy chain of CD98 surface antigen and for the cationic amino acid transporter-1 were similarly stimulated (2.8-fold and 2.6-fold, respectively). In contrast, none of the mRNA levels for light chains of CD98 (system L-amino acid transporter-1, system L-amino acid transporter-2, system y+L-amino acid transporter-1 and system y+L-amino acid transporter-2) studied nor for the cationic amino acid transporter-2B were altered.
The molecular basis of amino acid transport in vertebrate cells is currently a topic of much research (Palacin et al. 1998). Particularly striking are recent observations showing that some transporters are monomeric whereas others (a rapidly expanding group) are heterodimeric (Palacin, 1994). These include transporters responsible for system L (Broer et al. 1998; Kanai et al. 1998; Mannion et al. 1998; Mastroberardino et al. 1998; Pineda et al. 1999), for system y+L (Broer et al. 1998; Mastroberardino et al. 1998; Torrents et al. 1998; Pfeiffer et al. 1999) and for system XC− (Sato et al. 1999). Such transporters are composed of a common heavy chain (heavy chain of CD98 surface antigen (CD98hc)) and one of a family of related light chains (system L-amino acid transporter-1 (LAT-1), system L-amino acid transporter-2 (LAT-2), system y+L-amino acid transporter-1 (y+LAT-1), system y+L-amino acid transporter-2 (y+LAT-2) and system XC−-amino acid transporter (xCT)). Mastroberardino and colleagues (Mastroberardino et al. 1998) have proposed that in Xenopus oocytes it is expression of the heavy chain that is necessary for maturation, transport and/or surface residence of heterologous expressed light chain subunits; additionally, in epithelia one role of the heavy chain may be to determine the polarity of transporter expression (Pfeiffer et al. 1999; Pineda et al. 1999). In contrast other transporters (e.g. system y+) are composed of only a single chain (for system y+, a member of the cationic amino acid transporter (CAT) gene family).
In the placenta it has been known since the work of Smith and colleagues (Smith et al. 1973) that induction of transport activity follows in vitro culture. For one heterodimeric transporter (system y+L), Fei and colleagues (Fei et al. 1995) showed that following anti-sense depletion of the heavy chain of CD98 from total placental mRNA there was no longer expression of this transport function. This experiment importantly shows that the heavy chain is necessary for heterodimeric function; it does not, however, show whether under normal conditions it is expression of the heavy or of the light chain or of both that is altered, and that which therefore may be rate limiting. We have now looked at the pattern of transporter mRNA expression following in vitro culture of this tissue in order to discover for two heterodimeric transporters (systems L and y+L) how subunit mRNA abundance relates to transport function in this human tissue.
METHODS
Culture of villous tissue
Normal term placentae were obtained (with ethical committee approval) within 15 min from delivery and chilled on ice. The placenta was cut into cotyledons and the decidual surface was removed. The tissue was washed three times with ice-cold phosphate-buffered saline (PBS) containing 100 unit ml−1 penicillin and 100 unit ml−1 streptomycin, and chorionic villi were dissected into small pieces (a single piece was approximately 5 mg). All these procedures were carried out below 4°C. Three pieces of chorionic villi were placed on a polyester mesh (Netwell 500 μm mesh, Costar, NY, USA) and cultured in a 35 mm plastic culture dish at 37°C in an atmosphere of 5 % CO2 and 95 % air for 48 h with a change of medium 24 h after starting incubation. The culture medium used was RPMI medium 1640 with addition of 5 % fetal bovine serum (FCS), 100 unit ml−1 penicillin and 100 unit ml−1 streptomycin. Cultures were conducted in triplicate for each set of experiments to assess reproducibility. The number of placentae used in each study is mentioned in the figure legends.
Amino acid influx studies
Chorionic villi were washed twice with pre-warmed (37°C) PBS. The influx of amino acid was initiated by placing tissue in pre-warmed PBS containing 2 μM labelled amino acid, followed by further incubation at 37°C. Other additions are described in the figure legends. When Na+-free PBS was required for determination of influx in the absence of Na+, the following, i.e. NaCl, NaHCO3 and NaH2PO4, were replaced by choline chloride, choline bicarbonate and KH2PO4, respectively. Chorionic villi were quickly washed with ice-cold PBS with 10 mm unlabelled substrate amino acid, suspended in PBS and disrupted by sonication for 30 s in an ice bath at a power of 100 W. Aliquots were taken for liquid scintillation counting and protein determination.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Amino acid transporter relative mRNA abundance was analysed by semi-quantitative RT-PCR using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal standard. Total RNA was extracted from either fresh or cultured explant of villous tissue with a QuickPrep Total RNA Extraction Kit (Amersham Pharmacia Biotech, St Albans, UK) according to the manufacturer's protocol. RNA samples were treated with DNase I (Promega, Madison, WI, USA) before RT-PCR to remove any contaminating DNA. The primers used in the subsequent RT-PCR and the expected size of the PCR products are summarised in Table 1. One microgram RNA was reverse transcribed into cDNA using an oligo(dT)12–18 primer (Gibco BRL, Grand Island, NY, USA). The reverse transcription reaction, containing 500 μM dNTP (Gibco BRL), 25 μg ml−1 oligo(dT)12–18 primer, 10 unit μl−1 M-MLV reverse transcriptase (Gibco BRL), 3 mm MgCl2, 75 mm KCl, 10 mm dithiothreitol and 50 mm Tris-HCl (pH 8.3), was sequentially incubated at 25°C for 10 min, at 42°C for 50 min and at 70°C for 15 min, and then cooled on ice. As a negative control for the absence of exogenous DNA contamination, reactions run without RNA or with RNA in the absence of the reverse transcriptase revealed no amplified product (data not shown). The synthesised cDNA (0.05 μg equivalent to RNA) was used for PCR amplification in a reaction mixture containing 200 μM dNTP, 1 μM forward and backward primers, 0.05 unit μl−1Taq DNA polymerase (Gibco BRL), 1.5 mm MgCl2, 50 mm KCl and 20 mm Tris-HCl (pH 8.4). The PCR conditions were: 94°C for 3 min, 60°C for 1 min and 72°C for 2 min; then 26 cycles (for amino acid transporters) and 20 cycles (for GAPDH) of 94°C for 1 min, 60°C for 1 min and 72°C for 2 min; followed by a 10 min final extension at 72°C. The amount of template cDNA and the number of cycles were determined experimentally so that quantitative comparison could be made during the exponential phase of the amplification process for both the target and reference gene. PCR products were separated on a 2 % agarose gel. Gels were stained with ethidium bromide. A single band for each gene was observed at the expected size except for y+LAT-2 which was not detected. The intensity of both the transporter and the GAPDH band for each sample was quantified using a gel documentation and analysis system (GDS8000, Ultra-Violet Products, Cambridge, UK) and the ratio of the two was used as a normalised value for expression of each transporter gene. All assays were conducted in triplicate.
Table 1.
Primers used in PCR amplification
| Gene | Oligonucleotide sequence | Nucleotide number | Product size (bp) | Reference |
|---|---|---|---|---|
| CD98hc | forward: 5′-GAATGAGTTAGAGCCCGAGA-3′ | 151–170 | 269 | Quackenbush et al. (1987) |
| backward: 5′-CGATTATGACCACGGCACCA-3′ | 400–419 | |||
| LAT-1 | forward: 5′-TGTGCTGGCATTATACAGCG-3′ | 781–800 | 231 | Mastroberardino et al. (1998) |
| backward: 5′-AGGTGATAGTTCCCGAAGTC-3′ | 992–1011 | |||
| LAT-2 | forward: 5′-TTTCCAGGAACCTGACATCG-3′ | 896–915 | 200 | Pineda et al. (1999) |
| backward: 5′-ACATTGCAGTGACATAAGCG-3′ | 1076–1095 | |||
| y+LAT-1 | forward: 5′-CAGCACTGAGTATGAAGTGG-3′ | 295–314 | 186 | Torrents et al. (1998) |
| backward: 5′-TATATGAGCACACCCTTGGG-3′ | 461–480 | |||
| y+LAT-2 | forward: 5′-ACCCACCTACCATCTTGTCC-3′ | 291–310 | 119 | Nagase et al. (1996) |
| backward: 5′-CATTCAGCAGGGAGATCTCC-3′ | 390–409 | |||
| CAT-1 | forward: 5′-CCAACGTCAATGATAGGACC-3′ | 1274–1293 | 620 | Yoshimoto et al. (1991) |
| backward: 5′-GTCCAGCTGCATCATGAGAT-3′ | 1874–1893 | |||
| CAT-2B | forward: 5′-GCTACTTTATCATCGGGTGC-3′ | 1168–1187 | 438 | Closs et al. (1997) |
| backward: 5′-AACAAGAAACAGCGCGAGGA-3′ | 1586–1605 | |||
| GAPDH | forward: 5′-CGGGAAGCTTGTGATCAATGG-3′ | 225–245 | 358 | Tso et al. (1985) |
| backward: 5′-GGCAGTGATGGCATGGACTG-3′ | 563–582 |
CD98hc, heavy chain of CD98 surface antigen; LAT-1, system L-amino acid transporter-1; LAT-2, system L-amino acid transporter-2; y+LAT-1, system y+L-amino acid transporter-1; y+LAT-2, system y+L-amino acid transporter-2; CAT-1, cationic amino acid transporter-1; CAT-2B, cationic amino acid transporter-2B; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Differences between groups were analysed using Student's t test and results were considered statistically significant at P < 0.05.
RESULTS
Figure 1 compares 2 μM leucine influx into fresh and cultured microdissected chorionic villous explants prepared from term human placentae under initial rate conditions at 37°C. Total Na+-independent mediated transport was stimulated approximately 2-fold following 48 h culture. In order to separate the transport pathways contributing to total Na+-independent flux, the synthetic amino acid 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) (Christensen, 1979), a system-L-specific analogue, was added at 2 mm. The extent of the inhibition by BCH was the same as that found with 2 mm leucine suggesting that system L accounts for virtually all of the leucine flux into both fresh and cultured chorionic villi. It follows therefore that system L is increased 1.9-fold by culture. Figure 2 shows the equivalent experiment on fresh and cultured villi for lysine influx either in the presence or in the absence of Na+. Mediated lysine influx was lower than that for leucine; however, it too was markedly stimulated by culture. By studying the effect of low concentrations of leucine (100 μM) with or without Na+ it is possible to fractionate total mediated lysine influx into that through system y+L and that through system y+. (There is no evidence for participation of systems b0, + or B0, + since the inhibition by leucine is Na+ dependent and the total fluxes in both fresh and in cultured tissue are not altered by the presence of Na+.) In the fresh tissue leucine inhibited very substantially in the presence but not in the absence of Na+. This suggests that system y+L is dominant and that there is very little system y+ activity. Following culture, system y+L (leucine sensitive in the presence of Na+) and system y+ (leucine resistant in the presence of Na+) were both increased (2.6-fold and 3.2-fold, respectively) accounting for the observed stimulation of total flux. Figure 3 shows that relative mRNA abundance of the light chains of the heterodimeric transporters LAT-1, LAT-2 and y+LAT-1 were not altered following 48 h culture. In contrast, relative mRNA abundance of the heavy chain (CD98hc) was increased approximately 3-fold when compared with that before culture. For the monomeric system y+ transporter there was enhanced relative mRNA abundance of the cationic amino acid transporter-1 (CAT-1) but not of the cationic amino acid transporter-2B (CAT-2B) gene following culture.
Figure 1. Effect of in vitro culture on L-leucine influx in placental explants.

L-Leucine influx in either fresh or cultured explant of villous tissue over a 30 s period was measured in a medium containing 2 μM L-[3H]leucine (2 μCi ml−1 or 74 KBq ml−1) with or without unlabelled amino acid (2 mm for BCH or L-leucine, final concentration) in the absence (choline) of Na+ as described in Methods. Data show carrier-mediated influx rate defined by subtracting the diffusional component from the total influx. The diffusional component was determined by measuring the influx of L-[3H]leucine in the presence of 20 mm unlabelled L-leucine. Data represent the mean ± s.d. of three separate experiments from three placentae. The mediated BCH-sensitive fluxes in fresh and in cultured tissue are, respectively, 9.91 ± 1.51 and 18.64 ± 3.52 pmol (mg protein)−1 (30 s)−1. These are significantly different (P = 0.025). BCH, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid.
Figure 2. Effect of in vitro culture on L-lysine influx in placental explants.
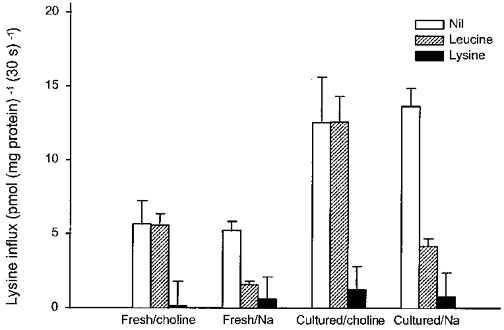
L-Lysine influx in either fresh or cultured explant of villous tissue over a 30 s period was measured in a medium containing 2 μM L-[3H]lysine (2 μCi ml−1 or 74 KBq ml−1) with or without unlabelled amino acid (100 μM for L-leucine or 2 mm for L-lysine, final concentration) in the presence or absence (choline) of Na+ as described in Methods. Data show carrier-mediated influx rate defined by subtracting the diffusional component from the total influx in either the presence or absence of Na+. The diffusional component was determined by measuring the influx of L-[3H]lysine in the presence of 20 mm unlabelled L-lysine. Data represent the mean ± s.d. of three separate experiments from three placentae. The mediated L-leucine-sensitive fluxes in the presence of Na+ in fresh and in cultured tissue are, respectively, 3.66 ± 0.61 and 9.49 ± 1.24 pmol (mg protein)−1 (30 s)−1. These are significantly different (P = 0.026). The mediated L-leucine-insensitive fluxes in the presence of Na+ in fresh and in cultured tissue are, respectively, 0.95 ± 0.21 and 3.01 ± 0.01 pmol (mg protein)−1 (30 s)−1. These are significantly different (P = 0.014).
Figure 3. Effect of in vitro culture on the relative abundance of the amino acid transporter mRNA in placental explants.
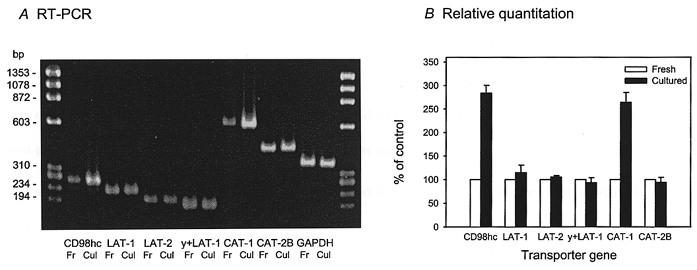
A, RT-PCR. The relative abundance of amino acid transporter mRNA and GAPDH mRNA were analysed by RT-PCR as described in Methods. The results presented are from a single representative experiment. Fr, fresh explant; Cul, cultured explant. B, relative quantification of the amino acid transporter mRNA. The intensity of both the amino acid transporter and the GAPDH band was quantified by using a gel documentation and analysis system of PCR products and the ratio of the two was used as a normalised relative abundance value of each transporter gene. Data represent the mean ± s.d. of three separate experiments from three placentae, expressed as a percentage of control (i.e. values for fresh explant). For CD98hc and for CAT-1 the observed changes were highly significant (P < 0.01). CD98hc, heavy chain of CD98 surface antigen; LAT-1, system L-amino acid transporter-1; LAT-2, system L-amino acid transporter-2; y+LAT-1, system y+L-amino acid transporter-1; CAT-1, cationic amino acid transporter-1; CAT-2B, cationic amino acid transporter-2B; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
DISCUSSION
It has been known for many years (Smith et al. 1973) that alterations in amino acid transport follow in vitro culture of tissues including placenta. Using explants of villous tissue (Watson et al. 1995) from normal term human placentae we have confirmed that there is indeed an increase in transport through amino acid transport systems y+, y+L and L. Although the mechanism of this process is currently poorly understood, the phenomenon allows us to ask novel questions concerning the expression of molecules responsible for these transport systems. In particular we can for the first time seek to establish whether induction of those amino acid transport systems that are now known to result from heteromeric protein interactions are limited by expression of one or of both of the relevant subunits. These results on heteromeric transport systems (y+L, L) can be compared with findings on system y+ which are more straightforward to interpret since they require only expression of a (family) of homomeric proteins (CAT gene products).
Our experiments show that relative mRNA abundance for CD98hc and for CAT-1 are markedly stimulated (2.8-fold and 2.6-fold, respectively) following explant culture in RPMI medium 1640 supplemented with 5 % FCS for 48 h: this is not seen for any of the four CD98 light chains (LAT-1, LAT-2, y+LAT-1 and y+LAT-2) studied, nor for CAT2-B. Since there is a concomitant increase in transport through sytems y+L and L (and also through system y+) our results show that for the heterodimeric transporters it is the single heavy chain (CD98hc), not the light chains, whose expression correlates with function. Possible mechanisms underlying this will require future studies aimed at determining protein abundances, subcellular localisations and stabilities; all of these will require appropriate antibodies (or ligands) to be developed. Nevertheless the current finding raises the possibility that CD98hc expression is rate limiting for transport phenotype.
Previous work (Mastroberardino et al. 1998; Pfeiffer et al. 1999; Pineda et al. 1999) has suggested that in heterologous expression systems CD98hc and rBAT (both of which are single transmembrane proteins that associate with a variety of 12 transmembrane light chains to produce a wide spectrum of different amino acid transport systems) regulate delivery of functional heterodimeric protein to the cell surface. Our results on an intact human tissue are compatible with such a model and suggest that in this explant system there may similarly be an intracellular pool of light chains available for delivery to the cell surface at a rate that depends on the rate of expression of the heavy chain. It should be pointed out that in other work (Gaugitsch et al. 1992; Spindler et al. 1997) expression of light chain following mitogen or hormonal stimulation was clearly observed, showing that in these systems it is not only the heavy chain whose transcription is physiologically regulated.
Since we have looked in parallel at expression of two such heteromeric systems (L and y+L) we can also examine an associated issue: what determines the relative distribution of a (limited) amount of heavy chain between an (excess) of differing light chains? Our data, following in vitro culture, show that there is a similar increment in function of both system L (1.9-fold) and system y+L (2.6-fold) without a change in relative mRNA abundance of associated light chains; this suggests that it is the relative molar proportion of available light chains that determines the relative level of different heteromeric transporter expression.
In native syncytiotrophoblasts, amino acid transport to the fetus is facilitated by polarised distribution of different transporters. The changes in transport function that we observed are compatible with the explant of villous tissue showing a reversal of this polarisation. In the less differentiated cytotrophoblast and in the basal membrane but not in the apical membrane of the mature syncytiotrophoblast system y+L will contribute to polarity of amino acid delivery (Eleno et al. 1994; Ayuk et al. 1998). The results reported here are in keeping with the reversal of cell phenotype seen within 48 h incubation of the first trimester human placental explants (Watson et al. 1995).
References
- Ayuk P, Glazier J, Sides K, D'Souza S, Sibley CP. L-Arginine transport in human placental syncytiotrophoblast microvillous and basal plasma membrane vesicles: membrane polarization and gestational regulation. The Journal of Physiology. 1998;509.P:78. P. [Google Scholar]
- Broer A, Hamprecht B, Broer S. Discrimination of two amino acid transport activities in 4F2 heavy chain-expressing Xenopus laevis oocytes. Biochemical Journal. 1998;333:549–554. doi: 10.1042/bj3330549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen HN. Exploiting amino acid structure to learn about membrane transport. Advances in Enzymology. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Closs EI, Graf P, Habermeier A, Cunningham JM, Forstermann U. Human cationic amino acid transporters hCAT-1, hCAT-2A, and hCAT-2B: three related carriers with distinct transport properties. Biochemistry. 1997;36:6462–6468. doi: 10.1021/bi962829p. [DOI] [PubMed] [Google Scholar]
- Eleno N, Devés R, Boyd CAR. Membrane potential dependence of the kinetics of cationic amino acid transport systems in human placenta. The Journal of Physiology. 1994;479:291–300. doi: 10.1113/jphysiol.1994.sp020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei YJ, Prasad PD, Leibach FH, Ganapathy V. The amino acid transport system y+L induced in Xenopus laevis oocytes by human choriocarcinoma cell (JAR) mRNA is functionally related to the heavy chain of the 4F2 cell surface antigen. Biochemistry. 1995;34:8744–8751. doi: 10.1021/bi00027a025. [DOI] [PubMed] [Google Scholar]
- Gaugitsch HW, Prieschl EE, Kalthoff F, Huber NE, Baumruker T. A novel transiently expressed, integral membrane protein linked to cell activation. Molecular cloning via the rapid degradation signal AUUUA. Journal of Biological Chemistry. 1992;267:11267–11273. [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) Journal of Biological Chemistry. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Mannion BA, Kolesnikova TV, Lin SH, Wang S, Thompson NL, Hemler ME. The light chain of CD98 is identified as E16/TA1 protein. Journal of Biological Chemistry. 1998;273:33127–33129. doi: 10.1074/jbc.273.50.33127. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Nagase T, Seki N, Ishikawa K, Ohira M, Kawarabayasi Y, Ohara O, Tanaka A, Kotani H, Miyajima N, Nomura N. Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Research. 1996;3:321–329. doi: 10.1093/dnares/3.5.321. [DOI] [PubMed] [Google Scholar]
- Palacin M. A new family of proteins (rBAT and 4F2hc) involved in cationic and zwitterionic amino acid transport: a tale of two proteins in search of a transport function. Journal of Experimental Biology. 1994;196:123–137. doi: 10.1242/jeb.196.1.123. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiological Reviews. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO Journal. 1999;18:49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. Journal of Biological Chemistry. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Quackenbush E, Clabby M, Gottesdiener KM, Barbosa J, Jones NH, Strominger JL, Speck S, Leiden JM. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proceedings of the National Academy of Sciences of the USA. 1987;84:6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Tamba T, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. Journal of Biological Chemistry. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Smith CH, Adcock EW, III, Teasdale F, Meschia G, Battaglia FC. Placental amino acid uptake: tissue preparation, kinetics, and preincubation effect. American Journal of Physiology. 1973;224:558–564. doi: 10.1152/ajplegacy.1973.224.3.558. [DOI] [PubMed] [Google Scholar]
- Spindler B, Mastroberardino L, Custer M, Verrey F. Characterization of early aldosterone-induced RNAs identified in A6 kidney epithelia. Pflügers Archiv. 1997;434:323–331. doi: 10.1007/s004240050403. [DOI] [PubMed] [Google Scholar]
- Torrents D, Estevez R, Pineda M, Fernandez E, Lloberas J, Shi YB, Zorzano A, Palacin M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. Journal of Biological Chemistry. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Research. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AL, Palmer ME, Burton G. Human chorionic gonadotrophin release and tissue viability in placental organ culture. Human Reproduction. 1995;10:2159–2164. doi: 10.1093/oxfordjournals.humrep.a136253. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yoshimoto E, Meruelo D. Molecular cloning and characterization of a novel human gene homologous to the murine ecotropic retroviral receptor. Virology. 1991;185:10–17. doi: 10.1016/0042-6822(91)90748-z. [DOI] [PubMed] [Google Scholar]


