Abstract
We have examined the responses of neurones in the suprachiasmatic nuclei (SCN) of the rat to retinal illumination under photopic and scotopic conditions to identify the types of photoreceptor input to these nuclei.
The majority of visually responsive SCN neurones studied under dark adaptation received rod input (48 of 52, 92 %). The action spectrum conformed to the sensitivity of rhodopsin, with maximal sensitivity at around 505 nm.
When also studied under light adaptation, most visually responsive SCN neurones (20 out of 26, 77 %) responded to input from cones. The action spectra conformed to the spectrum of green cone opsin, with a main sensitivity peak at 510 nm and a significant secondary peak in the near-ultraviolet region of the spectrum.
The frequency of spontaneous activity was typically low under scotopic conditions (range 0.2–17.2 Hz) and higher under photopic conditions (range 0.6–40 Hz) for any given neurone. The most common response under scotopic conditions was an ‘on-excitation’ (32 of 48, 62.5 %), which changed under photopic conditions to an on-excitation followed by a more prominent off-inhibition.
Responses also changed due to endogenous ultradian cycles. Depending on the phase, responses could be altogether absent and even reverted from excitation to inhibition on opposite phases of a cycle. Ultradian cycles had a circadian dependence and were most common at around the light phase:dark phase (L:D) and D:L transition points of the circadian cycle.
Under photopic conditions, SCN neurones showed rhythmic electrical activity, with a preferred firing interval that had a value between 18 and 39 ms. This rhythmic activity was probably the result of endogenous subthreshold membrane potential oscillations.
In conclusion, light acting either via rod or cone pathways could have powerful, opposing actions on SCN neurones. These actions were state dependent. The presence of these neuronal responses suggests a role for rod and cone photoreceptors in SCN function.
The suprachiasmatic nuclei (SCN) of the anterior hypothalamus have been identified as a major pacemaker of the circadian system in mammals. The endogenous circadian rhythmicity of their neural activity can be directly entrained to the environmental light-dark cycle via the retina and the retinohypothalamic tract. Neurophysiological studies using both single-unit and multi-unit recordings have shown that a subpopulation of SCN cells is influenced by retinal illumination (Groos & Mason, 1980) with a responsiveness to changes in both duration and intensity of illumination (Meijer et al. 1986). The identity of the photoreceptor type that mediates the responsiveness of the circadian system of rodents to light remains, however, unknown. Involvement of either a cone opsin-based mechanism (Nelson & Takahashi, 1991) or rhodopsin (Bronstein et al. 1987) has been suggested. An involvement of at least one photoreceptor with cone-like characteristics has been proposed (Provencio & Foster, 1995; David-Gray et al. 1998) and recently the possibility of non-rod, non-cone photoreceptors has also been suggested (Freedman et al. 1997; reviewed by Foster, 1998). A previous study from this laboratory demonstrated an input via both retinal rod and cone pathways to the pineal gland of mammals (Thiele & Meissl, 1987), raising the prospect that more than one photoreceptor may be involved in transmitting a photic signal to the circadian system. In the present study we have examined the spectral sensitivity and other response characteristics of SCN neurones under scotopic and photopic conditions to evaluate the possible mechanisms underlying photoreception in the circadian system.
Recently, a novel cone photopigment has been identified using immunohistochemical methods (Szel & Röhlich, 1992). From electroretinographic recordings it appears that it has a maximal sensitivity in the near ultraviolet (UV) part of the spectrum and minimal sensitivity in the ‘visible’ part (Deegan & Jacobs, 1993). Whether this photopigment is used in visual processing remains unclear. It may have a local homeostatic function in the retina and may not necessarily be involved in the transmission of visual signals to brain centres. In that case, central neurones should show no spectral sensitivity changes in response to short wavelength (SW) or long wavelength (LW) chromatic adaptation. Therefore, an additional aim of this study was to determine whether SCN neurones had any unusual properties in the ultraviolet range that might indicate that the novel SW photopigment is used in higher visual processes.
METHODS
All animal experiments reported in this manuscript were conducted in accordance with German animal welfare guidelines. The experiments were carried out in 20 adult pigmented rats (Brown Norway and lean Zucker, 220–390 g body weight), anaesthetised by an intraperitoneal injection of 25 % w/v urethane (1.2 g (kg body weight)−1). Another set of 12 rats were anaesthetised with sodium pentobarbitone (Nembutal; initial dose 90 mg (kg body weight)−1i.p., supplemented i.v. as and when necessary). Limb withdrawal reflexes were monitored to ensure adequate levels of general anaesthesia. The optic chiasm was exposed by a ventral craniotomy. The eyelids were retracted and diffusers were placed over the eyes to achieve a uniform illumination of the retina. The rats were placed in a light-sealed isolated chamber within a light-sealed laboratory and both retinae were illuminated with 0.1–1 s flashes of narrow-waveband light over a range of 340–674 nm from a xenon arc light source.
Experiments were conducted under scotopic or photopic conditions. During scotopic conditions, the photon flux due to background illumination was less than 109 photons m−2 s−1. A period of dark adaptation of at least 30 min preceded any testing. Therefore the recorded scotopic thresholds are about 3–4 log units above the absolute scotopic threshold for the rat (Dodt & Echte, 1961). During photopic conditions, the animals were tested under full-spectrum white background adaptation (up to 1019 photons m−2 s−1) and also under chromatic adaptation with long wavelength background adaptation (infrared to green with a cutoff wavelength at 500 nm, irradiance range 1.3 × 1018 to 5.5 × 1018 photons m−2 s−1) and short wavelength background adaptation (ultraviolet A to blue with a cutoff at 456 nm, irradiance range 8.5 × 1017 to 1.1 × 1018 photons m−2 s−1) so as to differentiate between possibly different photopic photoreceptor processes. Under these photopic lighting conditions, rhodopsin is completely bleached and rods are incapable of responding to light.
Extracellular single-unit recordings were obtained from photosensory neurones of the suprachiasmatic nuclei using microcapillary glass micropipettes filled with 2 M NaCl.
Response thresholds of individual neurones to retinal illumination were determined across the range 340–674 nm and were corrected for transmittance efficiency of the rat lens (Gorgels & van Norren, 1992). They were subsequently converted to response sensitivity according to the simple formula:
where T represents threshold, in units of photon flux.
The spectral sensitivity measurements were compared to Dartnall nomograms (Dartnall, 1953) to estimate whether sensitivity was compatible with the spectra of known photopigments in the retina of the rat. Dartnall nomograms are empirical nomograms that approximate the spectral sensitivity of opsin-based photopigments near their peak sensitivity region in the ‘visible’ region of the electromagnetic spectrum. There is no available statistical test for fitting a Dartnall nomogram to raw data. The Dartnall nomograms can be empirically approximated by a set of equations (Lamb, 1995), which were also used to evaluate the spectral sensitivities of SCN neurones.
After a cell had been fully tested, the recording electrode was removed and an electrode containing a saturated solution of silver nitrate was inserted at the same position and depth. Silver nitrate was then injected iontophoretically using 10 ms square wave pulses. The polarity was changed from positive to negative every 1–2 min at 5–20 μA for at least 10 min. Silver oxidises in situ to form an extremely avid stain that turns dark brown with exposure to light. It was not included in the recording micropipette as it can cause an increase in electrode resistance following oxidation after contact with sodium chloride or tissue fluids. Additionally, nitrate is thought to be toxic, so that no further recordings were made after an iontophoretic injection of silver nitrate. On the other hand, this new method of marking the approximate recording location was very reliable and the silver sediment was well localised and very resistant to fixation and further histological processing. Traces of reactive gliosis caused by the electrode penetrations were an additional aid in localising nearby electrode tracks in relation to the reference injection of silver nitrate. At the end of each experiment animals were overdosed with sodium pentobarbitone (Nembutal).
All values, unless stated otherwise, are presented as means ± s.e.m.
RESULTS
The action spectra of 61 units from the suprachiasmatic nuclei (SCN) that had electrical responses to retinal illumination were measured. Most responding units (57/61; 93 %) were recorded under urethane anaesthesia. The range of response latency of the 61 units examined was 25–150 ms at near-maximal stimulation (median, 60 ms). Histological reconstruction of the electrode tracks indicated that all these recordings were from the SCN.
Input from scotopic and photopic pathways
Under dark adaptation SCN neurones showed electrical responses to retinal illumination that were slow to adapt. The light threshold for eliciting a response improved with dark adaptation. The action spectrum of each studied unit conformed to a Dartnall nomogram with a single peak in the vicinity of 505 nm (Fig. 1A and B). It also conformed to the Lamb equation for the rod photoreceptor sensitivity with peak sensitivity (λmax) around 500–505 nm. Under the experimental conditions, scotopic threshold at around 505 nm was at a photon flux of 1.80 × 1010 ± 5.88 × 109 photons m−2 s−1 (number of units, n = 35) after a minimum of 30 min of dark adaptation. The threshold in the UV range, around 375 nm, was 1.41 × 1012 ± 3.55 × 101 1 photons m−2 s−1 (n = 33). Sensitivity continuously improved with extended periods of dark adaptation and, after 5–7 h of dark adaptation, thresholds of up to two orders of magnitude lower were attained.
Figure 1. Action spectra from SCN neurones.
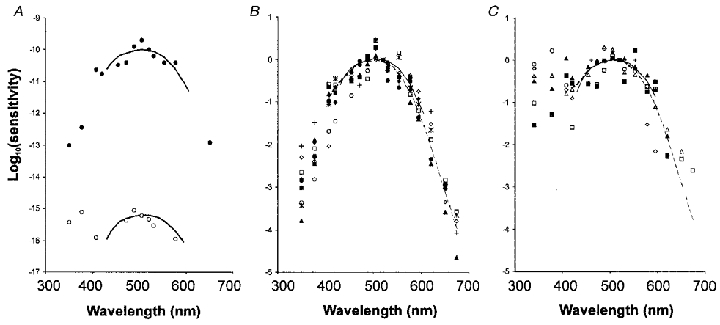
A, scotopic and photopic response sensitivity of a neurone in the SCN. Dartnall nomograms have been superimposed on the data points with a peak at 505 nm for the scotopic action spectrum (•) and 510 nm for the photopic action spectrum (○). The threshold for the photopic response is about 5 log units higher than the scotopic response threshold. The Y-axis is logarithmic. The scotopic threshold of about −10 corresponds to 10−10 photons m−2 s−1. B, data points for scotopic sensitivity from 10 different neurones arbitrarily normalised at the 520 nm value. For comparison, the Dartnall nomogram (continuous line, λmax≈505 nm) of the rat rhodopsin and the Lamb equation (dashed line) for the rat rhodopsin have been superimposed on the data points, with the 520 nm value equal to zero. C, data points for photopic sensitivity from 6 different neurones normalised at the 520 nm value. The Dartnall nomogram (continuous line, λmax≈510 nm) for the rat green cone opsin and the Lamb equation (dashed line) for the rat green cone opsin have been superimposed on the data points, with the 520 nm value equal to zero.
The spectral sensitivity of several of these neurones was also tested under light adaptation (1.3 × 1018 to 5.5 × 1018 photons m−2 s−1). Most neurones (20/26, 77 %) examined under both photopic and scotopic background illumination had inputs from both scotopic and photopic pathways for at least part of the duration that they were studied. The photopic responses were, however, fast adapting such that neurones adapted to any change in background irradiance within seconds. The scotopic and photopic thresholds differed for a given neurone by several log units and the sensitivity distributions also differed in their shape in the short wavelength region of the spectrum (Fig. 1A). Under white light adaptation, photopic responses had a λmax at around 510 nm with a response threshold at 4.3 × 1014 ± 2.1 × 1014 photons m−2 s−1 as established from those neurones that lacked scotopic input (n = 4). A secondary sensitivity peak (β-band) occurred at around 375 nm, with a mean threshold at 1.4 × 1014 ± 7.4 × 1013 photons m−2 s−1. The lowest thresholds for the photopic response of SCN neurones recorded during these experiments were in the range of 2.5 × 1012 photons m−2 s−1 (Fig. 1). As the λmax in the green spectrum around 505 nm was in the photopic range and about 4 log units lower than the sensitivity of the scotopic curve, in addition to their shape the sensitivity of these two curves further indicates separate photopic and scotopic input mechanisms.
Examination of the photopic responses under chromatic adaptation produced similar spectral sensitivity curves as when a white background was present. Under broad band short wavelength light adaptation, there was a main peak at 510 nm (threshold, 9.9 × 1013 ± 4.1 × 1013 photons m−2 s−1, n = 13) and a smaller peak at around 375 nm (threshold, 1.7 × 1014 ± 3.8 × 1013 photons m−2 s−1), in accordance with the known spectrum for the rat green cone opsin (Fig. 1C). The secondary peak in the UV range varied in relative size partly depending on background irradiance from 0 to 1.3 log units below the 510 nm peak. Also under broad band long wavelength light adaptation, most neurones had similar spectral sensitivity responses with a main peak at 510 nm (threshold, 3.2 × 1014 ± 9.5 × 1013 photons m−2 s−1). There was, however, greater variance in the relative size of the UV peak under these conditions (threshold, 4.0 × 1014 ± 1.8 × 1014 photons m−2 s−1, n = 13). Whereas in most cases of chromatic adaptation the chromatic sensitivity curves were identical under all conditions, indicating input from a single photoreceptor, in some cases of long wavelength adaptation the two peaks in the green and UV spectral regions were of equivalent size (Fig. 2A). This suggests that most SCN neurones receive photopic input from a single photoreceptor under differing conditions of chromatic adaptation, whose characteristics agree with the known characteristics of the rat green cone. However, some neurones seem to receive convergent input from an additional photoreceptor with enhanced UV sensitivity.
Figure 2. Chromatic conditioning of SCN neurones.
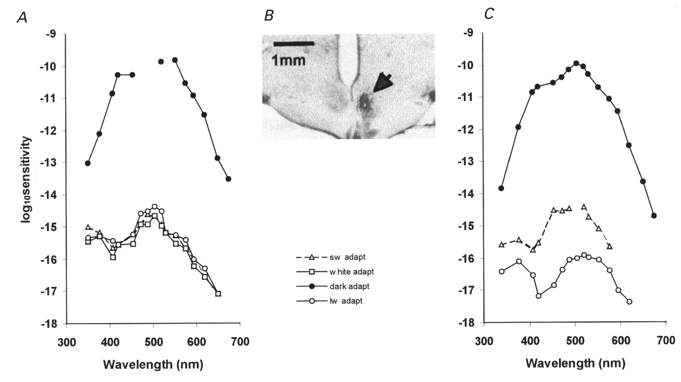
A, this neurone had a convergence from rods (filled symbols) and cones (open symbols). The cone responses were due to the common type of green cone photoreceptor and had a sensitivity peak at 510 nm with a secondary peak at around 375 nm under white light adaptation (white adapt). Chromatic adaptation with SW light (sw adapt) or LW light (lw adapt) failed to demonstrate independent component mechanisms. The sensitivity curve remained the same under all chromatic adaptation conditions. B, a Nissl-stained coronal section through the SCN showing the location of the recorded unit in A in the right SCN. The injection site (white spot in the lower centre of the nucleus) is surrounded by a silver nitrate sediment (arrow). Compare with the SCN nucleus on the left side. C, another neurone recorded from the SCN with rod- (filled symbols) and cone- (open symbols) mediated responses. The cone response sensitivity peaks could be independently modulated by chromatic adaptation. The green peak was suppressed under LW adaptation, indicating a contribution from another cone photoreceptor with greater sensitivity in the UV region than the typical green cone.
In five units for which complete sensitivity curves under both short wavelength and long wavelength adaptation were available, the difference in sensitivity in log units between the UV and green peaks was measured under these two conditions. The mean difference was higher under short wavelength (0.59 ± 0.20 log units) than long wavelength adaptation (0.17 ± 0.16 log units), indicating an enhancement of the sensitivity to UV under long wavelength adaptation (paired t test, P < 0.05).
Types of neuronal responses
Of those neurones tested under scotopic conditions, four showed no evoked responses at the time of stimulation with stimuli sufficient to activate rod but not cone pathways. The remaining 48 neurones tested under dark adaptation showed predominantly excitatory responses. The most common response was a pure sustained on-excitation (24/48, 50 %; Fig. 3A). Other responses included six units with transient and sustained on-excitation (6/48, 12.5 %; Fig. 3B), seven units with mixed transient and sustained excitatory effects, followed by a small off-inhibition (7/48, 15 %), five units with on-inhibition, followed by an off-excitation (5/48, 10 %; Fig. 3C) and several units showing a convergence of transient and sustained inhibitory and excitatory on- and/or off-inputs. Pure transient on/off responses without sustained components were very rare. Although these were the predominant responses under dark adaptation, they were commonly labile and could be changed by increasing levels of background adaptation, increasing intensity of the light stimulus and/or ‘spontaneous’ fluctuations in neuronal excitability as detailed below and could have been possibly also influenced by circadian time.
Figure 3. Types of neuronal responses.
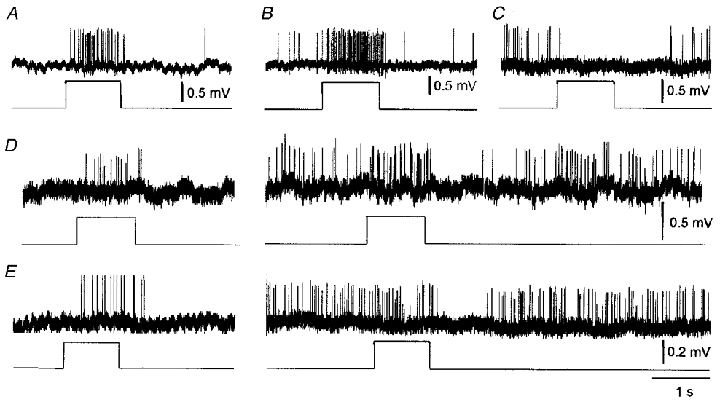
A, on-excitation. B, on-excitation with both transient and sustained components. C, on-inhibition. D, a neurone with on-excitation under dark adaptation (left) but on-excitation followed by a large off-inhibition under light adaptation (right). E, same as D but with only a transient on-excitatory response remaining under light adaptation, the off-inhibition being the most pronounced effect.
The sustained responses of SCN neurones to light could generally not be reduced to transient responses with near-threshold stimuli. As frequency of firing during a stimulus presentation in these cases was fairly constant, these sustained responses may be considered non-adapting for the time scales under study.
Scotopic-photopic antagonism
Some of the responses of SCN neurones changed under light adaptation. In particular, the neurones that had responded with pure sustained on-excitation under scotopic conditions showed an additional inhibitory off-response (Fig. 3D) when tested under photopic conditions (n = 8). Moreover, as their overall background firing rate increased under photopic conditions from 5.36 ± 1.36 to 21.05 ± 3.78 Hz (paired t test, probability of no difference < 0.01, n = 8) the net effect often was a just-discernible on-excitation followed by an often powerful off-inhibition. Similar effects were seen in the units with a transient component preceding the sustained on-excitation (Fig. 3E).
A different kind of response reversal was seen with some of the units showing primarily an inhibitory on-response at scotopic conditions with near-threshold scotopic stimuli (Fig. 4A). Increasing the stimulus intensity so as to begin to activate cone photoreceptors reversed the sign of the response from an overall inhibition to an on-excitation, followed by a small off-inhibition (Fig. 4B). At intermediate stimulus strengths, falling between scotopic and photopic levels, the two opposing drives cancelled each other and no clear effect was discernible on presentation of the stimulus.
Figure 4. Response reversal in an SCN neurone to light stimuli at scotopic and photopic irradiance levels.
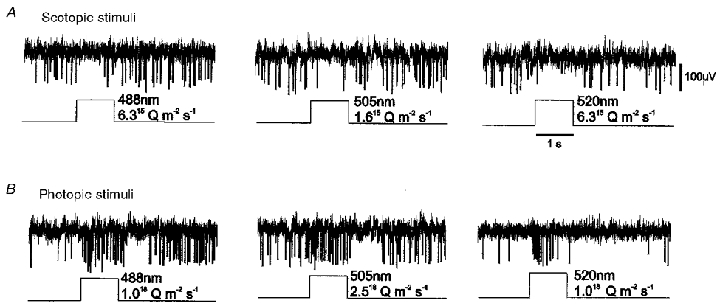
A, illumination of the retina at an irradiance sufficient to only activate rod photoreceptors caused an on-inhibition in this neurone. B, increasing the irradiance to also activate cone photoreceptors reversed the response sign to an on-excitation, followed by an off-inhibition.
Ordered interspike intervals
Autocorrelograms of the neuronal activity were made from all neurones tested under either scotopic or photopic conditions. These demonstrated a ubiquitous electrical ‘bistability’ in spontaneous discharge dependent on these two conditions. Figure 5 shows one such neurone with a typical on-excitatory response under scotopic conditions (Fig. 5A) changing to an on-excitation followed by off-inhibition under photopic conditions (Fig. 5B). Another characteristic aspect of this bistable behaviour was that the spontaneous firing pattern of these neurones changed under the two conditions of adaptation. Whereas under dark adaptation the firing of these neurones was totally random (Fig. 5C), under photopic conditions all light-responding suprachiasmatic neurones were displaying highly autocorrelated activity, such that a spike had a higher probability of occurring at a certain time after another spike (Fig. 5D). Interspike interval histograms best display the probability of occurrence of a subsequent spike. Under scotopic conditions, the neurone was discharging irregularly and the probability of a subsequent spike was exponentially decaying with time (Fig. 5E). This irregular firing was present even when spontaneous discharge rate was high. Irregularity in the pattern of discharge was not linked to a low spontaneous firing rate.
Figure 5. Discharge patterns in a visually responsive SCN neurone.
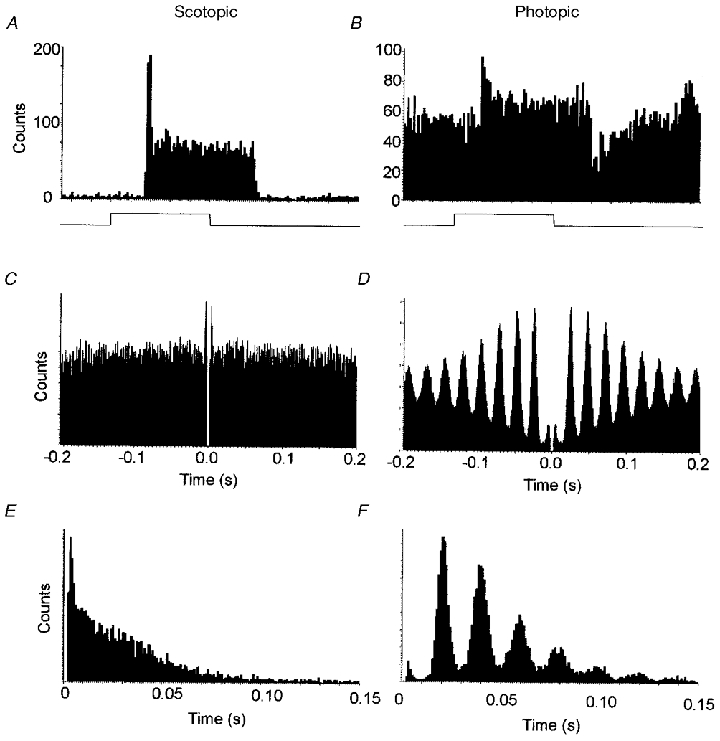
A, under dark adaptation, this neurone had a low firing rate (mean, 0.9 Hz) and responded to a light stimulus (indicated by the square waveform ramp at the lower trace) by an on-excitation that had both transient and sustained components. Average of 60 responses. B, under white light adaptation, this neurone showed a typically high spontaneous firing rate (mean, 26 Hz) and responded with an on-excitation followed by an off-inhibition. These responses have been averaged over several stimulus presentations. Average of 20 responses. C, autocorrelogram under dark adaptation, showing the distribution of neighbouring spikes to every spike sampled. The autocorrelogram of this neurone's spontaneous activity suggests that the electrical discharge pattern was completely random over a 20 min period. D, autocorrelogram under light adaptation from the same neurone, showing the patterning of activity at discrete intervals, over a 20 min sampling period. Interspike interval histograms: E, under dark adaptation, the interspike intervals are randomly distributed and frequency decays approximately exponentially with interval length; F, under light adaptation, however, the spike intervals occur at preferred frequencies which are multiples/harmonics of a basic frequency at 22 ms.
Under photopic conditions there was an ordered distribution of interspike intervals in histograms. There was without exception a tendency for spikes to be separated by an interval with a value between 18 and 39 ms (median, 24.5 ms, number of spikes, n = 24) or a higher harmonic, such as twice or three times the shortest possible interspike interval (Fig. 5F). This shortest interval varied sometimes with background irradiance but not greatly and was in all neurones but one and under all conditions between 18 and 30 ms. In two units, similar harmonically ordered interspike intervals were seen also under scotopic conditions but were very long (40 and 68 ms, respectively).
Ultradian excitability fluctuations and response gating
SCN units often had apparently spontaneous so-called isoperiodic or ultradian cycles in their firing. Under our experimental conditions these usually consisted of a sharp rise in spike frequency followed by a slower decay leading to a series of rapid alternations of active and quiet intervals (Fig. 6A). A quiet period sometimes ensued before the neuronal activity spontaneously repeated this pattern. The duration of a complete ultradian cycle was to a certain extent variable and on average between 110 and 350 s (median, 210 s). These ultradian activity cycles were most often seen under scotopic conditions and relatively rarely seen under photopic conditions, regardless of circadian phase. In some cases they were apparently elicited by changes in illumination levels from photopic to scotopic or vice versa. Nevertheless, the frequency of observation of these ultradian cycles had an underlying circadian pattern. A circadian plot of the frequency of neurones with ultradian cycles versus non-oscillating neurones is shown in Fig. 6B. The frequency was 1 (maximal) only at around the circadian transition points. Unfortunately, not enough recordings were available from the late circadian light (L) phase to determine whether or not the expression of ultradian cycles was less common during the L phase as a whole.
Figure 6. Ultradian cycles.
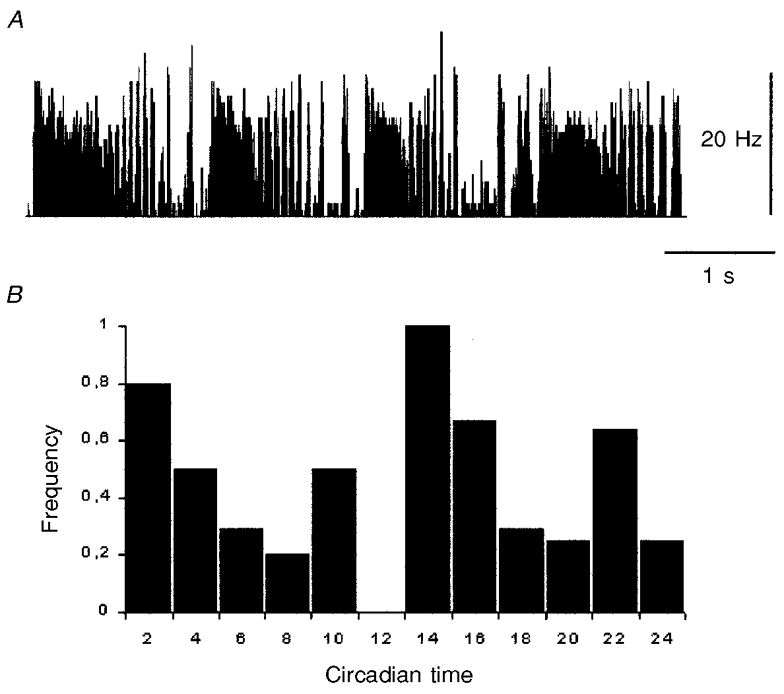
A, typical ultradian cycles with an initial sustained period of excitation followed by a period of alternating high and low frequency of firing in a suprachiasmatic neurone. B, frequency of neurones showing ultradian cycles at different circadian times (n = 25). Highest frequencies occurred at the L:D and D:L transition points. Circadian times 10–12 are represented less accurately by only two studied neurones.
These cycles had several consequences on the ability of a neurone to respond to retinal input. When a stimulus was presented during the low phase of this cycle a much stronger response could be elicited than when the same stimulus was presented during the high phase of the ultradian cycle. In other neurones, a complete reversal of the sign of the response was possible, depending on whether the stimulus was presented during the high or low phase of the ultradian rhythm. Figure 7 shows part of a long term recording from such a neurone, during late subjective night, to demonstrate this phenomenon. This neurone was studied first under darkness (Fig. 7A) and at circadium time (CT) 20:20 the animal was light adapted. Almost immediately, photopic illumination elicited in this neurone ultradian oscillations (Fig. 7B). During this period it responded to light flashes with sustained excitation during the low phase of the rhythm (Fig. 7E) but sustained inhibition during the high phase (Fig. 7F). At intermediate points in the circadian rhythm, intermediate mixed excitatory/inhibitory responses or no response at all were possible (Fig. 7G), even when the stimulus strength remained constant. These response reversals were independent of wavelength.
Figure 7. Input gating.
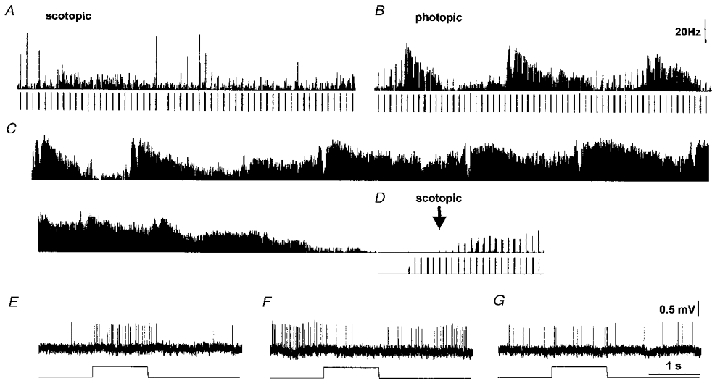
The times of the light stimuli are shown below the traces, when administered. Duration is 1 s per stimulus, period is 10 s. A, a non-oscillating SCN neurone responds with on-excitation (upper trace, integrated firing frequency) with a variety of stimuli (lower trace) under dark adaptation. B, light adaptation at CT20:20 elicits a series of ultradian cycles with a mean period of about 2.5 min. Stimulation with green narrow waveband light (520 nm, 1020 photons m−2 s−1) elicits an on-excitatory response at low phase but an on-inhibitory response at high phase. C, record of spontaneous firing, showing the ultradian rhythmicity. D, at CT22, the neurone has entered a quiet period, has completely stopped firing spontaneously altogether and cannot be induced to fire by maximal light stimulation. Responses return gradually after switching off the background adapting beam (arrow). Cone responses never recovered. E, on-excitatory response at low phase during an ultradian cycle under light adaptation. F, on-inhibitory response at high phase of the ultradian cycle. G, intermediate response, possibly a weak transient on-excitation here, at an intermediate place in the ultradian cycle.
A further significant finding was that after a period of about 80 min of displaying this ultradian rhythmicity, mean spontaneous firing rate became reduced until spontaneous firing completely ceased (Fig. 7D). In this new state, photopic stimuli of even the highest irradiance possible, which were until recently capable of eliciting powerful excitatory responses at low phase, no longer activated this neurone. The neurone became once again gradually active only after the background irradiance had been reduced to scotopic levels for several minutes at CT22. The responses to light flashes within scotopic levels returned seemingly unaffected. The neurone had become, nevertheless, entirely refractory to photopic input. Neither did it show a new period of ultradian oscillations when the background irradiance was brought back into photopic levels at CT23.
Long term recordings were rarely feasible and it was not possible to determine the extent to which conditioning with a period of photopic stimulation led to a time-dependent refractoriness to further photopic input. Nevertheless, in shorter recordings there were some cases of neurones not chosen for study because their responsiveness to light was of abnormally high threshold or disappeared during the experiment, possibly as a result of experimental history or perhaps due to circadian time. There was also a case when at around the onset of circadian dark phase, a previously non-responsive neurone started responding to light stimuli.
DISCUSSION
We have demonstrated a convergence of a scotopic and a photopic input to suprachiasmatic neurones. The spectral sensitivity, thresholds, dark and light adaptation times of these two inputs are compatible with the properties of retinal rods and cones. These two inputs can commonly have net effects of opposing signs and this presumed rod-cone antagonism is a novel type of antagonistic process in the visual system. This antagonism may be one possible mechanism for the entrainment of SCN activity to the environmental photoperiod. This finding would be compatible with an involvement of both rod and cone pathways in photoentrainment with the determining breaking point based around the net algebraic sum of the opposing influences from cone and rod inputs.
Changing lighting conditions and experimental and/or environmental history may also influence the processing of the visual information by the SCN. Specifically, visual input could condition its own action. A scotopic background irradiance reduced spontaneous firing rate in most SCN neurones, against which background short stimulus ramps caused on-excitatory responses. Conversely, a photopic background irradiance increased spontaneous firing rate in most SCN neurones, against which background short stimulus ramps caused predominantly off-inhibitory responses. As an additional level of control, the responsiveness of some neurones to light appeared to change during an experiment and it may also be of some relevance that input from cone pathways could be on one instance gated independently from input from rods.
Furthermore, the exact response to uniform retinal illumination, whether sustained or transient, ‘on’ or ‘off’, inhibitory or excitatory, monophasic, biphasic or multiphasic, as well as response intensity and threshold, may vary as neuronal state varies due to light adaptation, dark adaptation, the presence of ultradian oscillations or due to experimental/environmental history. In addition, other factors such as circadian time (Cui & Dyball, 1996; Meijer et al. 1998) also seem to influence the type of responses obtained in the SCN. This emerging concept of an SCN neurone producing a state-dependent or state-conditioned output, as opposed to a stereotyped excitation or inhibition, is likely to be an important aspect of the entraining function these neurones perform. The importance of many inputs to the nucleus may be in their ability to switch or gate SCN neuronal responsiveness to critical entraining stimuli, in addition to or in lieu of any short term excitatory or inhibitory electrical responses they may elicit.
Photopigments involved in the transmission of visual signals to the SCN
The action spectra of neuronal responses to light, when corrected for absorbance by the refractive media of the eyes (mostly due to lens absorbance) should superimpose onto the spectral sensitivity of the visual pigments from which they originated. The majority of the spectra obtained under short wavelength or white light adaptation in our experiments conformed adequately to the sensitivity of the rat green cone opsin with a λmax of 510 nm. Some neurones, at least at the time of recording, had input only from photopic pathways and the threshold corresponded to the threshold of retinal cones to light. Most SCN neurones that responded to light had under all chromatic adaptation conditions one type of spectral response, such as in the example in Fig. 2A. This spectral sensitivity curve has a strong UV component and it was the only type of photopic sensitivity spectrum seen in SCN neurones under short wavelength (purple) chromatic adaptation.
The small suppression in some neurones of the main sensitivity peak at 505 nm by long wavelength chromatic adaptation suggests converging inputs from two photopic mechanisms, such as the green cone pigment and a cone photopigment preferentially absorbing in the short wavelength range (UV), as recently proposed for the retina of several rodent species. No neurones were found with an exclusive input from the short wavelength photopigment; however, that would have raised sensitivity in the UV well above the ‘visible’ spectrum.
Scotopic responses were rather insensitive in the UV range. In pigeons, rods are thought to also have high absorbance in the UV range (Palacios & Goldsmith, 1993) but this may not be the case in mammals. This low sensitivity of the scotopic responses to UV may indicate greater filtering of short wavelength light by the eye at low illumination levels. It is, however, unlikely that this would explain the whole extent of the insensitivity to UV under scotopic conditions, especially as the filtering of UV by the rat lens has been already corrected for and the contribution of the other ocular media and self-screening would have to account for this difference. It would seem, therefore, that the scotopic response is relatively UV insensitive, has a single sensitivity peak at 500–505 nm and conforms to the hydroxylamine difference spectrum of rhodopsin. The slow change in threshold during dark adaptation and the response sensitivity after several hours of dark adaptation also agree with the properties of rhodopsin.
The present finding of a common input from rods may require a reassessment of the value of scotopic input in the function of the circadian system. The photoreceptor layer of the rat contains over 99 % rods. The rat being a nocturnal species, relies on a rod-based visual system that is primarily active under scotopic conditions, such as prevail when the sun is approximately ≤ 12 deg below the horizon. This is reflected in the electroretinogram, which in the rat is long established to be much smaller under photopic conditions. Considering their overwhelming predominance, the rods may provide the main source of retinal input against which other circadian information could be compared and this is borne out to the extent that a somewhat greater proportion of light-responsive SCN neurones could be activated by rod input than cone input (92 %vs. 77 %). Indeed, the concept of the light-dark cycle may be inseparable from a physiological rod-cone activity cycle.
Nevertheless, the retinal channel via which light information can entrain the endogenous circadian rhythm has not yet been determined. Recent studies in mice lacking cones (cl) or both rods and cones (rdta/cl) showed unattenuated phase-shifting responses to light, indicating that neither rods nor cones are required for photoentrainment (Freedman et al. 1999). Despite the reported loss of all known retinal photoreceptors, these rdta/cl mice also showed a normal suppression of pineal melatonin in response to light which was interpreted as an indication that mammals possess additional ocular photoreceptors that are used for the regulation of temporal physiology (Lucas et al. 1999). These non-rod, non-cone photoreceptors that are capable of regulating circadian behavioural responses to light probably use a vitamin A-based photopigment (Takahashi et al. 1984; Provencio & Foster, 1995). Our methods were best suited to identifying photoreceptor inputs from rod and cone pathways. Our findings seem to fulfil the alternative hypothesis of Takahashi et al. (1984) for two circadian opsin-based photoreceptors. We cannot exclude the possibility that there are two types of novel photoreceptors in the retina of the rat with characteristics similar to rods and cones but of presumably different morphology. Given, however, the resemblance of the scotopic and photopic responses with those of rods and cones, respectively, in terms of spectral sensitivity, threshold, adaptation characteristics, their mutual antagonism and the fact that not all neurones always show responses from both types, the most parsimonious explanation of our findings would be that rods and cones are a source of visual input to the SCN.
Our findings do not necessarily rule out input from other visual pathways to the SCN. Such input as may exist was not possible to detect using electrophysiological methods under our conditions of retinal illumination. For example we have not been able to determine the action spectra in neurones at times when they showed ultradian oscillations, since the variability in the response under these conditions made threshold determinations by spike counting impossible. The ultradian oscillations are electrical phenomena in themselves, possibly initiated by a photoreceptor input, which we have also not examined. It is also possible that a novel photoreceptor may be non-adapting or that it may have a threshold in the high photopic range; in either case it would have been missed. It will be a challenging task to evaluate the role and relative contribution of the classical rod/cone pathway and of the novel circadian photoreceptors in the physiology of the SCN.
The use of anaesthesia
It has been suggested that general anaesthesia may suppress the photoentrainment of the circadian system (Colwell et al. 1993). Nevertheless, urethane, which was used in this study was excluded by Colwell et al. on the basis of their studies of c-fos induction in the SCN and phase advance of the circadian locomotor rhythm. The scotopic and photopic response thresholds of SCN neurones to light and the shape of the action spectra also indicate that SCN neurones were highly sensitive to retinal input at the time of these experiments. We have also carried out experiments in 12 animals anaesthetised with sodium pentobarbitone. It was not normally possible to elicit reliable responses to retinal illumination in SCN neurones in these animals. This differential effect of the anaesthetics would also be compatible with a role for rods and cones in the photoentrainment of the SCN activity rhythm.
Ordered interspike intervals
Intracellular studies (Pennartz et al. 1997) have disclosed an endogenous depolarising potential that imparts a regular repetitive type of spontaneous firing activity to many SCN neurones. This generator potential depends on the resting membrane potential and may be the driving force behind our own observations of interspike time-ordered intervals in SCN neurones clustered around a single interval and its higher order harmonics. This leads to autocorrelated activity within SCN neurones under photopic light adaptation under general anaesthesia. Such autocorrelated activity has been first observed in the visual system of the cat (Neuenschwander & Singer, 1996) and although the frequency differs, the underlying mechanisms may be similar. A completely regular discharge pattern may emerge in SCN neurones were anaesthesia to be withdrawn and has been already noted in recordings made from SCN neurones in vitro (Thomson et al. 1984). This has prompted some investigators to refer to these regularly discharging SCN units as ‘clock neurones’. The assumption, however, that the regular firing in these neurones may have a time-keeping function is not guaranteed.
Under dark adaptation, this autocorrelated activity disappears and the neurones discharge irregularly and at an overall lower firing rate, somewhat resembling in these respects the cluster II-type neurones from the study of Pennartz et al. (1998). The two different neuronal states are likely to be represented by changes in membrane properties such as ionic mechanisms and overall resting membrane potential. These neurones are another example of a ‘bistable’ neuronal type that can operate under two different states to produce completely different outputs. This phenomenon was first observed in the lateral geniculate nucleus of the thalamus (Leresche et al. 1991) and may be a more widespread neuronal property than previously thought.
Ultradian cycles
Ultradian cycles have been in the past noted in the activity of SCN neurones (Meijer et al. 1997; Yamazaki et al. 1998). Three types of ultradian cycles have been described: long cycles with a period of about 80 min, short cycles with a period of about 120 s and intermediate cycles with a period of about 14 min. In this study, the shortest type, also called isoperiodic ultradian cycles (Miller & Fuller, 1992), were seen during our relatively short-term recordings. In good agreement with the study of Miller & Fuller, these ultradian cycles were never seen in the activity of optic fibres or outside the boundary of the SCN. They were observed during both the circadian dark phase and light phase but the frequency of observation was highest at the circadian L:D and D:L transition points. They were also more commonly observed after a transition from photopic to scotopic conditions and sometimes vice versa but were not elicited by scotopic-intensity light flashes under scotopic conditions or photopic-intensity light flashes under photopic conditions. The L:D and D:L transition points are well established to be the circadian times when light stimuli can, respectively, advance or delay endogenous circadian rhythms via the SCN (Hastings et al. 1996). As the effect of visual input (whether excitatory, inhibitory or other) largely depended on the phase of ultradian cycles, they may be one of the electrical phenomena correlated to the entrainment of the circadian clock. Further examination of the initiation and neuromodulation of these ultradian cycles in vitro and in vivo may be necessary to establish their function.
Ultraviolet sensitivity
It was not possible to record action spectra in SCN units under chromatic adaptation closely resembling the SW cone photopigment spectrum proposed on the basis of the electroretinogram of rats (Deegan & Jacobs, 1993). SCN neurones appeared no more selective and may have been less selective for SW cone input than retinal ganglion cells. Neurones that had a chromatic modulation of their sensitivity to UV compared to green, only did so to a limited extent. However, as the number of SW cones in the retina is a small percentage of the total number of cones, the frequency of exclusive SW-responding neurones or neurones with SW/LW antagonism would have been expected to be low at best. This question, however, could not be systematically addressed in these experiments, because of the limitation of the available equipment in ultraviolet spectral resolution and light output. As the number of SW cones is small in rodents, their use for high resolution spatial vision would seem impossible, making a circadian function seem a priori amore likely role.
Regardless of what the role of the SW cones may be, the high UV sensitivity of the green cone may be sufficient to mediate the well-documented effects of UV light on the circadian system (Brainard et al. 1986; Amir & Robinson, 1995).
Conclusion
The present electrophysiological study from rat SCN neurones clearly shows that, under normal photopic and scotopic conditions, both rod and cone pathways contribute to the responses of SCN neurones. Responses under dark adaptation conformed to the sensitivity of rhodopsin whereas responses under light adaptation conformed to input from green cones. These pathways could have opposing or non-opposing actions on SCN neurones. Responses are state dependent, could be conditioned by the light input itself and were affected by endogenous ultradian cycles that were dependent on the state of adaptation and also on the circadian cycle.
References
- Amir S, Robinson B. Ultraviolet light entrains rodent suprachiasmatic nucleus pacemaker. Neuroscience. 1995;69:1005–1011. doi: 10.1016/0306-4522(95)00393-w. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Podolin PL, Leivy SW, Rollag MD, Cole C, Barker FM. Near-ultraviolet radiation suppresses pineal melatonin content. Endocrinology. 1986;119:2201–2205. doi: 10.1210/endo-119-5-2201. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Jacobs GH, Haak KA, Neitz J, Lytle LD. Action spectra of the retinal mechanism mediating nocturnal light-induced suppression of rat pineal gland N-acetyltransferase. Brain Research. 1987;406:352–356. doi: 10.1016/0006-8993(87)90806-7. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Kaufman CM, Menaker M, Ralph MR. Light-induced phase shifts and fos expression in the hamster circadian system: the effects of anesthetics. Journal of Biological Rhythms. 1993;8:179–188. doi: 10.1177/074873049300800301. [DOI] [PubMed] [Google Scholar]
- Cui LN, Dyball REJ. Synaptic input from the retina to the suprachiasmatic nucleus changes with the light-dark cycle in the Syrian hamster. The Journal of Physiology. 1996;497:483–493. doi: 10.1113/jphysiol.1996.sp021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall HJA. Interpretation of spectral sensitivity curves. British Medical Bulletin. 1953;9:24–30. doi: 10.1093/oxfordjournals.bmb.a074302. [DOI] [PubMed] [Google Scholar]
- David-Gray ZK, Janssen JWH, DeGrip WJ, Nevo E, Foster RG. Light detection in a ‘blind’ mammal. Nature Neuroscience. 1998;1:655–656. doi: 10.1038/3656. [DOI] [PubMed] [Google Scholar]
- Deegan JS, Jacobs GH. On the identity of the cone types of the rat retina. Experimental Eye Research. 1993;56:375–377. doi: 10.1006/exer.1993.1049. [DOI] [PubMed] [Google Scholar]
- Dodt E, Echte K. Dark and light adaptation in pigmented and white rat as measured by electroretinogram threshold. Journal of Neurophysiology. 1961;24:427–445. doi: 10.1152/jn.1961.24.4.427. [DOI] [PubMed] [Google Scholar]
- Foster RG. Shedding light on the biological clock. Neuron. 1998;20:829–832. doi: 10.1016/s0896-6273(00)80464-x. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Sony B, van Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Freedman MS, van Schantz M, Soni BG, Foster RG. Molecular dissection of the coneless transgenic mouse retina. Investigative Ophthalmology and Visual Science. 1997;38:S322. [Google Scholar]
- Gorgels TGMF, van Norren D. Spectral transmittance of the rat lens. Vision Research. 1992;32:1509–1512. doi: 10.1016/0042-6989(92)90206-x. [DOI] [PubMed] [Google Scholar]
- Groos GA, Mason R. The visual properties of rat and cat suprachiasmatic neurones. Journal of Comparative Physiology. 1980;135:349–356. [Google Scholar]
- Hastings MH, Best JD, Ebling FJ, Maywood ES, McNulty S, Schurov I, Selvage D, Sloper P, Smith KL. Entrainment of the circadian clock. Progress in Brain Research. 1996;111:147–174. doi: 10.1016/s0079-6123(08)60406-9. [DOI] [PubMed] [Google Scholar]
- Lamb TD. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Research. 1995;35:3083–3091. doi: 10.1016/0042-6989(95)00114-f. [DOI] [PubMed] [Google Scholar]
- Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. The Journal of Physiology. 1991;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernandez J-M, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Research. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Schaap J, Watanabe K, Albus H. Multiunit activity recordings in the suprachiasmatic nuclei: in vivo versus in vitro models. Brain Research. 1997;753:322–327. doi: 10.1016/s0006-8993(97)00150-9. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Détári L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. Journal of Neuroscience. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Fuller CA. Isoperiodic neuronal activity in suprachiasmatic nucleus of the rat. American Journal of Physiology. 1992;263:R51–58. doi: 10.1152/ajpregu.1992.263.1.R51. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) The Journal of Physiology. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379:728–733. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- Palacios AG, Goldsmith TH. Photocurrents in retinal rods of pigeons (Columba livia): kinetics and spectral sensitivity. The Journal of Physiology. 1993;471:817–829. doi: 10.1113/jphysiol.1993.sp019930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Bierlaagh MA, Geurtsen AMS. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. Journal of Neurophysiology. 1997;78:1811–1825. doi: 10.1152/jn.1997.78.4.1811. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, De-Jeu MTG, Geurtsen AMS, Sluiter AA, Hermes MLHJ. Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. The Journal of Physiology. 1998;506:775–793. doi: 10.1111/j.1469-7793.1998.775bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Research. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- Szel A, Röhlich P. Two cone types of rat retina detected by anti-visual pigment antibodies. Experimental Eye Research. 1992;55:47–52. doi: 10.1016/0014-4835(92)90090-f. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Thiele G, Meissl H. Action spectra of the lateral eyes recorded from mammalian pineal glands. Brain Research. 1987;424:10–16. doi: 10.1016/0006-8993(87)91187-5. [DOI] [PubMed] [Google Scholar]
- Thomson A, West DC, Vlachonikolis IG. Regular firing patterns of suprachiasmatic neurons maintained in vitro. Neuroscience Letters. 1984;52:329–334. doi: 10.1016/0304-3940(84)90183-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. Journal of Neuroscience. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


