Abstract
States of peripheral autonomic arousal accompany emotional behaviour, physical exercise and cognitive effort, and their central representation may influence decision making and the regulation of social and emotional behaviours. However, the cerebral functional neuroanatomy representing and mediating peripheral autonomic responses in humans is poorly understood.
Six healthy volunteer subjects underwent H215O positron emission tomography (PET) scanning while performing isometric exercise and mental arithmetic stressor tasks, and during corresponding control tasks. Mean arterial blood pressure (MAP) and heart rate (HR) were monitored during scanning.
Data were analysed using statistical parametric mapping (SPM99). Conjunction analyses were used to determine significant changes in regional cerebral blood flow (rCBF) during states of cardiovascular arousal common to both exercise and mental stressor tasks.
Exercise and mental stressor tasks, relative to their control tasks, were associated with significantly (P < 0.001) increased MAP and HR. Significant common activations (increased rCBF) were observed in cerebellar vermis, brainstem and right anterior cingulate. In both exercise and mental stress tasks, increased rCBF in cerebellar vermis, right anterior cingulate and right insula covaried with MAP; rCBF in pons, cerebellum and right insula covaried with HR. Cardiovascular arousal in both categorical and covariance analyses was associated with decreased rCBF in prefrontal and medial temporal regions.
Neural responses in discrete brain regions accompany peripheral cardiovascular arousal. We provide evidence for the involvement of areas previously implicated in cognitive and emotional behaviours in the representation of peripheral autonomic states, consistent with a functional organization that produces integrated cardiovascular response patterns in the service of volitional and emotional behaviours.
Exercise, mental effort and emotional states are accompanied by reproducible changes in peripheral cardiovascular function affecting regional and systemic perfusion. The sympathetic and parasympathetic axes of the autonomic nervous system act to produce these integrated cardiovascular response patterns necessary for the metabolic support of behaviour, and are controlled directly by central autonomic nuclei within the brainstem and cerebellum. These autonomic regions receive afferent inputs from cortical and subcortical systems implicated in emotional and volitional behaviours. Peripheral autonomic responses may be an integral component of learning within cortical and subcortical systems (apparent in classical fear conditioning), and feedback of such responses may also influence emotional behaviour and decision making (Damasio et al. 1991). There is, as yet, only limited understanding of how ‘higher’ brain areas control and represent altered peripheral autonomic states in humans.
Studies in experimental animals have helped to identify components of the ‘central autonomic network’ and have enhanced our understanding of the functional relationships between cortical and subcortical centres in cardiovascular control (reviewed in Cechetto & Saper, 1990; Bennarroch, 1997). Changes in heart rate and blood pressure have been reported to result from electrical or chemical stimulation of a set of discrete brain areas, including regions implicated in (1) attention, motivation, decision making and episodic memory – anterior cingulate, ventromedial prefrontal cortex and hippocampus (Kaada, 1951; Buchanan et al. 1985; Neafsey, 1990); (2) representation of aversive emotions – amygdaloid complex (Kaada, 1951; Gelsema et al. 1989); (3) initiation and control of limb movements – motor cortex, nigrostriatal tract, neostriatum and cerebellum (Kaada, 1951; Delgado, 1960; Bradley et al. 1987, 1991; Angyan, 1994; Lin & Yang, 1994); (4) representation of internal sensory, somatic and endocrine states – insula, dorsomedial and lateral hypothalamus, and nucleus tractus solitarii (Oppenheimer & Cechetto, 1990; DiMicco et al. 1992; Allen & Cechetto, 1992; Spyer, 1999) and (5) brainstem sympathetic and parasympathetic nuclei (e.g. Willette et al. 1984). In addition, electrophysiological recordings indicate that these putative efferent autonomic centres also receive afferent information concerning peripheral autonomic states (e.g. Cechetto & Saper, 1987; reviewed in Cechetto & Saper, 1990).
There have been a limited number of similar studies in humans; stimulation of the insula (Oppenheimer et al. 1992), medial prefrontal cortex and anterior cingulate (Pool & Ransohoff, 1949), and medial temporal lobe (Fish et al. 1993) elicit changes in blood pressure and heart rate (occasionally accompanied by subjective mood changes). Lesions of discrete brain areas may also modulate autonomic responsivity. Thus, orbitofrontal damage reduces anticipatory arousal to emotive stimuli (Damasio et al. 1990), while lesions of the amygdala block autonomic responses that accompany conditioning (Bechara et al. 1995). Lesions to these areas are also associated with marked changes in social and emotional behaviour, suggesting that feedback of altered autonomic arousal (represented in cortical regions such as the orbital/ventromedial prefrontal cortex) may directly influence social behaviour and decision making (Damasio et al. 1991). There is a long history to the notion that autonomic feedback influences emotions; the James-Lange theory of emotion (James, 1894) proposed that subjective emotional experience was the by-product of perceiving visceral responses that are the essence of emotion.
Functional imaging studies have been used to investigate the central control of the cardiovascular system. In a single-photon emission tomography study, Williamson et al. (1997) reported activation of the left insula during dynamic exercise (cycling) but not passive exercise (cycling movements induced by moving pedals independently), suggesting involvement in autonomic cardiovascular regulation. Using positron emission tomography (PET), Nowak et al. (1999) showed that activation of sensory motor cortex accompanied effortful handgrip exercise, an activity pattern that was unaffected by removing afferent feedback from the arm by anaesthesia. Mental effort, a common component of many cognitive studies in functional imaging, has been associated with increased activity in anterior cingulate (Paus et al. 1998), and hyperactivity of anterior cingulate during mental stress has been reported in patients with coronary artery disease, relative to controls (Soufer et al. 1998). Using functional magnetic resonance imaging (fMRI), Harper et al. (1998) reported increased activity of orbitofrontal cortex, amygdalo-hippocampal complex, hypothalamus and cerebellum during hypertension elicited by cold pressor stimuli and performance of the Valsalva manoeuvre (breathing against a closed glottis), manipulations that undoubtedly engender altered autonomic states.
Thus, animal experiments have implicated diverse brain regions in the central control of blood pressure and heart rate. In humans, similar regions are implicated in the performance of complex cognitive, emotional or physical behaviours. Functional imaging techniques allow the in vivo measurement of changes in regional brain activity during the performance of behavioural tasks, though few studies have examined the representation and control of autonomic responses in the cardiovascular system. In the present study, we investigated the functional neuroanatomy of central cardiovascular control, using PET in volunteer subjects, to identify brain regions that respond to changes in heart rate and blood pressure common to exercise- and mental effort-induced states of autonomic arousal.
METHODS
Subjects
Six healthy right-handed male volunteers (mean age ± s.d., 31 ± 3 years) were recruited after medical screening to exclude disorders or medication which might affect brain function or perfusion. Subjects gave full written informed consent to take part in the study which was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee.
Experimental design
Subjects performed three repetitions of four tasks which replicated clinical stressor tests for assessing autonomic function, namely isometric exercise and mental arithmetic tasks. Subjects were familiarized with the procedures before scanning. For each subject, the three repetitions of the four tasks were presented in a unique pseudorandom order. Blood flow measurements were obtained using PET (see below) during each repetition of the four conditions. Immediately after the performance of each task, subjects were debriefed by asking them about the experience, and in the case of the (covert) mathematics tasks, what number they had reached. This provided a qualitative means of rating subjective stress and ensuring that the mathematics tasks were performed appropriately.
Experimental conditions
‘Effortful’ isometric exercise
Subjects held a pressure bulb (attached to a sphygmomanometer) in their right hand. Prior to the scanning session, maximal squeeze strength was measured for each individual (mean ± s.d., 196 ± 20 mmHg). When prompted by a display on a video monitor, subjects were required to squeeze to 40 % of their maximal squeeze strength (video display: ‘Squeeze to 80’) and to maintain this squeeze for ∼2.5 min. The feedback necessary for maintaining the squeeze pressure was presented visually to the subject by the video monitor. Performing this level of isometric exercise was associated with subjective tiredness and difficulty.
‘Effortless’ isometric exercise
Using identical methods and apparatus to the hard isometric exercise task, subjects were prompted to perform a minimal squeeze of 20 mmHg by the video monitor (display: ‘Squeeze to 20’) and to maintain this low level of pressure for ∼2.5 min. This level of exercise was not associated with subjective strain, tiredness or difficulty. In this way we controlled for the sensory and attentional aspects of the task that were not directly related to exercise difficulty.
‘Effortful’ mental arithmetic
Subjects were required to covertly (subvocally) perform serial subtractions of numbers as rapidly as possible, and to aim to reach as low a number as possible at the end of the scanning window. Just before the onset of the scanning window subjects were asked which number they had reached, to which they replied aloud. They were then told that they were going too slowly, and needed to go faster. The initial calculation was presented on a video display for the first 10 s of the start of the task (e.g. ‘879 – 17’ would require the second calculation to be 862 – 13, then 845 – 13 … 828 – 13 … 811 – 17, etc.). Different number pairs were used on each repetition. Subjects were told not to use other strategies for calculations, and after the scan were asked what number they had reached. This task was associated with subjective difficulty and stress.
‘Effortless’ mental arithmetic
Subjects were prompted to count in ones at a steady rate (∼1 Hz) from a number presented on the video display (e.g. ‘Count from 34’) over ∼2.5 min. This level of mental arithmetic was not associated with subjective difficulty and stress, but controlled for the inner speech and attentional components of the effortful mental arithmetic task.
Physiological monitoring and derivation of covariates of interest
Portapres 2.0 apparatus (TND Biomedical Instrumentation Research Unit, Amsterdam, The Netherlands) was used to measure heart rate and blood pressure on a beat-to-beat basis via a probe on the left index finger, throughout the scanning session. Analog output from Portapres of the pulse waveform was monitored on-line using Spike2 software (Cambridge Electronic Design, Cambridge, UK) on an IBM-compatible computer. The averages of mean arterial blood pressure (MAP, calculated as the true arithmetic mean of systolic and diastolic pressure on a beat-to-beat basis) and heart rate (HR) were calculated from the first 60 s of the PET scanning window for each task in each subject. The change from baseline in MAP and HR was calculated for each subject over each 60 s period (Fig. 1).
Figure 1. Diagram of experimental design for acquisition of H215O-PET rCBF and physiological (Portapres) data.
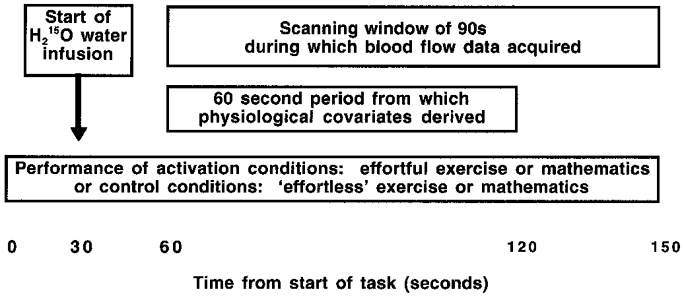
Subjects underwent 12 scans, representing 3 repetitions of 4 task conditions which were pseudorandomized in order within and between subjects: (1) effortful isometric exercise, (2) effortless isometric exercise control task, (3) effortful mental arithmetic (serial subtractions), and (4) effortless mental arithmetic control task (counting).
PET scan acquisition and analysis
Scans of the distribution of H215O were obtained using a Siemens/CPS ECAT EXACT HR+ PET Scanner operated in high sensitivity 3-D mode. Subjects received a total of 350 MBq of H215O over 20 s through a right antecubital cannula for each of the 12 scans, and activity was measured during a 90 s time window while the subjects performed the tasks. The PET images comprised i, j and k voxels (2 mm × 2 mm × 3 mm) with a 6.4 mm transaxial and 5.7 mm axial resolution (full width at half-maximum). The data were analysed with statistical parametric mapping (SPM99, Wellcome Department of Cognitive Neurology) implemented in Matlab (Mathworks, Sherborn, MA, USA). Structural MRIs from each subject were co-registered to the PET data following realignment of the PET time series. All the scans were then transformed into a standard stereotactic space (Talairach & Tournoux, 1988; Friston et al. 1995a). The scans were smoothed using a Gaussian filter set at 12 mm full width at half-maximum. The regional cerebral blood flow (rCBF) measurements were adjusted to a global mean of 50 ml dl−1 min−1.
Data were analysed using two statistical models. Firstly, a design matrix for the analysis of subject-by-task interactions was constructed to allow computation of contrasts for each subject performing each task. Global CBF was treated as a confounding covariate. Significant regional activation associated with the effects of effortful vs. effortless exercise and effortful vs. effortless mental arithmetic were computed across the subject group. Analysis of the conjunction of these tasks was used to determine which brain areas were commonly activated during both effortful vs. effortless exercise and effortful vs. effortless mathematics. Secondly, a design matrix was constructed for the analysis of subject-by-task interactions to determine brain areas where rCBF activity covaried with blood pressure and heart rate. In order to maximize the sensitivity of the analysis, effortful and effortless tasks were combined, providing four condition-specific covariates of interest: (1) changes in MAP over all exercise tasks; (2) changes in HR over all exercise tasks; (3) changes in MAP over all mathematics tasks; and (4) changes in HR over all mathematics tasks. These covariates were scaled to individual subject means. Conjunction analyses of these task-specific covariates were used to determine brain areas where activity covaried positively or negatively with MAP or HR in both exercise and mathematics tasks. The general methods employed by SPM have been described in detail by Friston et al. (1995a,b).
The central question of which brain areas are implicated in the central control of autonomic responses led us to predict, on the basis of previous animal and human data, the involvement of specific brain regions. In humans, the anterior cingulate and insula have been implicated in autonomic regulation by stimulation studies (Pool & Ransohoff, 1949; Oppenheimer et al. 1992) and functional imaging studies (Williamson et al. 1997; Fredrickson et al. 1998; Soufer et al. 1998). Similarly, lesion studies in humans have implicated the ventromedial prefrontal/orbitofrontal cortex (Damasio et al. 1990; Tranel & Damasio, 1994) and amygdalo-hippocampal complex (Bechara et al. 1995) in autonomic correlates of sympathetic arousal. Additionally there is a wealth of animal literature implicating the hypothalamus, brainstem and cerebellum in autonomic regulation (e.g. Willette et al. 1984; Cechetto & Saper 1990; Bradley etal. 1991; Allen & Cechetto, 1992; DiMicco et al. 1992; Gelsema et al. 1989; Spyer, 1999). As SPM corrects for the entire volume, and to avoid type 2 errors for these a priori regions of interest, we accepted uncorrected significance levels (i.e. voxel level of significance uncorrected for multiple analyses over the whole brain; Z-scores > 3.09, significant to P < 0.001). We report activations in other brain regions only when criteria for entire brain volume corrected significance were met. Significant activations are reported for cluster sizes of greater than 10 voxels.
RESULTS
Physiological arousal of the cardiovascular system and subjective experience during isometric exercise and mental stress
Compared to their respective control tasks (effortless isometric exercise and mental arithmetic), effortful isometric exercise and effortful mental arithmetic evoked significant (P < 0.001) increases in MAP and HR (see Fig. 2). Subjects reported subjective difficulty when performing the effortful exercise task (e.g. difficulty in maintaining squeeze pressure, transient aching of hand or arm) and effortful mental arithmetic (e.g. difficulty in keeping track of numbers, ‘feeling of stress’ in trying to perform the task quickly). The effortless exercise and arithmetic tasks were not associated with subjective tiredness or performance difficulty. Although we did not ask subjects to provide quantitative data on their subjective experiences, reports of performance difficulty broadly corresponded with the degree of physiological arousal observed.
Figure 2. Changes in physiological measures during control tasks and during effortful isometric exercise and effortful mental arithmetic (mental stress) tasks.
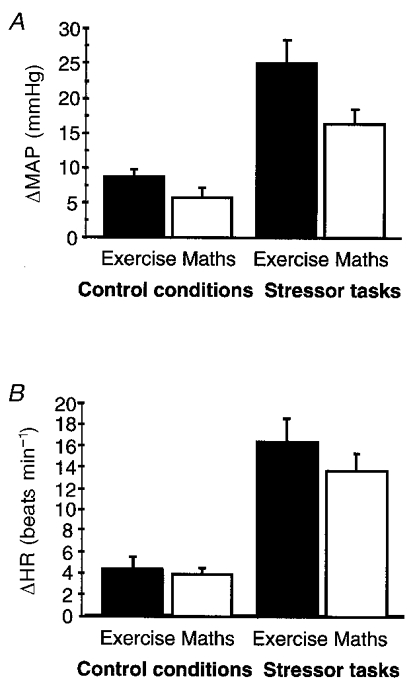
A, bar plot (means +s.d.) of changes in MAP (ΔMAP) for the 4 task conditions (increases in MAP from baseline during isometric exercise: effortful task, 24.9 ± 15.0 mmHg; effortless task, 8.5 ± 5.6 mmHg; P < 0.001, Student's t test; during mental arithmetic: effortful task, 16.2 ± 9.3 mmHg; effortless task, 5.6 ± 6.3 mmHg; P < 0.001). B, bar plot (means +s.d.) of changes in HR (ΔHR) for the 4 conditions (increases in HR from baseline during isometric exercise: effortful task, 16.2 ± 10.4 beats min−1; effortless task, 4.2 ± 5.6 beats min−1; P < 0.001; during mental arithmetic: effortful task, 13.6 ± 7.1 beats min−1; effortless task, 3.7 ± 2.9 beats min−1; P < 0.001).
rCBF activity related to task performance
Individual analyses of rCBF changes did not reveal marked individual variation in the location and lateralization of activity associated with the performance of stressor tasks. Variation in the spatial extent, but not lateralization, of rCBF responses covarying with HR and MAP was noted from individual analyses.
Significantly increased rCBF in the left somatic sensorimotor cortex, cerebellum and brainstem was evident during effortful isometric exercise relative to its control condition (P < 0.05, corrected). During effortful mental arithmetic vs. its control condition (counting), there was significantly increased rCBF in the right anterior cingulate and cerebellum (both P < 0.05, corrected). In the conjunction of these two contrasts, there were significant increases in rCBF in midline cerebellum (P < 0.05, corrected), brainstem (in the region of the pontine reticular nuclei) and right dorsal cingulate (at the junction of rostral and caudal regions of the anterior cingulate cortex; see Paus et al. 1998) (both P < 0.001, uncorrected). Activity in lateral regions of cerebellar cortex was also apparent at this lower level of significance (Table 1 and Fig. 3).
Table 1.
Areas activated by both isometric exercise and mental stress tasks compared to control tasks
| A. Increased rCBF during stressor tasks compared to control tasks | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Cerebellum (vermis) | L | −2, −52, −20 | 453 | 4.77 * |
| Brainstem (pons) | R | 16, −30, −42 | 78 | 3.44 |
| Anterior cingulate (32) | R | 14, 10, 42 | 32 | 3.32 |
| B. Increased rCBF during control (low stress) tasks compared to stressor tasks | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Amygdala | L | −30, 4, −18 | 242 | 4.94 * |
| Superior frontal gyrus (8) | R | 8, 38, 50 | 917 | 4.69 * |
| Middle frontal gyrus (8) | L | −32, 26, 52 | 409 | 4.65 * |
| Middle temporal gyrus (21/20) | L | −58, −16, −22 | 201 | 4.55 * |
| Brainstem (pons) | R | 14, −18, −22 | 157 | 4.25 |
| Brainstem (pons) | L | −16, −24, −14 | 129 | 3.75 |
| Brainstem (pons) | L | −6, −20, −34 | 10 | 3.28 |
| Hippocampus | R | 30, −30, −8 | 100 | 3.92 |
| Orbitofrontal cortex (11/47) | L | −38, 28, −12 | 48 | 3.73 |
| Insula | L | −58, −6, 14 | 53 | 3.67 |
| Subgenual cingulate (24/32) | L | −8, 24, −4 | 57 | 3.66 |
Significant regional blood flow changes during performance of stressor tasks. The brain regions listed showed significant increases in rCBF in the conjunction of exercise and mathematics conditions to P < 0.05 (corrected) or in regions of interest predicted a priori to P < 0.001 (uncorrected). L, left; R, right; Tal, Talairach co-ordinates (Talairach & Tournoux, 1988). The number of voxels per cluster is given (total search volume, 200 641 voxels).
Significance surviving correction for multiple comparisons.
Figure 3. Brain areas showing increased CBF during both stressor tasks compared to control conditions.
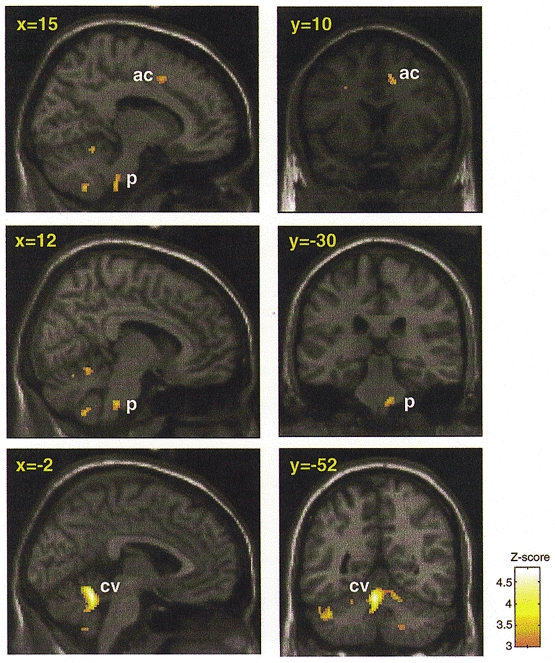
Localized increases in rCBF common to effortful isometric exercise and effortful mental arithmetic conditions (minus their respective control conditions). Conjunction analysis of effortful vs. effortless exercise with effortful vs. effortless mental arithmetic was used to determine common increases in rCBF associated with performing the effortful tasks. Areas of significant common activation above P < 0.001 are colour scaled according to the Z-score (scale given in figure). Group data are presented on parasaggital and coronal slices of a standard template T1-weighted structural image derived from one subject, normalized to standard space (Talairach & Tournoux, 1988). Talairach co-ordinates in the x-dimension and y-dimension are given for parasaggital and coronal slices, respectively. The following areas are labelled: ac, cingulate area 32; p, pons; and cv, cerebellum (vermis).
The left amygdala, right superior frontal gyrus, left middle frontal gyrus and left middle temporal gyrus showed significant (P < 0.05, corrected) increases in rCBF in the conjunction of control conditions relative to the effortful stress conditions (i.e. relatively reduced rCBF during both isometric exercise and mental stress). Significant (P < 0.001, uncorrected) increases in rCBF were also evident in pons, right hippocampus, left orbitofrontal cortex, left insula and left anterior cingulate in the same contrast.
rCBF activity related to measures of autonomic activity
MAP
Activity in the right anterior cingulate (area 32, extending to area 24), left postcentral gyrus (both P < 0.05, corrected), bilateral cerebellum and cerebellar vermis, right posterior insula and adjacent transverse temporal gyrus, and right orbitofrontal cortex showed significant positive covariation (P < 0.001, uncorrected) with MAP in both isometric exercise and mental arithmetic tasks (i.e. activity in these regions was greater when blood pressure was raised, independent of the nature of the task) (Table 2 and Fig. 4). Significant negative covariation between rCBF activity and MAP was evident in right middle temporal gyrus (P < 0.05, corrected), left uncus, left hippocampus, bilateral ventromedial prefrontal cortex, right cerebellum, right parahippocampal gyrus and left dorsal cingulate (P < 0.001, uncorrected) (i.e. activity in these regions decreased with increasing MAP).
Table 2.
Areas covarying with MAP during both isometric exercise and mental stress
| A. Greater rCBF at higher MAP | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Anterior cingulate (32) | R | 16, 20, 38 | 767 | 4.93* |
| Anterior cingulate (32) | R | 10, 4, 40 | — | 4.00 |
| Anterior cingulate (24) | R | 12, 26, 20 | — | 3.83 |
| Postcentral gyrus (2) | L | −30, −32, 48 | 1222 | 4.66* |
| Cerebellum (vermis) | — | 0, −52, −20 | 109 | 4.06 |
| Cerebellum | R | 16, −38, −26 | 40 | 3.72 |
| Cerebellum | L | −20, −68, −32 | 45 | 3.32 |
| Posterior insula and transverse temporal gyrus | R | 32, −32, 12 | 130 | 3.79 |
| Posterior insula and transverse temporal gyrus | R | 36, −34, 20 | — | 3.50 |
| Insula | R | 36, −10, −14 | 12 | 3.33 |
| Orbitofrontal cortex (10) | R | 44, 54, −4 | 10 | 3.23 |
| B. Greater rCBF at lower MAP | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Middle temporal gyrus (21) | R | 60, 0, −12 | 211 | 4.65* |
| Uncus | L | −28, 6, −20 | 57 | 4.03 |
| Hippocampus | L | −46, −24, −12 | 161 | 4.02 |
| Medial frontal gyrus (8) | L | −14, 36, 42 | 60 | 4.00 |
| Orbitofrontal cortex (11) | L | −6, 36, −18 | 57 | 3.38 |
| Orbitofrontal cortex (11) | R | 6, 46, −28 | 13 | 3.23 |
| Cerebellum | R | 40, −80, −36 | 41 | 3.31 |
| Parahippocampal gyrus | R | 22, −18, −14 | 43 | 3.29 |
| Parahippocampal gyrus | R | 14, −24, 18 | — | 3.07 |
| Cingulate (23) | L | −6, −54, 10 | 19 | 3.15 |
Significant regional blood flow changes covarying with MAP in the conjunction of exercise and mathematics conditions to P < 0.05 (corrected) and P < 0.001 (uncorrected). The number of voxels per cluster is given (total search volume, 200 641 voxels). Cluster size is not given for peaks of activation subsumed within a preceding larger cluster for the same region.
Significant to correction, P < 0.05.
Figure 4. Right anterior cingulate activity showing positive covariance with MAP in exercise and mental arithmetic tasks.
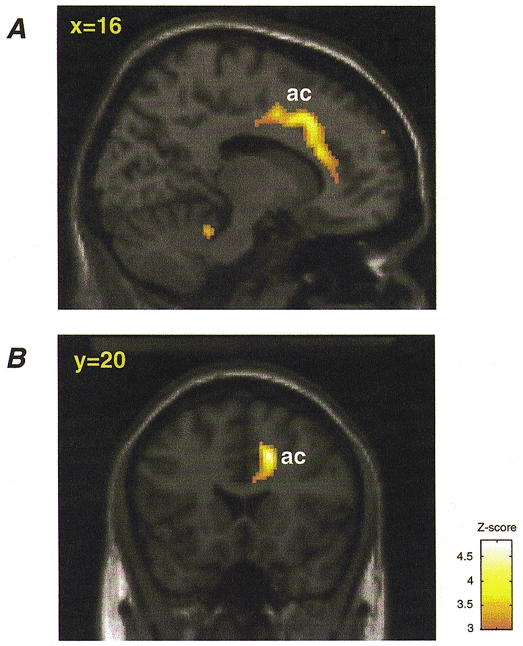
Activity in the right anterior cingulate (ac) covaried significantly with increasing blood pressure. For all subjects regional activity covarying with MAP was computed for isometric exercise and mental arithmetic tasks. A conjunction analysis was then performed to identify brain areas in which rCBF positively covaried with MAP in both exercise and mental stress tasks. Voxels showing significant (P < 0.001, uncorrected) activity are depicted on a template T1-weighted structural image of a single subject. A, parasaggital view. B, coronal view. Regional rCBF activity (above P < 0.001, uncorrected) is colour scaled according to the Z-score (scale depicted in figure).
HR
A significant positive correlation between rCBF and HR (P < 0.001, uncorrected) was evident in brainstem (pons), midline and left cerebellum, and right insula, independent of task. Significant negative covariation between rCBF and HR was evident in right middle frontal gyrus (P < 0.05, corrected), right anterior and posterior cingulate, bilateral insula, bilateral orbitofrontal cortex, left cerebellum and left amygdala (P < 0.001, uncorrected) (i.e. lower activity in these areas when HR was raised; Table 3).
Table 3.
Areas covarying with HR during both isometric exercise and mental stress
| A. Greater rCBF at higher HR | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Pons/inferior cerebellar peduncle | R | 16, −48, −50 | 91 | 4.22 |
| Pons/middle cerebellar peduncle | L | −14, −34, −42 | 18 | 3.17 |
| Pons | R | 2, −28, −30 | 10 | 3.13 |
| Cerebellum | L | −6, −78, −34 | 49 | 3.43 |
| Cerebellum | L | −28, −56, −48 | 73 | 3.29 |
| Cerebellum | L | −28, −52, −32 | — | 3.22 |
| Insula | R | 28, 14, 6 | 48 | 3.55 |
| Insula | R | 62, 6, −14 | 10 | 3.22 |
| B. Greater rCBF at lower HR | ||||
|---|---|---|---|---|
| Area (Brodmann area) | Side | Tal (x, y, z) | No. voxels | Z-score |
| Middle frontal gyrus (8) | R | 18, 22, 48 | 427 | 5.07* |
| Cingulate (23) | R | 6, −26, 32 | 104 | 3.65 |
| Cingulate (24) | R | 12, −14, 36 | — | 3.47 |
| Cingulate (31) | R | 20, −22, 46 | 40 | 3.47 |
| Posterior insula/transverse temporal gyrus | L | −38, −26, 14 | 1187 | 4.08 |
| Insula | R | 40, 2, −16 | 108 | 3.64 |
| Insula | R | 42, −14, 4 | 25 | 3.17 |
| Cerebellum | L | −30, −38, −20 | 42 | 3.48 |
| Orbitofrontal cortex (11) | L | −34, 28, −12 | 26 | 3.40 |
| Orbitofrontal cortex (11) | R | 30, 34, −8 | 10 | 3.26 |
| Amygdala | L | −22, −8, −12 | 70 | 3.19 |
Significant regional blood flow changes covarying with HR in the conjunction of exercise and mathematics tasks to P < 0.05 (corrected) and P < 0.001 (uncorrected). The number of voxels per cluster is given (total search volume, 200 641 voxels). Cluster size is not given for peaks of activation subsumed within a preceding larger cluster.
Significant to correction, P < 0.05.
DISCUSSION
We have demonstrated significant changes in rCBF in discrete cortical and subcortical brain regions associated with states of altered peripheral cardiovascular arousal. The findings provide functional evidence for the involvement of areas previously implicated in cognitive and emotional behaviours in the central generation or representation of peripheral cardiovascular arousal. The data are consistent with a functional organization of the central nervous system designed to produce integrated cardiovascular response patterns for the metabolic support of volitional and emotional behaviours.
Although we were able to define brain areas associated with peripheral cardiovascular arousal, the methodology we have used does not allow us to differentiate between the generation, maintenance or representation (through feedback) of different states of autonomic arousal. Additionally, due to the prolonged nature of the activation/scanning conditions, the study may fail to identify brain regions that are transiently activated during short-term changes and fluctuations in physiological responses. For example, in fear conditioning, transient activity of the amygdala is seen in early, but not late, phases of conditioning (Buchel et al. 1998). Nonetheless, there is independent evidence suggesting that both efferent and afferent autonomic responses are represented in areas such as the anterior cingulate and insula (Cechetto & Saper, 1990) – implying that it may be difficult to dissociate efferent activity from afferent representation. Despite limitations in temporal resolution of our PET technique the use of conjunction analyses, across two dissimilar tasks, provides a powerful means of identifying brain areas involved in autonomic responses. We identified commonalities in regional brain activation associated with cardiovascular states induced by exercise and mental stress, and our results consequently identify components of a central autonomic control system that are likely to be involved in both generating and representing peripheral cardiovascular arousal across a range of behavioural states.
An important finding of our study was that changes in systemic blood pressure are reflected in activity changes of right anterior cingulate. The anterior cingulate is a large cortical structure located around the rostral corpus callosum that is frequently activated during functional imaging studies involving difficult cognitive tasks (Paus et al. 1998). The human anterior cingulate is anatomically divisible into distinct sub-areas, and is implicated in both cognitive and affective processes: attentional control, motor and cognitive executive functions; willed action and response selection; declarative short-term memory; subjective emotional states, anxiety and painful experience; involuntary and autonomic changes during emotional states, and affective and social behaviour (reviewed in Devinsky et al. 1995). Our data suggest that peripheral changes in blood pressure are reflected in activity within a distinct region of right anterior cingulate, Brodmann area 32 extending caudally into area 24. This region may be important for integrating peripheral cardiovascular changes with cognitive effort, motor preparedness and emotional states. More posterior cingulate regions (Brodmann areas 23, 24 and 31) showed reduced activity with increasing heart rate and blood pressure, perhaps consistent with a proposed behavioural dissociation of anterior and posterior cingulate functions (Bussey et al. 1997).
The cerebellum is an important component in a central autonomic network (Spyer, 1999) but is underemphasized in many neurological models of autonomic control (e.g. Benarroch, 1997). A range of autonomic functions appear to involve pathways through the cerebellum, including representation of cardiovascular responses (Lisander & Martner, 1975; Bradley et al. 1987, 1991; Harper et al. 1998), postural control of blood pressure and heart rate (Nisimaru et al. 1998), conditioned cardiovascular responses (Gherlarducci et al. 1996), and modulation of autonomic components of emotional behaviour (Martner, 1975). Moreover, cerebellar pathology is a feature of multiple system atrophy in which there is central autonomic dysregulation (Smith & Mathias, 1996). In healthy individuals, the cerebellum, like the anterior cingulate, is frequently activated in functional imaging studies of sensorimotor, cognitive or emotional processes. Recently, the cerebellum has been implicated in mood and cognition – executive deficits and affective changes follow cerebellar damage – indicating the importance of pathways linking the cerebellum with prefrontal and anterior cingulate regions implicated in emotional and cognitive processes (Schmahmann & Sherman, 1998). We observed midline cerebellar activity during the performance of difficult, arousing exercise and mental stress, together with some lateral cerebellar cortical activity at a lower level of significance. Previous studies have directly implicated cerebellar vermis areas in cardiovascular control and cardiovascular responses during aversive conditioning (a model for emotional learning (Bradley et al. 1987, 1991; Ghelarducci et al. 1996). The activity we observed in lateral cerebellar cortex may indicate that changes in somatic physiology may also influence cerebellar regions subserving other functions, e.g. motor co-ordination. Together, our findings suggest peripheral states of cardiovascular arousal are represented in cerebellum, which may serve to integrate cardiovascular responses with on-going cognitive or motor behaviour. Thus, the cerebellum may act as a functional relay between cortex and brainstem through which brainstem autonomic nuclei are modulated by cortical activity related to cognitive, motor and emotional behaviours.
Our findings also confirmed the role of brainstem structures in the representation of autonomic responses. Increased activity in discrete areas within the pons was apparent in the analysis of effortful vs. effortless task performance, e.g. activity in the region of pontine reticular nuclei was associated with the performance of effortful (compared to effortless) tasks. Moreover, increases in heart rate covaried notably with midline and lateral pontine activity. However our analyses were not able to identify an association between changes in cardiovascular states and activity in the medulla, despite evidence for the important role played by structures within the medulla (e.g. nucleus of the solitary tract) in homeostatic mechanisms, such as autonomic control of the cardiovascular system (e.g. Benarroch, 1997; Spyer, 1999). Among the possible reasons that may contribute to our failure to find medulla activation in association with cardiovascular arousal is that increased cardiac- (and respiratory-) related, pulsatile motion of the brainstem leads to greater residual variance in measurable activity and consequent reduced sensitivity. Consistent with this, very few functional imaging studies have been able to detail activity within the medulla compared to activity within the pons, which is both larger and less affected by pulsatile motion. Techniques to overcome this problem are developing in some imaging modalities (e.g. cardiac gating in fMRI), but were not available for use in our study.
The role of the insula in the control and representation of autonomic states has been well established from stimulation and electrophysiological studies in animals (reviewed in Cechetto & Saper, 1990; Benarroch, 1997). The insula is anatomically and functionally connected with ‘autonomic’ centres such as the amygdala, orbital and ventromedial prefrontal cortex, and the hypothalamus. In humans, intra-operative electrical stimulation of the insula elicits changes in cardiovascular function which appear to be lateralized; tachycardia and hypertension result from stimulation of the right insula, and bradycardia and hypotension from stimulation of the left (Oppenheimer et al. 1992). However, despite the apparent association between the right insula and sympathetic arousal, functional imaging studies have not reported a consistent lateralization of insula activity elicited by emotive and aversive stimuli (e.g. Phillips et al. 1997; Buchel et al. 1998). In our study, increases in MAP and HR were associated with right insula activity, whereas low stress conditions and decreasing MAP and HR were associated with left insula activity. Thus, our findings are consistent with lateralization of cardiovascular control within the insula, as proposed by Oppenheimer et al. (1992). However, we note that there was also some right insula activation with decreasing heart rate.
One difficulty in interpreting our results is whether increases in activity associated with the performance of effortless vs. effortful tasks, or with relatively lower HR and MAP, reflect the representation of parasympathetic activity, or a deactivation of brain regions (involved in other representations) during sympathetic arousal. We take the view that areas predicted apriori to be involved in autonomic representations are likely to reflect autonomic activation. Thus, medial temporal lobe structures (amygdala, uncus, hippocampus and parahippocampal gyrus), orbitofrontal and ventromedial prefrontal cortices and some insula areas appear to preferentially represent states of low sympathetic arousal and high parasympathetic tone, manifest as decreased MAP and HR. This finding is of interest since these brain areas are reported to be activated during emotional stress, anxiety and the processing of emotive stimuli (e.g. McGuire et al. 1994; Morris et al. 1996; Buchel et al. 1998) which are typically associated with increased HR and blood pressure. However, activity within these brain areas is often context dependent, and it may be that an interaction between affective processing and systemic arousal potentiates the activity of these regions, perhaps to enable the interruption of on-going behaviours. Nevertheless, bradycardia accompanies anticipatory arousal to threatening stimuli (Roozendaal et al. 1990) and strongly emotive stimuli elicit patterns of parasympathetic activity, e.g. fear-induced bradycardia, vasovagal syncope and ‘freezing’. Our results suggest that these states of parasympathetic activity may be represented in medial temporal lobe regions.
The findings reported may have important clinical implications; central autonomic failure is a core feature of multiple-system atrophy (MSA, Shy-Drager syndrome), but may also occur in other degenerative disorders, e.g. cortical Lewy-body disease and Alzheimer-type dementia. Our study identifies a set of brain areas whose integrity is important for peripheral cardiovascular control, and which may be compromised by neurodegenerative conditions such as MSA (Mathias & Bannister, 1999). Moreover, many of these brain (e.g. limbic and paralimbic) areas are also involved in cognitive and behavioural functions that are dysfunctional in neurodegenerative conditions.
Our study also has relevance for the interpretation of brain-imaging findings across a range of experimental designs. Our data indicate that some brain areas are involved in representing states of cardiovascular arousal independently of how the arousal is engendered. Activity in regions such as right anterior cingulate (often attributed to cognitive, anticipatory or emotional processing) might occur whenever a difficult or arousing task – associated with increases in blood pressure – is contrasted with a low-level task that does not induce cardiovascular changes. Consistent with this, Paus et al. (1998) demonstrated a relationship between reported anterior cingulate PET (rCBF) activity and task difficulty. Other measures of arousal, e.g. skin conductance, have also been correlated with cingulate (and right insula) activity during the processing of emotive stimuli (Fredrikson et al. 1998), and increased anterior cingulate activity is associated with the induction of subjective mood states, which combines attentional effort with emotional processing (e.g. Lane et al. 1997). However, although right anterior cingulate activity did covary with MAP in both tasks, it is unlikely that this region acts simply as a generic cardiovascular monitor or response generator. The emphasis placed on attentional and emotional representations within the anterior cingulate (e.g. Devinsky et al. 1995), suggests that it is specialized for the integration of autonomic responses with cognitive and affective processes. However, in the anterior cingulate, and in regions such as the insula and amygdala, activity associated with processing emotive material remains difficult to disentangle from concurrent changes in peripheral cardiovascular status. Measurement of cardiovascular arousal during performance of cognitive and emotional tasks, or the use of peripherally acting drugs to diminish autonomic responses to test stimuli, may be a useful means of overcoming ambiguity in the interpretation of task-related activity in putative autonomic regions.
In summary, we have described activity, independently of whether arousal was induced by exercise or cognition, in right anterior cingulate, right insula, cerebellum and brainstem during peripheral cardiovascular arousal (and hence peripheral sympathetic autonomic activity). Activity in the amygdala, hippocampus, orbitofrontal/ventromedial prefrontal cortex, left insula and regions of cingulate, cerebellum and brainstem reflect decreased cardiovascular arousal, corresponding perhaps to parasympathetic autonomic activity. Thus, we describe a network of brain centres in which peripheral cardiovascular changes are generated and represented. It is also through these brain areas that cognitive, somatomotor and affective brain systems are integrated with the autonomic nervous system to provide the metabolic support for thought, action and emotion.
References
- Allen GV, Cechetto DF. Functional and neuroanatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. Journal of Comparative Neurology. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- Angyan L. Somatomotor and cardiorespiratory responses to basal ganglia stimulation in cats. Physiology and Behavior. 1994;56:167–173. doi: 10.1016/0031-9384(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;267:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bennarroch EE. Central Autonomic Network: Functional Organization and Clinical Correlations. Armonk, NY, USA: Futura Publishing Company Inc.; 1997. Functional anatomy of the central autonomic network; pp. 29–60. [Google Scholar]
- Bradley DJ, Ghelarducci B, Spyer KM. The role of the posterior cerebellar vermis in cardiovascular control. Neuroscience Research. 1991;12:45–56. doi: 10.1016/0168-0102(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Paton JF, Spyer KM. Cardiovascular responses evoked from the fastigial region of the cerebellum in anaesthetized and decerebrate rabbits. The Journal of Physiology. 1987;392:475–479. doi: 10.1113/jphysiol.1987.sp016792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan SL, Valentine J, Powell DA. Autonomic responses are elicited by electrical stimulation of the medial but not lateral frontal cortex in rabbits. Behavioral Brain Research. 1985;18:51–62. doi: 10.1016/0166-4328(85)90168-8. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan R, Friston K. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. Journal of Comparative Neurology. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Cechetto DR, Saper CB. Role of the cerebral cortex in autonomic function. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford, UK: Oxford University Press; 1990. pp. 208–223. [Google Scholar]
- Damasio AR, Tranel D, Damasio HC. Individuals with sociopathic behaviour caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: Theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton LB, editors. Frontal Lobe Function and Dysfunction. Oxford, UK: Oxford University Press; 1991. pp. 217–229. chap. 11. [Google Scholar]
- Delgado JM. Circulatory effects of cortical stimulation. Physiological Reviews. 1960;40(suppl. 4):146–178. [PubMed] [Google Scholar]
- Devinsky O, Morrel MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:276–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Soltis RP, Anderson JJ, Wible JH. Hypothalamic mechanisms and the cardiovascular response to stress. In: Kunos G, Ciriello J, editors. Central Neural Mechanisms in Cardiovascular Regulation. Vol. 2. Boston, MA, USA: Birkhauser; 1992. pp. 52–79. [Google Scholar]
- Fish DR, Gloor P, Quesney FL, Olivier A. Clinical responses to electrical brain stimulation of temporal and frontal lobes in patients with epilepsy. Pathophysiological implications. Brain. 1993;116:397–414. doi: 10.1093/brain/116.2.397. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T, Olsson MT, Fischer H, Andersson J, Langstrom B. Functional neuroanatomical correlates of electrodermal activity: a positron emission tomographic study. Psychophysiolgy. 1998;35:179–185. [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995a;2:165–189. [Google Scholar]
- Friston K, Holmes AP, Worsley K, Poline J-B, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995b;2:189–210. [Google Scholar]
- Gelsema AJ, Agarwal SK, Calaresu FR. Cardiovascular responses and changes in neural activity in the rostral ventrolateral medulla elicited by electrical stimulation of the amygdala of the rat. Journal of the Autonomic Nervous System. 1989;27:91–100. doi: 10.1016/0165-1838(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B, Salamone D, Simoni A, Sebastiani L. Effects of early cerebellar removal on the classically conditioned bradycardia of adult rabbits. Experimental Brain Research. 1996;111:417–423. doi: 10.1007/BF00228730. [DOI] [PubMed] [Google Scholar]
- Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, Alger J. Regional brain activation in humans during respiratory and blood pressure challenges. Clinical Experimental Pharmacology and Physiology. 1998;25:483–486. doi: 10.1111/j.1440-1681.1998.tb02240.x. [DOI] [PubMed] [Google Scholar]
- James W. Physical basis of emotion. Psychological Reviews. 1894;1:516–529. Reprinted (1994) in Psychological Reviews 101, 205–210. [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat and dog. Acta Physiologica Scandinavica. 1951;24(suppl. 83):1–285. [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lin MT, Yang JJ. Stimulation of the nigrostriatal dopamine system produces hypertension and tachycardia in rats. American Journal of Physiology. 1994;266:H2489–2496. doi: 10.1152/ajpheart.1994.266.6.H2489. [DOI] [PubMed] [Google Scholar]
- Lisander B, Martner J. Integrated somatomotor, cardiovascular and gastrointestinal adjustments induced from the cerebellar fastigial nucleus. Acta Physiologica Scandinavica. 1975;94:358–367. doi: 10.1111/j.1748-1716.1975.tb05895.x. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. British Journal of Psychiatry. 1994;164:459–468. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- Martner J. Cerebellar influences on autonomic mechanisms. Acta Physiologica Scandinavica. 1975;(suppl. 425):1–43. [PubMed] [Google Scholar]
- Mathias CJ. In: Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Bannister R, editor. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: Anatomical and physiological observations. Progress in Brain Research. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- Nisimaru N, Okahara K, Yanai S. Cerebellar control of cardiovascular responses during postural changes in conscious rabbits. Neuroscience Research. 1998;32:267–271. doi: 10.1016/s0168-0102(98)00094-7. [DOI] [PubMed] [Google Scholar]
- Nowak M, Olsen KS, Law I, Holm S, Paulson OB, Secher NH. Command-related distribution of regional cerebral blood flow during attempted handgrip. Journal of Applied Physiology. 1999;86:819–824. doi: 10.1152/jappl.1999.86.3.819. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Research. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. NeuroReport. 1998;9:R37–47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:496–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pool JL, Ransohoff J. Autonomic effects on stimulating the rostral portion of the cingulate gyri in man. Journal of Neurophysiology. 1949;12:385–392. doi: 10.1152/jn.1949.12.6.385. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Differential effect of lesioning of the central amygdala on the bradycardiac and behavioral response of the rat in relation to conditioned social and solitary stress. Behavioral Brain Research. 1990;41:39–48. doi: 10.1016/0166-4328(90)90052-g. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Smith GD, Mathias CJ. Differences in cardiovascular responses to supine exercise and to standing after exercise in two clinical subgroups of Shy-Drager syndrome (multiple system atrophy) Journal of Neurology, Neurosurgery and Psychiatry. 1996;61:297–303. doi: 10.1136/jnnp.61.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, Goldman-Rakic P. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proceedings of the National Academy of Sciences of the USA. 1998;95:6454–6459. doi: 10.1073/pnas.95.11.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM. Central nervous control of the cardiovascular system. In: Mathias CJ, Bannister R, editors. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford, UK: Oxford University Press; 1999. pp. 45–55. chap. 6. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Theime; 1988. [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:427–438. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Willette RN, Punnen S, Krieger AJ, Sapru HN. Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Research. 1984;321:169–174. doi: 10.1016/0006-8993(84)90696-6. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, McColl R, Mathews D, Winchester P, Friberg L, Mitchell JH. Activation of the insular cortex during dynamic exercise in humans. The Journal of Physiology. 1997;503:277–283. doi: 10.1111/j.1469-7793.1997.277bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


