Abstract
This study investigated the effects of fatigue, induced by production of maximal isometric force for 60 s with four fingers, upon indices of multifinger co-ordination.
Measurements of individual finger forces were performed during single- and multifinger maximal force production (maximal voluntary contraction, MVC) for two sites of force application, the middle of the distal or the middle of the proximal phalanxes. Two fatiguing exercises were used, involving force production at the distal phalanxes and at the proximal phalanxes. Fourteen subjects were tested.
The total force in four-finger tasks dropped by about 43 % when it was produced at the site involved in the fatiguing exercise. During force production at the other site, MVC dropped by 23 %. During single-finger MVC tests, force drop with fatigue was similar across all four fingers (about −25 % of their corresponding MVCs).
Force production by one finger was accompanied by involuntary force production by other fingers (enslaving). Enslaving remained unchanged by fatigue when measured during force generation at the site involved in the fatiguing exercise, but increased during force production at the other site.
The total MVC of four fingers acting in parallel was smaller than the sum of the MVCs of these fingers in single-finger tasks (force deficit). The force deficit increased with fatigue. Force-sharing patterns during four-finger tasks showed only minor changes under fatigue.
These results indicate that the effects of fatigue were not limited to changes in the force-generating capabilities of the muscles. In particular, fatigue could lead to a reorganisation at a neural level that defines commands to individual fingers.
Prolonged voluntary muscle contraction leads to an inability to maintain a required level of muscle force commonly termed ‘fatigue’. Fatigue has been shown to involve changes at different levels of the system for muscle force production (for a review see Enoka & Stuart, 1992). These include changes in the contractile muscle properties (Bigland-Ritchie & Woods, 1984) and segmental reflexes (Woods et al. 1987; Hagbarth et al. 1995), as well as at higher neurophysiological (Belhaj-Saif et al. 1996) and psychological (Belanger & McComas, 1981) levels of the generation of voluntary motor command. The results of most studies involving direct electrical stimulation of a muscle during its prolonged isometric contraction have suggested that the drop in muscle force is primarily due to changes within the muscle and/or neuromuscular synapses (McKenzie et al. 1992; Sheean et al. 1997; Allen et al. 1997; however, see Gandevia et al. 1996; Kent-Braun, 1999).
In most everyday movements, large groups of muscles are used in a co-ordinated manner. There have been reports suggesting that the central organisation of multimuscle systems may change under fatigue (Lucidi & Lehman, 1992; Bonnard et al. 1994; Forestier & Nougier, 1998). However, a detailed analysis of fatigue-associated changes in the co-ordination of multimuscle systems has been lacking.
In a recent series of studies (Latash et al. 1998a,b; Li et al. 1998a,b; Zatsiorsky et al. 1998), force production by a set of fingers acting in parallel has been investigated. An interdependence of forces produced by individual fingers has been described in such tasks. In particular, total force was shared among fingers relatively independently of the total force magnitude, possibly reflecting the principle of minimisation of rotational moments about the longitudinal functional axis of the hand (principle of minimisation of secondary moments; Li et al. 1998a,b). According to this principle, the central nervous system (CNS) tends to minimise the sum of all individual moments created by finger forces with respect to the longitudinal axis of the hand. Force production by one finger has been shown to be accompanied by involuntary force production by other fingers; this effect was termed enslaving (Li et al. 1998b; for similar findings see also Kilbreath & Gandevia, 1994). MVC by several fingers acting in parallel has been shown to be smaller than the sum of the MVCs of these fingers in single-finger tasks; this effect was called force deficit (Li et al. 1998b; for similar findings see also Ohtsuki, 1981; Kinoshita et al. 1996).
The main purpose of the present study was to investigate the possibility of changes in the central organisation involved in force production by a set of fingers under fatigue. This object of study is particularly attractive because of the relatively simple recording of forces produced by individual digits. Besides, the different anatomical points of attachment of extrinsic and intrinsic muscles (Kendall et al. 1971; Close & Ralston, 1973; Basmajian & De Luca, 1985) present an opportunity to vary the relative involvement of these muscle groups by changing the point of force application.
Effects of fatigue on multifinger force production have been reported extensively for handgrip strength (Loscher & Gallasch, 1993; Bembem et al. 1994; West et al. 1995). However, these studies focused on total force changes rather than on possible changes in the interaction of forces produced by individual fingers. Within this study, we asked questions about changes in force-sharing patterns, enslaving and force deficit under fatigue. In particular, we expected the basic rule of force sharing to be valid under fatigue so that the principle of minimisation of secondary moments holds. Prior to fatigue, force deficit during multidigit force production tasks may be expected to result from a lack of recruitment of the largest motor units (size principle; Henneman et al. 1965; Fig. 1). During a fatiguing exercise involving all four fingers, these large motor units (F3 in Fig. 1) will not work because of the force deficit phenomenon. So, the next largest group of motor units (F2 in Fig. 1) is expected to fatigue. As illustrated by Fig. 1, this may lead to a preserved force deficit in absolute units and an increase in the relative force deficit quantitatively related to the fatigue-induced drop in the total force.
Figure 1. An illustration of a possible mechanism leading to changes in force deficit under fatigue.
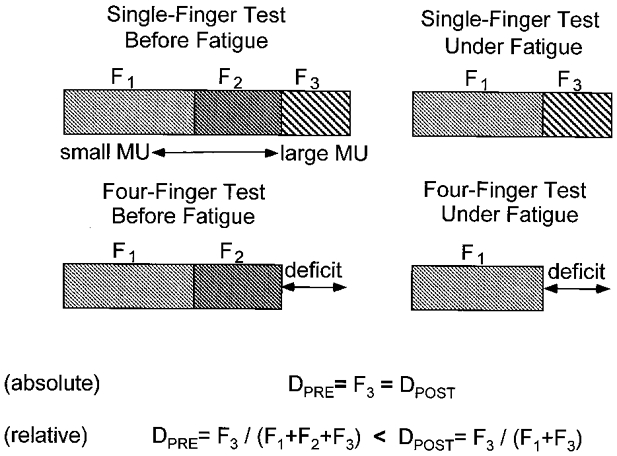
Total force (shown by the horizontal bars) is produced by small motor units (MUs; F1), medium-size MUs (F2) and large MUs (F3). Force deficit during four-finger tests prior to fatigue (Dpre) is due to the lack of recruitment of the largest MUs (cf. the upper and lower left drawings). Fatigue leads to derecruitment of medium-size MUs (upper right drawing). Absolute force deficit is expected to be constant, whereas relative force deficit is expected to increase under fatigue (Dpost; lower right drawing).
METHODS
Subjects
Fourteen right-handed subjects participated in this experiment (9 men and 5 women), aged 28.2 ± 8.7 years. The subjects had no previous history of neuropathies or trauma to the upper extremities. All subjects gave informed consent according to the procedures approved by the Compliance Office of the Pennsylvania State University. They were informed on the design of the study and the exact number of trials they were expected to perform.
Apparatus
Four unidirectional piezoelectric force sensors (208A03, PCB Piezotronics, Inc., Depew, NY, USA) were used for force measurement. The sensors were each connected in series with wire cables that were suspended by swivel attachments from slots in the top plate of the inverted U-shaped frame of the experimental device (see Fig. 2a for a schematic illustration of the experimental set-up). The slots were placed 30 mm apart from each other in the medio-lateral direction and allowed fore-aft adjustment of the wires to accommodate an individual subject's anatomical differences in finger length. The fingers applied force to rubber-coated loops located at the bottom of each wire. These loops could be placed either in the middle of the distal phalanxes or in the middle of the proximal phalanxes. A hand fixation device was located at the bottom of the frame and used to stabilise the palm of the hand and to ensure a constant hand configuration throughout the experiment. The subjects were instructed to keep their forearms flat on the supporting surface, which was at the same height as the support point of the hand fixation device. The subjects were seated in a chair facing the test apparatus with the upper arm at approximately 45 deg of abduction in the frontal plane and 45 deg of flexion in the sagittal plane, and the elbow at approximately 45 deg of flexion (full extension being 0 deg). The wrist was fixed at 20 deg of extension (hand and forearm aligned corresponding to 0 deg) and the fingers were positioned so that there was 20 deg of flexion at the metacarpophalangeal joints. In addition, to make certain that the subjects produced only vertical forces, the wires that were connected in series with the load cells had to maintain a vertical orientation (no major medio-lateral or fore-aft movement was allowed). Due to the employed experimental procedure all four finger forces were parallel to each other.
Figure 2. Method.
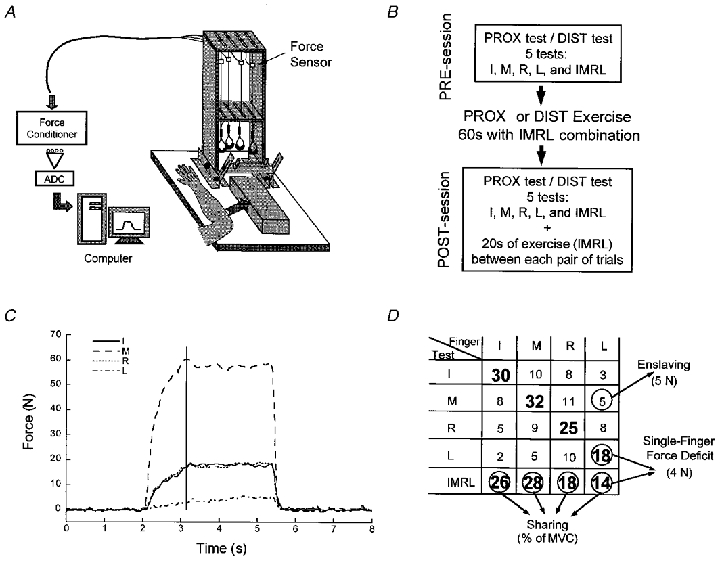
A, schematic drawing of the experimental set-up (adapted with permission from Li, 1998). ADC, analog-to-digital converter. B, schematic illustration of the protocol. Abbreviations: PROX, proximal; DIST, distal; I, index finger; M, middle finger; R, ring finger; L, little finger; IMRL, all four fingers acting together. C, a typical trial by a representative subject at a MVC test using the middle finger. The vertical line indicates the moment of maximal force production by the middle finger when all the measurements were taken. Note the simultaneous force production (enslaving) by the other fingers. D, a typical set of data recorded during a mini-session. For each test, the force of each finger is reported at the time of MVC. Numbers in bold represent the forces produced by the master fingers; other numbers are the forces produced by the slave fingers. The figure illustrates the definitions of enslaving, force deficit and sharing (see text for more details).
Procedure
In each trial, the subjects were asked to press as hard as possible (maximal voluntary contraction, MVC) with one particular finger or with all four fingers during a period of about 2 s. The rate of force production to reach the MVC was not explicitly prescribed, but the subjects had to reach it within 2 s. All the individual finger forces and their sum were displayed on-line on the screen in front of the subject. Two vertical lines were drawn on the screen to indicate to the subject when he or she had to generate a MVC. At the beginning of each trial, prior to force production, the software automatically adjusted the initial value of the sensor signals to zero, so that the weight of the hand and other passive forces were not part of the recorded forces. The total duration of each trial was approximately 5 s. During single-finger MVC tasks, the subject was asked to pay no attention to forces produced by the other fingers as long as the explicitly involved finger generated its maximal force. The explicitly involved fingers will be termed master fingers while other force-producing fingers will be termed slave fingers. The subjects were given several practice trials before testing began.
Three experimental factors were manipulated. (1) Measurements before and after fatiguing exercise were used to evaluate the effects of fatigue (fatigue factor). The fatiguing exercise consisted of 60 s at 100 % of MVC with all four fingers acting together. (2) The protocol was repeated on different days for two sites of force application during the fatiguing exercise, the middle of the proximal phalanxes and the middle of the distal phalanxes (exercise site factor). (3) During tests prior to and after the fatiguing exercise, MVCs were recorded during force application at the distal and at the proximal phalanxes (test site factor).
The organisation of the experimental sessions is illustrated in Fig. 2B. Each session (before and after exercise) consisted of 10 trials organised into two mini-sessions. Within a mini-session, MVCs were performed successively for single-finger tasks, index (I), middle (M), ring (R) and little (L), and in the four-finger task (IMRL). The order of the tests was randomised. Each session included one mini-session with the loops positioned at the proximal phalanxes and one with the loops at the distal phalanxes. To avoid fatigue in the pre-session (before fatiguing exercise), individual trials were separated by 20 s of rest (cf. Valero-Cuevas et al. 1998). In order to maintain the level of fatigue during the post-session (after the fatiguing exercise), at the end of each trial the subjects had to repeat the same fatiguing exercise for an additional period of 20 s. This kept force recovery during the post-session to under 10 %. The total duration of one test (pre-session, fatiguing exercise, and post-session) was about 30 min. There was at least 1 day of rest between two tests that involved fatiguing exercises at the proximal site and at the distal site of force application. For a given subject, and a given fatiguing exercise, the order of proximal and distal tests was the same in the pre- and post-session. However, this order was pseudorandomised (balanced) across subjects, and across the types of fatiguing exercise (distal and proximal). In particular, half of the subjects started the experiment with the fatiguing exercise at the proximal site of force production, and the other half started with the fatiguing exercise at the distal site of force production.
Data acquisition and processing
The output of the sensors was sent to four separate AC/DC signal conditioners (M482M66, PCB Piezotronics). A 12-bit analog-to-digital converter was used for digitising analog output. A Gateway (Pentium 133) microcomputer was used to control the experiment, acquire and process the data (see Fig. 2). A LabView program was used to collect the force signals from each force transducer for 5 s at the sampling frequency of 66 Hz. The digital signals were then converted into force values. The collected data were stored on the computer for off-line processing. The corresponding files were processed using a Matlab program. The force data were first digitally low-pass filtered with a second-order Butterworth filter at 5 Hz. Then, as shown in Fig. 2C, within each trial, the force value for each finger was extracted at the moment when the maximal force value was reached for the explicit task.
A typical set of data recorded during a mini-session is presented in Fig. 2D. Within each mini-session several dependent variables were calculated, as described below.
Force deficit
The maximal force produced by four fingers acting in parallel is smaller than the sum of the maximal forces of each finger during single-finger force production. Force deficit for four fingers was defined as the difference between the sum of MVCs during the single-finger tasks and the maximal total force in the four-finger task (absolute force deficit). Force deficit was further expressed as a percentage of the former value (relative force deficit); the contribution of one finger, L, to force deficit is indicated in Fig. 2D. Force deficit for a single finger was defined as the difference between that finger's MVC in a single-finger task and its maximal force in a four-finger task. Force deficit could be expressed in absolute units (N) and as a percentage of MVC of that particular finger in the single-finger task.
Force sharing
The force share of a finger in a four-finger task was defined as the percentage of force generated by this finger compared to the total force in the task (see Fig. 2D). Note that the sum of the four individual shares is equal to 100 %.
Enslaving
Twelve enslaving forces were produced within a mini-session (see the non-bold numbers in Fig. 2D). Enslaving was expressed as the mean value of all these forces, so it represents the mean amount of force produced by a slave finger (in N) during a single-finger task. It was further expressed as the mean percentage of MVC exhibited by each slave finger during a single-finger task.
The position of the neutral line
The neutral line is an imaginary line parallel to the longitudinal axis of the hand with respect to which the sum of the four moments generated by individual finger forces is zero. The position of the neutral line was expressed in centimetres from the M finger (positive values indicate that the neutral line is between M and L, whereas negative values indicate that it is between I and M). We assumed point force application for each individual finger, no horizontal forces and a constant interval of 3 cm between each pair of fingers. Therefore the mathematical expression of the neutral line position (N, expressed in cm) based on individual finger forces (Fi, where i = I, M, R, L) is as follows:
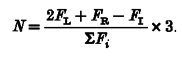 |
Given that each cable could move ±1 mm laterally, the maximal estimated error resulting from this computation of the neutral line position is 1 mm.
Statistical analysis
Repeated-measures three-factor ANOVAs were used; the factors (test site, fatigue and exercise site) are described earlier in the text.
Sharing patterns were compared using multivariate ANOVAs (MANOVAs) including the same three factors. Rao's R was used to assess the significance of these tests. Because the four individual shares did not constitute a set of independent variables (since their sum was always 100 %), sharing patterns were compared using three shares only (for M, R and L).
All statistical analyses were performed using Statistica software.
RESULTS
Changes in MVC
There were no significant differences in most of the studied indices between the male and female subgroups (with the obvious exception of higher MVCs demonstrated by males). Therefore, we present analyses of pooled data across all the subjects. After fatiguing exercise, MVC in four-finger tasks dropped in all subjects, the mean MVC across all conditions being 139.9 versus 89.7 N, respectively, for the pre- and post-session (see Table 1). Higher MVCs were produced during force generation at the proximal site than at the distal site (137.2 versus 92.4 N, respectively; a main effect of test site, F1,13 = 12.89, P < 0.05). The drop in MVC was always higher when measured at the site of the fatiguing exercise. For instance, as illustrated in Fig. 3, when the fatiguing exercise was performed at the proximal site, the mean force drop across individual subjects was 42.6 % during force production at the proximal site, and 20.3 % during force production at the distal site. When the fatiguing exercise was performed at the distal site, the effects were reversed, i.e. the mean force drop was 43.2 % versus 25.4 % at the distal and proximal site, respectively.
Table 1.
MVCs across all experimental conditions and tests
| Exercise | Prox | Dist | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Pre | Post | Pre | Post | ||||||
| Test | Prox | Dist | Prox | Dist | Prox | Dist | Prox | Dist | P < 0.05 | |
| IMRL | Mean | 172.4 | 105.2 | 92.9 | 83.4 | 164.4 | 117.5 | 119.1 | 63.5 | F, T, FT, EFT |
| s.e. | 24.2 | 9.6 | 10.0 | 7.8 | 22.6 | 11.1 | 14.2 | 3.8 | ||
| I | Mean | 68.1 | 41.4 | 50.0 | 36.4 | 57.9 | 43.6 | 46.1 | 33.2 | F, T, FT, EFT |
| s.e. | 9.7 | 3.4 | 7.3 | 2.8 | 6.4 | 3.1 | 5.1 | 2.1 | ||
| M | Mean | 57.9 | 33.6 | 37.5 | 30.9 | 52.1 | 36.1 | 40.3 | 27.1 | F, T, FT, EFT |
| s.e. | 7.9 | 2.7 | 3.8 | 2.8 | 6.5 | 2.7 | 5.0 | 2.2 | ||
| R | Mean | 38.4 | 21.6 | 27.9 | 18.6 | 36.7 | 23.6 | 28.9 | 16.9 | F, T |
| s.e. | 4.6 | 2.1 | 3.7 | 2.0 | 5.0 | 2.4 | 4.6 | 1.5 | ||
| L | Mean | 37.4 | 22.4 | 27.0 | 17.6 | 37.3 | 23.1 | 31.1 | 16.9 | F, T, EFT |
| s.e. | 4.4 | 2.2 | 3.7 | 1.7 | 5.1 | 2.8 | 4.8 | 1.9 | ||
| I+M+R+L | Mean | 201.6 | 119.0 | 142.4 | 103.5 | 184.0 | 126.4 | 146.4 | 94.1 | F, T, FT, EFT |
| s.e. | 26.5 | 10.5 | 18.5 | 9.4 | 22.9 | 11.0 | 19.6 | 7.8 | ||
Means and s.e. across subjects are presented (in N). Abbreviations: Prox, proximal; Dist, distal; I, index finger; M, middle finger; R, ring finger; L, little finger; IMRL, all four fingers acting together; I + M + R + L, sum of the forces generated by individual fingers in single-digit MVC tests. The last column shows statistically significant main effects and interactions (P < 0.05) in corresponding ANOVAs: E, exercise site factor; F, fatigue factor; T, test site factor.
Figure 3. Drop in MVC induced by the fatiguing exercise.
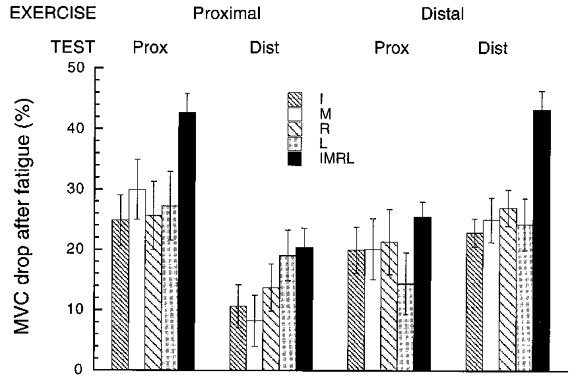
Averaged values across subjects are presented for each task, exercise site and test site of force production. Error bars show s.e. For abbreviations see Fig. 2B.
When individual finger MVCs were compared during the pre- and post-sessions, all MVCs dropped on average from 39.5 to 30.4 N. MANOVAs confirmed significant main effects of fatigue (R4,10 = 17.20, P < 0.05) and test site (R4,10 = 5.59, P < 0.05). ANOVAs of individual MVCs produced similar results. For each finger, we found significant main effects of fatigue (F1,13 > 19.5, P < 0.001) and test site (F1,13 > 9.33, P < 0.01).
An additional ANOVA (exercise site/test site/finger) showed that the magnitudes of the force drop induced by fatigue in single-finger tasks were comparable across fingers (see Fig. 3), i.e. all fingers were similarly affected by fatigue (F3,39 < 0.40, P > 0.05). We also found a significant interaction between test site and exercise site (F1,13 = 20.19, P < 0.05) demonstrating a higher drop in the MVC at the site of the fatiguing exercise. More specifically, we observed a drop in the MVC at the distal site after a prolonged exercise at the proximal site, and at the proximal site after a prolonged exercise at the distal site. The magnitude of these effects of the spread of fatigue was expressed as the ratio of the drop measured at the non-exercise site to the drop seen at the site of the exercise. The mean magnitude of this ratio was 0.48 ± 0.18 (s.d. is across the fingers) for the exercise at the proximal site, and 0.78 ± 0.12 for the exercise at the distal site.
Force deficit
As illustrated in Table 2 (see also Fig. 4), the force deficit in the four-finger tasks increased with fatigue. This increase was significant when the force deficit was expressed both as a percentage of the corresponding MVC and in absolute units (N) (F1,13 > 9.05, P < 0.05). Across all conditions, the values of the mean force deficit in the pre- and post-sessions were 12.7 and 25.4 %, respectively (or 19 and 32.3 N, respectively). The effect of fatigue was larger when measured at the site that was involved in the fatiguing exercise. For instance, after the exercise at the proximal site, the relative force deficit increased by 122 % when measured at the proximal site, while it only increased by 59 % when measured at the distal site.
Table 2.
Force deficit, enslaving and neutral line position values across all experimental conditions
| Exercise | Prox | Dist | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Pre | Post | Pre | Post | ||||||
| Test | Prox | Dist | Prox | Dist | Prox | Dist | Prox | Dist | P < 0.05 | |
| Force deficit (N) | Mean | 29.2 | 13.8 | 49.5 | 20.1 | 19.6 | 8.9 | 27.2 | 30.6 | F, T, ET, EFT |
| s.e. | 7.2 | 3.1 | 8.0 | 2.9 | 5.5 | 3.0 | 9.1 | 4.4 | ||
| Force deficit (%) | Mean | 15.0 | 12.4 | 33.3 | 19.7 | 13.1 | 10.3 | 17.0 | 31.5 | F, ET, EFT |
| s.e. | 3.2 | 2.1 | 2.6 | 2.5 | 3.0 | 2.8 | 3.9 | 2.2 | ||
| Enslaving (N) | Mean | 11.7 | 5.2 | 7.1 | 4.4 | 10.1 | 5.5 | 7.4 | 3.4 | F, T, FT, EFT |
| s.e. | 2.6 | 1.0 | 1.4 | 0.9 | 2.0 | 1.3 | 1.1 | 0.5 | ||
| Enslaving (%) | Mean | 22.9 | 18.3 | 20.2 | 25.1 | 21.4 | 16.8 | 28.0 | 15.1 | T, ET, EFT |
| s.e. | 2.5 | 2.6 | 2.1 | 3.3 | 2.2 | 2.2 | 3.4 | 1.4 | ||
| Neutral line (cm) | Mean | 0.89 | 0.75 | 0.96 | 0.55 | 0.81 | 0.80 | 0.91 | 0.61 | T |
| s.e. | 0.09 | 0.09 | 0.16 | 0.06 | 0.10 | 0.06 | 0.12 | 0.11 | ||
Means and s.e. across subjects are presented. Abbreviations as in Table 1.
Figure 4. Force deficit in the four-finger tests.
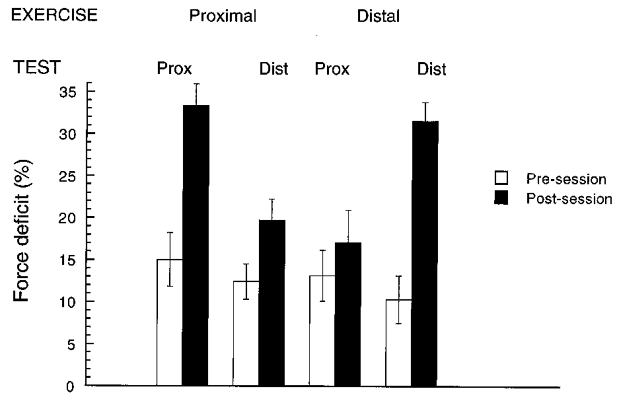
Averaged values across subjects are presented for each exercise site and test site of force production. Error bars show s.e. For abbreviations see Fig. 2B.
Enslaving
When enslaving was quantified as the mean force produced by slave fingers in single-finger tasks, there was a significant drop in enslaving after exercise (F1,13 = 10.82, P < 0.05; 8.1 versus 5.6 N). Larger enslaving effects were seen during force production at the proximal site than at the distal site (F1,13 = 23.76, P < 0.05; 9.1 versus 4.6 N). However, given that all MVCs dropped after fatigue, it appeared necessary to consider the enslaving effect after normalisation to the actual MVCs observed prior to and after the fatiguing exercise. After this procedure, the effect of fatigue almost reversed (F1,13 = 3.60, P = 0.08), indicating a tendency for higher enslaving under fatigue (19.8 versus 22.1 %). The enslaving effect remained unchanged when measured at the site involved in the fatiguing exercise (F1,13 = 2.17, P > 0.05), whereas it increased when measured at the other site (F1,13 = 14.44, P < 0.05; 19.9 versus 26.6 %). After normalisation, larger enslaving effects were seen during force production at the proximal site than at the distal site (F1,13 = 6.93, P < 0.05; 23.1 versus 18.8 %).
Sharing patterns
Sharing patterns are illustrated in Fig. 5. There were no effects of fatigue (R3,11 = 2.31, P > 0.05), whether the exercise was performed at the proximal or at the distal site (R3,11 = 0.93, P > 0.05). However, sharing patterns were significantly different when measured at the distal and at the proximal phalanxes (R3,11 = 5.35, P < 0.05). In fact, there was an effect of fatigue on the sharing patterns, but this effect depended on the site of force production during the pre- and post-sessions, as shown by a significant interaction between fatigue and test site (R3,11 = 6.44, P < 0.05). When data recorded at the proximal and distal sites were analysed separately, a significant effect of fatigue was found for both sites (R3,11 > 4.57, P < 0.05). However, as illustrated by Fig. 5, the magnitude of this effect was rather small: the mean change in a finger share induced by fatigue was 1.6 %.
Figure 5. Force-sharing patterns in the four-finger tests.
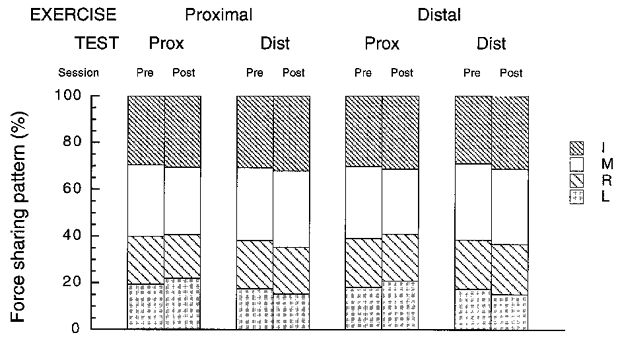
Averaged values across subjects are presented for each exercise site and test site of force production. Note the very small changes across all experimental conditions. For abbreviations see Fig. 2B.
Neutral line
There was no effect of fatigue on the position of the neutral line. One-way ANOVAs revealed no significant difference in the neutral line position between force production at the proximal and distal sites at the pre-session (F1,13 = 0.99, P > 0.05), whereas there was a difference during the post-session (F1,13 = 13.52, P < 0.05) (see Table 2). The magnitude of the observed effects was rather small: the largest change induced by fatigue was 0.2 cm.
DISCUSSION
After the fatiguing exercise, we observed an approximately 40 % drop in MVC when the subjects were asked to produce force with all four fingers by the same phalanxes (distal or proximal) that were used during the exercise. This observation is similar to the 40 % drop in grip strength described by Little & Johnson (1986) after a 90 s exercise at 80 % of the MVC. Effects of fatigue on the maximal force produced by all four fingers were also seen when the subjects were asked to produce MVC at the other site, i.e. at the distal phalanxes after a fatiguing exercise at the proximal phalanxes, or vice versa. These effects were smaller by about 50 % as compared to the effects seen at the site of the exercise.
A number of findings suggest that fatigue-induced effects were not limited to changes in the force-producing capabilities of individual muscles. Changes at a neural level of finger co-ordination may be inferred from a number of observations considered later in the Discussion.
Effects of fatigue on the sharing pattern
There were only minor changes in the sharing pattern after the fatiguing exercise; maximal changes in a finger's share were under 2.5 %. Hence, we feel it is safe to conclude that the solution used by the subjects' CNS to the ill-posed problem of force distribution among the fingers was basically unchanged under fatigue. With respect to the sharing pattern, fatigue may be viewed as a natural ‘perturbation’ changing the force-generating capabilities of the muscles. In our experiments, all the fingers showed similar drops in their MVCs with fatigue despite the fact that they produced different percentages of their individual MVCs (measured in single-digit tasks) during the fatiguing exercise. The preservation of the sharing pattern in such conditions is a non-trivial outcome particularly when compared to substantial changes in other indices of multifinger co-ordination such as force deficit.
In previous papers (Li et al. 1998a,b), the sharing pattern was viewed as partly defined by the principle of minimisation of secondary moments (see Introduction). Very small changes in the position of the neutral line under fatigue provide additional support for the principle of minimisation of secondary moments and demonstrate that the principle holds under fatigue. Although the level of fatigue was homogeneous across fingers (as revealed by similar relative drops in the MVCs measured in single-finger tasks), we found slight changes in the sharing pattern, so minor adjustments at the level of the synergy formation cannot be excluded.
To our knowledge, only one other study has addressed possible changes in the force-sharing pattern under fatigue (Sparto et al. 1997). In this study, the authors investigated a repetitive lifting task and found that, despite changes in the kinematics, the load sharing between the hip and the spine was relatively unchanged. These findings are compatible with an assumption that the same general rule of force sharing is used after a fatiguing exercise, i.e. that the synergy is stable against fatigue.
Effects of fatigue on force deficit
In previous studies (Li et al. 1998a,b; Zatsiorsky et al. 1998; Latash et al. 1998a) we suggested that effects of force deficit and enslaving could be attributed to either peripheral factors (such as the presence of multidigit muscles and tendinous connections) or a particular central organisation of descending commands, or both. In a later study, however, we described the effects of force deficit of comparable magnitudes during force application at the proximal phalanxes (Li et al. 2000). Note that these effects were also observed in the present study. The insertion points of intrinsic flexor muscles make them prime movers for force generation at the proximal phalanxes. These muscles, however, are digit specific, and the strength of their tendinous connections is relatively low (Schieber, 1991; Kilbreath & Gandevia, 1994). Therefore, one may assume that the mentioned peripheral factors are unlikely to play a major role in defining the observed interdependence of individual finger forces. In particular, force deficit is more likely to emerge at a central, neural level defining the distribution of commands to individual muscles (see Zatsiorsky et al. 1998).
In the Introduction, we suggested a simple scheme (Fig. 1) that predicted an increase in force deficit under fatigue based on the size principle of motor unit recruitment (Denny-Brown & Pennybacker, 1938; Henneman et al. 1965). Though rather simplified, this scheme has received support in a recent study (Fuglevand et al. 1999) conducted on hand muscles, which demonstrated that fatigue effects were larger in motor units initially exerting large forces. Note, however, that violations of the size principle during prolonged force production at submaximal levels have been reported (Nordstrom & Miles, 1991), as has the possibility of a turnover in motor units (Zijdewind et al. 1995). The scheme illustrated in Fig. 1 predicts a quantitative relationship between a drop in MVC and an increase in relative force deficit under fatigue. According to this scheme, a drop in single-finger MVC by 25 % (a typical result in our study) is expected to lead to an increase in relative force deficit by about 33 %. Actual changes in force deficit in the study ranged from 60 to 120 %, i.e. they were two- to fourfold higher than those predicted based solely on the size principle. The same scheme predicts no changes in the force deficit measured in absolute units. However, the force deficit increased significantly under fatigue (by approximately +60 %) when measured in absolute units (N).
This observation suggests that there must be other causes for the increase in force deficit under fatigue. Originally (Li et al. 1998b), the force deficit was viewed as resulting from a ceiling effect on the central neural drive to all the muscles involved in the task: the CNS was assumed to be unable to activate maximally all muscles or muscle compartments at the same time. In the present experiments, the ceiling effect remains the only candidate to support quantitative change in the force deficit (see also below), and we believe that this effect may be directly modified by the level of fatigue.
Effects of fatigue on enslaving
Enslaving may be viewed as an index of an inability to produce force by individual digits without involving other digits of the hand (see Methods). The changes in enslaving with fatigue were most unexpected. No significant changes were observed when the test was performed at the site that had produced force during the fatiguing exercise. However, when subjects tried to produce MVCs with individual fingers at the other site, the enslaving effects increased corresponding to less-selective control of individual digits. Note that other effects of fatigue, such as the drop in MVC and an increase in the force deficit, were larger when measured at the same site that had been involved in the fatiguing exercise than when measured at the other phalanxes. These observations suggest changes under fatigue in central neural mechanisms that define co-ordination of muscles and muscle compartments serving individual fingers.
Central versus peripheral changes with fatigue
Earlier studies reported that a drop in muscle force after a prolonged sustained contraction at a high force level was due to peripheral changes in the ability of the muscle to generate force (for references see Introduction). Note that virtually all these studies were performed using tasks that did not involve an explicit problem of multielement co-ordination. A number of findings of our study suggest that the effects of fatigue were not limited to changes in the force-generating capabilities of the muscles. These involve, in particular, (1) the large spread of fatigue across sites of force production (cf. Harding et al. 1993; Li et al. 2000), (2) the disproportionate increase in the force deficit, and (3) the counter-intuitive or even puzzling changes in the enslaving.
Recent studies (Sacco et al. 1997; Kent-Braun, 1999) point in the same direction: a loss in force is not necessarily related to force-generating muscle properties, while more complex neural mechanisms can be involved. In particular, Sacco et al. (1997) showed a spread of fatigue over synergistic muscles, e.g. the medial and lateral gastrocnemius. After a prolonged electrical stimulation of the lateral gastrocnemius only, these authors observed a drop of 52 % in the EMG for the lateral gastrocnemius, but also a 29 % drop in the EMG for the medial gastrocnemius, which was not subjected to the stimulation.
Interestingly, changes at the central neural levels of control were accompanied by relatively unchanged characteristics of force sharing among the fingers. Apparently, the redundancy of the system is large enough to allow for adjustments with fatigue that do not violate the basic organisation of the synergy, in particular the principle of minimisation of secondary moments.
Limitations of the study and concluding comments
Probably the most significant limitation of this study is the lack of control of muscle activation levels using EMG. In particular, this limits our ability to interpret the data in terms of the involvement of intrinsic and extrinsic muscles and in terms of the relative contribution of the active and passive forces during enslaving (Kilbreath & Gandevia, 1994; Leijnse, 1998). This limitation is not easy to overcome since intramuscular EMG is likely to make MVC trials uncomfortable and interfere with natural performance. Note, however, that Kilbreath & Gandevia (1994) have shown that, as soon as a finger produces a force greater than 20 % of its MVC, EMG activity can be recorded in muscles serving adjacent fingers. This supports an involvement of central neural mechanisms in the enslaving effects during our single-finger MVC trials.
Fatigue of all four fingers acting in parallel may be viewed as a relatively natural phenomenon associated with prolonged grasping tasks. It is not unexpected, therefore, that changes at a central neural level induced by fatigue are organised to preserve the basic synergy defining the pattern of force sharing among the fingers. We would predict, however, that fatigue in a less natural task could lead to a major modification of the synergy, reflected in grossly changed sharing patterns and violations of the principle of minimisation of secondary moments. Such a study, when only one of the fingers will participate in a fatiguing exercise, is included in our immediate plans.
Acknowledgments
Frederic Danion was supported by the Fyssen foundation. The study was in part supported by a NIH grant NS-35032. The comments by the reviewing editor and the two expert referees are greatly appreciated.
References
- Allen GA, Gandevia SC, Middleton J. Quantitative assessments of elbow flexor muscle performance using twitch interpolation in post-polio patients: no evidence for deterioration. Brain. 1997;120:663–672. doi: 10.1093/brain/120.4.663. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles Alive. 5. Baltimore, MD, USA: Williams & Wilkins; 1985. [Google Scholar]
- Belanger AY, Mccomas AJ. Extent of motor unit activation during effort. Journal of Applied Physiology. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Belhaj-Saif A, Fourment A, Maton B. Adaptation of the precentral cortical command to elbow muscle fatigue. Experimental Brain Research. 1996;111:405–416. doi: 10.1007/BF00228729. [DOI] [PubMed] [Google Scholar]
- Bembem MG, Massey BH, Bembem DA, Misner JE, Boileau RA. Isometric intermittent endurance of four muscle groups in men aged 20–74 yr. Medicine and Science in Sports and Exercise. 1994;28:145–154. doi: 10.1097/00005768-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscle fatigue. Muscle and Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Bonnard M, Sirin AV, Oddson L, Thorstensson A. Different strategies to compensate for the effects of fatigue revealed by neuromuscular adaptation processes in humans. Neuroscience Letters. 1994;166:101–105. doi: 10.1016/0304-3940(94)90850-8. [DOI] [PubMed] [Google Scholar]
- Close JR, Ralston HJ. Functional Anatomy of the Extremities: Some Electronic and Kinematic Methods of Study. Springfield, IL, USA: Charles Thomas; 1973. [Google Scholar]
- Denny-Brown D, Pennybacker JB. Fibrillation and fasciculation in voluntary muscle. Brain. 1938;61:311–334. [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. Journal of Applied Physiology. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Forestier N, Nougier V. The effects of muscular fatigue on the coordination of a multijoint movement in human. Neuroscience Letters. 1998;252:187–190. doi: 10.1016/s0304-3940(98)00584-9. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. Journal of Neurophysiology. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. The Journal of Physiology. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Bongiovanni LG, Nordin M. Reduced servo-control of fatigued human finger extensor and flexor muscles. The Journal of Physiology. 1995;485:865–872. doi: 10.1113/jphysiol.1995.sp020776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding DC, Brandt KD, Hillberry BM. Finger joint force minimisation in pianists using optimization techniques. Journal of Biomechanics. 1993;26:1403–1412. doi: 10.1016/0021-9290(93)90091-r. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibility of motoneurones of different sizes. Journal of Neurophysiology. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Kendall HO, Kendall FP, Wadsworth GE. Muscles: Testing and Function. Baltimore, MD, USA: Williams & Wilkins; 1971. [Google Scholar]
- Kent-Braun JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. European Journal of Applied Physiology. 1999;80:57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. The Journal of Physiology. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Murase T, Bandou T. Grip posture and forces during holding cylindrical objects with circular grips. Ergonomics. 1996;39:1163–1176. doi: 10.1080/00140139608964536. [DOI] [PubMed] [Google Scholar]
- Latash ML, Gelfand IM, Li ZM, Zatsiorsky VM. Changes in the force-sharing pattern induced by modifications of visual feedback during force production by a set of fingers. Experimental Brain Research. 1998a;123:255–262. doi: 10.1007/s002210050567. [DOI] [PubMed] [Google Scholar]
- Latash ML, Li ZM, Zatsiorsky VM. A principle of error compensation studied within a task of force production by a redundant set of fingers. Experimental Brain Research. 1998b;122:131–138. doi: 10.1007/s002210050500. [DOI] [PubMed] [Google Scholar]
- Leijnse JN. A method and device for measuring force transfers between the deep flexors in the musician's hand. Journal of Biomechanics. 1998;31:773–779. doi: 10.1016/s0021-9290(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Li ZM. PhD thesis. Penn State University, PA, USA: 1998. Control of the finger flexor muscles in static tasks. [Google Scholar]
- Li ZM, Latash ML, Newell KM, Zatsiorsky VM. Motor redundancy during maximal voluntary contraction in four-finger tasks. Experimental Brain Research. 1998a;122:71–78. doi: 10.1007/s002210050492. [DOI] [PubMed] [Google Scholar]
- Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Experimental Brain Research. 1998b;119:276–286. doi: 10.1007/s002210050343. [DOI] [PubMed] [Google Scholar]
- Li ZM, Zatsiorsky VM, Latash ML. The effect of finger extensor mechanism on the flexor force during isometric tasks. Journal of Clinical Biomechanics. 2000. in the Press. [DOI] [PubMed]
- Little MA, Johnson BR., Jr Grip strength, muscle fatigue, and body composition in nomadic Turkana pastoralists. American Journal of Physiological Anthropology. 1986;69:335–344. doi: 10.1002/ajpa.1330690306. [DOI] [PubMed] [Google Scholar]
- Loscher NW, Gallasch E. Myo-electric signals from two extrinsic hand muscles and force tremor during isometric handgrip. European Journal of Applied Physiology. 1993;67:99–105. doi: 10.1007/BF00376651. [DOI] [PubMed] [Google Scholar]
- Lucidi CA, Lehman SL. Adaptation to fatigue of long duration in human wrist movements. Journal of Applied Physiology. 1992;73:2596–2603. doi: 10.1152/jappl.1992.73.6.2596. [DOI] [PubMed] [Google Scholar]
- Mckenzie DK, Bigland-Ritchie B, Gorman RB, Gandevia SC. Central and peripheral fatigue of human diaphragm and limb muscles assessed by twitch interpolation. The Journal of Physiology. 1992;454:643–656. doi: 10.1113/jphysiol.1992.sp019284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Miles TS. Instability of motor units firing rates during prolonged isometric contractions in human masseter. Brain Research. 1991;549:268–274. doi: 10.1016/0006-8993(91)90467-a. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T. Inhibition of individual fingers during grip strength exertion. Ergonomics. 1981;24:21–36. doi: 10.1080/00140138108924827. [DOI] [PubMed] [Google Scholar]
- Sacco P, Newberry R, Mcfadden L, Brown T, Mccomas AJ. Depression of human electromyographic activity by fatigue of a synergistic muscle. Muscle and Nerve. 1997;20:710–717. doi: 10.1002/(sici)1097-4598(199706)20:6<710::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated movements of rhesus monkey: means of quantifying the independence of digits. Journal of Neurophysiology. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- Sheean GL, Murray NMF, Rothwell JC, Miller DH, Thompson AJ. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain. 1997;120:299–315. doi: 10.1093/brain/120.2.299. [DOI] [PubMed] [Google Scholar]
- Sparto PJ, Parnianpour M, Reinsel TE, Simon S. The effect of fatigue on multijoint kinematics and load sharing during a repetitive lifting test. Spine. 1997;22:2647–2654. doi: 10.1097/00007632-199711150-00013. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation. Journal of Biomechanics. 1998;31:693–703. doi: 10.1016/s0021-9290(98)00082-7. [DOI] [PubMed] [Google Scholar]
- West W, Hicks A, Clement L, Dowling J. The relationship between voluntary electromyogram, endurance time, and intensity of effort in isometric handgrip exercise. European Journal of Applied Physiology. 1995;71:301–305. doi: 10.1007/BF00240408. [DOI] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. Journal of Neurophysiology. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biological Cybernetics. 1998;79:139–150. doi: 10.1007/s004220050466. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D, Kukulka CG. Spatial differences in fatigue-associated electromyographic behaviour of the human first dorsal interosseus muscle. The Journal of Physiology. 1995;483:499–509. doi: 10.1113/jphysiol.1995.sp020601. [DOI] [PMC free article] [PubMed] [Google Scholar]


